Abstract
Multiple endocrine neoplasia type 1 (MEN1) is an autosomal dominant disorder associated mainly with tumors of multiple endocrine organs. Mutations in the MEN1 gene that encodes for the menin protein are the predominant cause for hereditary MEN1 syndrome. Though menin is a tumor suppressor, its molecular mechanism of action has not been defined. Here we report that menin interacts with AKT1 in vitro and in vivo. Menin downregulates the level of active AKT and its kinase activity. Through interaction with AKT1, menin suppresses both AKT1 induced proliferation and anti-apoptosis in non-endocrine and endocrine cells. Confocal microscopy analysis revealed that menin regulates AKT1 in part by reducing the translocation of AKT1 from the cytoplasm to the plasma membrane during growth factor stimulation. Our findings may be generalizable to other cancers, insofar as we found that loss of menin expression was also associated with AKT activation in a mouse model of pancreatic islet adenoma. Together, our results suggest menin as an important novel negative regulator of AKT kinase activity.
Keywords: Multiple endocrine neoplasia I (MEN1), Menin, Tumor suppressor, AKT, cellular localization
Introduction
Multiple endocrine neoplasia type 1 (MEN1) is an autosomal dominant disorder characterized by tumors predominantly in endocrine organs including the parathyroid glands, the pancreatic islets and the pituitary gland (1). In 1997, the MEN1 gene was identified and germline mutations in the gene were found in over 70% of MEN1 patients (2). Similar mutations were also found in many sporadic tumors. To date, over 1000 germline and somatic MEN1 mutations have been identified (3). Therefore, MEN1 mutations are the main cause for the MEN1 syndrome.
Menin is the protein encoded by the MEN1 gene. Even though neoplasia of MEN1 is tissue selective, menin is ubiquitously expressed. It contains three nuclear localization signals (NLS) in its carboxyl-terminus. The functions of these NLS are not only to drive the nuclear localization of menin but also to coordinate regulation of its target gene transcription (4). More recently, two functional nuclear export signals (NES) have been identified in menin that have been shown to direct β-catenin out of the nucleus and thus reduce its transcriptional activity (5).
It is difficult to study the function of menin because it has no homology with any known protein. Identification of proteins that interact with menin may help to decipher its potential biological role. Until now, more than 20 proteins have been documented to interact with menin; these include transcription factors: JunD , NF-κB, smad3, Pem, ERα, β-catenin and MLL complex; proteins involved in regulation of DNA repair: RPA2, FANCD2; kinases: ASK, nm23H1; and cytoskeletal proteins: non-muscle myosin heavy chain IIA, GFAP, and vimentin (6). It has been reported that menin, through association with some of its partners, may regulate gene transcription, cell proliferation, apoptosis, and genome stability. However, the precise molecular mechanism of menin as a tumor suppressor needs to be further investigated
The phosphoinositide 3-kinase (PI3K) signaling pathway plays a central role in regulating cell proliferation, cell growth, apoptosis, cell migration and metabolism (7). Upon growth factor stimulation, PI3K becomes active and converts the plasma membrane lipid phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2] (PIP2) to phosphatidylinositol-3,4,5-trisphosphate [PI(3,4,5)P3] (PIP3) which can recruit signaling proteins with pleckstrin-homology (PH) domains to the inner face of the plasma membrane. Among these PH domain containing proteins, the most important protein is the serine-threonine kinases AKT (protein kinase B - PKB). The AKT family contains three highly conserved members: AKT1, AKT2 and AKT3. When PI3K is activated, all three isoforms of AKT are translocated from the cytoplasm to the plasma membrane and are phosphorylated by phosphoinositide-dependent kinase 1 (PDK1) and potential PDK2, respectively at two conserved residues, corresponding to Thr308 (T308) within the active loop and Ser473 (S473) within the hydrophobic motif of AKT1, thereby transforming all AKT isoforms to their active form (7, 8). Active AKT further phosphorylates and activates several downstream effectors.
The deregulation of AKT signaling has been implicated in many human cancers, including breast cancer, pancreatic cancer, thyroid cancer, and gastric carcinoma (7). Interestingly, AKT activity has been found in human pituitary tumors (9); overexpression of constitutively active AKT1 in β-cells of transgenic mice induces cell proliferation, growth and survival (10, 11); RET-mediated cell transformation in multiple endocrine neoplasia type 2 (MEN2) is critically dependent on the activation of the PI3K/AKT pathway (12). However, many aspects of the molecular mechanisms of PI3K/AKT pathway involved in the regulation of endocrine cell proliferation, apoptosis and growth still remain a mystery.
Here, we demonstrate that menin suppresses AKT signaling in non-endocrine and endocrine cells. Our studies are consistent with menin terminating AKT activity partially through blocking its translocation from the cytoplasm to the plasma membrane. These studies support a unique and novel function of menin as a negative regulator of AKT.
Materials and Methods
Antibodies
The menin antibody SQV has been described previously (13). Other anti-menin antibodies were from Bethyl Laboratories. Antibodies against α-tubulin and p84 were from GeneTex. Anti-FLAG antibody was from Sigma. Fluorescent secondary antibodies FITC and Texas red-conjugated anti-rabbit or anti-mouse IgG were from Invitrogen. All other antibodies were from Cell Signaling.
Cell culture, cell transfection and animal use
HEK-293 (ATCC), MIN6 and mouse embryonic fibroblasts cells (MEFs) were cultured in DMEM supplemented with 10% FBS. Menin-null MEFs were as previously described (14). Lipofectamine 2000 (Invitrogen) was used for transient transfections.
Men1+/− mice were generated and genotyped as previously described (15). The animals were handled according to National Institutes of Health and American Association of Laboratory Animal Care guidelines.
Production of recombinant adenoviruses
Menin expressing (Ad-Men1) and green fluorescent protein (GFP) expressing (Ad-GFP) (control) recombinant adenovirus were produced based on the pAdEasy system (16). WT HA-tagged AKT1 (Ad-HA-AKT1), HA-tagged constitutively active AKT1 (Myr-AKT1), HA-tagged dominant-negative AKT1 (Dn-AKT1) (both T308 and S473 were mutated to alanine) and β-gal (negative control) recombinant adenoviruses were from Vector BioLabs.
Plasmid construction
The plasmids of FLAG-tagged wild type (WT) menin (FM) and FLAG-tagged menin mutations derived from MEN1 patients were generated as previously described (17). The menin deletion mutants were generated by PCR using human menin cDNA as a template and cloned into the HindIII/EcoRI site of pFLAG-CMV2 (Sigma). WT HA-tagged AKT1 (HA-AKT1) and AKT1 deletions were generated by PCR using human AKT1 cDNA as a template and cloned into the EcoRI/XhoI site of pCMV-HA (Clontech).
Immunoprecipitation and pull down assay
Immunoprecipitation (IP) experiments were performed using Protein G Agarose Immunoprecipitation Kit (KPL).
In the pull down assay, FLAG-menin and FLAG-BAP (Bacterial Alkaline phosphatase, as control) plasmids were generated, expressed, purified and verified as previously reported (18). GST and GST-AKT1 fusion proteins were from NOVUS Biologicals. GST-AKT1 kinase was from Cell Signaling.
