Abstract
There is increasing evidence that the physical environment is a critical mediator of tumor behavior. Hepatocellular carcinoma (HCC) develops within an altered biomechanical environment and increasing matrix stiffness is a strong predictor of HCC development. The aim of this study was to establish whether changes in matrix stiffness, which are characteristic of inflammation and fibrosis, regulate HCC cell proliferation and chemotherapeutic response. Using an in vitro system of “mechanically-tunable” matrix-coated polyacrylamide gels, matrix stiffness was modeled across a pathophysiologically-relevant range, corresponding to values encountered in normal and fibrotic livers.
Results
Increasing matrix stiffness was found to promote HCC cell proliferation. The proliferative index (assessed by Ki67 staining) of Huh7 and HepG2 cells was 2.7-fold and 12.2-fold higher, respectively, when the cells were cultured on stiff (12kPa) versus soft (1kPa) supports. This was associated with stiffness-dependent regulation of basal and HGF-stimulated mitogenic signaling through extracellular regulated kinase (ERK), protein kinase B (PKB/Akt) and signal transducer and activator of transcription 3 (STAT3). β1-integrin and focal adhesion kinase (FAK) were found to modulate stiffness-dependent HCC cell proliferation. Following treatment with cisplatin, we observed reduced apoptosis in HCC cells cultured on a stiff versus soft (physiological) supports. Interestingly, however, surviving cells from soft supports had significantly higher clonogenic capacity than surviving cells from a stiff microenvironment. This was associated with enhanced expression of cancer stem cell markers, including CD44, CD133, c-kit, CXCR4, octamer-4 (OCT4) and NANOG.
Conclusion
Increasing matrix stiffness promotes proliferation and chemotherapeutic resistance, whereas a soft environment induces reversible cellular dormancy and stem cell characteristics in HCC. This has implications for both the treatment of primary HCC and the prevention of tumor outgrowth from disseminated tumor cells.
Keywords: Liver fibrosis, Mechanotransduction, Cancer Stem Cells, Metastasis, Microenvironment
Introduction
Hepatocellular carcinoma (HCC) is the third-most common cause of cancer related mortality world-wide (1). The majority (80%) of HCCs develop in the context of advanced liver fibrosis or cirrhosis and liver cirrhosis is the single most important risk factor for HCC development (2). Liver fibrosis is defined by stereotypical changes in both the biochemical and physical properties of the cellular microenvironment. However, the role of mechanical factors in modulating the growth and progression of HCC remain poorly defined. Recent studies involving ultrasound elastography (FibroScan) demonstrate that liver stiffness measurements are a strong predictor of HCC development (3). Furthermore, once established, tumor development is associated with further increases in matrix stiffness to values greater than those of the surrounding hepatic parenchyma (4). It is therefore evident that HCC develops in a niche with mechanical properties distinct from those encountered in the normal liver.
Cancer development and progression is dependent upon both intrinsic genetic abnormalities and external structural determinants (5). Matrix stiffness has recently been directly implicated in aiding tumor development. Increases in matrix stiffness that enhance cell contractility have been found to be sufficient to enhance the transformation of mammary epithelial cells (6). Conversely, a reduction in tissue stiffness by inhibition of collagen cross-linking impedes malignant growth and tumor development in a murine model of breast cancer (7). Cellular stiffness-sensing relies upon intracellular tension, which is determined by a dynamic equilibrium between forces generated by a contractile cytoskeleton and the elastic resistance (stiffness) provided by the ECM. In this context, cancer progression (tumor growth, invasion and dissemination) is accompanied by changes in both the mechanical properties of the cancer cell niche and changes in cellular contractility (modified by genetic and epigenetic factors) that regulate tumor cell behavior.
HCC continues to have a poor prognosis (median survival less than 12 months), reflecting its late presentation and lack of effective therapies (8, 9). The effectiveness of both hepatic resection and liver transplantation for HCC is limited by tumor recurrence, which can occur months or years following resection of a primary tumor (10). Furthermore, systemic chemotherapy has proved ineffective both for the treatment of advanced HCC and in an adjuvant/ neoadjuvant setting for the eradication of disseminated (dormant) tumor cells, the progenitors of clinical metastases. The mechanisms underlying chemotherapy resistance in HCC have not been fully elucidated. Although it has previously been demonstrated that the composition of the matrix can enhance chemotherapy resistance in a range of epithelial cancers (11, 12), the role of matrix stiffness has not been specifically addressed for HCC or other epithelial cancers.
Here we demonstrate that mechanical factors regulate both the proliferation and chemotherapeutic response of HCC cells. In addition, we show that both tumor cell differentiation and cancer stem cell characteristics are influenced by the mechanical properties (stiffness) of the cancer cell niche.
Materials and Methods
Cell Culture and Microscopy
Human HCC cell lines Huh7 and HepG2 (kindly provided by S. Wigmore, Edinburgh, UK) were cultured in Dulbecco’s Modified Eagle Medium (Gibco, Paisley, UK) supplemented with 10% fetal calf serum, penicillin/ streptomycin and L-glutamine. For all experiments cells were plated at semi-confluent density in 1% fetal calf serum. Chemical reagents were purchased from Sigma (Poole, UK) unless otherwise stated. Transforming growth factor-beta (TGFβ) and hepatocyte growth factor (HGF) (Peprotech, London, UK) were used at concentrations of 5ng/ml and 10ng/ml, respectively. Anti-β1-integrin, clone 6S6 (Millipore, Watford, UK) and control IgG1 immunoglobulin (AbD Serotec, Oxford, UK) were used for cell culture experiments at 50µg/ml. Echistatin (Tocris, Bristol, UK) was used in cell culture experiments at 100nM concentration. The chemical FAK inhibitor, PF573228 (Tocris, Bristol, UK) was solubilized in dimethyl-sulfoxide (DMSO) and used for cell culture experiments at a concentration of 1–5µM. A detailed description of microscopy and morphological analysis can be found in Supplementary Methods online.
Preparation of Polyacrylamide Gel Supports
Polyacrylamide (PA) gels of variable stiffness were prepared on glass coverslips using modifications (13) to the method initially described by Pelham and Wang (14). A detailed description can be found in the Supplementary Methods online.
Immunofluorescence Staining
Cells were fixed in 4% paraformaldehyde in PBS and permeabilized with 0.2% Triton-X-100 in PBS. Slides were stained with anti-Ki67 (Novocastra, Newcastle, UK) and anti-vinculin (Sigma, Poole, UK); corresponding Alexa Fluor-555 secondary antibodies were used for detection (Invitrogen, Paisley, UK). Actin stress fibers were stained with Alexa-488 Phalloidin (Invitrogen, Paisley, UK). Nuclear DNA was counterstained with 4’, 6’-diamidino-2-phenyl-indole dihydrochloride (DAPI) (Dako, Ely, UK). Cellular proliferative index (Ki67 positive cells/total cells) was calculated by direct cell counting from 15 randomly selected high magnification photomicrographs from Ki67-stained slides (n=3).
Western Blot
A detailed description of western blotting and a complete list of antibodies are provided in the supplementary methods online.
Immunohistochemistry
Human HCC specimens were obtained from archived tissue held by Tayside Tissue Bank and the Department of Pathology, University Medical Center Hamburg-Eppendorf with appropriate ethical approval (UK-LREC: TR000216). Immunohistochemistry was performed as previously described (15). The primary antibodies and antigen retrieval regimes used were anti-pFAK (pY397) (Invitrogen, Paisley, UK/ Microwave pH9) and anti-β1-integrin (Abcam, Cambridge, UK/ Microwave pH9). Negative controls with isotype immunoglobulins (Santa Cruz, Heidelberg, Germany) and species-specific serum alone showed no specific staining.
