SUMMARY
Animals reared in hypoxic environments frequently exhibit smaller body sizes than when reared under normal atmospheric oxygen concentrations. The mechanisms responsible for this widely documented pattern of body size plasticity are poorly known. We studied the ontogeny of responses of Drosophila melanogaster adult body size to hypoxic exposure. We hypothesized that there may be critical oxygen-sensitive periods during D. melanogaster development that are primarily responsive to body size regulation. Instead, our results showed that exposure to hypoxia (an atmospheric partial pressure of oxygen of 10 kPa) during any developmental stage (embryo, larvae and pupae) leads to smaller adult size. However, short hypoxic exposures during the late larval and early pupal stages had the greatest effects on adult size. We then investigated whether the observed reductions in size induced by hypoxia at various developmental stages were the result of a decrease in cell size or cell number. Abdominal epithelial cells of flies reared continuously in hypoxia were smaller in mean diameter and were size-limited compared with cells of flies reared in normoxia. Flies reared in hypoxia during the embryonic, larval or pupal stage, or during their entire development, had smaller wing areas than flies reared in normoxia. Flies reared during the pupal stage, or throughout development in hypoxia had smaller wing cells, even after controlling for the effect of wing size. These results suggest that hypoxia effects on the body size of D. melanogaster probably occur by multiple mechanisms operating at various developmental stages.
KEY WORDS: oxygen, development, size
INTRODUCTION
Animals are exposed to hypoxia in a variety of natural environments, including high altitude, eutrophic water, flooding and when living within dense organic substrates such as soil, dung or other organisms (Hoback and Stanley, 2001). Hypoxic exposure leads to depressed growth rates and extends development times in a wide range of vertebrate and invertebrate animals, including humans (Frisancho and Paul, 1970; Harrison et al., 2006; Owerkowicz et al., 2009). Drosophila melanogaster body mass is positively and linearly correlated with atmospheric oxygen level from 4 to 21 kPa atmospheric partial pressure of oxygen (PO2) (Peck and Maddrell, 2005; Klok et al., 2009; Harrison and Haddad, 2011). In addition to reducing overall body mass, hypoxic exposure has also been shown to reduce thorax length and wing size (Frazier et al., 2001). The regulatory mechanisms responsible for these hypoxia-mediated effects on growth and body size remain unclear, but have become an area of increasing research interest, particularly in genetic model organisms such as Mus musculus and D. melanogaster (Dekanty et al., 2005; Aragones et al., 2008). Identification of a critical hypoxiasensitive stage during development may substantially speed the search for the mechanisms responsible for the hypoxia sensitivity of growth. In this study we examined the effect of hypoxia (10 kPa PO2) during specific developmental stages and ages on body and cell size in D. melanogaster.
Exposure to hypoxia may affect growth and body size characteristics through multiple overlapping mechanisms, including direct limitation of ATP production (either generally or in specific tissues), via signaling pathways that affect behaviors such as feeding and cellular growth control systems, or by inducing cardiorespiratory remodeling with costs to growth of other tissues (Harrison and Haddad, 2011). In D. melanogaster, direct suppression of whole-body metabolic rate is unlikely at 10 kPa PO2 because critical PO2 values (the oxygen value at which function becomes oxygen limited) for metabolic rate and locomotion are below 4 kPa PO2 in both larvae and adults (Klok et al., 2010; Van Voorhies, 2009). Metabolic rates of larval and adult flies reared for seven generations in 10 kPa PO2 are similar to those reared in normoxia (Klok et al., 2010). Thus, the suppressing effects of moderate hypoxia on growth and development in D. melanogaster seem to be the result of signal transduction pathways, not ATP limitations.
A variety of signaling pathways that affect cell and body size are known to be hypoxia sensitive in animals. One relatively well-studied growth regulating system is the insulin pathway, which can interact with hypoxia-inducible factors (HIFs) to suppress growth, cell size and cell number during hypoxia (Brugarolas et al., 2004; Gorr et al., 2006; Dekanty et al., 2005). Flies reared in 7.5 kPa PO2 have smaller wing cells and reduced wing cell numbers compared with normoxia-reared flies, suggesting that such a mechanism functions at moderate levels of hypoxia (Peck and Maddrell, 2005). In D. melanogaster HIF is involved in the reduction of cellular growth of somatic tissues by at least two mechanisms. First, HIF acts to block the ability of insulin to stimulate protein synthesis, reducing growth and cell size at the whole body level (Reiling and Hafen, 2004). Second, stimulation of cellular growth by the complex of cyclin D and cyclin-dependent protein kinase 4 was found to operate via the activation of HIF prolyl hydroxylase (encoded by the gene Hph; also known as fatiga) (Frei and Edgar, 2004). Unambiguous demonstration of the role of HIF in this growth control was demonstrated by showing that overexpression of the HIF-α gene [i.e. similar (sima) in Drosophila] is sufficient to cause a marked autonomous reduction of cell size in the fat body (Centanin et al., 2005; Dekanty et al., 2005). Low oxygen levels reduce the capacity of prolyl hydroxylase to hydroxylate SIMA protein, resulting in stabilization of HIF and depression of cell growth (Centanin et al., 2005). Stabilized HIF can also suppress tissue growth and cell size by causing expression of the genes scylla and charybdis. These are genes that inhibit growth by downregulating protein synthesis (Reiling and Hafen, 2004).
To identify the mechanisms responsible for influencing body size in hypoxia, it would be useful to determine the temporal sensitivity of this regulation. Peck and Maddrell reported that immature D. melanogaster switched from hypoxic to normoxic environments during development achieve normal adult size unless switched during the pupal period, suggesting that the pupal stage is the critical ‘oxygen-sensitive’ period for determining adult size in D. melanogaster (Peck and Maddrell, 2005). Together with the observation that hypoxia-reared adults had fewer and smaller cells, these findings suggest that exposure to low PO2 influences adult size in D. melanogaster by causing a decrease in pupal tissue PO2, with subsequent effects on cell-cycle activity. However, in another study, larval mass was reduced under hypoxic conditions (10 kPa PO2), suggesting that growth in the larval stage is also sensitive to hypoxia (Henry and Harrison, 2004). Feeding rate was also suppressed for D. melanogaster larvae in 10 kPa PO2 (Frazier et al., 2001), again, suggesting that the larval stage should be sensitive to hypoxia. Because the resources available for imaginal disc generation and adult size are acquired only during larval development, the effects of PO2 on adult size could be mediated by effects on larval feeding, instar length, ATP production or cell cycle activity.