Immunoblot analysis
Whole cells were solubilized in protein lysis buffer (Cell Signaling). Cytoplasmic and nuclear extracts were prepared with the NE-PER kit (Pierce). Antibodies against α-tubulin or p84 were used as loading control for whole cell/cytoplasmic extracts and nuclear extracts, respectively.
Quantitative RT- PCR
RNA extraction, reverse transcription and quantitative PCR were performed as previously described (19) using a SYBR Green PCR Master Mix kit (Applied Biosystems). The primers specific for mouse AKT1 were: forward, 5 ’-TCCCAGTAAGTGCGGGTCAT-3’ and reverse, 5’-CCAATCGGTAGTAGCGACGG-3’.
Confocal microscopy
After transfection and IGF-I treatment, HEK-293 cells were cultured on coverslides. Immunofluorescence staining was performed as previously described (19). Images were acquired at room temperature using a confocal microscope (LSM 510 META; Carl Zeiss). Images were acquired in different channels (405, 488, and 594 nm) and processed using Photoshop 8.0. Line scans of fluorescence intensity were performed using the intensity profile functions of the software (Carl Zeiss).
In vitro kinase assay
After treatment, the proteins from KO and KOM cells were extracted and active AKT was immunoprecipitated by p-AKT (S473) antibody following incubation with purified GST-GSK-3 fusion protein for in vitro AKT kinase assay (Cell Signaling).
Apoptosis/necrosis detection
The levels of apoptosis/necrosis were determined using the Cell Death Detection ELISAplus kit (Roche). Caspase3 colorimetric assay was also performed (Cell Signaling).
Cell proliferation assay
1000 menin-null cells were seeded in a 96-well plate and infected with different combination of adenoviruses for 24 hours. BrdU was then added to the culture medium for 24 hours before the cells were harvested. Incorporation of BrdU was determined by ELISA assay (Roche).
Immunohistochemistry
Immunohistological analysis of mouse pancreas was performed as previously described on 5µm sections (20). The sections were examined under bright-field microscopy with an Axioskop microscope coupled to an AxioCam MRc5 camera with Axiovision AC software (Carl Zeiss). Images were captured at room temperature and processed using Photoshop 8.0.
Statistical analysis
Unpaired two-tail t test and correlation analysis were performed using the statistical analysis functions in GraphPad Prism 4.0 program (GraphPad). All results in histograms showed the mean ± SEM of at least 3 independent experiments for each condition (* p<0.05; ** p<0.01; *** p<0.001).
Results
Menin associates with AKT1 in vitro and in vivo
To determine if menin and AKT1 interact with each other, we performed IP of FM or HA-AKT1 in HEK-293 cells, which resulted in the co-IP of AKT1 or menin, respectively, suggesting that the two proteins associate with each other (Fig. 1A). We also confirmed that overexpressed HA-AKT1 in HEK-293 cells bound to purified FLAG-menin (Fig. 1A) by a pull down assay.
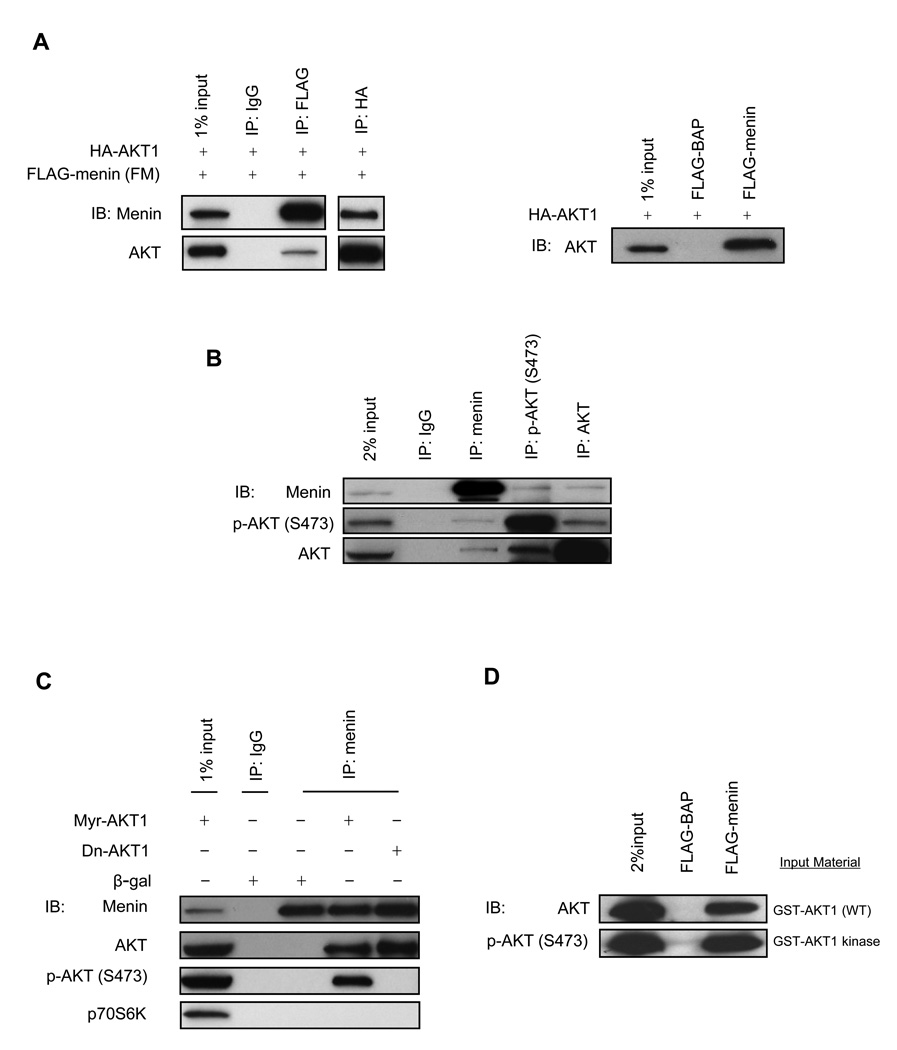
Figure 1. AKT1 interacts with menin.
(A) HEK-293 cells were cotransfected with FM and HA-AKT1 followed by co-IP (left panel). Whole cell lysates from HEK-293 cells transfected with HA-AKT1 were used for FLAG pull down assay (right panel). (B) The pancreas of three-month-old mice was used for co-IP. (C) Whole cell lysates from HEK-293 cells infected with various adenovirus (10 MOI) (multiplicity of infection) were used for co-IP. (D) Purified GST-AKT1 or GST-AKT1 kinase was used for FLAG pull down assay.
To determine whether the interaction between endogenous menin and AKT can occur in normal tissues under physiological conditions, the whole pancreata of three-month-old mice were used in co-IP studies. Endogenous phospho-AKT (S473) (p-AKT (S473)) and total AKT co-immunoprecipitated with endogenous menin in mouse pancreas. This result was further confirmed by a reciprocal IP experiment (Fig. 1B).