Clonogenic Assay
Cells were plated at semi-confluent density onto PA gels. After 48h cells either received cisplatin (HepG2 10µM/ Huh7 20µM) or 5-fluorouracil (5FU/ 25µM) or were left untreated in plating medium. After 24h the medium was changed to normal culture medium and the cells were incubated further for 48h, for a total of 5 days of culture. Cells were then retrieved by trypsinization, counted and plated at clonal density (10,000 cells/well) into 12-well plates in normal culture medium. Cells were fixed at between 5–10 days in 4% paraformaldehyde and stained with 0.5% crystal violet solution. Colonies were visualized with a VersaDoc system and analyzed with Quantify-One (BioRad, Hercules, USA).
Flow-Cytometric Analysis
Cells were harvested by trypsinization and single cell suspension generated by passing cells through a 40µm cell strainer. Cells were stained with the following antibodies: CD44-PE, CD117-PE (c-kit), CD133-PE, CD184-PE (CXCR-4) and corresponding PE-labelled isotype controls (E-Bioscience, Hatfield, UK). After staining, cells were washed, post-fixed in 1% paraformaldehyde and analyzed on a FACScan (BD Biosciences, Franklin Lakes, USA). Data analysis was performed using FlowJo software (Tree-Star Inc, Ashland, USA).
cDNA Synthesis and Real-Time PCR
Relative mRNA expression for genes of interest was determined by Real-Time PCR using an Applied Biosystems 7700 Sequence Detection System. Primer sequences for the genes of interest and the 18S housekeeping gene were purchased from Applied Biosystems (Warrington, UK).
Statistics
Data are expressed as mean +/− standard error of the mean (SEM) of at least three independent experiments unless stated otherwise. Comparisons between groups were performed using a two-tailed student t-test.
Results
Increasing Matrix Stiffness is Associated with a Mesenchymal Shift in HCC Cells
The response of HCC cells to alterations in matrix stiffness was investigated using a system of mechanically-tunable ligand-coated PA gels (13, 14). In this system, matrix stiffness is altered by modulating the bis-acrylamide crosslink density of thin PA gels without altering the surface composition or density of ligands to which the cells are exposed (13). Matrix stiffness (expressed as shear modulus, G’) was modeled across a range of pathophysiologically-relevant stiffness values (1–12kPa) corresponding to values encountered in normal and fibrotic livers (16). The PA gels used in this study were coated with collagen-I, representing the predominant ECM protein encountered in the fibrotic liver.
For both Huh7 and HepG2 cells we observed a consistent morphological response to changes in support stiffness. HCC cells on soft (1kPa) supports were small and rounded in contrast to the well-spread and flattened cells seen on stiff (12kPa) supports (Figure 1). Differences in cellular spreading as a function of support stiffness develop rapidly (within 1 hour) and are maximal at 24 hours (Figure S1). Confocal microscopy showed that increasing matrix stiffness was associated with the development of prominent actin stress fibers and mature (vinculin-positive) focal adhesions (Figure 2). These features were absent in cells cultured on soft supports. The presence of stress fibers is linked to acquisition of mesenchymal properties (mesenchymal-shift) and de-differentiation in epithelial cells. In accordance with this we demonstrated upregulation of the mesenchymal markers N-cadherin (Huh7/HepG2) and vimentin (shown for Huh7; vimentin is not expressed in HepG2 cells under either condition) in HCC cells cultured on stiff supports (Figure 3A). There was no change in the expression of the epithelial marker E-cadherin. HepG2 and Huh7 cells cultured on soft supports expressed higher levels of albumin, hepatocyte nuclear factor-4α (HNF4α), α1-antitrypsin and alpha-fetoprotein (AFP) than cells cultured on stiff supports (Figure 3B). This suggests that a soft environment promotes a differentiated hepatocyte phenotype, whereas increasing support stiffness is associated with cellular de-differentiation towards a mesenchymal phenotype.
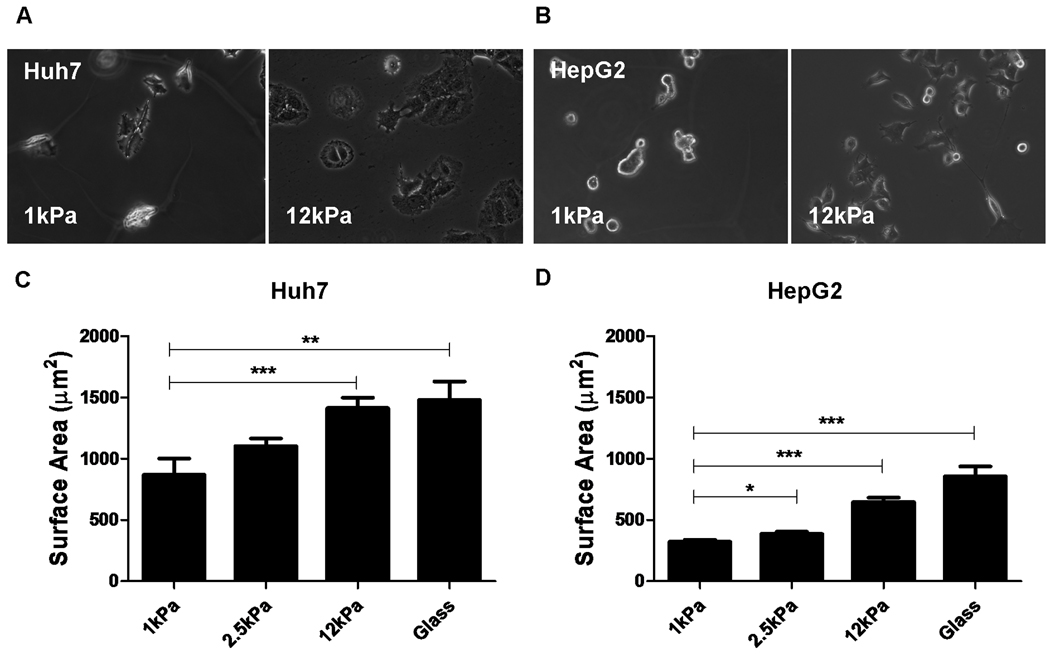
Figure 1. Changes in matrix stiffness regulate HCC cell morphology and spreading.
Huh7 and HepG2 cells were cultured on collagen-I-coated polyacrylamide gels with “tunable stiffness” (expressed as shear modulus, G’) in the range of 1–12kPa and collagen-I-coated glass. The stiffness values of the polyacrylamide gel supports used were selected in order to reflect range of stiffness values encountered in normal and fibrotic livers. Phase-contrast photomicrographs demonstrate the regulation of cellular morphology by support stiffness in both (A) Huh7 and (B) HepG2 cells. The surface area (square microns) of (C) Huh7 and (D) HepG2 cells was calculated by digital image analysis of phase-contrast images of cells on polyacrylamide gel supports. In each case, values reflect the mean (±SEM) of measurements from 50 cells in 3 independent experiments (*p<0.05, **p<0.01 and ***p<0.001).
Figure 2. Changes in matrix stiffness regulate the formation of actin stress fibers and focal adhesion maturation in HCC cells.