The seemingly conflicting identification of larval vs pupal stages as critical oxygen-sensitive periods (Peck and Maddrell, 2005; Harrison and Henry, 2004) may result from differences in experimental design. In the Peck and Maddrell study, changes in oxygen treatment occurred at set days rather than at particular developmental landmarks, making it difficult to determine the exact developmental stages at which the animals were exposed (Peck and Maddrell, 2005). Also, although the experiments of Peck and Maddrell suggested that larvae are insensitive to hypoxia, these authors were careful to point out that their findings did not demonstrate that larval growth was unaffected by hypoxia, only that they were able to recover from the early exposure to hypoxia. To resolve these issues, we repeated the Peck and Maddrell experimental design, and also developed some additional experiments to tease apart the oxygen-sensitivity of the various ages and stages of this insect (Peck and Maddrell, 2005).
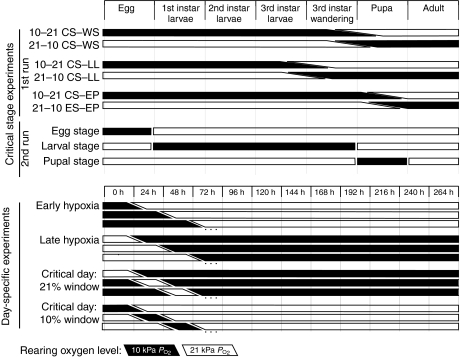
We conducted four types of experiments (Fig. 1) to distinguish the temporal stages during development in which sensitivity to PO2 may influence adult body size. As performed by Peck and Maddrell, we switched D. melanogaster from a hypoxic to a normoxic environment at various ages; a procedure we designate as the early hypoxia (EH) experiments (Peck and Maddrell, 2005). We also conducted the reverse experiment, switching D. melanogaster from a normoxic to a hypoxic environment; experiments we designate as late hypoxia (LH) experiments. We also conducted experiments in which PO2 was switched at specific developmental stages, so that hypoxia was only experienced during the embryonic, larval or pupal period [designated critical stage (CS) experiments]. Finally, we reared flies in normoxia (21 kPa PO2) or hypoxia (10 kPa) with the exception of a single day during development when they were reared at the alternative PO2 [critical day (CD) experiments]. All experiments focused on determining the effect of hypoxic exposure on adult body mass. In addition, we determined how hypoxic exposure affects epithelial cell size and number in the D. melanogaster wing and abdomen.
Fig. 1.
Diagram outlining the basic protocols for each experimental setup. Black bars indicate periods of hypoxic exposure and white bars indicate normoxic exposure. Sloping transitions between PO2 values indicate that in some experiments, animals varied in their developmental stage at the point of atmospheric transition because of the inter-individual variation in development rates within a population of flies. In the first run of the critical stage (CS) experiments, three protocols were performed. (1) The CS–WS (CS–wandering stage switch) experiments: animals were reared in either hypoxia or normoxia and switched to the converse oxygen treatment when approximately half of the larvae were exhibiting wandering behavior. (2) The CS–LL (CS–late larval switch) experiment: animals were switched when wandering behavior was observed, but no pupae were present. (3) The CS–EP (CS–early pupal switch) experiment: only animals that had pupated were switched, and any remaining larvae were discarded. In the second run of the CS experiments, animals were transferred individually to minimize variation in stages of exposure, and they experienced hypoxia during either the embryonic, larval or pupal stage. The numbers beside the treatment indicate the first and second atmospheric oxygen levels, respectively; for example the 10–21 CS–WS group experienced 10 kPa PO2 up to the late larval stage and 21 kPa PO2 during the pupal stage. In the early and late hypoxia (EH and LH) experiments, animals began development in either hypoxia or normoxia, and groups were switched to the converse oxygen treatment at varying time periods. In the critical day (CD) experiments, flies were reared throughout development in either hypoxia or normoxia, but, at various time periods, experienced 24 h exposures to the converse oxygen treatment. For the CD experiments, only the first three treatment groups of each experiment type are shown; ellipses indicate that each day of development was tested.
MATERIALS AND METHODS
Animals and general rearing conditions
Wild-type Drosophila melanogaster Meigen (Oregon-R; Carolina Biological Supply, Burlington, NC, USA) were reared for two generations on a standard yeast-based diet containing tetracycline and rifampicin to eliminate possible Wolbacchia infection. Fly stocks were maintained in 178 ml plastic bottles with 50 ml of medium or 35 ml glass vials with 25 mm of medium. Tegocept solution (methyl paraben; Genesee Scientific, San Diego, CA, USA) was incorporated into the medium to inhibit fungal growth. All stocks were reared in incubators at a temperature of 25±1°C and with 14 h:10 h light:dark photoperiod.
Experimental rearing of D. melanogaster in either hypoxia or normoxia took place in 76×45×45 cm polycarbonate polymer environmental chambers. PO2 within chambers was determined approximately every 30 min with a paramagnetic oxygen sensor and regulated by controlling the flow rate of oxygen or nitrogen into the chambers (ROXY-8, Sable Systems Inc., Las Vegas, NV, USA). Humidities in the environmental chambers were >95% (Hobo data logger, Onset Computer Corp., Bourne, MA, USA). To consistently determine adult masses, adults were anaesthetized with CO2 and weighed within 2 days of eclosion on a microbalance with a precision of 0.001 mg (Mettler MX 5, Toledo, Columbus, OH, USA). Because of the time limitations of these experiments (over 2000 flies were weighed), we were only able to analyze the responses of both sexes in a few of these experiments; for the others, we focused on males as these showed a more reliable change in mass with hypoxia.
Egg laying conditions and the control of larval density
For the first run of the CS experiments and the EH–LH experiments, three females and five males were collected from the stock culture and placed in glass vials with medium for 2 days, for mating and egg laying. During mating and egg-laying periods, flies were maintained in normoxic environments. Adults were discarded and vials containing progeny (late embryonic stage or early first instar) were transferred to controlled atmospheric oxygen conditions.
For the second run of the CS experiments and the CD experiments, approximately 300 flies were removed from our laboratory culture and placed into 0.5 l glass jars containing Petri dishes with a grape juice-based nutritional medium (Frazier et al., 2001). Flies were given 3 h to lay eggs on the medium, after which eggs were individually transferred into bottles with standard medium and separated randomly into treatment groups.
Variation in larval rearing density can significantly affect adult Drosophila body mass (Miller and Thomas, 1958). As a result, we took steps to maintain uniform larval density. In the second run of the CS experiment and all of the CD experiments, this was done by individually transferring equal numbers of eggs into each original treatment bottle. In other experiments, we controlled larval density by keeping the number of mating pairs per bottle and the duration of egg laying periods constant. These approaches typically left us with 50–100 larvae per bottle. Variation in larval density, from 5 to 500 larvae/bottle did not affect adult size (Rascón and Harrison, 2010), so it is unlikely that variation in larval density affected our experimental results.