AKT1 exists as inactive and active forms in cells. We wondered whether menin could interact with only o n e f orm of AKT1 or both forms. Menin was immunoprecipitated with the menin SQV antibody from HEK-293 cells infected with adenovirus containing β-gal, Myr-AKT1 or Dn-AKT1. Both overexpressed active AKT1 and overexpressed inactive AKT1 can coprecipitate with endogenous menin. There were undetectable interactions between endogenous menin and p70S6K (a mitogen activated Ser/Thr protein kinase) in HEK-293 cells (Fig. 1C).
To test whether the protein interaction between menin and AKT1 is direct or indirect, in vitro expressed and purified FLAG-menin was used to pull down in vitro expressed and purified GST-AKT1 (WT) or GST-AKT1 kinase (phosphorylated at both T308 and S473). The pull down assay showed that both GST-AKT1 and GST-AKT1 kinase can interact with FLAG-menin but not with FLAG-BAP (Fig. 1D).
Menin suppresses AKT kinase activity
To investigate if menin can regulate AKT signaling by inhibiting the kinase activity of AKT, immortalized menin-null MEFs, which were stably infected with either empty vectors (KO cells) or menin-expressing retroviruses (KOM cells), were used (21). The cells were serum starved overnight and then incubated with or without 20% fetal bovine serum (FBS) for 30 minutes and the cytoplasmic proteins were analyzed by immunoblot (IB). The p-AKT (S473) level in the cytoplasm increased about 3.6 fold in KO cells after FBS treatment. However, in KOM cells, the cytoplasmic p-AKT (S473) level increased to only two fold after serum stimulation (Fig. 2A). In contrast, neither the mRNA level of AKT1 (Supplementary Fig. S1A) nor the total protein level of AKT (Fig. 2A) showed any significant difference in KO or KOM cells.
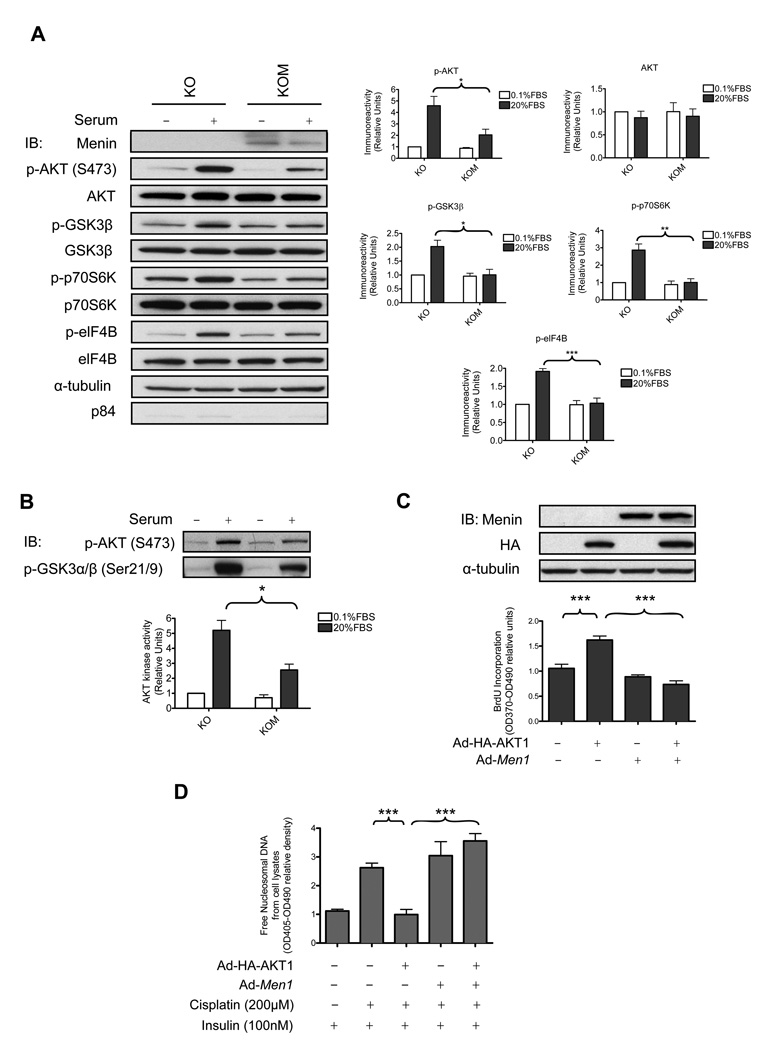
Figure 2. Menin suppresses AKT kinase activity and AKT1 mediated effects.
(A) The cytoplasmic extracts from 3×105 KO and KOM cells were subjected to IB. The quantified results from four independent experiments are shown in the graphs. The protein levels were normalized against the respective total protein and α-tubulin levels. (B) An in vitro kinase assay was performed with endogenous active AKT (S473) immunoprecipitated from the cytoplasmic extracts of KO or KOM cells. The quantified results from three independent experiments are shown on the bottom. (C) Menin-null MEFs were infected with various adenovirus (100 MOI) followed by cell proliferation assay. Cell lysates from the cells were subjected to IB. (D) 1×104 menin-null MEFs were seeded in 6-well plates and infected with various adenovirus (100 MOI) followed with cisplatin and insulin treatment and processed to detect release of free nucleosomal DNA.
We next examined some downstream effectors of AKT. IB (Fig. 2A) showed that the ability of AKT to phosphorylate its direct or indirect substrates GSK3β, p70S6K and elF4B decreased around 50%, 65% and 44%, respectively in KOM cells compared with KO cells under serum stimulation.
We examined whether menin might inhibit the kinase activity of AKT in vitro using purified GST-GSK3 fusion protein as the substrate. The amount of p-AKT (S473) immunoprecipitated from serum-stimulated KO cells was much higher than that immunoprecipitated from serum-stimulated KOM cells. p-AKT (S473) immunoprecipitated from serum-stimulated KO cells can induce phosphorylation of the GST-GSK3 fusion protein by 5.2 fold, but p-AKT (S473) immunoprecipitated from KOM cells under serum stimulation can induce phosphorylation of GST-GSK3 fusion protein by only 2.6 fold (Fig. 2B). These results indicate that menin downregulates the active form of AKT and thus suppresses AKT kinase activity. We further confirmed that menin inhibits AKT kinase activity in an overexpression system (Supplementary Fig. S1B).
Menin inhibits AKT1 mediated cell proliferation and anti-apoptosis effects
We evaluated whether menin interferes with the downstream functions of AKT in cell proliferation and anti-apoptosis. We infected menin-null MEFs with control adenoviruses or adenoviruses expressing HA-AKT1 and/or menin. Expression of HAAKT1 led to a 60% increase in cell proliferation (Fig. 2C). Coexpression of menin with HA-AKT1 suppressed AKT1 mediated cell proliferation about 55%. It has been reported that cisplatin, an antitumor agent, can induce apoptosis in various cell lines including MEFs and the endocrine cell line MIN6 (22, 23). We confirmed that cisplatin could induce a 1.6 fold increase of free nucleosomal DNA, a late-stage marker for apoptotic cells, in menin-null MEFs compared with DMSO control (Fig. 2D). Furthermore, we did not observe any significant difference of cisplatin treatment on cell necrosis (Supplementary Fig. S2A). Surprisingly, expression of HA-AKT1, but not expression of menin or coexpression of HA-AKT1 and menin, suppressed cisplatin-induced release of free nucleosomal DNA (62% downregulation) (Fig. 2D). Collectively, these results suggest that menin can suppress AKT1 mediated cell proliferation and anti-apoptotic effects.