Confocal microscopy (×320 magnification) of (A) Huh7 and (B) HepG2 cultured on soft (1kPa) and stiff (12kPa) collagen-I-coated polyacrylamide supports as indicated. The photomicrographs displayed are of cells stained for the presence of actin stress fibers (phalloidin-green), mature focal adhesions (anti-vinculin-red) and nuclear DNA (4’, 6’-diamidino-2-phenyl-indole dihydrochloride (DAPI-blue). The merged image (right panel) demonstrates the spatial relationship between actin stress fibers and mature focal adhesions. Inserts display high magnification images for Huh7 and HepG2 cells cultured on stiff (12kPa) supports demonstrating the insertion of actin stress fibers into mature focal adhesions. In each image, the scale bars represent 20 microns.
Figure 3. Increased matrix stiffness is associated with mesenchymal shift in HCC cells.
(A). Western blot from whole cell lysates showing expression of E-cadherin, N-cadherin and vimentin in Huh7 and HepG2 cells cultured on soft (1kPa) and stiff (12kPa) collagen-I-coated polyacrylamide gel supports (as indicated). (B). Western blots from whole cell lysates showing expression of albumin, hepatocyte nuclear factor 4 alpha (HNF4α), α1-antitrypsin and alpha-fetoprotein (AFP) in Huh7 and HepG2 cells. (C). Western blots from whole cell lysates from Huh7 cells showing the expression of phospho-Smad2, phospho-Smad3 and total Smad2/3 following stimulation with transforming growth factor beta (TGFβ) (5ng/ml). In each western blot equal quantities of protein were loaded and equal loading confirmed in relation to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. In each case the western blots shown are representative examples from 3 independent experiments. (D) The line graphs show a schematic representation of densitometry analysis of phosphorylated Smad2 and Smad3, expressed relative to GAPDH. Each time-point represents the mean of three independent experiments.
TGFβ is a potent inducer of mesenchymal changes in both primary and transformed epithelial cells. We therefore investigated whether support stiffness regulated TGFβ-induced Smad signaling activity in HCC cells. The Huh7 cell line demonstrated increased basal activity of the TGFβ signaling pathway (as indicated by increased Smad3 phosphorylation) in cells cultured on stiff supports (Figure 3C/D). In addition, upon stimulation with TGFβ there was enhanced Smad2 and Smad3 phosphorylation in cells from stiff supports.
Increasing Matrix Stiffness Promotes HCC Proliferation
In both HCC cell lines, matrix stiffness regulated HCC cell proliferation (Figure 4A). The proliferative indices of Huh7 and HepG2 cells (assessed by nuclear localization of Ki67) were 2.7-fold (p<0.001) and 12.2-fold (p<0.001) higher, respectively, when the cells were cultured on stiff (12kPa) versus soft (1kPa) supports. Maximal proliferative index was seen when cells were cultured on collagen-I-coated glass, which has a shear modulus several orders of magnitude higher than any physiological matrix. Both MTT assay (Figure S2) and direct cell counting (data not shown) confirmed an increase in total cell number with increasing support stiffness. A similar trend for cellular proliferation was observed in primary mouse hepatocytes (Figure S3). Matrix stiffness had a corresponding effect on the expression of cell cycle regulators of G1 progression (Figure 4B/C). We observed a strong reduction in the expression of cyclin-D1 and cyclin-D3 in cells cultured on soft supports. There was no evidence of upregulation of the cyclin-dependent kinase inhibitors p21cip or p27kip on soft gels and indeed a moderate down-regulation of p27kip was observed on soft gels. Induction of terminal senescence on soft supports was excluded by showing that upon transfer to a stiff matrix, cells resumed proliferation to levels comparable to cells coming from a stiff matrix (Figure S4A). Furthermore, cells on both soft and stiff supports showed no evidence of beta-galactosidase accumulation (data not shown). In each cell line, differences in cellular proliferation as a function of stiffness were evident across a wide range of plating densities (Figure S5).
Figure 4. Increased matrix stiffness promotes HCC cell proliferation.
(A) Graphs showing the mean proliferative index (Ki67 positivity) of Huh7 and HepG2 cells cultured on collagen-I-coated polyacrylamide gel supports across a range of stiffness values (1–12kPa), as indicated, and collagen-I-coated glass (n=3). (B). Western blots from whole cell lysates showing expression of cyclin-D1 and cyclin-D3 in Huh7 and HepG2 cells cultured on soft (1kPa) and stiff (12kPa) supports, as indicated. (C) Western blots from whole cell lysates showing expression of cyclin-dependent kinase inhibitors, p21cip and p27kip in Huh7 and HepG2, cultured on soft (1kPa) and stiff (12kPa) supports, as indicated. In each western blot, equal quantities of protein were loaded and equal loading confirmed in relation to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. The western blots shown are representative examples from 3–6 independent experiments. (D). Graphs showing the mean proliferative index (Ki67 positivity) of Huh7 (left panel) and HepG2 (right panel) cells cultured on both soft (1kPa) and stiff (12kPa) polyacrylamide supports coated with collagen-I, collagen-IV, laminin or fibronectin (n=3). In each case, error bars represent SEM, *p<0.05, **p<0.01 and ***p<0.001.
In order to exclude a specific effect related to collagen-I, we investigated the effect of different ECM coatings on HCC cell proliferation on PA gels (Figure 4D). While minor differences were observed with respect to cellular morphology and spreading (Figure S6) when cells were plated on collagen-I, collagen-IV, laminin and fibronectin-coated gels, the biochemical composition of the surface coating did not significantly alter the stiffness-dependent regulation of cell proliferation. In other words, the physical rather than the biochemical properties of the PA gels exerted the predominant effect on HCC cell proliferation.
Matrix Stiffness Modulates Basal and HGF-induced Signaling Responses
Using immunoblotting with phosphorylation-specific antibodies we analyzed stiffness-dependent differences in the activity of critical mitogenic signaling pathways. Growth on stiff (12kPa) versus soft (1kPa) supports was associated with enhanced focal adhesion kinase (FAK), extracellular-regulated-kinase (ERK), protein kinase B (PKB/Akt) (Huh7 cells only) and signal transducer and activator of transcription-3 (STAT3) phosphorylation (Figure 5A). Substrate stiffness significantly modulated growth factor-induced mitogenic signaling in response to HGF. Upon stimulating cells plated on both soft and stiff PA gels with HGF we observed an increase in the magnitude of ERK, PKB/Akt and STAT3 activation in cells cultured on stiff gels (Figures 5C/S7). Substrate stiffness also modulated cyclin-D1 expression in response to HGF stimulation. Following HGF stimulation in HepG2 and Huh7 cells, there was upregulation of cyclin-D1 expression in cells cultured on both soft and stiff supports (Figure 5B). Importantly, the magnitude of cyclin-D1 induction following HGF stimulation was substantially higher in cells cultured on stiff supports.
Figure 5. Matrix stiffness regulates mitogenic signaling in HCC.
(A) Western blots showing basal expression of phosphorylated and total focal adhesion kinase (FAK), extracellular-regulated kinase ERK, protein kinase B (PKB/Akt) and signal transducer and activator of transcription 3 (STAT3) in Huh7 and HepG2 cells cultured on soft (1kPa) and stiff (12kPa) supports, as indicated. (B). Western blots showing cyclin D1 expression in Huh7 and HepG2 cells cultured for 24 hours on soft (1kPa) and stiff (12kPa) supports in the presence (+) or absence (−) of hepatocyte growth factor (HGF) (10ng/ml). (C). Western blots showing a time-course analysis for expression of phosphorylated and total ERK, PKB/ Akt and STAT3 in Huh7 cells cultured on soft (1kPa) and stiff (12kPa) supports. Whole cell lysates were harvested at baseline and specific timepoints (as indicated) following the addition of HGF (10ng/ml) to culture media. In each western blot, equal quantities of protein were loaded and equal loading confirmed in relation to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. The line graphs (right panel) show a schematic representation of densitometry analysis of phosphorylated ERK, PKB/ Akt and STAT3 expressed relative to GAPDH. Each time-point represents the mean of three independent experiments.