Ontogeny of hypoxic sensitivity: CS experiments
The CS experiments exposed flies to acute periods of hypoxia during specific developmental stages (Fig. 1). Two runs of the CS experiments were performed. In the first run, all eggs were laid in normoxia, and only the oxygen sensitivity of the larval and pupal stages was tested. In the second run, oxygen sensitivity of embryonic, larval and pupal stages were investigated. All eggs were laid in normoxia except the constant-hypoxia and embryonic-hypoxia treatment groups, for which eggs were laid in 10 kPa PO2.
In the first run of the CS experiments, because individuals within a bottle exhibit variation in the time to pupation, we used three different protocols for switching the PO2 between 10 and 21 kPa PO2. In the CS–wandering stage switch (CS–WS) experiments, we switched the PO2 when approximately half of the individuals in the bottle exhibited wandering behavior, with the period before the switch designated ‘larval’ and the period after the switch designated ‘pupal’ (Fig. 1). The CS–WS experiments could not unequivocally distinguish larval and pupal oxygen sensitivities because some individuals were larvae and some had pupariated when the switch was made. Therefore, we also conducted CS–late larval switch (CS–LL) experiments in which we switched the PO2 when a majority of flies were exhibiting wandering behavior, but before any had initiated pupariation (Fig. 1). This ensured that none of the adults we measured experienced the test PO2 as pupae. We also conducted CS–early pupal switch (CS–EP) experiments, designed to ensure that none of the adults we measured experienced the test PO2 as larvae (Fig. 1). In the CS–EP experiments, the individuals were switched between treatment PO2 chambers when a large fraction of the flies in the vial were in pupal cases, but some larvae were still present. Prior to the change in oxygen levels, all wandering larvae were killed, and hot paraffin wax was poured over the medium to encapsulate remaining larvae.
In the second run of the CS experiments, we included examination of the sensitivity of the embryonic stage to PO2, including the PO2 experienced by the females during egg laying. We had treatment groups that experienced embryonic, larval or pupal hypoxia (10 kPa PO2), with their remaining development time spent in normoxia (Fig. 1). We also had control groups that experienced continuous hypoxia (during egg laying up to measurement of adult mass), or continuous normoxia. Instead of transferring whole vials of flies as occurred in the first CS run, animals were transferred individually as embryos or larvae, to ensure that each individual experienced the desired treatment conditions.
In the second run of the CS experiment, embryos were transferred in groups of 70 to culture bottles. Each treatment group contained three bottles. Twelve hours after egg laying, any early-hatching larvae were removed and discarded to ensure that all embryos utilized in the experiment were fertilized and laid during the experimental egg-laying period, and to ensure that embryos experienced at least 24 h of hypoxia. The remaining embryos were left in 10 kPa PO2 and switched to 21 kPa PO2 individually as they hatched into larvae. Bottles were checked at ≤12 h intervals to ensure that hatched larvae spent less than 12 h in hypoxia.
For the larval hypoxia group, hatched first instar larvae were transferred individually from bottles kept in normoxia to bottles kept in hypoxia using small metal spatulas. Bottles were checked at ≤12 h intervals to ensure that hatched larvae spent less than 12 h in normoxia. The first 35 larvae (half of the total population in the bottle) to pupate were discarded. The remaining larvae were transferred to a new bottle and placed back into normoxia until adult eclosion. For the pupal hypoxia group, 35 wandering-stage larvae per bottle were transferred from normoxia to hypoxia, in which they developed until eclosion.
Ontogeny of hypoxic sensitivity: EH vs LH experiments
In these experiments, after 2 days for egg laying, adult flies were removed from the vials and the progeny were reared first in either hypoxia (10 kPa PO2) or normoxia (21 kPa PO2). They were subsequently switched to the alternative PO2 after a specific period of time. The EH experiments had a very similar protocol to that used by Peck and Maddrell (Peck and Maddrell, 2005). Flies were reared for a varying number of hours in hypoxic conditions before being switched to normoxia (Fig. 1). For the LH experiments, flies were reared for a varying number of hours in normoxia and then were switched to hypoxia (Fig. 1). One vial (∼30 flies) was switched at each of the eight switch times: 12, 24, 72, 120, 168, 216, 264 and 312 h following the removal of the adults. Control flies were also reared continuously in either hypoxia or normoxia.
Ontogeny of hypoxic sensitivity: CD experiments
To test for critical periods, particularly within the larval or pupal stage, we examined the effect of changing PO2 for 24 h periods during development. Flies were reared at constant normoxia except for one 24 h period of 10 kPa PO2, or at constant 10 kPa PO2 with one 24 h period of normoxia (Fig. 1). Each treatment had three replicates, with 100 eggs per replicate bottle.
Bottles were transferred to the test PO2 quickly after embryos were collected; the maximum age of any embryo before oxygen treatment was 3 h. At the beginning and end of each of the test 24 h period, the flies were observed and the approximate developmental stage for the treatment groups (embryo, larval or pupal) was noted.
Hypoxic rearing effects on abdominal epithelial cell size
Approximately 50 adult flies were placed into each of ten bottles, with five bottles kept in normoxia and five bottles kept in 10 kPa PO2. Adults were removed after 2 days of mating and egg laying. Two days after new adults eclosed, they were transferred to fresh bottles for an additional 2 days, so that the measurements were made on 4- to 5-day-old adults. From each treatment group, 25 males were cold-anesthetized, weighed and stored overnight at 4°C. The next day, the abdomens were removed and fixed in alcoholic Bouin's fixative (with 1% dimethylsulphoxide). After 3 days of fixation, specimens were washed with 95% ethanol and dehydrated using 95% and absolute n-butanol then cleared with xylene, infiltrated with paraffin and embedded in labeled paraffin blocks. Dorsoventral sections (5 μm thick) were cut with a Surgipath microtome and stained with Hematoxylin, Toluidine Blue and Eosin. Cells were imaged using a compound microscope with a ×100 objective and a digital camera attachment. Regions of the continuous row of abdominal epithelial cells were measured (Able Image Analyzer; Mu Labs, Ljubljana, Slovenia) and divided by the number of cells to estimate average cell width.