Domains of menin and AKT1 necessary for their interaction
To determine the domains of menin for interaction with AKT1, we generated FLAG-tagged menin deletions containing either the NH2 terminus (FM1), the middle region (FM2) or the C-terminus (FM3) of menin (Fig. 3A). We observed that AKT1 could co-immunoprecipitate with FM, FM2 and FM3. However, no co-IP was observed between FM1 and AKT1 (Fig. 3A). These results indicate that the middle region and the C-terminus of menin are sufficient for binding to AKT1. Expression of HA-AKT1 and various fusion proteins of menin were confirmed by IB (Fig. 3A).
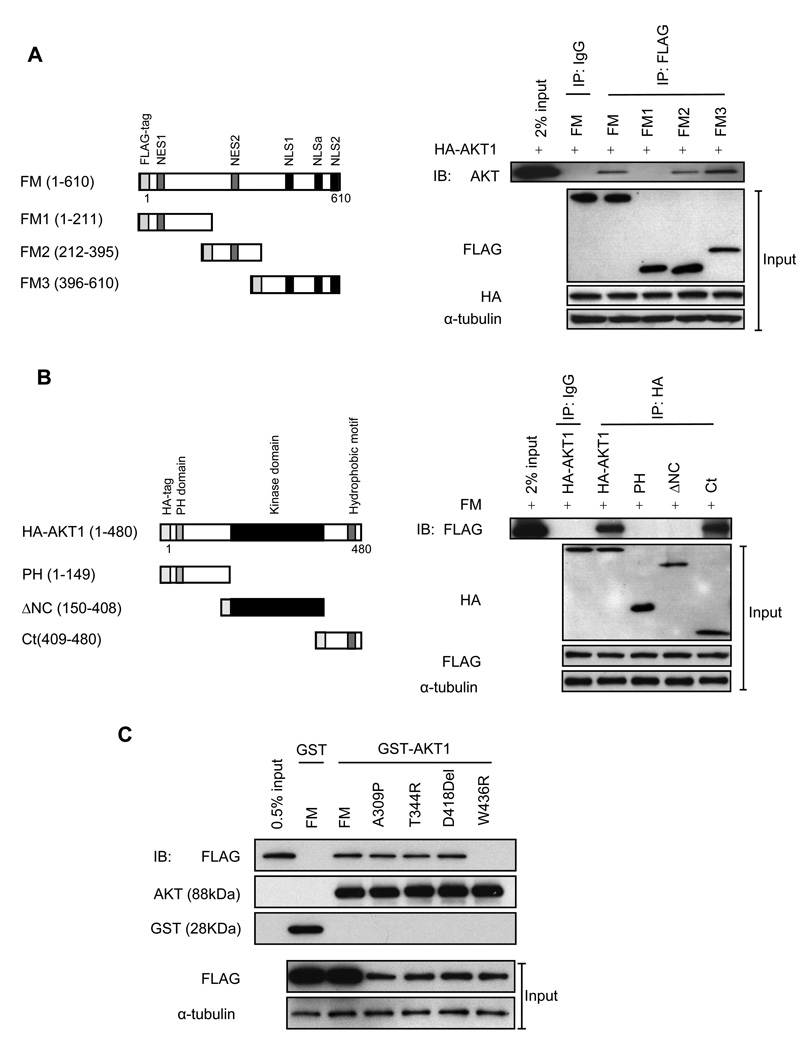
Figure 3. The regions of menin, AKT1 and menin mutants sufficient for their interaction.
(A) Diagram of menin deletions. NES: nuclear export signals. NLS: nuclear localization signals (left panel). Immunoprecipitates from HEK-293 cells were used for co-IP; expression of transfected menin and AKT respectively, from the input proteins is also shown (right panels). (B) Diagram of AKT1 deletions (left panle). Immunoprecipitates from HEK-293 cells were used for co-IP; expression of transfected menin and AKT respectively, from the input proteins is also shown (right panels). Normal mouse IgG was used as a negative control in (A) and (B). (C) GST pull down assay was performed as indicated. Expression of transfected menin from the input proteins is shown (bottom panels).
To identify the domains of AKT1 that interact with menin, HA-tagged PH domain (PH), kinase domain (ΔNC) or C-terminus (Ct) of AKT1 were coexpressed with FM in HEK-293 cells. Menin specifically interacted with the C-terminus of AKT1 (amino acids 409 to 480), but did not interact with other regions of AKT1 (Fig. 3B). These results indicate that the C-terminus of AKT1 is sufficient for binding to menin. Expression of FM and various fusion proteins of AKT1 were confirmed by IB (Fig. 3B).
MEN1 W436R fails to interact with AKT1
Based on the observation that amino acids 212 to 610 of menin are sufficient for binding to AKT1, we explored whether menin mutants in this region may disrupt the interaction between menin and AKT1. We tested the interaction of FLAG-tagged MEN1 disease-related menin mutations A309P, T344R, D418Del and W436R in HEK-293 cells in a GST-AKT1 pull down assay. FM and menin mutants A309P, T344R, D418Del interacted normally with AKT1; however, the menin mutant W436R did not interact with AKT1 (Fig. 3C). The levels of GST-AKT1, GST and the expression levels of FLAG-tagged menin mutants were confirmed by IB (Fig. 3C). Although the expression of menin mutants was lower compared with WT menin, these fusion proteins were pulled down by GST-AKT1 at similar levels.
MEN1 W436R can not suppress AKT1 kinase activity
We examined whether the menin mutants could suppress the phosphorylation of AKT and AKT kinase activity. HEK-293 cells were cotransfected with HA-AKT1 and different menin mutants. The cells were serum stimulated for 30 minutes after overnight serum starvation and the cytoplasmic proteins were extracted for IB. WT menin suppressed the phosphorylation of AKT at S473 by 30% and decreased p-GSK3β level by 39% induced by overexpression of AKT1 (Fig. 4A). The menin mutants A309P, T344R and D418Del behaved similar to WT menin to downregulate p-AKT (S473) and p-GSK3β levels (Fig. 4A). However, p-AKT (S473) and p-GSK3β levels remained at similar levels in the cells overexpressing AKT1 or in the cells cotransfected with AKT1 and W436R. Because we previously demonstrated that W436R could not interact with AKT1 (Fig. 3C), this suggests that the negative regulation of AKT signaling by menin is in part through its interaction with AKT1. The above results also indicate that AKT is not the only target of menin. How menin regulate tumorigenesis in endocrine tissues still need to be further investigated.