β1-Integrin and phospho-FAK are expressed in human HCC tumors and regulate the stiffness-dependent proliferation of human HCC cells in vitro
Integrins and integrin-associated focal adhesions are known to be important mediators of mechanotransduction. We therefore used immunohistochemistry to investigate the prevalence of β1-integrin and phospho-FAKTyr397 expression in HCC tissue from an unselected cohort of 15 HCC specimens obtained at the time of tumor resection or biopsy (Figure 6A). β1-integrin was expressed in tumor tissue in 15/15 HCC specimens tested. In addition we found upregulation of FAK expression in tumor tissue relative to the surrounding parenchyma in 8/15 (53%) HCC specimens tested. These results are consistent with published histological studies (17, 18). We subsequently investigated the effect of the β1-integrin inhibition on HCC cell proliferation in vitro using a function blocking anti-β1-integrin antibody (6S6) and the dysintegrin echistatin (Figure 6C). Anti-β1-integrin antibody and echistatin promoted cellular rounding in both the Huh7 and HepG2 cells cultured on collagen-I-coated 12kPa (stiff) supports. Huh7 cell proliferation was reduced by treatment with both 6S6 antibody (38% reduction, p<0.05) and echistatin (29% reduction, p=0.07) relative to relevant controls. Similarly, in HepG2 cells, cell proliferation was reduced by treatment with both 6S6 antibody (92% reduction, p<0.001) and echistatin (21% reduction, p<0.01). The effect of FAK activation on HCC cell proliferation was investigated in experiments with the small molecular FAK inhibitor PF573228 (Figure 6B/C). Treatment with PF573228 (5µM) was associated with a reduction in the proliferation of both Huh7 (42% reduction, p<0.01) and HepG2 cells (45% reduction, p<0.001) cultured on collagen-I-coated 12kPa polyacrylamide gels. Furthermore, inhibition of β1-integrin or FAK expression in HepG2 cells with siRNA resulted in a significant reduction in cellular proliferation relative to control siRNA transfection (Figure S8). A similar trend in respect to cellular proliferation was observed following siRNA-dependent inhibition of β1-integrin or FAK expression in Huh7 cells, although in this case the reduction was not statistically significant.
Figure 6. β1 Integrin and phospho-FAK are expressed in human HCC tumors and regulate the stiffness-dependent proliferation of human HCC cells in vitro.
(A) Low magnification (×50) photomicrographs from a human HCC resection specimen stained with haematoxylin and eosin (left panel), anti-β1-integrin (middle panel) and anti-phospho-FAK (right panel). Negative control staining is represented by the indented images in the top right-hand corner of each image. β1-integrin is expressed in both the HCC tumor (HCC) and surrounding hepatic parenchyma (P), as indicated. Phospho-FAKTyr397 is strongly expressed in the HCC tissue relative to the hepatic parenchyma. Scale bars represent 200 microns. (B) Western blots showing the expression of phospho-FAKTyr397 in Huh7 and HepG2 cells either left untreated or treated for 24 hours with the focal adhesion kinase (FAK) inhibitor PF573228 at concentrations of 1µM and 5µM, as indicated. In each western blot, equal quantities of protein were loaded and equal loading confirmed in relation to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. (C) Graphs showing the mean proliferative index (Ki67 positivity) of Huh7 and HepG2 cells cultured on 12kPa (stiff) collagen-I-coated polyacrylamide supports. Cells were treated with the anti-β1-integrin antibody 6S6 (50µg/ml), isotype control IgG1 antibody (50µg/ml), echistatin (100nm), PF573228 (1µM), PF573228 (5µM), DMSO (vehicle control) or left in media alone (untreated control) for 24 hours, as indicated (n=3–5). In each case, error bars represent SEM, *p<0.05, **p<0.01 and ***p<0.001.
Matrix Stiffness Modulates HCC Apoptosis and Clone-forming Capability Following Chemotherapy
HCC is resistant to treatment with conventional chemotherapeutic agents. We therefore investigated whether the stiffness of the cancer cell niche regulates the susceptibility of HCC cells to chemotherapy-induced apoptosis. In both cell lines, there was decreased apoptosis – as indicated by reduced Poly-ADP-ribose polymerase (PARP) cleavage – in cells cultured on stiff supports (Figure 7A). There was a non-significant trend towards increased numbers of surviving cells on stiff supports (data not shown). We also performed a series of clonogenic assays to investigate whether changes in matrix stiffness modulate the survival and behavior of tumor-initiating cells after chemotherapy. Following cisplatin treatment, the surviving cell population included an increased frequency of clone-initiating cells for both HepG2 (2.4-fold, p<0.001) and Huh7 cells (2.2-fold, p<0.05) cultured on soft (1kPa) versus stiff (12kPa) supports (Figure 7B). In addition, there was a non-significant trend towards an absolute increase in the total number of clone-forming cells from soft supports (data not shown). To assess the validity of this finding, experiments were repeated using a second, unrelated chemotherapeutic agent, 5-fluorouracil (5-FU). Consistent with our findings with cisplatin, following 5-FU chemotherapy there was an increased frequency of clone-initiating cells from HepG2 (3.6-fold, p<0.001) and Huh7 cells (1.9-fold, p<0.05) cultured on soft versus stiff supports. There was no difference in the frequency of clone-initiating cells in untreated HepG2 or Huh7 cells after culture on soft or stiff supports in the absence of chemotherapy. The paradoxical increase in the clone-initiating capability of chemotherapy-treated cells from a low stiffness environment could be explained by selective enrichment for clone-initiating cells with stem cell characteristics. We therefore performed flow-cytometric analyses for putative cancer stem cell markers in HCC cells cultured on soft (1kPa) and stiff (12kPa) supports, both without and following cisplatin treatment (Figure 8A). Culture on soft versus stiff supports was associated with an enrichment for the cell surface markers CD133 (1.5-fold, p<0.001), c-kit (1.3-fold, p=0.78), CD44 (6.4-fold, p<0.001) and CXCR4 (2.9-fold, p<0.01). Following cisplatin treatment, there was statistically significant upregulation of CD44 (1.7-fold, p<0.01), CD133 (1.6-fold, p<0.01) and c-kit (15.8-fold, p<0.01) for cells maintained on soft but not stiff supports. Additionally, real-time PCR demonstrated up-regulation of stem cell-associated transcription factors OCT4 and NANOG in HepG2 cells cultured on soft versus stiff supports, both in untreated controls (OCT4 2.0-fold increase, p<0.05; NANOG 2.7-fold increase, p<0.05) and following cisplatin treatment (OCT4 2.0-fold increase, p<0.05; NANOG 3.4-fold increase, p<0.05) (Figure 8B).
Figure 7. Matrix stiffness regulates apoptosis and clonogenic capacity following chemotherapy.