Effect of developmental stage on the response of wing cell size and cell number to hypoxia
To test the effect of hypoxia on cell size and number during specific developmental stages, we used the flies from the second run of the CS experiments. Wings were removed and mounted onto glass microscope slides using Cytoseal™ low viscosity mounting medium (Richard Allan Scientific, Kalamazoo, MI, USA). Images were taken at ×250 and ×400 magnification using a compound microscope (Carl Zeiss Axio Imager D1 Digital Imaging Microscope; Carl Zeiss Microimaging, Inc., Thornwood, NY, USA). Wing area and wing cell area were determined using a graticule calibration using ImageJ. Outlines of the entire wing were used to determine wing area. Trichome densities were measured to find the average cell size in the second posterior distal wing cell (Partridge et al., 1994). Because each hair on the D. melanogaster wing corresponds to a single cell, the area of a box drawn in the second posterior distal region was measured and divided by the number of hairs within the box to calculate average cell areas. To find the average cell number, the total area of the second posterior distal wing cell was divided by the average cell area for that region. Cell counts, box areas and average wing cell size and number were measured twice for each individual, and the average value for each individual was used as a datum.
Statistics
Statistical analyses to test for differences in adult mass were carried out with significance set at P=0.05 (STATISTICA 9, StatSoft, Tulsa, OK, USA). Data for male and female flies were analyzed separately because of their distinctly different masses. For all experiments (CS, CD, EH and LH), we first tested for an overall oxygen treatment effect with ANOVA and then tested whether each treatment differed from rearing in continuous normoxia or hypoxia using Bonferroni-corrected a priori comparisons.
When examining the effect of oxygen on abdominal epithelial cell size, we first tested for an effect of body mass on cell widths. As there was no effect of body size, we tested for an oxygen treatment effect on estimated cell diameters using ANOVA.
For the experiment examining the effect of developmental stage when hypoxia was administered on wing cell size and number, there was a strong overall effect of body mass and wing area on cell number and size. Therefore, to determine specific effects of developmental stage on cell size and number that were independent of mass, we used wing area as a covariate in the analysis. We first tested for overall experimental effect with ANCOVA, followed by Duncan's multiple range tests to determine differences among the treatment groups.
RESULTS
Ontogeny of hypoxic sensitivity: CS experiments
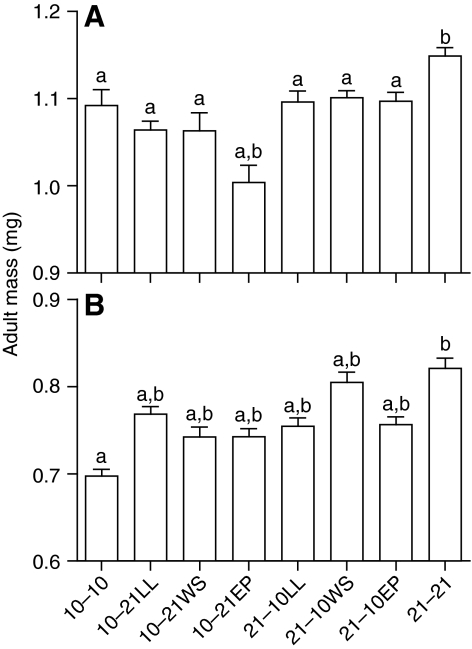
Run 1, CS experiments: larval vs pupal hypoxic sensitivity For analysis of larval vs pupal sensitivity as determined in the first run of the CS experiments, we focused on the CS–WS experiments because these had the largest sample sizes. In general, either larval or pupal hypoxia reduced adult size. There was a highly significant treatment effect on female adult body size (Fig. 2A; F6,626=16.4, P<0.001). All the groups that experienced hypoxia (10–10, 10–21, 21–10; the first number is the PO2 during the larval stage and the second number the PO2 during the pupal stage) were significantly smaller than the 21–21 group. For females, either larval or pupal hypoxia was sufficient to create a small adult; and neither the 10–21 nor the 21–10 differed significantly from the 10–10 group. However, larval hypoxia was more effective at reducing adult size; animals that experienced larval hypoxia (10–21) were significantly smaller than animals that experienced pupal hypoxia (21–10; P=0.009).
Fig. 2.
Results from the first run of the CS experiments in (A) female and (B) male Drosophila melanogaster, indicating that both larval and pupal stages are oxygen sensitive. The larval to pupal oxygen switch occurred using three different protocols described in the text, with switches at the wandering stage (WS), late larval (LL) or early pupal (EP) stages. Letters indicate significant differences from 21–21 (a) or 10–10 (b) by an a priori Bonferroni-corrected test. In this and subsequent figures, means and 95% confidence intervals are shown. For both males and females, exposure to hypoxia at either the larval or pupal stage, regardless of switching protocol, caused a significant reduction in adult body mass compared with flies reared constantly in 21 kPa PO2. Additionally, female flies in the 10–21 EP group were significantly smaller than flies reared constantly in hypoxia.
There was also a highly significant treatment effect on body size for males (Fig. 2B; F6,561=30.4, P<0.001). All of the groups that experienced hypoxia (10–10, 10–21, 21–10) were significantly smaller than the 21–21 group. Larval or pupal hypoxia created flies that were smaller than normal, but these flies were not as small as those reared for their entire development in hypoxia. Larval and pupal hypoxia were equally effective at reducing size in male flies.
Tests of the effect of larval to pupal switch method: comparisons of the CS–LL, CS–EP and CS–WS treatment groups
In general, similar results were obtained for the CS–LL, CS–EP and CS–WS experiments, suggesting that the specific switch method was not crucial to the overall conclusions. However, there were some significant treatment effects, which were partly predictable from the duration of exposure to hypoxia (Fig. 2). Female flies in the CS–EP treatment were significantly smaller than the other treatments, probably because these vials were exposed to hypoxia longer than the LL and WS groups. For males the 10–21 CS–LL animals were significantly larger than the CS–WS treatment flies, perhaps because these vials spent less time in hypoxia.
For the 21–10 treatments, female adult mass did not differ in the three treatment groups; flies in all groups were smaller than those reared continuously in normoxia and statistically the same size as flies reared continuously in hypoxia. For males, flies in all treatment groups were intermediate in size between the continuous normoxia and the continuous hypoxia group, though the WS flies tended to be larger than those in the other two treatments.
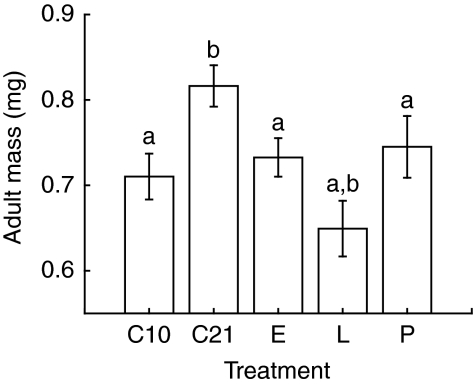
Effects of hypoxia during embryonic, larval or pupal stage (CS, run 2 experiments)
In these experiments, male flies that experienced hypoxia during any stage of their development had significantly smaller adult body masses than those kept in a constant 21 kPa PO2 throughout their entire development (Fig. 3; overall ANOVA F4,146=18.59, P<0.0001). Flies that experienced hypoxia for the entire larval stage were also significantly smaller than flies that experienced hypoxia during their entire development.