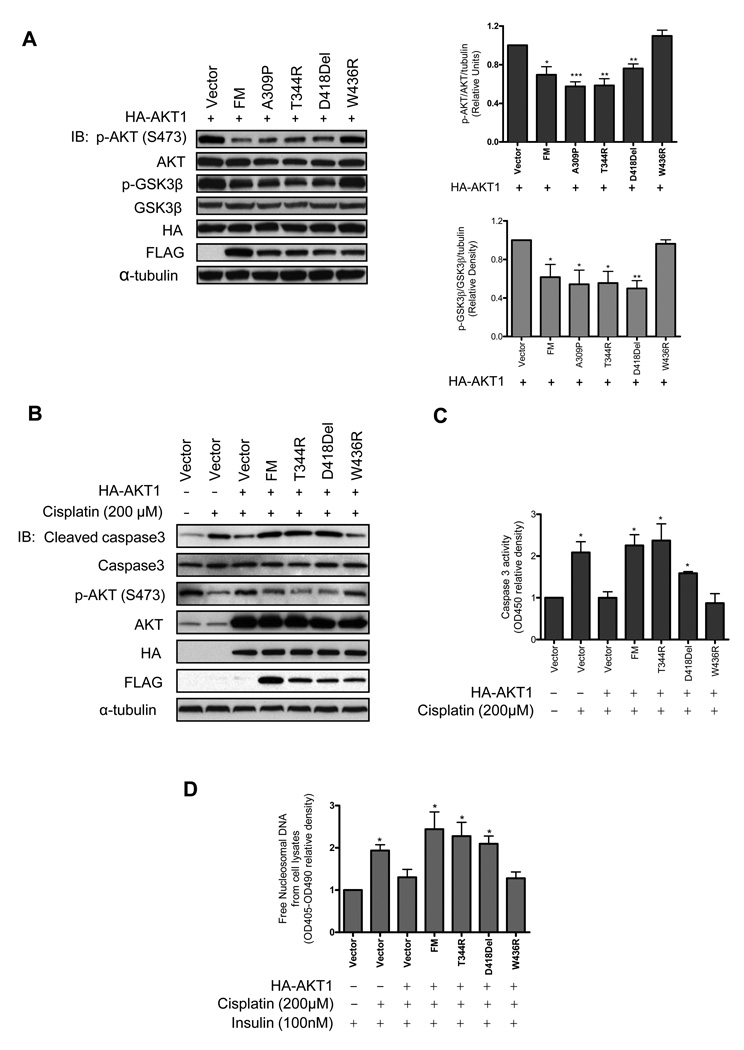
Figure 4. Association of menin and AKT1 is essential for menin to regulate AKT activation.
(A) The cytoplasmic extracts of HEK-293 transfected with various plasmids were subjected to IB. The quantified results from three independent experiments are shown on the graphs. (B) The cell lysates from 5×106 MIN6 transfected with various plasmids were subjected to IB. (C) The cell lysates from the same treatment as (B) were used for caspase3 activity ELISA assay. (D) 2×104 MIN6 cells transfected with various plasmids were processed to detect release of free nucleosomal DNA. The cells cotransfected with HA-AKT1 and vector in (A), (C) and (D) were used as controls for statistical analysis.
MEN1 W436R fails to inhibit AKT1 mediated anti-apoptosis effect in endocrine cells
We investigated whether menin regulates the AKT signaling pathway in endocrine cells. MIN6 insulinoma cells were cotransfected with HA-AKT1 and different menin mutants (Fig. 4B). After the cells were starved overnight for serum and glucose, the cells were treated with cisplatin for 24 hours. We first examined the level of cleaved caspase3. As expected, in the vector control cells, cleaved caspase3 level was dramatically increased in the cells treated with cisplatin compared with DMSO treatment (Fig. 4B). In contrast, increase of cleaved caspase3 level by cisplatin was blocked by overexpression of AKT1 and this blockage was rescued by cotransfecting the cells with WT menin, T344R, and D418Del but not by W436R. We ruled out the possibility that either WT menin or the menin mutants alone can impair the effect of cisplatin on caspase3 cleavage (Supplementary Fig. S2B). We also observed similar results by caspase3 activity ELISA assay (Fig. 4C).
Interestingly, p-AKT (S473) level was largely decreased in the cells treated with cisplatin and it reached high levels when AKT1 was overexpressed (Fig. 4B). On the other hand, overexpression of WT menin, T344R and D418Del but not W436R suppressed the elevation of p-AKT (S473) level induced by cotransfecting the cells with AKT1 (Fig. 4B).
The level of cleaved caspase3 is a marker for an early event of apoptosis. We examined another marker of apoptosis, the release of free nucleosomal DNA in MIN6 cells. We observed that cisplatin treatment results in 50% increase in the release of free nucleosomal DNA, but overexpression of AKT1 reduced the effect of cisplatin on the release of free nucleosomal DNA by around 33% (Fig. 4D). This reduction was only observed in cells cotransfected with AKT1 and W436R but not in other cotransfectants. However, cisplatin treatment had similar effects on cell necrosis as shown in Supplementary Fig. S2D, suggesting that AKT or menin does not impair cell necrosis under cisplatin treatment. Cell death ELISA assay was also performed on W436R single transfectant to rule out the effect of W436R on cisplatin induced DNA fragmentation (Supplementary Fig. S2C). Collectively, the results indicate that first, AKT1 signaling pathway plays a critical role in maintaining survival of these endocrine cells; second, menin can promote apoptosis through suppression of the level of p-AKT/AKT1 kinase activity in these endocrine cells.
Menin blocks translocation of AKT1 to the plasma membrane
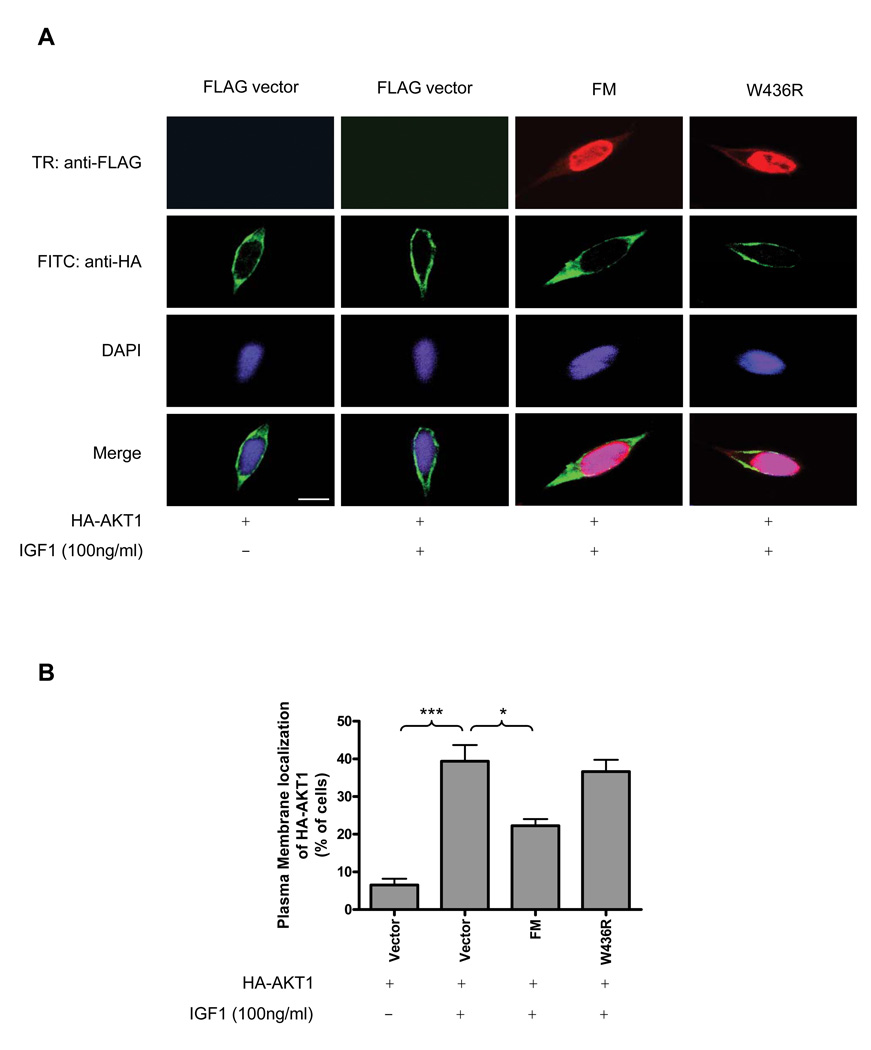
