Abstract
Testis brain RNA-binding protein (TB-RBP), the mouse orthologue of the human protein Translin, is a widely expressed and highly conserved protein with proposed functions in chromosomal translocations, mitotic cell division, and mRNA transport, stabilization, and storage. Targeted inactivation of TB-RBP leads to abnormalities in fertility and behavior. A testis-enriched kinesin KIF17b coimmunoprecipitates with TB-RBP in a RNA–protein complex containing specific cAMP-responsive element modulator (CREM)-regulated mRNAs. The specificity of this interaction is confirmed by in vivo RNA–protein crosslinking and transfections of hippocampal neurons. Combining in situ hybridization and immunohistochemistry at the electron microscope level, a temporally sequential dissociation of KIF17b and TB-RBP from specific mRNAs is detected with TB-RBP release coincident with the time of mRNA translation, indicating a separation of the processes of transport and translation. We conclude that KIF17b serves as a molecular motor component of a TB-RBP–mouse ribonucleoprotein complex transporting a group of specific CREM-regulated mRNAs in mammalian male postmeiotic germ cells. Because KIF17b has been reported to control CREM-dependent transcription in male germ cells by regulating the intracellular location of the transcriptional coactivator activator of CREM in testis, this indicates that one kinesin links the processes of transcription and transport of specific mRNAs in mammalian male germ cells.
During spermatogenesis, male germ cells undergo massive differentiation and rapid polarization that require transport and specific localization of vesicles, proteins, mRNAs, and organelles. The need to maintain phenotypic equivalence of the X or Y chromosome bearing haploid male germ cells further necessitates the sharing of specific mRNAs through intercellular bridges (1, 2). A number of kinesin and dynein superfamily motor proteins have been proposed to carry out transport processes in the testis (3). The mRNA binding protein TB-RBP, the mouse orthologue of human Translin (4), binds specific mRNAs in postmeiotic germ cells and in neurons, two of the highly polarized cell types in which mRNA transport and localization are essential. In male germ cells TB-RBP transports specific mRNAs intracellularly and intercellularly in a mouse ribonucleoprotein complex containing the Ter ATPase (5). Microtubule reconstitution experiments reveal that specific translationally delayed mRNAs are linked to microtubules through TB-RBP (6). TB-RBP binds brain mRNAs such as α-Ca2+-calmodulin-dependent protein kinase II mRNA and ligatin (7, 8) and a number of translationally delayed and X chromosome-encoded mRNAs that are solely expressed in male postmeiotic germ cells (5). In our investigation of the components of the TB-RBP–mRNA complexes involved in mRNA transport, we have identified a kinesin by coimmunoprecipitation and matrix-assisted laser desorption/ionization–time-of-flight (MALDI-TOF) mass spectroscopy. This kinesin, KIF17b, is an isoform of the neuronal kinesin KIF17, which has been implicated in the transport of N-methyl-d-aspartate receptor-containing vesicles through its interaction with mLin10 (9). In the nucleus, KIF17b has been demonstrated to be responsible for the nucleocytoplasmic localization and hence the transcriptional coactivation of activator of cremin testis (ACT), the activator of cAMP-responsive element modulator (CREM) in postmeiotic male germ cells (10). The testicular target mRNAs recognized by TB-RBP are dependent on CREM for their transcription and undergo the translational delay/silencing similar to the silencing seen during the transport of neuronal mRNAs. Thus, this communication represents the first demonstration of the direct association of one motor protein controlling both the transcription and transport of a specific population of mRNAs. Moreover, we demonstrate that KIF17b dissociates from the mRNA–protein complex before the release of TB-RBP, coincident with the initiation of mRNA translation. Thus, for this population of germ cell mRNAs, translation does not occur during mRNA transport.
Materials and Methods
Coimmunoprecipitation and MALDI-TOF Mass Mapping of TB-RBP-Associated Proteins. Affinity-purified anti-TB-RBP antibodies were used to immunoprecipitate TB-RBP and associated proteins as described (11). Briefly, mouse testis and brain cytoplasmic S100 extracts (10 mg) were precleared by incubation at 4°C for 1 h with 200 μl of protein A-agarose beads in a final volume of 10 ml of immunoprecipitation buffer (TBS, 0.1% Tween 20, 0.1% Empigen BB) in the presence of proteinase inhibitor mixture (Sigma). After centrifugation at 600 × g for 5 min at 4°C, the supernatant was incubated overnight at 4°C with 20 μg of affinity-purified anti-TB-RBP antibodies and 50 μl of protein A-agarose beads. The beads were then collected by centrifugation at 400 × g for 2 min and washed six times with 10 ml of each immunoprecipitate buffer. The washed beads were resuspended in 50 μl of 2× SDS gel loading buffer and boiled for 3 min, and the proteins were separated by SDS/4–12% PAGE. After electrophoresis, the gels were stained with Coomassie brilliant blue or silver, and protein bands, specifically coimmunoprecipitated with TB-RBP, were excised and mass-mapped by MALDI-TOF mass spectrometry at the Core Lab for Protein Microsequencing of the Worcester Foundation for Biomedical Research (Worcester, MA).
Cloning of hKIF17b cDNA by RNA Ligase-Mediated RACE. A partial human EST clone (GenBank accession no. AF009624) of 937 bp encoding the C terminus of a kinesin superfamily gene matching the peptide sequences derived from the MALDI-TOF mass spectrometric analysis was amplified by RT-PCR from human testis RNA (Clontech). The sequence of another cDNA derived from an incompletely spliced human brain mRNA clone (KIAA1405) was then used to amplify a 2-kb partial cDNA clone from human testis RNA. RNA ligation mediated 5′ RACE using the First Choice RLM-RACE kit (Ambion, Austin, TX) was used to obtain the complete 5′ end of the cDNA from human testis RNA. The complete ORF (3,069 bp) was then amplified by overlapping PCR. The sequence was confirmed by comparison with the human genomic locus. The cDNA sequence has been deposited in the GenBank database (accession no. AY484427).