Fig. 3.
Results from the second run of the CS experiments, indicating that hypoxia during the embryonic, larval or pupal stage reduced adult mass in male D. melanogaster. Treatment groups: C10, constant 10 kPa PO2; C21, constant 21 kPa PO2; E, embryos exposed to hypoxia; L, larvae exposed to hypoxia; P, pupae exposed to hypoxia. Letters indicate significant difference from constant 21 kPa PO2 (a) or from constant 10 kPa PO2 (b) by an a priori Bonferroni-corrected test.
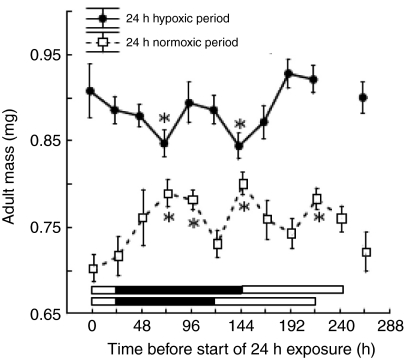
CD experiments
The adult mass of male flies exposed to 24 h of normoxia with their remaining development in hypoxia increased significantly when the normoxic period was provided during the late larval to early pupal period (start of normoxia 72 or 96 h after egg laying) and mid pupal stage (start of normoxia 144 h after egg laying; Fig. 4). Similarly, flies reared in normoxia except for a 24 h period of hypoxic exposure during the late larval (hypoxia beginning at 72 h) or mid pupal (hypoxia beginning at 144 h) period were significantly smaller than flies kept at constant 21 kPa PO2. Thus the late larval and early to mid pupal periods of Drosophila development are developmental periods when brief changes in atmospheric oxygen level have the strongest effects on adult size.
Fig. 4.
CD experiments. Top graph: CD experiments in which male D. melanogaster were reared in 21 kPa PO2 except for a 24 h period of 10 kPa PO2. Bottom graph: CD experiments in which flies were reared in 10 kPa PO2 except for 24 h of 21 kPa PO2. The x-axis labels indicate the number of hours after egg laying. The separate right-most data points, are from flies that were reared in either 21 kPa PO2 (top) or 10 kPa (bottom) PO2 continuously throughout their entire development. Adult flies were weighed 2 weeks after egg laying. Any values marked with an asterisk are significantly different from the constant normoxia (top right-most data point) or constant hypoxia (bottom right-most data point) groups by an a priori test with a Bonferroni-corrected P-value. The typical developmental timeline of the experimental flies was: 0–24 h, embryonic stage (left-most white bar at bottom); 24–96 h, larval stage (black bar at bottom); 96–192 h, pupal stage (right-most white bar).
EH experiments
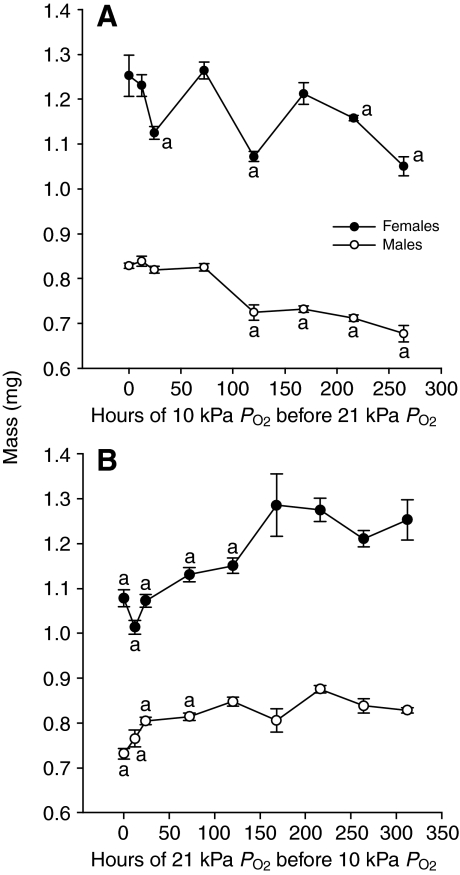
For both females (F7,74=22.8, P<0.0001) and males (F8,108=25.0, P<0.0001), there was a significant effect of switch day on mass in the EH experiments. When flies were switched from hypoxia to normoxia during the pupal stage, mass of adult males decreased significantly (overall ANOVA, F7,74=22.8, P<0.0001). Masses of animals switched during the embryonic or larval stage were equivalent to those of animals kept continuously in 21 kPa, and animals switched during the pupal stage had masses equivalent to those kept continuously in 10 kPa PO2 (Fig. 5A). Masses of females varied significantly (overall ANOVA, F8,113=8.7, P<0.0001); animals shifted after 72, 120, 216 and 264 h had masses lower than those raised in 21 kPa and identical to those raised continuously in 10 kPa.
Fig. 5.
EH and LH experiments. (A) EH experiment, in which D. melanogaster began life in 10 kPa PO2 and were moved to 21 kPa PO2 after a set number of hours of development, indicated on the x-axis. (B) LH experiment, in which flies began life in 21 kPa PO2 and were moved to 10 kPa PO2 after the number of hours of development shown on the x-axis. Groups marked with ‘a’ are significantly different from those that experienced constant normoxia, by a Bonferroni-corrected a priori comparison.
LH experiments
For both females (F7,71=9.97, P<0.001) and males (F8,79=18.2, P<0.0001) there was a significant effect of switch day on adult mass. When flies were switched from normoxia to hypoxia during the larval stage, adult masses were smaller than those of flies reared continuously in normoxia, but if the PO2 was switched from normoxia to hypoxia during the pupal stage, adult sizes were similar to those of normoxia-reared animals (Fig. 5B).