Based on the observations in the present study that 1) menin suppresses AKT kinase activity through downregulation of p-AKT (S473); 2) menin interacts with inactive AKT1 which is exclusively localized in the cytoplasm, we hypothesized that menin can impair the ability of inactive AKT to translocate from the cytoplasm to the plasma membrane, thereby blocking the phosphorylation of AKT at the plasma membrane. To test this hypothesis, HEK-293 cells were cotransfected with HA-AKT1 and empty vector, FM, or FLAG-tagged menin mutant W436R. After serum starvation, the cells were treated with IGF-1 for 5 minutes and then followed by immunofluorescence staining (Fig. 5A). In unstimulated cells, HA-AKT1 localized to the cytoplasm in most of the cells and was located at the plasma membrane in only 6% of the cells. However, HA-AKT1 was highly concentrated at the plasma membrane in IGF-1 treated cells (39% of the cells). Overexpression of HA-AKT1 with FM together resulted in changing the cellular localization of HA-AKT1 from the plasma membrane to the cytoplasm and HA-AKT1 was only located at the plasma membrane in 22% of the cells. We also observed that FM was mainly expressed in the nucleus as reported by many groups, but it was also found to moderately colocalize with HA-AKT1 in the cytoplasm (Fig. 5A). In contrast, overexpression of HA-AKT1 with W436R did not change the cellular localization of HA-AKT1 as WT menin did and HA-AKT1 was found to localize to the plasma membrane in 37% of the cells (Fig. 5B). These results indicate that menin can regulate the cellular localization of AKT1 through interacting with it and this is one possible mechanism by which it inhibits AKT kinase activity.
Figure 5. Menin promotes AKT1 accumulation in cytoplasm upon growth factor stimulation.
(A) The cellular localization of tagged proteins was assessed in HEK-293 cells and the representative results are shown. FITC (Fluorescein Isothiocyanate): anti-HA (rabbit). TR (Texas red): anti-FLAG (mouse). Bar=25µm. (B) Quantification of the percentage of HA-AKT1 located at the plasma membrane from Figure 5A. Bars represent average of four experiments in which at least 200 transfected cells were visually counted for each experiment.
Menin absent and AKT activated in mouse islet adenomas
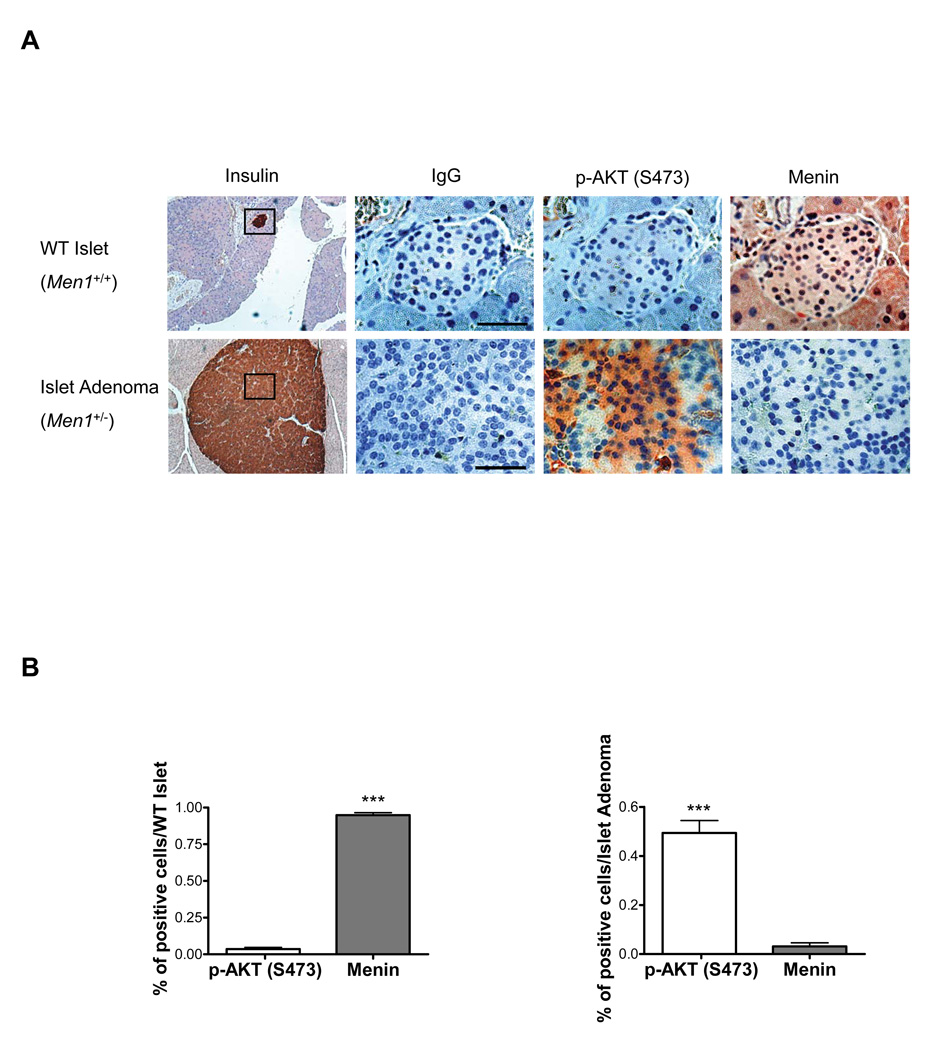
The regulation of AKT kinase activity by menin suggests that decrease or absence of menin expression would correlate with an increase in expression of active AKT in tumors. To confirm our findings in tumors of animal models, we examined the expression level of menin and p-AKT (S473) by immunohistochemistry in 30 normal islets of four WT mice and 16 islet adenomas of four Men1+/− mice (Fig. 6A). The percentage of menin and p-AKT (S473) positive cells were counted in each WT islet/ islet adenoma of serial sections. In 30 WT islets, menin was highly expressed (>95%) (Fig. 6B) in all islets but p-AKT (S473) was only moderately expressed (10–25%) in 8 islets and absent in 22 islets. In 16 islet adenomas, menin was absent (11 islet adenoma) or expressed (5 islet adenomas) at a very low level (5–20%); however, p-AKT (S473) was detected at high level (>50%) in all islet adenomas (Fig. 6B). To further confirm this finding, the peptide blocker of p-AKT (S473) was used to specifically block the p-AKT (S473) antibody used in this study to show that p-AKT (S473) antibody was specific for p-AKT (S473) (Supplementary Fig. S3A). Moreover, menin and p-AKT (S473) remained at similar levels in pancreatic exocrine tissues of WT mice compared with Men1+/− mice (Fig. 6A, Supplementary Fig. S3B). The above results demonstrate a clear negative correlation between menin and p-AKT (S473) and this correlation is statistically significant in each category (in the WT islets: pearson r= −.61, p<0.0005; in the islet adenomas: pearson r= −0.8, p<0.0005).