(A) Western blot showing full-length (116kDa) and cleaved poly-ADP-ribose polymerase (PARP) (89kDa) expression in Huh7 and HepG2 cells following treatment with cisplatin on soft (1kPa) and stiff (12kPa) supports, as indicated. In each western blot, equal quantities of protein were loaded and equal loading confirmed in relation to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. The western blots shown are representative examples from 3 independent experiments. (B) Colony formation potential of Huh7 and HepG2 cells following chemotherapy. Huh7 and HepG2 cells were cultured for 48 hours on either soft (1kPa) or stiff (12kPa) polyacrylamide supports. Cells were then left untreated (left panel) or treated with cisplatin (middle panel) or 5-fluorouracil (5-FU) (right panel) for 24 hours prior to media change. After a further 48 hours in culture, the cells were trypsinized and equal numbers re-plated at clonal density in 12-well plates. Clonogenic capacity was calculated by direct counting of the resulting colonies. The results are expressed as the percentage colony formation relative to the number of colonies obtained from 1kPa supports from three independent experiments.
Figure 8. Matrix stiffness and chemotherapy regulate stem cell marker expression in HepG2 cells.
(A) Quantification by flow cytometric analysis of putative cancer stem cell markers CD133, c-kit, CD44 and CXCR-4 in HepG2 cells cultured for 5 days on soft (1kPa) or stiff (12kPa) supports. Cells were either left untreated (black) or treated for 24 hours with cisplatin (white). Results are representative of three independent experiments. (B) Real-time quantitative PCR analysis of octamer-4 (OCT4) (left panel) and NANOG (right panel) expression in HepG2 cells cultured for 5-days on soft (1kPa) or stiff (12kPa) supports. Cells were either left untreated (black) or treated for 24 hours with cisplatin (white). Expression is relative to the 18S housekeeping gene. In each case, error bars represent SEM, *p<0.05, **p<0.01 and ***p<0.001.
Discussion
In this study we have demonstrated that the stiffness of the sub-cellular matrix profoundly alters the phenotype and behavior of HCC cells in vitro. Pathophysiological increases in matrix stiffness – as encountered in fibrotic and cirrhotic livers (19) – promote the proliferation of HCC cells. Our work defines novel mechanisms linking the physical properties of the fibrotic liver and the malignant behavior of HCC. Our data is consistent with in vivo evidence, not only of de novo HCC development and progression against a background of cirrhosis, but also animal studies showing that the induction of liver fibrosis is associated with accelerated tumor growth following orthotopic HCC implantation (20, 21). Furthermore, histological examination of human HCC specimens demonstrates a significant association between the presence of hepatic fibrosis and enhanced tumor cell proliferation (22). Critically, our findings suggest that a reduction in the stiffness of the cancer cell niche – as would be encountered by a disseminated tumor cell entering an unaffected secondary site – would be sufficient to promote reversible cellular quiescence and cancer cell dormancy.
It has previously been demonstrated that matrix stiffness can regulate proliferation in non-transformed cells. More recently increased matrix stiffness has been shown to promote cellular proliferation in glioma cells (23). We have extended these findings to a range of epithelial malignancies, including HCC (Figure S9). Furthermore, we have shown that β1-integrin and FAK (the canonical mediator of integrin-related signaling) regulate stiffness-dependent proliferation in HCC cells. In both fibroblasts and non-transformed mammary epithelial cells, a critical role for ERK-induced cyclin-D induction has been established in the stiffness-dependent regulation of cell proliferation (24). In accordance with these findings we demonstrate both an upregulation of cyclin-D levels and increased mitogenic signaling through ERK in HCC cells on stiff substrates. Interestingly, reduced ERK activation has previously been linked to cellular quiescence in a cell line-specific model of cancer dormancy (25). Additionally, for the first time, we demonstrate a role for matrix stiffness in modulating the activation of the STAT3 pathway. STAT3 has recently been identified as a central component in tumor progression and a potential target of cancer therapy in HCC and other epithelial malignancies (26). The STAT3 pathway is activated in response to multiple cytokines and growth factors during cancer-associated inflammation (e.g. interleukin-6, interleukin-10, epidermal growth factor and HGF). Our findings demonstrate that matrix stiffness has a substantial impact upon the intrinsic and extrinsic (growth factor-induced) activation of the STAT3 pathway. This indicates an additional role for biophysical factors in regulating this critical signaling pathway. Interactions between pathways conveying information from both soluble mediators and the ECM are integrated at the level of the cytoskeleton. In this context, the role of cytoskeletal tension has been likened to a cellular rheostat, acting to dampen or augment the responses of multiple signaling pathways to growth factor stimulation, thereby blocking or facilitating mitogenic responses.
It has been proposed that the ECM is a critical regulator of cellular dormancy (27); however the role of matrix stiffness in regulating this process has not been specifically addressed. The growth, invasion and dissemination of tumor cells are accompanied by dramatic changes in the mechanical properties (stiffness) of the cancer cell niche. The bone marrow, a common reservoir site for disseminated tumor cells, provides a microenvironment with stiffness significantly lower than that encountered in most epithelial tumors (28). Our findings suggest that a reduction in the stiffness of the cancer cell niche would be sufficient to promote reversible cellular quiescence (dormancy). Furthermore, increases in environmental stiffness (as may occur with inflammation, surgery or stromal reaction to tumor) or alteration in the stiffness-sensing machinery of the cell (as a result of genomic instability) might facilitate reactivation. Indeed, early work on cancer cell dormancy in animal models established inflammation and surgical trauma as a mechanism of reactivation of dormant cells (29, 30). More recently, fibrosis-associated collagen-I has been linked to reactivation of tumor cells in an in vivo model of cancer cell dormancy (31). With respect to the liver, tumor growth and intrahepatic metastasis have been shown to be enhanced in a fibrotic environment (20–22).
The precise phenotype of disseminated tumor cells derived from epithelial malignancies remains poorly defined (32). During tumor dissemination, tumor cells are believed to acquire mesenchymal properties, enabling them to migrate through and invade surrounding tissues and enter the bloodstream (33). However, at secondary sites, tumor cells are primarily detected by their epithelial characteristics and outgrowing metastases recapitulate the epithelial phenotype of the primary tumor (34). Despite our increasing understanding of the regulation of epithelial-to-mesenchymal-transition, the reverse process – mesenchymal-to-epithelial-transition – is largely uncharacterized. We have demonstrated that HCC cells lose mesenchymal features; including stress-fibers, N-cadherin and vimentin expression, and up-regulate markers of hepatocyte differentiation when maintained in a soft environment. This is consistent with previous findings showing that non-transformed mammary epithelial cells revert to an organized epithelial phenotype in a soft environment (6), and that hepatocytes retain an epithelial phenotype on soft collagen gels (35). FAK activation has been implicated in the process of epithelial-to-mesenchymal-transition and responsiveness to TGFβ (36). It remains unclear whether reduced FAK activation and TGFβ signaling in cells in a low stiffness environment is a mechanistic link to mesenchymal-to-epithelial-transition.
The high rate of chemotherapy resistance in HCC is a major obstacle in treating patients with advanced disease. Identifying the mechanism of this resistance has the potential to reveal new treatment options for this group of patients. We have provided evidence that increasing ECM stiffness – as encountered by cells within an established tumor (4) – reduces chemotherapy-induced-apoptosis. However, the clinical utility of systemic chemotherapy is also limited by the failure of adjuvant/neoadjuvant chemotherapy to target disseminated tumor cells that give rise to late tumor recurrence and metastases (32, 37). Intriguingly, we have been able to demonstrate an increase in clone-initiating capability following chemotherapy in cells from a low stiffness environment. This was accompanied by an increase of cells positive for cancer stem cell markers (CD44, CD133, c-kit, CXCR-4, OCT4 and NANOG) (38). This provides a potential mechanism for long-term survival and clone-initiating capability of disseminated tumor cells in a soft environment (e.g. bone marrow) following chemotherapy. Whether the higher frequency of cells with a cancer stem cell phenotype is due to positive selection or active induction of cancer stem cell characteristics needs to be determined.