Hypoxic rearing effects on abdominal epithelial cell size
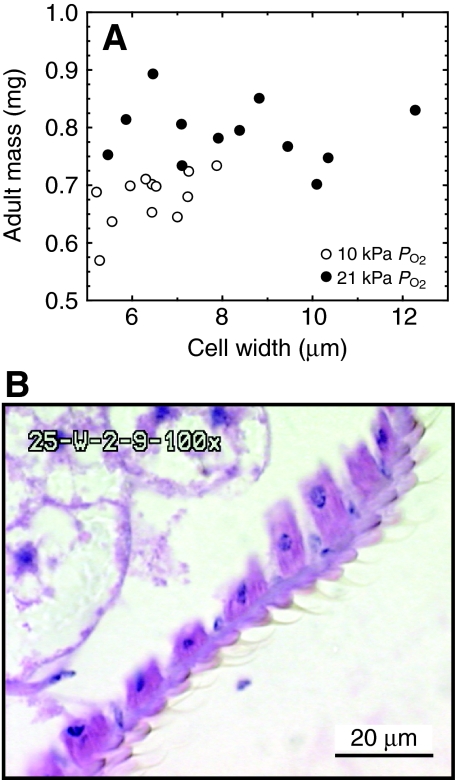
Flies reared in 10 kPa PO2 had significantly smaller body masses (ANOVA, F1,24=27.3, P<0.0001) and cell widths (ANOVA, F1,24=11.86, P<0.002) than the 21 kPa group (Fig. 6). The flies reared in 10 kPa did not have any cells wider than 8 μm. There was no significant correlation between body mass and abdominal cell width within treatment groups.
Fig. 6.
(A) Abdominal epithelial cell size vs body mass for D. melanogaster reared at 10 or 21 kPa PO2. Body mass was not related to cell width, but rearing in hypoxia reduced cell widths. (B) Stained abdominal epithelial cells.
Effect of developmental stage on the hypoxic response of wing cell size and cell number
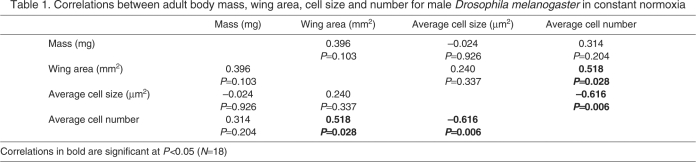
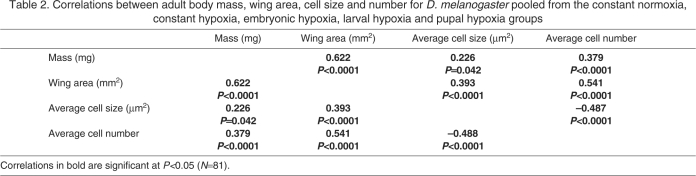
For flies reared in normoxia, there was no significant correlation between body mass and wing area, but flies with larger wings had more cells, and cell size and number were negatively correlated (Table 1). When all treatment groups were pooled, mass and wing area were positively correlated, and larger wings were positively correlated with both cell number and cell size (Table 2). However, cell size and number were strongly negatively correlated (Table 1).
Table 1.
Correlations between adult body mass, wing area, cell size and number for male Drosophila melanogaster in constant normoxia
Table 2.
Correlations between adult body mass, wing area, cell size and number for D. melanogaster pooled from the constant normoxia, constant hypoxia, embryonic hypoxia, larval hypoxia and pupal hypoxia groups
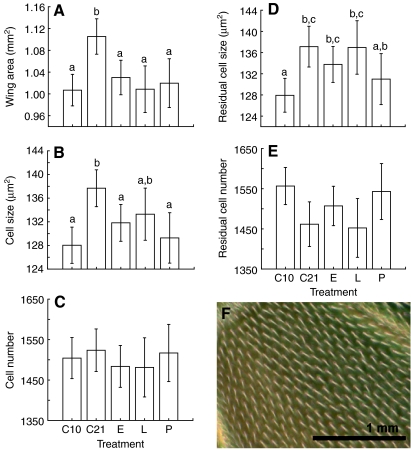
Wing area was significantly reduced in all treatment groups that experienced hypoxia and this effect was primarily due to a reduction in average cell size (Fig. 7A–C). To determine whether there were effects of hypoxia on cell size or number independently of general effects of wing size, we used a general linear model with wing area as a covariate. After controlling for the effect of wing area, we found a significant effect of treatment on cell size (Fig. 7D; ANCOVA, F4,76=4.18, P=0.004). Compared with normoxia-reared flies, the wingarea specific cell size was significantly smaller in the continuous hypoxia group and the pupal hypoxia group. After controlling for wing area, there was no significant treatment effect on cell number (Fig. 7E; ANCOVA, F4,76=2.45, P=0.0532), though there was a trend for rearing in hypoxia during the pupal stage or throughout development to produce flies with relatively more cells for their wing size.
Fig. 7.
(A) Wing areas of D. melanogaster after various oxygen treatments (C10, constant 10 kPa PO2; C21, constant 21 kPa PO2; E, 10 kPa PO2 during the embryo stage; L, 10 kPa PO2 during larval stage; P, 10 kPa PO2 during pupal stage). (B) Average cell area in the second posterior distal wing region. (C) Average number of cells in the second posterior distal wing region. (D) Area of the cells of the second posterior distal wing region, corrected for the linear effect of wing size across all groups. (E) Number of cells in the second posterior distal wing region, corrected for the linear effect of wing size across all groups. In A, B and D, a common letter indicates that groups do not differ significantly (Duncan's multiple range tests). (F) Image of a typical second posterior distal wing cell; each cell has a single trichome.
DISCUSSION
Exposure to hypoxia (10 kPa PO2) reduces the mass of adult Drosophila by an average of 7% regardless of whether the exposure occurs during the embryonic, larval or pupal stages. This effect is present even with relatively short-term hypoxic exposures (24 h, less than 10% of total development time) if the exposure occurs during the late larval or early pupal stage. We found that rearing in hypoxia can reduce abdominal epithelial cell size and wing cell size, especially when hypoxia occurs in the pupal stage. The diversity of developmental stages affected by hypoxia and the stage-specific responses of cell size suggest that multiple mechanisms may be responsible for the effects of hypoxia on adult body size in D. melanogaster.
Ontogeny of hypoxia sensitivity in D. melanogaster
Our more detailed and extensive experiments lead to a different conclusion than that of Peck and Maddrell, who suggested that the pupal stage might be the only developmental period in which hypoxia could produce small adults (Peck and Maddrell, 2005). When we repeated the experimental protocols used by Peck and Maddrell (the EH experiments) we found the same pattern: that apparently only flies switched from hypoxia to normoxia during the pupal stage were significantly smaller (Fig. 5A). However, when we reversed the direction of the experiment (LH experiments, Fig. 5B), we found the opposite result: that flies switched to hypoxia after the onset of pupation were similar in size to flies reared in normoxia, whereas flies switched to hypoxia during the larval stage were smaller. At first glance, this is a very confusing result that suggests that the sequence of exposure to atmospheric oxygen level is crucial to the effect on adult size. Such hysteresis may be a component of the mechanism for this pattern, however, we feel that a more likely explanation is that developmental stage was not precisely controlled in the EH and LH experiments, and the most sensitive period for hypoxia effects (late third instar and early pupal stage) occurred between switch times for these experiments (see below).