Northern Blot Analysis. Northern blot of RNA (10 μg) from various mouse tissues (brain, heart, kidney, liver, lung, spleen, and testis) and from purified germ cell populations were carried out as described (12). A partial KIF17b cDNA sequence of 1 kb, corresponding to the 5′ end of the gene, was used as probe for Northern analysis. The blots were rehybridized with β-actin cDNA as a loading control.
Generation of Anti-KIF17b Antibodies, Western Blot Analysis, and Reciprocal Coimmunoprecipitation of TB-RBP. A partial cDNA sequence of KIF17b, corresponding to AA736–AA800, was expressed as a 6-His-tagged fusion protein in Escherichia coli, purified by Ni-affinity, and used to raise rabbit polyclonal antibodies. Monospecific affinity-purified anti-KIF17b antibodies were used for Western analysis of protein samples from various mouse tissues and for immunoprecipitations as described above.
Quantitative RT-PCR Assay for KIF17b–TB-RBP Complex-Associated mRNAs. Specific TB-RBP- and KIF17b-associated mRNAs were immunoprecipitated from sexually mature mouse testis cytoplasmic extracts under RNase-free conditions with affinity-purified anti-TB-RBP or anti-KIF17b antibodies as described (5). Affinity-purified anti-KIF2b antibodies, a kinesin known not to interact with TB-RBP or RNA, were used as controls for the immunoprecipitations. Purified RNA from the immunoprecipitations was reverse-transcribed by using random primers after treatment with amplification-grade DNase I using the RETRO-Script reverse transcription kit (Ambion). The reverse-transcribed cDNAs were used in triplicate at dilutions of 1×, 1:10, and 1:100 in real-time PCR analysis by using the 7700 Real-Time Thermal Cycler (Applied Biosystems). Primer sets were designed by using 3′ UTR sequences for the genes analyzed with primer express software. All primers produce a single amplicon of the predicted size. Real-time PCR amplification of GAPDH was used as a control for normalization. SYBR green reagent (Applied Biosystems) was used to amplify the genes, and fold differences were calculated by using CT values.
In Vivo Crosslinking of Specific Germ Cell mRNAs with TB-RBP–Kinesin Complexes. The RNA immunoprecipitation protocol of Niranjanakumari et al. (13) was adapted to analyze the in vivo RNA–protein interactions of TB-RBP and its associated protein, KIF17b, in mouse germ cells. Briefly, testes from 25 wild-type or TB-RBP-null mice were washed in PBS buffer, decapsulated, suspended in 20 ml of DMEM, and treated with collagenase (500 μg/ml) for 20 min at 32°C. The samples were incubated with trypsin (1 mg/ml in DMEM) for 20 min at 32°C to prepare a single-cell suspension of mixed somatic and germ cells. The cells, after centrifugation at 2,000 rpm for 5 min at room temperature, were washed twice with PBS buffer and resuspended in 10 ml of PBS. Formaldehyde (AR grade, Fisher, 37% wt/wt) was added to a final concentration of 1% wt/vol and incubated at room temperature for 10 min with slow mixing. Crosslinking reactions were stopped by the addition of glycine (1 M, pH 7) to a final concentration of 0.25 M followed by incubation at room temperature for 5 min. The cells were washed twice with ice-cold PBS and resuspended in 4 ml of RIPA buffer (50 mM Tris-HCl, pH 7.4/1% Nonidet P-40/0.5% sodium deoxycholate/0.05% SDS/1 mM EDTA/150 mM NaCl) containing protease inhibitors (Sigma) and 50 units/ml Superasin RNase inhibitor (Ambion). The cell suspensions were sonicated with a 2-mm probe for three 10-s bursts at a setting of 1 with a Heat Systems/Ultrasonics Model W-220F Cell Disruptor. A small aliquot of the sample was examined under the microscope to monitor cell disruption. The sonicates were centrifuged at 13,000 rpm at 4°C for 10 min to remove insoluble material. The supernatants were diluted to 20 ml in RIPA buffer and precleared by incubation with 200 μl of protein A-agarose beads and 100 μg/ml E. coli tRNA for 1 h at 4°C followed by centrifugation at 2,000 rpm for 5 min. Aliquots of the precleared supernatants were incubated with 20 μg of affinity-purified anti-TB-RBP or anti-KIF17b antibodies and 50 μl each of protein A-agarose beads at 4°C for 4 h. The beads were collected by centrifugation at 1,000 rpm for 2 min and washed six times with 5 ml each of RIPA buffer, at alternating high-salt (1 M NaCl) and low-salt (150 mM NaCl) conditions. The beads after the final low-salt wash were suspended in 200 μl of 50 mM Tris-HCl, pH 7.0/5 mM EDTA/10 mM DTT/1% SDS followed by incubation at 70°C for 45 min to reverse the crosslinks. The immunoprecipitated RNAs were isolated with TRI Reagent (Sigma) according to the manufacturer's protocol. Aliquots of RNA were analyzed by RT-PCR after treatment with RNase-free amplification-grade DNase I (Stratagene) followed by heat-EDTA inactivation of DNase I. Reverse transcriptions with random decamer primers were carried out by using the RETROScript reverse transcription kit (Ambion). All PCR assays were done with gene-specific primers and used puReTaq Ready-To-Go PCR beads (Amersham Pharmacia).