Figure 6. Expression of p-AKT (S473) in islet adenomas correlates with loss of menin expression.
(A) Immunohistochemical staining of pancreas sections from 18-month-old WT mice with normal islets and from 18-month-old Men1+/− mice with islet adenomas with antibodies against p-AKT (S473) and menin corresponding to the black square region indicated in insulin staining sections. Normal rabbit IgG was used as a negative control. Representative results are shown in the figures; bar=50 µm. (B) Quantification of the percentage of p-AKT (S473) and menin expressed cells per islet from Figure 6A. n=30 (WT islets), n=16 (Islet adenomas).
Discussion
Until now, most of the proteins identified to interact with menin have been nuclear proteins (6). These results are consistent with the predominant expression of menin in the nucleus and are mainly related to the transcriptional regulatory function of menin. A modest portion of menin is also expressed in the cytoplasm in most of the tested cell lines, including HEK-293, MEF, NIH3T3, Hela cells and endocrine cell lines MIN6 and GH4C1 (24, 25). Menin also has been found to interact with GFAP and vimentin, which are cytosolic proteins (26). However, menin’s function in the cytoplasm is poorly understood. Interestingly, the majority of active AKT is at the plasma membrane although active AKT has been identified in the nucleus in response to a chronic growth factor stimulus; however, the subcellular localization of inactive AKT is exclusively in the cytoplasm. In the present study, we show that menin interacts with both active and inactive AKT1. This result may provide new evidence for a role of menin in the cytoplasm.
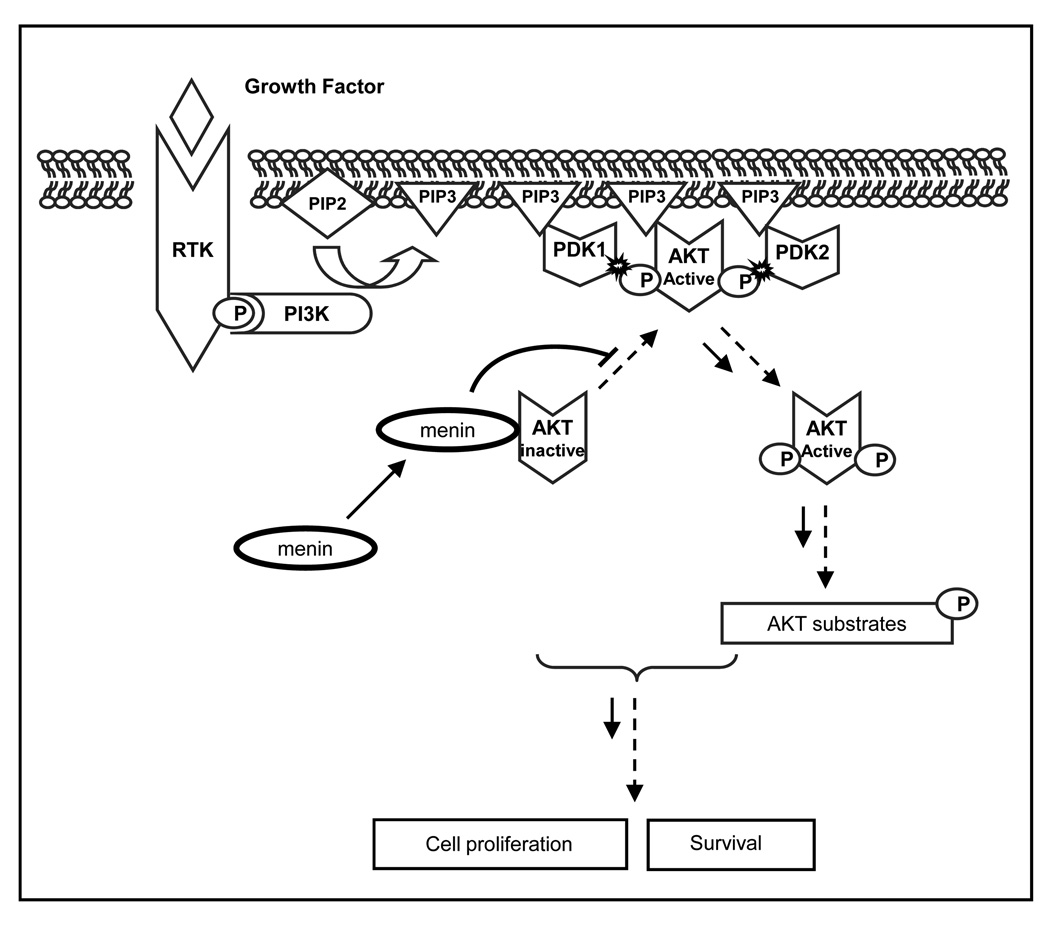
Based on our current study, we propose that menin acts as a scaffold protein to bind inactive AKT1, thereby sequestering it to the cytoplasm and preventing its activation. This results in inhibition of downstream functions, such as cell proliferation and anti-apoptosis (Fig. 7). Interestingly, the PH domain of AKT1, which is the docking site for PIP3 to the plasma membrane, is not necessary for binding to menin (Fig. 3B). This result suggests that menin retains inactive AKT1 in the cytoplasm not through blocking the PH domain of AKT1 but through an unclear mechanism.
Figure 7. Model for the negative regulation of menin in AKT signaling pathway.
Menin inhibits AKT signaling by association with AKT. Upon growth factor stimulation, menin interacts with AKT in the cytoplasm and subsequently reduces the translocation of AKT to the plasma membrane, thus inactivating AKT.
Hyperactivation of AKT has been observed in various cancers and it plays a critical role in regulation of cancer cell survival. The mechanisms of activating this kinase have been intensely studied in the past several decades. However, little is know about how the kinase activity is terminated. PTEN is the most important negative regulator of AKT and has been shown to be absent or functionally inactivated in many tumor types, thereby promoting AKT activation (27). Interestingly, both menin-null MEFs and islet adenomas examined had normal wild type PTEN expression (Supplementary Fig. S4A and B). Our data rules out the role of PTEN in regulation of AKT activity in both menin-null MEFs and mouse islet adenomas and suggests that menin is a novel negative regulator of AKT activity.