Our various stage-specific hypoxia tests clearly demonstrate that hypoxic exposure during the embryonic, larval or pupal stage creates small adults (Figs 2, 3). Interestingly, there appears to be a sex-dependent effect on the developmental sensitivity to oxygen. Hypoxia exposure only during larval or pupal stages produced females as small (or smaller) than flies reared continuously in 10 kPa PO2 (Fig. 2A). By contrast, for males, hypoxia experienced during the embryonic (Fig. 3), larval or pupal periods (Fig. 2B) sometimes produced adults with body masses intermediate between those reared continuously in normoxia or hypoxia, and only sometimes produced flies as small as those reared continuously in 10 kPa PO2 (Fig. 3). Thus, in males, there is some evidence for a cumulative effect of larval and pupal hypoxia on adult size; whereas for females it appears as if any significant hypoxic exposure causes adult flies to be smaller. Sex-based differences were also observed in a study on the evolutionary responses to rearing oxygen levels (Klok et al., 2009).
The CD experiments (Fig. 4), in which flies experienced only 1 day of an atmospheric oxygen level that differed from otherwise continuous normoxic or hypoxic rearing, indicated that flies are most sensitive to PO2 during the final portion of the larval stage (latter third instar) and the early-to-middle portion of the pupal stage. These results help explain the fact that changing atmospheric oxygen level more than 150 h after egg laying in the LH experiments had no effect on adult size (Fig. 5B). The high sensitivity of the late third instar larval stage is likely to be due to the fact that many insects gain most of their mass during this period (Nijhout et al., 2006). Exposure to 10 kPa PO2 reduces feeding rate of fly larvae (Frazier, 2007), possibly explaining the reduced size. Most imaginal disc development of adult tissue occurs during the early pupal stage of development, explaining why this early pupal period may also be particularly sensitive to hypoxia (Bainbridge and Bownes, 1981).
It is somewhat puzzling that the CD experiments (Fig. 4) suggest that short periods of altered oxygen can affect adult size, and that exposure to hypoxia during the embryonic stage reduced adult male size (Fig. 3), whereas the EH experiments (Fig. 5) showed that flies exposed to hypoxia for up to 72 h after egg laying had the same body masses as flies reared continuously in normoxia. The fact that the larval stage is most sensitive to hypoxia during the latter third instar (Fig. 3) may partially explain this pattern; the switch after 72 h probably preceded this sensitive period. The fact that hypoxia at embryonic stage did not affect body size in the EH experiments as it did in the experiments shown in Fig. 3 may be explained by discrepancies in experimental design. Only in the second run of the CS experiment were all flies exposed to hypoxia for the entirety of the embryonic stage. In other versions of this experiment, we focused on the larval-to-pupal transition and did not control for variation in embryonic development times. Accordingly, it is possible that many of the embryos in these treatment groups did not experience hypoxia for the entire embryonic stage. The embryonic stage lasts approximately 20 h in D. melanogaster reared at 25°C (Powsner, 1935), so perhaps exposure during the full embryonic stage is necessary for this effect to take place. Another possible explanation is that the effect of hypoxia during the embryonic stage is mediated by maternal effects, as egg laying took place in hypoxia in the CS, but not the EH, experiments.
Hypoxia effects on cell size and number
Although prior studies have found that wing cell sizes reflect the cell sizes in other parts of a fly when temperature changes (Partridge et al., 1994), we wanted to determine whether epithelial cells of the abdomen showed a similar response to oxygen as the easily measured wing cells. Indeed, abdominal epithelial cells were smaller when flies were reared in hypoxia (Fig. 6). In this sample, body mass was not related to cell width, so we could clearly show that the differences in cell size were due to oxygen, and were not simply a correlate of the difference in body size. It appears that hypoxia precludes the formation of cells of the larger size class, as there were no abdominal cells with widths greater than 8 μm. This finding is consistent with other studies that report an effect of hypoxia on the cell size and cycle in D. melanogaster (Douglas et al., 2005; Dekanty et al., 2005).
The effects of hypoxia on wing cell size and number depend on the developmental period when hypoxia is experienced. Flies reared continuously in hypoxia, or those that experience pupal hypoxia, had smaller cells, after correcting for their smaller wing area (Fig. 7D). This result suggests that the size of cells formed from the imaginal discs during metamorphosis were partially determined by local oxygen levels. In contrast, flies that only experienced hypoxia during the embryonic or larval period had smaller wings, but had cell sizes and numbers predictable from wing area. This result is consistent with the finding that flies that fed on low quality food were smaller as a result of a reduction in cell number and not of changes in cell size (Arendt, 2007).
The fact that larval hypoxia affects feeding (Frazier, 2007), which cannot occur during the pupal stage, and the partly differential responses of cell size to larval and pupal hypoxia, suggest that there may be at least two independent mechanisms by which hypoxia reduces adult body size. However, conceivably all of these effects could be mediated by similar oxygen-sensitive signaling pathways. Increased HIF signaling has been demonstrated to reduce cellular and organismal growth (Gorr et al., 2006), and modulate hypoxiasensitive behavior (Chang and Bargmann, 2008). Wingrove and O'Farrell reported that Drosophila exposed to hypoxia displayed exploratory behavior and experienced cell cycle arrest, and provided evidence for nitric oxide mediation of these effects (Wingrove and O'Farrell, 1999). Changes in nitric oxide or HIF signaling may modulate feeding behavior and cellular growth processes, causing effects on adult size. Future studies manipulating such signaling pathways will be necessary to test such possible mechanisms.
LIST OF ABBREVIATIONS
- CD
critical day experiment, in which flies were reared in either 10 or 21 kPa PO2 with one 24 h window of the converse oxygen treatment
- CS
critical stage experiments, including WS experiments and embryonic stage hypoxia experiments, designed to test for critical oxygen-sensitive developmental stages
- EH
early hypoxia experiments, in which flies were reared for a varying number of days in 10 kPa PO2, and then were switched to 21 kPa PO2
- LH
late hypoxia experiments, in which flies were reared for a varying number of days in 21 kPa PO2 and then were switched 10 kPa PO2
- PO2
atmospheric partial oxygen pressure
- WS
wandering stage experiments, designed to test for larval or pupal stage oxygen sensitivity
FOOTNOTES
This research was partially supported by NSF IBN 0419704 and NSF EAR 0746352 to J.F.H. as well as by the HHMI-funded ASU SOLUR program and the Arnold and Mabel Beckman Foundation. We thank Ben McKinley for his excellent efforts on the first run of the CS experiment and the EH and LH experiments. James Waters made excellent comments on the manuscript and was invaluable in figure preparation. Deposited in PMC for release after 12 months.