Alexa Fluor 546-Labeled Y and H Element RNA. In vitro transcription of linearized plasmid carrying the Y and H element sequences from the 3′ UTR of protamine 2 was performed as described (12). To generate fluorescent riboprobes for in vivo localization with TB-RBP or KIF17b, the plasmid was transcribed by using the Megascript transcription kit (Ambion) in the presence of Alexa Fluor 546-labeled UTP (Molecular Probes).
Colocalization of KIF17b, TB-RBP, and the Target Y and H Element RNA Sequence in Cultured Rat Hippocampal Neurons. Rat embryonic hippocampal neurons were cultured in B27/neurobasal medium as described by Brewer et al. (14). The cultured cells were transfected with plasmid vectors expressing enhanced GFP fusions of KIF17b or TB-RBP and a DSRed2 fusion of TB-RBP. The DNA samples were diluted by using Nupherin-neuron reagent (Biomol, Plymouth Meeting, PA) before transfection using FuGENE 6 (Roche, Indianapolis, IN) according to manufacturer's protocols. (Addition of the Nupherin-neuron was found to improve transfection efficiency of FuGENE 6 for hippocampal neurons ≈10-fold.) Cells that were transfected with pEGFP–TB-RBP or pEGFP–KIF17b were retransfected 36 h later with Alexa Fluor 546-labeled Y and H element RNA (0.3 μg per 2-cm culture dish) in the presence of RNAsin (50 units/ml, Promega) by using Nupherin-neuron and FuGENE 6 reagents. The cells were washed twice with Dulbecco's PBS, and fresh medium was added 30 min after the labeled RNA transfection. The cells were allowed to recover in the incubator (37°C, 5% CO2) for 15 min before live confocal imaging was performed with a Bio-Rad Multiphoton MRC-1024 microscope.
Results and Discussion
By using affinity-purified anti-TB-RBP antibodies, several proteins including TB-RBP, TRAX (11), and an unknown protein of ≈120 kDa were immunoprecipitated from a testis cytoplasmic S100 extract from sexually mature mice (Fig. 1A, lane 6). The 120-kDa band was not detected in a parallel immunoprecipitation with cytoplasmic S100 extract from brain (Fig. 1 A, lane 5) or in the preclearing controls (Fig. 1 A, lanes 3 and 4). MALDI-TOF mass mapping of the120-kDa protein revealed sequences identical to a partial human cDNA clone (GenBank accession no. AF009624), with extensive homology to the KIF3 family of motor proteins. The cDNA sequence together with the sequence of a homologous KIAA cDNA clone (KIAA1405, GenBank accession no. AB037826) were used to design primers for RT-PCR to generate a 2-kb partial cDNA clone from human testis RNA. This sequence was used to amplify the complete 5′ end of the cDNA by the RNA ligase-mediated RACE protocol. The complete sequence was highly homologous to mouse KIF17, a mouse brain-specific kinesin (9). While this research was in progress, the sequence of KIF17b, a mouse testis-specific isoform of KIF17 that regulates the localization of ACT protein, was reported (10). The four peptide sequences obtained from MALDI-TOF mass mapping of the 120-kDa testicular protein were identical to sequences in KIF17b (data not shown).
Fig. 1.
(A) Coimmunoprecipitation of KIF17b and TB-RBP from mouse testis extracts. A silver-stained SDS/polyacrylamide gel shows total extracts from brain (lane 1) and testis (lane 2), protein A-agarose beads preclearing controls of extracts from brain (lane 3) and testis (lane 4), and immunoprecipitated cytoplasmic S100 extracts from brain (lane 5) and testis (lane 6) using affinity-purified anti-TB-RBP. Excised protein bands were sequenced by MALDI-TOF mass spectrometry. M, marker proteins. (B) KIF17b expression is specific to postmeiotic male germ cells. Shown is a Northern blot of RNA (10 μg) from brain (lane 1), testis (lane 2), mixed germ cells (lane 3), pachytene spermatocytes (lane 4), round spermatids (lane 5), and elongating spermatids (lane 6). Robust expression of KIF17b mRNA is seen in the postmeiotic male germ cells. (C) Affinity-purified anti-KIF17b recognizes TB-RBP-associated KIF17b from testis extracts. A Western blot shows protein A-agarose beads preclearing of extracts from brain (lane 1) and testis (lane 2), brain S100 cytoplasmic extract (lane 3), anti-TB-RBP immunoprecipitation of extracts from brain (lane 4) and testis (lane 5), and the enrichment of KIF17b in the TB-RBP immunoprecipitate (lane 6). (D) Anti-KIF17b coimmunoprecipitates TB-RBP from testis extracts. Western blot of extract immunoprecipitated with anti-KIF17b antibodies shows TB-RBP in the immunoprecipitate. Lane 1, protein A-agarose bead preclearing; lane 2, anti-KIF17b immunoprecipitate.
As previously reported by Macho et al. (10), by the criterion of Northern blotting, KIF17b mRNA expression is restricted to the testis and is not detectable in brain (10) or in heart, kidney, lung, and spleen (data not shown). To confirm the tissue specificity of the TB-RBP-associated kinesin, we looked for KIF17b mRNA in brain. KIF17b mRNA was undetectable by RT-PCR in total RNA from human brain as well as from human hippocampal poly(A)+ RNA (data not shown). Thus, based on sequence and tissue specificity, we conclude that the mouse kinesin that immunoprecipitates with TB-RBP is KIF17b.