In summary, we have provided evidence that the biomechanical composition of the ECM is a critical regulator of HCC behavior. We suggest that the high stiffness environment encountered in chronic fibrotic liver disease fosters HCC progression by promoting cellular proliferation, a mesenchymal phenotype and resistance to chemotherapy. Conversely, a soft physiological environment (as might be encountered by a disseminated tumor cell) induces cellular dormancy, a stem cell phenotype and enhanced clonogenic capacity following chemotherapy. Indeed, we propose that alterations in the stiffness of the cancer cell niche are responsible for regulating cancer cell proliferation and phenotype throughout the natural history of HCC. Manipulation of environmental stiffness or interference with the stiffness-sensing apparatus of HCC cells has the potential to impede both tumor growth and reactivation of dormant tumor cells, thereby limiting recurrence. In concert with future in vivo models of HCC, these findings will provide a platform for the future design of therapies targeting the biomechanical properties of the cancer cell niche.
Supplementary Material
Graphs showing a time-course analysis of cellular spreading in Huh7 (A) and HepG2 (B) cells cultured on soft (1kPa) and stiff (12kPa) collagen-I-coated polyacrylamide gel supports in low serum conditions. In each case, the surface area (square microns) of cells was calculated by digital image analysis of phase-contrast images of cells on polyacrylamide gel supports. In each case, values reflect the mean (±SEM) of measurements from 50 cells in three independent experiments (*p<0.05, **p<0.01 and ***p<0.001). In a control experiment, cellular spreading was measured in the presence of low dose mitomycin c (as indicated) in order to assess whether differences in cellular proliferation between polyacrylamide gel supports were sufficient to account for the observed differences in cellular spreading. The dose of mitomycin c used (Huh7 100nM/ HepG2 200nM) was informed by dose-finding experiments (data not shown).
Graphs showing the MTT assay measurements of Huh7 (A) and HepG2 (B) cells cultured for 5-days on collagen-I-coated polyacrylamide gel supports across a range of stiffness values (1–12kPa), as indicated and collagen-I-coated glass (n=3). Error bars are SEM, *p<0.05. In each case, error bars represent SEM, *p<0.05, **p<0.01 and ***p<0.001.
Primary murine hepatocytes were cultured on collagen-I-coated polyacrylamide gels across a range of stiffness values (1–12kPa) corresponding to the range of stiffness values encountered in normal and fibrotic livers. The photomicrographs show phase-contrast images (×100 magnification) of primary murine hepatocytes cultured on (A) soft (1kPa) and (B) stiff (12kPa) supports. (C) Graph showing the effect of matrix stiffness on the proliferation of primary murine hepatocytes. Proliferation was assessed by measurement of the mean number of Ki67 positive cells per low power field after 48 hours in culture. Cells were cultured on both soft (1kPa-white) and stiff (12kPa-black) collagen-I-coated polyacrylamide supports. Error bars represent SEM, p values are as indicated.
Graph showing the mean proliferative index (Ki67 positivity) of Huh7 and HepG2 cells re-plated on tissue culture plastic following 5 days culture on either soft (1kPa- white) or stiff (12kPa-black) collagen-I-coated polyacrylamide gel supports (n=3). Proliferative index (Ki67-positive cells/ total cells) was measured 2 days after re-plating. In each case, error bars are SEM, ns indicates not statistically significant. Upon transfer to a stiff matrix (tissue culture plastic) HCC cells from a soft (1kPa) support resume proliferation to levels comparable to cells coming from a stiff (12kPa) support.
Graphs showing the effect of cell plating density on the mean proliferative index (Ki67 positivity) of Huh7 (A) and HepG2 (B) cells cultured on soft (1kPa- white) or stiff (12kPa-black) collagen-I-coated polyacrylamide gel supports. Huh7 and HepG2 cells were plated at 3 different plating densities (1000–25,000cm2), as indicated and the proliferative index was calculated following 48 hours culture (n=3 for each condition). Error bars are SEM, *p<0.05. In each case, error bars represent SEM, *p<0.05, **p<0.01 and ***p<0.001.
Graphs showing the projected surface area (square microns) of (A) Huh7 and (B) HepG2 cells on soft (1kPa- white) and stiff (12kPa- black) polyacrylamide gel supports coated with different ECM components: collagen-I, collagen-IV, laminin and fibronectin (as indicated). Projected surface area was calculated by digital image analysis of phase-contrast images. In each case, values reflect the mean (±SEM) of measurements from 50 cells in three independent experiments (*p<0.05, **p<0.01 and ***p<0.001).
Western blots showing a time-course analysis for expression of phosphorylated and total extracellular-regulated kinase (ERK), protein kinase B (PKB/ Akt) and signal transducer and activator of transcription 3 (STAT3) in HepG2 cells cultured on soft (1kPa) and stiff (12kPa) supports. Whole cell lysates were harvested at baseline and at specific timepoints (as indicated) following the addition of hepatocyte growth factor (HGF) (10ng/ml) to culture media. The line graphs (right panel) show a schematic representation of densitometry analysis of phosphorylated ERK, PKB/ Akt and STAT3 expressed relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Each time-point represents the mean of three independent experiments.
Western blots showing expression of β1-integrin and focal adhesion kinase (FAK) in (A) Huh7 and (B) HepG2 cells that were either left untreated or transfected with siRNA for β1-integrin, FAK or control (scrambled) sequences (as indicated). Western blots show the relevant protein expression at 72 hours following transfection. Graphs showing the mean proliferative index (Ki67-positivity) of (C) Huh7 and (D) HepG2 cells cultured on soft (1kPa) and stiff (12kPa) collagen-I-coated polyacrylamide gel supports following transfection procedure (as indicated). Transfected cells and untreated controls were trypsinized and transferred to polyacrylamide gel supports at 48 hours following the transfection procedure. Cells were fixed at 72 hours and proliferation assessed using nuclear localization of Ki67 antigen (n=5). In each case, values reflect the mean (±SEM), ns= not significant, *p<0.05, **p<0.01 and ***p<0.001.
(A) Graph showing projected surface area (square microns) of LNCap (prostate), A549 (lung adenocarcinoma), MCF7 (breast), MDA (breast), HepG2 (HCC) and Huh7 (HCC) epithelial cancer cells calculated by digital image analysis of phase-contrast images of cells on soft (1kPa- white) and stiff (12kPa- black) polyacrylamide gel supports. In each case, values reflect the mean (±SEM) of measurements from 50 cells in three independent experiments (*p<0.05, **p<0.01 and ***p<0.001). (B) Graph showing the mean proliferative index (Ki67 positivity) of human epithelial cancer cell lines (as detailed above) cultured on soft (1kPa- white) and stiff (12kPa- black) collagen-I-coated polyacrylamide supports. In each case, values reflect the mean (±SEM) of measurements from three independent experiments (***p<0.001).
Acknowledgments
Financial support:
This study was supported by a fellowship from the Deutsche Forschungsgemeinschaft to JS (SCHR1213/1), by grants from the Medical Research Council to TTGW (G07000582) and JPI (G0600033), by a grant from the NIH (DK 058123) to RGW and by a fellowship from the European Association for the Study of the Liver to MD.