REFERENCES
- Aragones J., Schneider M., Van Geyte K., Fraisl P., Dresselaers T., Mazzone M., Dirkx R., Zacchigna S., Lemieux H., Jeoung N. H., et al. (2008). Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat. Genet. 40, 170-180 [DOI] [PubMed] [Google Scholar]
- Arendt J. (2007). Ecological correlates of body size in relation to cell size and cell number: patterns in flies, fish, fruits and foliage. Biol. Rev. 82, 241-256 [DOI] [PubMed] [Google Scholar]
- Bainbridge S. P., Bownes M. (1981). Staging the metamorphosis of Drosophila melanogaster. Development 66, 57 [PubMed] [Google Scholar]
- Brugarolas J., Lei K., Hurley R. L., Manning B. D., Reiling J. H., Hafen E., Witters L. A., Ellisen L. W., Kaelin W. G. (2004). Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 18, 2893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanin L., Peter J., Ratcliffe P. J., Wappner P. (2005). Reversion of lethality and growth defects in Fatiga oxygen-sensor mutant flies by loss of Hypoxia-Inducible Factor-α/Sima. EMBO Rep. 6, 1070-1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Bargmann C. I. (2008). Hypoxia and the HIF-1 transcriptional pathway reorganize a neuronal circuit for oxygen-dependent behavior in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 105, 7321-7326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekanty A. S., Lavista-Llanos Irisarri M., Oldham S., Wappner P. (2005). The insulin-PI3K/TOR pathway induces a HIF-dependent transcriptional response in Drosophila by promoting nuclear localization of HIF-alpha/Sima. J. Cell Sci. 118, 5431-5441 [DOI] [PubMed] [Google Scholar]
- Douglas R. M., Farahani R., Morcillo P., Kanaan A., Xu T., Haddad G. G. (2005). Hypoxia induces major effects on cell cycle kinetics and protein expression in Drosophila melanogaster embryos. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R511-R521 [DOI] [PubMed] [Google Scholar]
- Frazier M. R. (2007). Alpine Insects: Physiology and Evolution in Cold, Thin Air. PhD thesis, University of Washington: Seattle, WA, USA: [Google Scholar]
- Frazier M. R., Woods H. A., Harrison J. (2001). Interactive effects of rearing temperature and oxygen on the development of Drosophila melanogaster. Physiol. Biochem. Zool. 74, 641-650 [DOI] [PubMed] [Google Scholar]
- Frei C., Edgar B. A. (2004). Drosophila cyclin D/Cdk4 requires Hif-1 prolyl hydroxylase to drive cell growth. Dev. Cell 6, 241-251 [DOI] [PubMed] [Google Scholar]
- Frisancho A. R., Paul T. B. (1970). Altitude and growth: a study of the patterns of physical growth of a high altitude Peruvian Quechua population. Am. J. Phys. Anthropol. 32, 279-292 [DOI] [PubMed] [Google Scholar]
- Gorr T. A., Gassmann M., Wappner P. (2006). Sensing and responding to hypoxia via HIF in model invertebrates. J. Insect Physiol. 52, 349-364 [DOI] [PubMed] [Google Scholar]
- Harrison J., Frazier M. R., Henry J. R., Kaiser A., Klok C. J., Rascon B. (2006). Responses of terrestrial insects to hypoxia or hyperoxia. Respir. Physiol. Neurobiol. 154, 4-17 [DOI] [PubMed] [Google Scholar]
- Harrison J. F., Haddad G. G. (2011). Effects of oxygen on growth and size: synthesis of molecular, organismal and evolutionary studies with Drosophila melanogaster. Ann. Rev. Physiol. 73, 95-113 [DOI] [PubMed] [Google Scholar]
- Henry J. R., Harrison J. F. (2004). Plastic and evolved responses of larval tracheae and mass to varying atmospheric oxygen content in Drosophila melanogaster. J. Exp. Biol. 207, 3559-3567 [DOI] [PubMed] [Google Scholar]
- Hoback W. W., Stanley D. W. (2001). Insects in hypoxia. J. Insect Physiol. 47, 533-542 [DOI] [PubMed] [Google Scholar]
- Klok C. J., Hubb A. J., Harrison J. F. (2009). Single and multigenerational responses of body mass to atmospheric oxygen concentrations in Drosophila melanogaster: evidence for roles of plasticity and evolution. J. Evol. Biol. 22, 2496-2504 [DOI] [PubMed] [Google Scholar]
- Klok C. J., Kaiser A., Lighton J. R. B., Harrison J. F. (2010). Critical oxygen partial pressures and maximal tracheal conductances for Drosophila melanogaster reared for multiple generations in hypoxia or hyperoxia. J. Insect Physiol. 56, 461-469 [DOI] [PubMed] [Google Scholar]
- Miller R. S., Thomas J. L. (1958). The effects of larval crowding and body size on the longevity of adult Drosophila melanogaster. Ecology 39, 118-125 [Google Scholar]
- Nijhout H. F., Davidowitz G., Roff D. A. (2006). A quantitative analysis of the mechanism that controls body size in Manduca sexta. J. Biol. 5, 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owerkowicz T., Elsey R. M., Hicks J. W. (2009). Atmospheric oxygen level affects growth trajectory, cardiopulmonary allometry and metabolic rate in the American alligator (Alligator mississippiensis). J. Exp. Biol. 212, 1237-1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L., Barrie B., Fowler K., French V. (1994). Evolution and development of body size and cell size in Drosophila melanogaster in response to temperature. Evolution 48, 1269-1276 [DOI] [PubMed] [Google Scholar]
- Peck L. S., Maddrell S. H. P. (2005). Limitation of size by hypoxia in the fruit fly Drosophila melanogaster. J. Exp. Zool. A Comp. Exp. Biol. 303A, 968-975 [DOI] [PubMed] [Google Scholar]
- Powsner L. (1935). The effects of temperature on the durations of the developmental stages of Drosophila melanogaster. Physiol. Zool. 8, 474-520 [Google Scholar]
- Rascón B., Harrison J. F. (2010). Lifespan and oxidative stress show a nonlinear response to atmospheric oxygen level in Drosophila. J. Exp. Biol. 213, 3441-3448 [DOI] [PubMed] [Google Scholar]
- Reiling J. H., Hafen E. (2004). The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by down-regulating S6K activity up stream of TSC in Drosophila. Genes Dev. 18, 2879-2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhies W. A. (2009). Metabolic function in Drosophila melanogaster in response to hypoxia and pure oxygen. J. Exp. Biol. 212, 3132-3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingrove J. A., O’Farrell P. H. (1999). Nitric oxide contributes to behavioral, cellular, and developmental responses to low oxygen in Drosophila. Cell 98, 105-114 [DOI] [PMC free article] [PubMed] [Google Scholar]