To determine whether KIF17b is expressed in the same testicular germ cells as CREM, ACT, and TB-RBP, we analyzed by Northern blotting total RNA from populations of isolated enriched germ cells. KIF17b mRNA was detected in total testis (Fig. 1B, lane 2), mixed germ cells (Fig. 1B, lane 3), and the postmeiotic round and elongating spermatids (Fig. 1B, lanes 5 and 6), the same germ cell types that express CREM, ACT, and TB-RBP (10). To confirm the tissue specificity of the KIF17b protein, affinity-purified antibodies were generated against AA736–AA800 of KIF17b. The antibodies specifically recognize the120-kDa protein band in testis extracts (Fig. 1C, lane 5) and after immunoprecipitation with anti-TB-RBP from cytoplasmic extracts of testis (Fig. 1C, lane 6). The addition of RNase to extracts before immunoprecipitation gave identical results, indicating that RNA is not serving as a linker. No 120-kDa protein or any protein was detected in brain extracts (Fig. 1C, lane 3) or in the pellets of anti-TB-RBP immunoprecipitates from brain confirming the selective expression of KIF17b (Fig. 1C, lane 4). No KIF17b was detected in any of the preclearing fractions (Fig. 1 C, lanes 1 and 2, and D, lane 1). Immunoprecipitation of testis extracts with anti-KIF17b coimmunoprecipitates TB-RBP further, establishing their association in a complex (Fig. 1D, lane 2).
To determine whether the TB-RBP and KIF17b complexes functionally associate with a predicted population of CREM-dependent male germ cell mRNAs, several approaches were taken. First, affinity-purified antibodies against TB-RBP and KIF17b were used to coprecipitate associated mouse ribonucleoprotein complexes under RNase-free conditions (5, 15). As a control for the specificity of immunoprecipitation, we used affinity-purified antibody to another germ cell-expressed but non-RNA-associated kinesin, KIF2b, in parallel precipitations. After RNA purification from the precipitates and DNase treatment, real-time RT-PCR was used to quantitate six known TB-RBP target and non-target mRNAs. There was significant enrichment of four target mRNAs encoding protamine 1 (2,072-fold), protamine 2 (5,800-fold), testis-specific GAPDH (GAPDS; 3,104-fold), and A kinase anchoring protein 4 (AKAP4) (2,000-fold) in the anti-TB-RBP immunoprecipitates when direct fold differences were compared with precipitated RNA from the immunoprecipitates of the non-RNA binding control kinesin KIF2b. Similar enrichments of the mRNAs encoding protamine 1 (800-fold), protamine 2 (512-fold), GAPDS (445-fold), and AKAP4 (128-fold) were seen with anti-KIF17b immunoprecipitates. As controls, mRNAs encoding phosphoglycerate kinase 2 (PGK2) and TB-RBP, two mRNAs lacking the Y and H elements and known to be poorly represented in the TB-RBP associated mouse ribonucleoproteins, were not significantly enriched in the immunoprecipitates with anti-TB-RBP (64- and 22-fold, respectively) or anti-KIF17b (10- and 2-fold, respectively). We reproducibly found less total mRNA in the KIF17b immunoprecipitates than in the TB-RBP immunoprecipitates. This could be because of the loss of TB-RBP–KIF17b interaction during the immunoprecipitation or more likely suggests that less KIF17b is associated with the mRNA–protein complexes than TB-RBP. Evidence for the second possibility demonstrating a temporally briefer association of KIF17b with the target mRNAs than the association of TB-RBP follows (see Fig. 3).
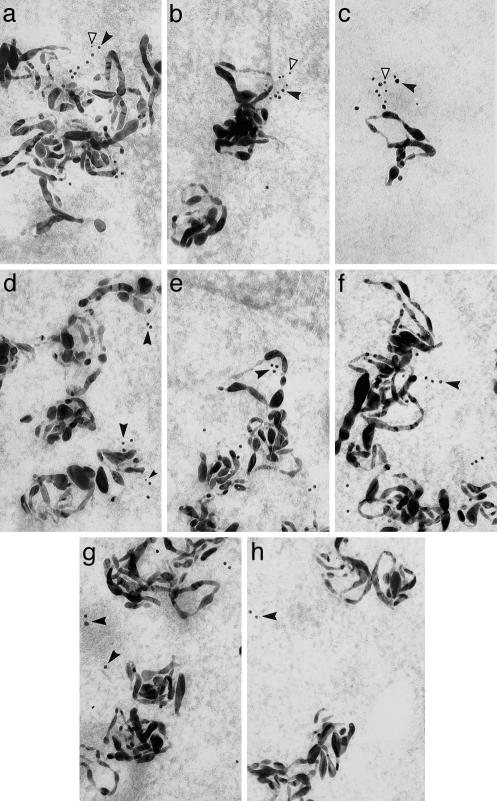
Fig. 3.
Colocalization of TB-RBP, KIF17b, and mRNAs by in situ hybridization and immunoradioautography. Shown are TB-RBP (15-nm gold particles, solid arrowheads) and KIF17b (10-nm gold particles, open arrowheads) in mouse seminiferous tubules hybridized in situ with antisense RNA probes (large filamentous silver grains) encoding AKAP4 (a, b, d, e, and g), protamine 2 (c and f), or PGK2 (h) In early (a, step 2) and late (b, step 7) round spermatids, AKAP4 silver grains are in close association with KIF17b and TB-RBP (10- and 15-nm colloidal gold particles (a, step 2). Protamine 2-generated silver grains are also in close association with KIF17b and TB-RBP in round spermatids (c, step 7) whereas PGK2-generated silver grains are not (h, step 7). During spermatid elongation, the KIF17b gold particles gradually dissociate from AKAP4 mRNAs (d, step 9, and e, step 11) and from the protamine mRNAs (f, step 11). In late step spermatids TB-RBP also dissociates from the AKAP4 mRNAs (g, step 15) and from the protamine 2 mRNAs (data not shown). (Magnification: ×100,000.)