List of Abbreviations
- HCC
Hepatocellular Carcinoma
- HGF
Hepatocyte Growth Factor
- ERK
Extracellular Regulated Kinase
- PKB/ Akt
Protein Kinase B
- STAT3
Signal Transducer and Activator of Transcription 3
- OCT4
Octamer-4
- ECM
Extracellular Matrix
- TGFβ
Transforming Growth Factor beta
- PA
Polyacrylamide
- DAPI
4’, 6’-diamidino-2-phenyl-indole dihydrochloride
- HNF4α
Hepatocyte Nuclear Factor 4 alpha
- AFP
Alpha fetoprotein
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- MTT
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
- FAK
Focal Adhesion Kinase
- PARP
Poly-ADP-ribose polymerase
- 5-FU
5-Fluorouracil
Contributor Information
Jörg Schrader, Email: jschrade@uke.uni-hamburg.de.
Timothy T Gordon-Walker, Email: timgordonwalker@yahoo.co.uk.
Rebecca L Aucott, Email: raucott@staffmail.ed.ac.uk.
Mariëlle van Deemter, Email: mvandeemter@gmail.com.
Alexander Quaas, Email: a.quaas@uke.de.
Shaun Walsh, Email: shaun.walsh@nhs.net.
Daniel Benten, Email: d.benten@gmx.net.
Stuart J Forbes, Email: stuart.forbes@ed.ac.uk.
Rebecca G Wells, Email: rgwells@mail.med.upenn.edu.
John P Iredale, Email: John.Iredale@ed.ac.uk.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94(2):153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5) Suppl 1:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Masuzaki R, Tateishi R, Yoshida H, Goto E, Sato T, Ohki T, et al. Prospective risk assessment for hepatocellular carcinoma development in patients with chronic hepatitis C by transient elastography. Hepatology. 2009;49(6):1954–1961. doi: 10.1002/hep.22870. [DOI] [PubMed] [Google Scholar]
- 4.Masuzaki R, Tateishi R, Yoshida H, Sato T, Ohki T, Goto T, et al. Assessing liver tumor stiffness by transient elastography. Hepatol Int. 2007;1(3):394–397. doi: 10.1007/s12072-007-9012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingber DE. Can cancer be reversed by engineering the tumor microenvironment? Semin Cancer Biol. 2008;18(5):356–364. doi: 10.1016/j.semcancer.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowak AK, Chow PK, Findlay M. Systemic therapy for advanced hepatocellular carcinoma: a review. Eur J Cancer. 2004;40(10):1474–1484. doi: 10.1016/j.ejca.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 9.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver; J Hepatol; 2001. pp. 421–430. [DOI] [PubMed] [Google Scholar]
- 10.Mathurin P, Raynard B, Dharancy S, Kirzin S, Fallik D, Pruvot FR, et al. Meta-analysis: evaluation of adjuvant therapy after curative liver resection for hepatocellular carcinoma. Aliment Pharmacol Ther. 2003;17(10):1247–1261. doi: 10.1046/j.1365-2036.2003.01580.x. [DOI] [PubMed] [Google Scholar]
- 11.Sethi T, Rintoul RC, Moore SM, MacKinnon AC, Salter D, Choo C, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med. 1999;5(6):662–668. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 12.Conti JA, Kendall TJ, Bateman A, Armstrong TA, Papa-Adams A, Xu Q, et al. The desmoplastic reaction surrounding hepatic colorectal adenocarcinoma metastases aids tumor growth and survival via alphav integrin ligation. Clin Cancer Res. 2008;14(20):6405–6413. doi: 10.1158/1078-0432.CCR-08-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86(1 Pt 1):617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94(25):13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kendall TJ, Hennedige S, Aucott RL, Hartland SN, Vernon MA, Benyon RC, et al. p75 Neurotrophin receptor signaling regulates hepatic myofibroblast proliferation and apoptosis in recovery from rodent liver fibrosis. Hepatology. 2009;49(3):901–910. doi: 10.1002/hep.22701. [DOI] [PubMed] [Google Scholar]
- 16.Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, et al. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293(6):G1147–G1154. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 17.Patriarca C, Roncalli M, Gambacorta M, Cominotti M, Coggi G, Viale G. Patterns of integrin common chain beta 1 and collagen IV immunoreactivity in hepatocellular carcinoma. Correlations with tumour growth rate, grade and size. J Pathol. 1993;171(1):5–11. doi: 10.1002/path.1711710104. [DOI] [PubMed] [Google Scholar]
- 18.Fujii T, Koshikawa K, Nomoto S, Okochi O, Kaneko T, Inoue S, et al. Focal adhesion kinase is overexpressed in hepatocellular carcinoma and can be served as an independent prognostic factor. J Hepatol. 2004;41(1):104–111. doi: 10.1016/j.jhep.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 19.Castera L, Vergniol J, Foucher J, Le BB, Chanteloup E, Haaser M, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128(2):343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Kornek M, Raskopf E, Tolba R, Becker U, Klockner M, Sauerbruch T, et al. Accelerated orthotopic hepatocellular carcinomas growth is linked to increased expression of pro-angiogenic and prometastatic factors in murine liver fibrosis. Liver Int. 2008;28(4):509–518. doi: 10.1111/j.1478-3231.2008.01670.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuriyama S, Yamazaki M, Mitoro A, Tsujimoto T, Kikukawa M, Tsujinoue H, et al. Hepatocellular carcinoma in an orthotopic mouse model metastasizes intrahepatically in cirrhotic but not in normal liver. Int J Cancer. 1999;80(3):471–476. doi: 10.1002/(sici)1097-0215(19990129)80:3<471::aid-ijc22>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Osada S, Kanematsu M, Imai H, Goshima S, Sugiyama Y. Hepatic fibrosis influences the growth of hepatocellular carcinoma. Hepatogastroenterology. 2008;55(81):184–187. [PubMed] [Google Scholar]
- 23.Ulrich TA, de Juan Pardo EM, Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009;69(10):4167–4174. doi: 10.1158/0008-5472.CAN-08-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein EA, Campbell LE, Kothapalli D, Fournier AK, Assoian RK. Joint requirement for Rac and ERK activities underlies the mid-G1 phase induction of cyclin D1 and S phase entry in both epithelial and mesenchymal cells. J Biol Chem. 2008;283(45):30911–30918. doi: 10.1074/jbc.M804537200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aguirre Ghiso JA, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol. 1999;147(1):89–104. doi: 10.1083/jcb.147.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedvat M, Huszar D, Herrmann A, Gozgit JM, Schroeder A, Sheehy A, et al. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 2009;16(6):487–497. doi: 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barkan D, Green JE, Chambers AF. Extracellular matrix: a gatekeeper in the transition from dormancy to metastatic growth. Eur J Cancer. 2010;46(7):1181–1188. doi: 10.1016/j.ejca.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levental I, Georges PC, Janmey PA. Soft biological materials and their influence on cell function. Soft Matter. 2007;3:299–306. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- 29.Fisher B, Fisher ER. Experimental evidence in support of the dormant tumor cell. Science. 1959;130:918–919. doi: 10.1126/science.130.3380.918. [DOI] [PubMed] [Google Scholar]
- 30.Agostino D, Cliffton EE. Organ localization and the effect of trauma on the fate of circulating cancer cells. Cancer Res. 1965;25(10):1728–1732. [PubMed] [Google Scholar]
- 31.Barkan D, El Touny LH, Michalowski AM, Smith JA, Chu I, Davis AS, et al. Metastatic Growth from Dormant Cells Induced by a Col-I-Enriched Fibrotic Environment. Cancer Res. 2010 doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vessella RL, Pantel K, Mohla S. Tumor cell dormancy: an NCI workshop report. Cancer Biol Ther. 2007;6(9):1496–1504. doi: 10.4161/cbt.6.9.4828. [DOI] [PubMed] [Google Scholar]