Loss of menin is the major cause for the development of MEN1 tumors, yet the role of menin in tumorigenesis is not clear. As a result, there are no specific drug targets for MEN1 related tumors (28). Our data reveal that active AKT is markedly increased in mouse islet adenomas but not in the surrounding exocrine tissues; on the other hand, menin expression is only lost in islet adenomas compared with exocrine tissues. These results indicate that activation of AKT might be a major determination of tumorigenesis in MEN1. Because AKT and menin are ubiquitous expressed, the role of menin to inhibit AKT activity may be insufficient to explain the endocrine-selective tumorigenesis in MEN1. How and why menin expression is lost only in the specific affected tissues in MEN1 remains a subject of intense investigation. However, dysregulation of AKT has been associated with human carcinogenesis in various cancers. Our findings provide new insight into the MEN1 syndrome and may lead to new therapeutic strategies for the rare MEN1 syndrome as well as some more common tumors.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. V. Hoffmann for dissections of mice and diagnosis of the islet adenomas, Dr. X. Hua for kindly providing KO and KOM cells, Dr. S. Wank for providing the confocal microscope, Dr. L. Burns for discussion and Dr. F. S. Collins for suggestions.
Financial Support: These studies were supported by the intramural program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Gagel RF, Marx SJ. Multiple Endocrine Neoplasia. In: Kronenberg HM, Melmed S, Polonsky KS, Largen PR, editors. Williams Textbook of Endocrinology 11th edition. Philadelphia: W.B. Saunders Company; 2007. pp. 1705–1746. [Google Scholar]
- 2.Chandrasekharappa SC, Guru SC, Manickam P, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404–407. doi: 10.1126/science.276.5311.404. [DOI] [PubMed] [Google Scholar]
- 3.Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008;29:22–32. doi: 10.1002/humu.20605. [DOI] [PubMed] [Google Scholar]
- 4.La P, Desmond A, Hou Z, Silva AC, Schnepp RW, Hua X. Tumor suppressor menin: the essential role of nuclear localization signal domains in coordinating gene expression. Oncogene. 2006;25:3537–3546. doi: 10.1038/sj.onc.1209400. [DOI] [PubMed] [Google Scholar]
- 5.Cao Y, Liu R, Jiang X, et al. Nuclear-cytoplasmic shuttling of menin regulates nuclear translocation of {beta}-catenin. Mol Cell Biol. 2009;29:5477–5487. doi: 10.1128/MCB.00335-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y, Hua X. In search of tumor suppressing functions of menin. Mol Cell Endocrinol. 2007;265–266:34–41. doi: 10.1016/j.mce.2006.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 8.Elghazi L, Balcazar N, Bernal-Mizrachi E. Emerging role of protein kinase B/Akt signaling in pancreatic beta-cell mass and function. Int J Biochem Cell Biol. 2006;38:157–163. doi: 10.1016/j.biocel.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Musat M, Korbonits M, Kola B, et al. Enhanced protein kinase B/Akt signalling in pituitary tumours. Endocr Relat Cancer. 2005;12:423–433. doi: 10.1677/erc.1.00949. [DOI] [PubMed] [Google Scholar]
- 10.Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. Islet beta cell expression of constitutively active Akt1/PKB alpha induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Invest. 2001;108:1631–1638. doi: 10.1172/JCI13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuttle RL, Gill NS, Pugh W, et al. Regulation of pancreatic beta-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat Med. 2001;7:1133–1137. doi: 10.1038/nm1001-1133. [DOI] [PubMed] [Google Scholar]
- 12.Segouffin-Cariou C, Billaud M. Transforming ability of MEN2A-RET requires activation of the phosphatidylinositol 3-kinase/AKT signaling pathway. J Biol Chem. 2000;275:3568–3576. doi: 10.1074/jbc.275.5.3568. [DOI] [PubMed] [Google Scholar]
- 13.Guru SC, Goldsmith PK, Burns AL, et al. Menin, the product of the MEN1 gene, is a nuclear protein. Proc Natl Acad Sci U S A. 1998;95:1630–1634. doi: 10.1073/pnas.95.4.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal SK, Novotny EA, Crabtree JS, et al. Transcription factor JunD, deprived of menin, switches from growth suppressor to growth promoter. Proc Natl Acad Sci U S A. 2003;100:10770–10775. doi: 10.1073/pnas.1834524100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crabtree JS, Scacheri PC, Ward JM, et al. A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors. Proc Natl Acad Sci U S A. 2001;98:1118–1123. doi: 10.1073/pnas.98.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heppner C, Bilimoria KY, Agarwal SK, et al. The tumor suppressor protein menin interacts with NF-kappaB proteins and inhibits NF-kappaB-mediated transactivation. Oncogene. 2001;20:4917–4925. doi: 10.1038/sj.onc.1204529. [DOI] [PubMed] [Google Scholar]
- 18.Sukhodolets KE, Hickman AB, Agarwal SK, et al. The 32-kilodalton subunit of replication protein A interacts with menin, the product of the MEN1 tumor suppressor gene. Mol Cell Biol. 2003;23:493–509. doi: 10.1128/MCB.23.2.493-509.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Culty M. Identification and distribution of a novel platelet-derived growth factor receptor beta variant: effect of retinoic acid and involvement in cell differentiation. Endocrinology. 2007;148:2233–2250. doi: 10.1210/en.2006-1206. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Thuillier R, Culty M. Prenatal estrogen exposure differentially affects estrogen receptor-associated proteins in rat testis gonocytes. Biol Reprod. 2004;71:1652–1664. doi: 10.1095/biolreprod.104.030205. [DOI] [PubMed] [Google Scholar]
- 21.Schnepp RW, Mao H, Sykes SM, et al. Menin induces apoptosis in murine embryonic fibroblasts. J Biol Chem. 2004;279:10685–10691. doi: 10.1074/jbc.M308073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banck MS, Beaven SW, Narla G, Walsh MJ, Friedman SL, Beutler AS. KLF6 degradation after apoptotic DNA damage. FEBS Lett. 2006;580:6981–6986. doi: 10.1016/j.febslet.2006.10.077. [DOI] [PubMed] [Google Scholar]
- 23.Muller D, Jones PM, Persaud SJ. Autocrine anti-apoptotic and proliferative effects of insulin in pancreatic beta-cells. FEBS Lett. 2006;580:6977–6980. doi: 10.1016/j.febslet.2006.11.066. [DOI] [PubMed] [Google Scholar]
- 24.Huang SC, Zhuang Z, Weil RJ, et al. Nuclear/cytoplasmic localization of the multiple endocrine neoplasia type 1 gene product, menin. Lab Invest. 1999;79:301–310. [PubMed] [Google Scholar]
- 25.Wautot V, Khodaei S, Frappart L, et al. Expression analysis of endogenous menin, the product of the multiple endocrine neoplasia type 1 gene, in cell lines and human tissues. Int J Cancer. 2000;85:877–881. doi: 10.1002/(sici)1097-0215(20000315)85:6<877::aid-ijc23>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Egido J, Cunningham J, Berg M, Oberg K, Bongcam-Rudloff E, Gobl A. Menin's interaction with glial fibrillary acidic protein and vimentin suggests a role for the intermediate filament network in regulating menin activity. Exp Cell Res. 2002;278:175–183. doi: 10.1006/excr.2002.5575. [DOI] [PubMed] [Google Scholar]
- 27.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 28.Marx SJ. Molecular genetics of multiple endocrine neoplasia types 1 and 2. Nat Rev Cancer. 2005;5:367–375. doi: 10.1038/nrc1610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.