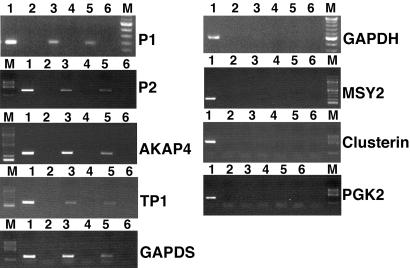
Because it is difficult to totally exclude all nonspecific associations among mRNAs, KIF17b, and TB-RBP in the immunoprecipitations described above, we performed in vivo crosslinking of RNA and protein using highly stringent immunoprecipitation conditions that maintain protein–RNA associations (13). As additional specificity controls, testis extracts from mice lacking TB-RBP were used. Single-cell preparations of dissociated male germ cells from wild-type and TB-RBP-null mice were crosslinked with formaldehyde, sonicated, and immunoprecipitated with anti-TB-RBP or anti-KIF17b antibodies. This allowed us to increase the stringency of the immunoprecipitations substantially and to determine whether KIF17b can directly bind mRNAs in the absence of TB-RBP. In the TB-RBP-null mice, TRAX protein is also absent, but KIF17b levels are normal (16). Five CREM-dependent TB-RBP target mRNAs encoding protamine 1, protamine 2, AKAP4, transition protein 1, and GAPDS were enriched in the anti-TB-RBP immunoprecipitates from wild-type testis extracts after formaldehyde crosslinking (Fig. 2, lane 3). All of these mRNAs contain TB-RBP binding Y and H sequence elements. In contrast, mRNAs of GAPDH, MSY2, clusterin, and PGK2, four mRNAs that do not contain Y and H elements and do not bind to TB-RBP, were not detectable in the TB-RBP immunoprecipitates (Fig. 2, lane 3). The absence of GAPDH mRNA in the TB-RBP complex after formaldehyde crosslinking and immunoprecipitation is an especially good indicator for the sequence specific binding of TB-RBP to RNA and for the stringency of the RIP assay procedure, because the GAPDH and GAPDS mRNA sequences are highly homologous and GAPDH and GAPDS are equally abundant in the testis. MSY2, clusterin, and PGK2 were chosen as additional negative control mRNAs, because they are expressed at very high levels in mouse testis and do not bind TB-RBP. Analyzing immunoprecipitates from TB-RBP-null testis to assess possible nonspecific IgG–RNA/ribonucleoprotein protein interaction, we did not detect any of the mRNAs in the precipitates (Fig. 2, lane 4). Immunoprecipitation of testis extracts from wild-type mice with anti-KIF17b antibodies gave results identical to those obtained with the anti-TB-RBP, i.e., strong signals for all of the mRNAs that are bound by TB-RBP, whereas the control mRNAs lacking Y and H sequences were not detectable in the precipitates (Fig. 2, lane 5). However, in the extracts prepared from TB-RBP-null mice, no mRNAs were detected in the KIF17b immunoprecipitates, demonstrating that KIF17b does not bind the mRNAs directly and TB-RBP and TRAX are essential components for KIF17b to bind in a complex with protamines 1 and 2, TP1, AKAP4, and GAPDS mRNAs (Fig. 2, lane 6).
Fig. 2.
Immunoprecipitation assay showing TB-RBP binding to specific germ cell mRNAs. RNA protein complexes immunoprecipitated after in vivo formaldehyde crosslinking in trypsinized testicular cells were analyzed by RT-PCR (35 cycles of amplification) using specific primers for each mRNA. Lane 1, total RNA from TB-RBP–/– testes; lane 2, preclearing of crosslinked extracts; lane 3, immunoprecipitate from wild-type testes with anti-TB-RBP; lane 4, immunoprecipitate from TB-RBP–/– testes with anti-TB-RBP; lane 5, immunoprecipitate from wild-type testes with anti-KIF17b; lane 6, immunoprecipitate from TB-RBP–/– testes with anti-KIF17b. RNAs were resolved on agarose gels and visualized with ethidium bromide staining.
Using the procedures of in situ hybridization immunohistochemistry and electron microscopy, we have demonstrated that specific postmeiotic mRNAs are transported intracellularly and intercellularly in association with TB-RBP and the Ter ATPase (5). Our results suggested that this process is energy-dependent, and, in view of the interactions among TB-RBP, KIF17b, and specific mRNAs, we reasoned that KIF17b could be the motor protein associated with the TB-RBP–mRNA complex in early postmeiotic cells. To determine whether we could detect the RNA–KIF17b–TB-RBP complexes in vivo, we used the same experimental approach. Mouse testicular tissues were hybridized with a 3H-labeled AKAP4 antisense RNA followed by double ImmunoGold labeling on the opposite surface of the radioautographic emulsion with the TB-RBP and KIF17b antibodies (Fig. 3). To visualize and discriminate between the two antibodies, the testicular sections were immunoreacted first with the TB-RBP antibody followed by incubation with a secondary antibody conjugated to 15-nm gold particles, and then with the KIF17b antibody followed by incubation with protein A conjugated to 10-nm gold particles. A close association between single molecules of AKAP4 mRNA (the filamentous radioautographic grains) and TB-RBP and KIF17b (the 10- and 15-nm colloidal gold particles, respectively) was observed in the cytoplasm of round spermatids (Fig. 3 a and b). A negative control, in situ hybridization of seminiferous tubules with the AKAP4 antisense RNA followed by ImmunoGold labeling with normal rabbit serum detected silver grains but no ImmunoGold labeling in the cytoplasm of round spermatids (data not shown).
To confirm the specificity of the germ cell mRNA, TB-RBP, and KIF17b complexes, we examined two additional mRNAs encoding protamine 2 and PGK2. Protamine 2 is an abundant postmeiotically expressed mRNA that is stored in the cytoplasm ≈7 days before it is translated in elongated spermatids (17). TB-RBP binds to conserved sequences in its 3′ UTR and in vitro specifically suppresses translation of target mRNAs (18). PGK2 mRNA is expressed at low levels in meiotic cells and at high levels in round spermatids and does not contain binding sites for TB-RBP or bind to TB-RBP (5). Identical clusters of TB-RBP and KIF17b in close association with the protamine 2 mRNA were detected in early-stage spermatids (Fig. 3c), whereas no association was seen between the PGK2 mRNA and the TB-RBP and KIF17b in any cell type (Fig. 3h).