- 33.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28(1–2):15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 34.Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39(3):305–318. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- 35.Godoy P, Hengstler JG, Ilkavets I, Meyer C, Bachmann A, Muller A, et al. Extracellular matrix modulates sensitivity of hepatocytes to fibroblastoid dedifferentiation and transforming growth factor beta-induced apoptosis. Hepatology. 2009;49(6):2031–2043. doi: 10.1002/hep.22880. [DOI] [PubMed] [Google Scholar]
- 36.Cicchini C, Laudadio I, Citarella F, Corazzari M, Steindler C, Conigliaro A, et al. TGFbeta-induced EMT requires focal adhesion kinase (FAK) signaling. Exp Cell Res. 2008;314(1):143–152. doi: 10.1016/j.yexcr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Naumov GN, Townson JL, MacDonald IC, Wilson SM, Bramwell VH, Groom AC, et al. Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late-developing metastases. Breast Cancer Res Treat. 2003;82(3):199–206. doi: 10.1023/B:BREA.0000004377.12288.3c. [DOI] [PubMed] [Google Scholar]
- 38.Lee TK, Castilho A, Ma S, Ng IO. Liver cancer stem cells: implications for a new therapeutic target. Liver Int. 2009;29(7):955–965. doi: 10.1111/j.1478-3231.2009.02040.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Graphs showing a time-course analysis of cellular spreading in Huh7 (A) and HepG2 (B) cells cultured on soft (1kPa) and stiff (12kPa) collagen-I-coated polyacrylamide gel supports in low serum conditions. In each case, the surface area (square microns) of cells was calculated by digital image analysis of phase-contrast images of cells on polyacrylamide gel supports. In each case, values reflect the mean (±SEM) of measurements from 50 cells in three independent experiments (*p<0.05, **p<0.01 and ***p<0.001). In a control experiment, cellular spreading was measured in the presence of low dose mitomycin c (as indicated) in order to assess whether differences in cellular proliferation between polyacrylamide gel supports were sufficient to account for the observed differences in cellular spreading. The dose of mitomycin c used (Huh7 100nM/ HepG2 200nM) was informed by dose-finding experiments (data not shown).
Graphs showing the MTT assay measurements of Huh7 (A) and HepG2 (B) cells cultured for 5-days on collagen-I-coated polyacrylamide gel supports across a range of stiffness values (1–12kPa), as indicated and collagen-I-coated glass (n=3). Error bars are SEM, *p<0.05. In each case, error bars represent SEM, *p<0.05, **p<0.01 and ***p<0.001.
Primary murine hepatocytes were cultured on collagen-I-coated polyacrylamide gels across a range of stiffness values (1–12kPa) corresponding to the range of stiffness values encountered in normal and fibrotic livers. The photomicrographs show phase-contrast images (×100 magnification) of primary murine hepatocytes cultured on (A) soft (1kPa) and (B) stiff (12kPa) supports. (C) Graph showing the effect of matrix stiffness on the proliferation of primary murine hepatocytes. Proliferation was assessed by measurement of the mean number of Ki67 positive cells per low power field after 48 hours in culture. Cells were cultured on both soft (1kPa-white) and stiff (12kPa-black) collagen-I-coated polyacrylamide supports. Error bars represent SEM, p values are as indicated.
Graph showing the mean proliferative index (Ki67 positivity) of Huh7 and HepG2 cells re-plated on tissue culture plastic following 5 days culture on either soft (1kPa- white) or stiff (12kPa-black) collagen-I-coated polyacrylamide gel supports (n=3). Proliferative index (Ki67-positive cells/ total cells) was measured 2 days after re-plating. In each case, error bars are SEM, ns indicates not statistically significant. Upon transfer to a stiff matrix (tissue culture plastic) HCC cells from a soft (1kPa) support resume proliferation to levels comparable to cells coming from a stiff (12kPa) support.
Graphs showing the effect of cell plating density on the mean proliferative index (Ki67 positivity) of Huh7 (A) and HepG2 (B) cells cultured on soft (1kPa- white) or stiff (12kPa-black) collagen-I-coated polyacrylamide gel supports. Huh7 and HepG2 cells were plated at 3 different plating densities (1000–25,000cm2), as indicated and the proliferative index was calculated following 48 hours culture (n=3 for each condition). Error bars are SEM, *p<0.05. In each case, error bars represent SEM, *p<0.05, **p<0.01 and ***p<0.001.
Graphs showing the projected surface area (square microns) of (A) Huh7 and (B) HepG2 cells on soft (1kPa- white) and stiff (12kPa- black) polyacrylamide gel supports coated with different ECM components: collagen-I, collagen-IV, laminin and fibronectin (as indicated). Projected surface area was calculated by digital image analysis of phase-contrast images. In each case, values reflect the mean (±SEM) of measurements from 50 cells in three independent experiments (*p<0.05, **p<0.01 and ***p<0.001).
Western blots showing a time-course analysis for expression of phosphorylated and total extracellular-regulated kinase (ERK), protein kinase B (PKB/ Akt) and signal transducer and activator of transcription 3 (STAT3) in HepG2 cells cultured on soft (1kPa) and stiff (12kPa) supports. Whole cell lysates were harvested at baseline and at specific timepoints (as indicated) following the addition of hepatocyte growth factor (HGF) (10ng/ml) to culture media. The line graphs (right panel) show a schematic representation of densitometry analysis of phosphorylated ERK, PKB/ Akt and STAT3 expressed relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Each time-point represents the mean of three independent experiments.
Western blots showing expression of β1-integrin and focal adhesion kinase (FAK) in (A) Huh7 and (B) HepG2 cells that were either left untreated or transfected with siRNA for β1-integrin, FAK or control (scrambled) sequences (as indicated). Western blots show the relevant protein expression at 72 hours following transfection. Graphs showing the mean proliferative index (Ki67-positivity) of (C) Huh7 and (D) HepG2 cells cultured on soft (1kPa) and stiff (12kPa) collagen-I-coated polyacrylamide gel supports following transfection procedure (as indicated). Transfected cells and untreated controls were trypsinized and transferred to polyacrylamide gel supports at 48 hours following the transfection procedure. Cells were fixed at 72 hours and proliferation assessed using nuclear localization of Ki67 antigen (n=5). In each case, values reflect the mean (±SEM), ns= not significant, *p<0.05, **p<0.01 and ***p<0.001.
(A) Graph showing projected surface area (square microns) of LNCap (prostate), A549 (lung adenocarcinoma), MCF7 (breast), MDA (breast), HepG2 (HCC) and Huh7 (HCC) epithelial cancer cells calculated by digital image analysis of phase-contrast images of cells on soft (1kPa- white) and stiff (12kPa- black) polyacrylamide gel supports. In each case, values reflect the mean (±SEM) of measurements from 50 cells in three independent experiments (*p<0.05, **p<0.01 and ***p<0.001). (B) Graph showing the mean proliferative index (Ki67 positivity) of human epithelial cancer cell lines (as detailed above) cultured on soft (1kPa- white) and stiff (12kPa- black) collagen-I-coated polyacrylamide supports. In each case, values reflect the mean (±SEM) of measurements from three independent experiments (***p<0.001).