The association of KIF17b and TB-RBP with AKAP4 mRNA is temporally transient, with KIF17b dissociating first from the complex in steps 10–11 spermatids (Fig. 3 d and e), and TB-RBP dissociating later during steps 12–15 (Fig. 3g). Although AKAP4 mRNA is transcribed in early-stage spermatids, AKAP4 protein is synthesized days later toward the end of spermatogenesis, coincident with the step in which TB-RBP dissociates from the AKAP4 mRNA (5). Because electron microscopy can be statistically selective, we have used a quantitative method (19) to more precisely measure the step-specific association/dissociation of TB-RBP and KIF17b from mRNAs (Table 1). We find in round spermatids that both TB-RBP and KIF17b form complexes with the AKAP4 and protamine 2 mRNAs, but not with the PGK2 mRNA. Moreover, KIF17b dissociates from the complex between steps 9 and 12, and TB-RBP dissociates between steps 15 and 16. These data suggest that TB-RBP serves as a linker, repressor, and/or stabilizer protein by binding to specific mRNAs that require translational delay, and KIF17b serves as a motor protein transporting the TB-RBP–mRNA complex (Fig. 3). The early release of KIF17b from the mRNA–protein complex suggests that, in postmeiotic male germ cells, the transport and translation of specific mRNAs are temporally distinct. We interpret these data to indicate that KIF17b transports mRNAs in a translationally suppressed form, and mRNA translation follows the release of TB-RBP.
Table 1. Temporal quantitation of complexes of AKAP4 mRNA with TB-RBP and KIF17b as spermatids mature.
| Steps 1-3 | Steps 4-7 | Steps 8-13 | Steps 14-16 | |
|---|---|---|---|---|
| Closely associated | 70 ± 5 | 75 ± 7* | 25 ± 7† | 5 ± 3† |
| Nearby | 15 ± 5 | 9 ± 6 | 10 ± 7† | 3 ± 3† |
| Not associated | 15 ± 5 | 20 ± 7 | 68 ± 5 | 91 ± 1 |
Values are expressed as percentages of silver grains (± SD) associated with both antibodies. The criteria defining the closely associated and nearby categories have been described (5).
Fifty percent of the ImmunoGold complex is composed of 10-nm gold particles (KIF17b).
Five percent of the ImmunoGold complex is composed of 10-nm gold particles
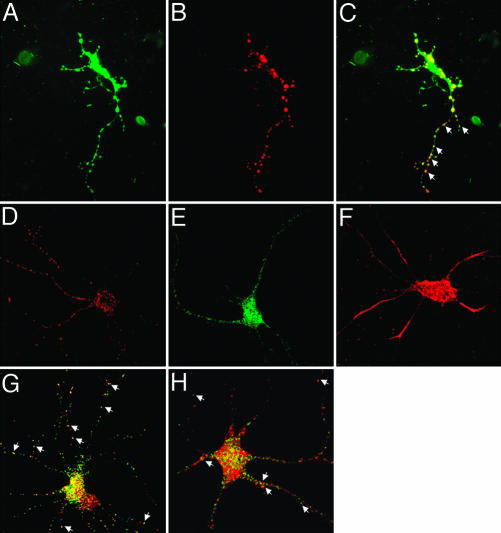
Cultured neurons have been extensively used to examine the transport of mRNA–protein complexes (20). Therefore, we have used cultures of rat hippocampal neurons to examine the targeting of mRNAs by TB-RBP and KIF17b to dendrites. To first establish whether TB-RBP and KIF17b colocalize in neurons, we transfected cultures of primary rat hippocampal neurons with GFP–KIF17b and TB-RBP–DsRed fusion proteins. By using epifluorescence or confocal microscopy, singly transfected cells expressing either TB-RBP or KIF17b showed punctate patterns of localization in the cell body and throughout their dendritic processes (Fig. 4 A–C). In neurons expressing both TB-RBP and KIF17b, there was a high degree of colocalization of TB-RBP (red) and KIF17b (green) (Fig. 4 A–C). To investigate whether the KIF17b–TB-RBP complex was associated with RNA, these cultures of hippocampal neurons were retransfected with Alexa Fluor 546-labeled RNA encoding a subclone of the 3′ UTR of protamine 2 containing the TB-RBP-binding sequence elements. Monitoring fluorescence within 30 min after transfection, the RNA was enriched in the cell body and showed limited punctate distribution in the dendritic processes (Fig. 4F). Introduction of the labeled RNA into cells that also expressed GFP–TB-RBP revealed a more punctate and widespread distribution of the RNA with extensive colocalization between the TB-RBP protein and the Y and H element riboprobe (Fig. 4G). Similarly, in neuronal cells expressing GFP–KIF17b, many punctate particles of the labeled RNA colocalized with KIF17b in the processes (Fig. 4H). Particles containing TB-RBP, KIF17b, or RNA alone were also detected. This likely reflects interactions between endogenous TB-RBP and endogenous kinesins with the Y and H element riboprobe or endogenous hippocampal mRNAs binding to the TB-RBP and KIF17b fusion proteins, because additional transport mechanisms for mRNAs containing Y and H elements and numerous unknown TB-RBP target mRNAs likely exist in neurons.
Fig. 4.
Transfected KIF17b colocalizes with TB-RBP and Y and H element containing RNA in rat hippocampal neurons. Plasmid constructs containing KIF17b and TB-RBP cDNAs cloned in frame in pEGFPc2 and pDsRed1 vectors, respectively, were transfected into cultured rat embryonic hippocampal neurons with Nupherin-neuron and FuGENE 6 reagent. (A) GFP–KIF17b fusion protein. (B) TB-RBP–DsRed fusion protein. (C) Merged image. Arrowheads indicate the colocalization of KIF17b and TB-RBP in the dendritic processes. (D–H) Transfected Y and H element RNA colocalizes with TB-RBP and KIF17b. Cells expressing GFP–TB-RBP or GFP–KIF17b were retransfected 36 h later with Alexa Fluor 546 RNA probe and show colocalization (arrows) of RNA with GFP–TB-RBP (G) and GFP–KIF17b (H). Control single transfections with TB-RBP–DsRed (D), GFP–KIF17b (E), and Alexa Fluor 546 (F) riboprobes. Dual-channel confocal imaging was performed within 30 min of transfection of the riboprobe.
Diverse functions, ranging from intracellular organelle and vesicle transport to cell division and chromosomal and spindle movements, have been proposed for members of the kinesin superfamily (21). In the brain, kinesins play prominent roles in intracellular transport (21). Recently, BC1 RNA was shown to be enriched in a staufen1–ribonucleoprotein complex that also contains kinesin heavy chain and α-Ca2+-calmodulin-dependent protein kinase II mRNA (22). The RNA-binding protein CPEB has been shown to bind specific mRNAs in a complex with dynein and kinesin heavy chain (20). Many RNA-binding proteins, including CPEB, staufen, and TB-RBP, bind to α-Ca2+-calmodulin-dependent protein kinase II mRNA, suggesting multiple components and pathways exist for transport, stabilization, and translational regulation of brain mRNAs.
In the testis, kinesins are notably essential for mitosis, meiosis, and organelle transport and assembly (23). The importance of kinesins in intramanchette transport and intraflagellar transport in elongating spermatids has also been proposed based on their localization to these cellular compartments (23). The differential distribution of mRNAs as round spermatids mature to the highly polarized late stage male germ cells could serve as molecular precursors to polarity in male gametes. Although establishing asymmetry through localized protein synthesis mediated by mRNA transport in round and elongating spermatids is an attractive possibility, it is unproven. Defects in mRNA localization in the testis and brain could account for the marked reduction in sperm count and behavioral abnormalities observed in TB-RBP-null mice (16). The release of KIF17b from the transported mRNA before TB-RBP dissociation suggests the separation of mRNA transport and translation processes.
The ability of KIF17b to coordinately regulate the transcription and transportation/localization of a group of mRNAs would represent a way for this kinesin isoform to establish mRNA localization in male germ cells. Linkage between transcription and mRNA export and transport/storage has been proposed for another germ cell DNA/RNA-binding protein, FRGY2, which activates transcription from a Y-box containing promoter, binds to nascent transcripts, and controls their translation in the cytoplasm (24). Similarly, the nucleocytoplasmic shuttling heterogeneous nuclear ribonucleoprotein human ribonucleoprotein A1 accompanies eukaryotic mRNAs from their active site of transcription to that of translation (25). In round spermatids TB-RBP binds to mRNAs in the nucleus (5), probably subsequent to splicing, because RT-PCR primers spanning introns do not detect protamine 1 or 2 pre-mRNAs in TB-RBP immunoprecipitates (data not shown).
Transcription during spermatogenesis is temporally regulated, with many genes selectively expressed in the diploid spermatogonia, during meiosis, or in the early differentiative stages of haploid spermatids (26). The transcriptional activation of CREM in spermatids is essential for normal spermatogenesis, and deletion of CREM prevents the synthesis of many essential mRNAs encoding structural proteins of the sperm nucleus and fibrous sheath (27, 28). Because transcription terminates in male germ cells during midspermiogenesis long before the synthesis of many of the spermatid and sperm proteins, many spermatid mRNAs are stored up to 7 days in the cytoplasm before their translation (26). Interestingly, the five translationally delayed mRNAs that specifically coprecipitate with TB-RBP and KIF17b (Fig. 2) are dependent on CREM for expression. Another RNA-binding protein, MIWI, the murine homologue of PIWI, associates with ACT and CREM transcribed mRNAs in the cytoplasm (15). Although there is no known direct interaction of MIWI with CREM or ACT, the absence of MIWI results in the destabilization of its target mRNAs. Although TB-RBP binds to many of the same mRNAs, their stability is not reduced in TB-RBP deficient mice (16). The existence of other testicular RNA-binding proteins such as MSY2 (29), MSY4 (30), and Tarbp2 (31) with overlapping specificities suggests that redundant pathways for mRNA transport and storage exist in the postmeiotic germ cells to ensure temporal and spatial regulation of gene expression. The observation that KIF17b serves as a regulator of CREM transcription and as a molecular motor for movement of the CREM transcribed mRNA–protein complexes links one kinesin to the processes of transcription and mRNA transport in the highly differentiated germ cells of the mammalian testis.
Acknowledgments
We thank S. Donlon for excellent technical assistance and M. Maronski and M. Dichter for neuronal cells. This work was supported by National Institutes of Health Grant HD 28832 and Mellon Foundation Junior Investigator Research Grant 10200675.
Abbreviations: ACT, activator of CREM in testis; CREM, cAMP-responsive element modulator; MALDI-TOF, matrix-assisted laser desorption/ionization–time-of-flight; GAPDS, testis-specific GAPDH; AKAP4, A kinase anchoring protein 4; PGK2, phosphoglycerate kinase 2.
References
- 1.Braun, R. E., Behringer, R. R., Peschon, J. J., Brinster, R. L. & Palmiter, R. D. (1989) Nature 337, 373–376. [DOI] [PubMed] [Google Scholar]
- 2.Caldwell, K. A. & Handel, M. A. (1991) Proc. Natl. Acad. Sci. USA 88, 2407–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou, Y., Millette, C. F. & Sperry, A. O. (2002) Biol. Reprod. 66, 843–855. [DOI] [PubMed] [Google Scholar]
- 4.Aoki, K., Suzuki, K., Sugano, T., Tasaka, T., Nakahara, K., Kuge, O., Omori, A. & Kasai, M. (1995) Nat. Genet. 10, 167–174. [DOI] [PubMed] [Google Scholar]
- 5.Morales, C. R., Lefrancois, S., Chennathukuzhi, V., El-Alfy, M., Wu, X., Yang, J., Gerton, G. L. & Hecht, N. B. (2002) Dev. Biol. 246, 480–494. [DOI] [PubMed] [Google Scholar]
- 6.Han, J. R., Yiu, G. K. & Hecht, N. B. (1995) Proc. Natl. Acad. Sci. USA 92, 9550–9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han, J. R., Gu, W. & Hecht, N. B. (1995) Biol. Reprod. 53, 707–717. [DOI] [PubMed] [Google Scholar]
- 8.Severt, W. L., Biber, T. U., Wu, X., Hecht, N. B., DeLorenzo, R. J. & Jakoi, E. R. (1999) J. Cell Sci. 112, 3691–3702. [DOI] [PubMed] [Google Scholar]
- 9.Setou, M., Nakagawa, T., Seog, D. H. & Hirokawa, N. (2000) Science 288, 1796–1802. [DOI] [PubMed] [Google Scholar]
- 10.Macho, B., Brancorsini, S., Fimia, G. M., Setou, M., Hirokawa, N. & Sassone-Corsi, P. (2002) Science 298, 2388–2390. [DOI] [PubMed] [Google Scholar]
- 11.Wu, X. Q., Lefrancois, S., Morales, C. R. & Hecht, N. B. (1999) Biochemistry 38, 11261–11270. [DOI] [PubMed] [Google Scholar]
- 12.Chennathukuzhi, V. M., Kurihara, Y., Bray, J. D. & Hecht, N. B. (2001) J. Biol. Chem. 276, 13256–13263. [DOI] [PubMed] [Google Scholar]
- 13.Niranjanakumari, S., Lasda, E., Brazas, R. & Garcia-Blanco, M. A. (2002) Methods 26, 182–190. [DOI] [PubMed] [Google Scholar]
- 14.Brewer, G. J., Torricelli, J. R., Evege, E. K. & Price, P. J. (1993) J Neurosci. Res. 35, 567–576. [DOI] [PubMed] [Google Scholar]
- 15.Deng, W. & Lin, H. (2002) Dev. Cell 2, 819–830. [DOI] [PubMed] [Google Scholar]
- 16.Chennathukuzhi, V., Stein, J. M., Abel, T., Donlon, S., Yang, S., Miller, J. P., Allman, D. M., Simmons, R. A. & Hecht, N. B. (2003) Mol. Cell. Biol. 23, 6419–6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fajardo, M. A., Butner, K. A., Lee, K. & Braun, R. E. (1994) Dev. Biol. 166, 643–653. [DOI] [PubMed] [Google Scholar]
- 18.Yang, J., Chennathukuzhi, V., Miki, K., O'Brien, D. A. & Hecht, N. B. (2003) Biol. Reprod. 68, 853–859. [DOI] [PubMed] [Google Scholar]
- 19.Haddad, A., Smith, M. D., Herscovics, A., Nadler, N. J. & Leblond, C. P. (1971) J. Cell Biol. 49, 856–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, Y. S., Carson, J. H., Barbarese, E. & Richter, J. D. (2003) Genes Dev. 17, 638–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirokawa, N., Noda, Y. & Okada, Y. (1998) Curr. Opin. Cell Biol. 10, 60–73. [DOI] [PubMed] [Google Scholar]
- 22.Mallardo, M., Deitinghoff, A., Muller, J., Goetze, B., Macchi, P., Peters, C. & Kiebler, M. A. (2003) Proc. Natl. Acad. Sci. USA 100, 2100–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navolanic, P. M. & Sperry, A. O. (2000) Biol. Reprod. 62, 1360–1369. [DOI] [PubMed] [Google Scholar]
- 24.Bouvet, P. & Wolffe, A. P. (1994) Cell 77, 931–941. [DOI] [PubMed] [Google Scholar]
- 25.Siomi, M. C., Eder, P. S., Kataoka, N., Wan, L., Liu, Q. & Dreyfuss, G. (1997) J. Cell Biol. 138, 1181–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hecht, N. B. (1998) Bioessays 20, 555–561. [DOI] [PubMed] [Google Scholar]
- 27.Blendy, J. A., Kaestner, K. H., Weinbauer, G. F., Nieschlag, E. & Schutz, G. (1996) Nature 380, 162–165. [DOI] [PubMed] [Google Scholar]
- 28.Nantel, F., Monaco, L., Foulkes, N. S., Masquilier, D., LeMeur, M., Henriksen, K., Dierich, A., Parvinen, M. & Sassone-Corsi, P. (1996) Nature 380, 159–162. [DOI] [PubMed] [Google Scholar]
- 29.Yu, J., Hecht, N. B. & Schultz, R. M. (2002) Biol. Reprod. 67, 1093–1098. [DOI] [PubMed] [Google Scholar]
- 30.Giorgini, F., Davies, H. G. & Braun, R. E. (2002) Development 129, 3669–3679. [DOI] [PubMed] [Google Scholar]
- 31.Zhong, J., Peters, A. H., Lee, K. & Braun, R. E. (1999) Nat. Genet. 22, 171–174. [DOI] [PubMed] [Google Scholar]