Abstract
Achieving anatomical graft placement remains a concern in anterior cruciate ligament (ACL) reconstruction. The purpose of this study was to quantify the effect of femoral graft placement on the ability of ACL reconstruction to restore normal knee kinematics under in vivo loading conditions. Two different groups of patients were studied: one in which the femoral tunnel was placed near the anterior and proximal border of the ACL (anteroproximal group, n=12) and another where the femoral tunnel was placed near the center of the ACL (anatomic group, n =10)
MR imaging and biplanar fluoroscopy were used to measure in vivo kinematics in these patients during a quasi-static lunge. Patients with anteroproximal graft placement had up to 3.4mm more anterior tibial translation, 1.1mm more medial tibial translation and 3.7° more internal tibial rotation compared to the contralateral side. Patients with anatomic graft placement had motion that more closely replicated that of the intact knee, with anterior tibial translation within 0.8mm, medial tibial translation within 0.5mm, and internal tibial rotation within 1°.
Grafts placed anteroproximally on the femur likely provide insufficient restraint to these motions due to a more vertical orientation. Anatomical femoral placement of the graft is more likely to reproduce normal ACL orientation, resulting in a more stable knee. Therefore, achieving anatomical graft placement on the femur is crucial to restoring normal knee function and may decrease the rates of joint degeneration after ACL reconstruction.
1. Introduction
Rupture of the anterior cruciate ligament (ACL) has been shown to alter native tibiofemoral motion (Zhang 2003; Andriacchi 2006; Barrance 2006; DeFrate 2006; Gao 2010). Specifically, past in vivo kinematic studies have shown ACL deficiency increases anterior translation, medial translation, and internal rotation of the tibia under various loading conditions (Georgoulis 2003; Andriacchi 2005; Andriacchi 2006; DeFrate 2006). These altered kinematics are thought to contribute to the degenerative changes observed after ACL injury (Georgoulis 2003; Andriacchi 2005; Andriacchi 2006; Barrance 2006; Li 2006). Despite advances in ACL reconstruction techniques, the development of osteoarthritis after surgery remains a concern (Fink 2001; Salmon 2006; Oiestad 2009), with some studies questioning the ability of reconstruction to prevent degeneration compared to patients who forego surgery (Lohmander 2004; von Porat 2004; Fithian 2005).
The inability of ACL reconstructions to restore normal knee motion has been thought to be an important factor contributing to the associated joint degeneration after ACL reconstruction (Logan 2004; Tashman 2004; Papannagari 2006). Anatomically placed grafts are believed to more closely reproduce native ACL function and knee kinematics (Arnold 2001; Heming 2007; Brophy 2009). However, recent studies have suggested that non-anatomic placement of the graft might be a frequent problem during ACL reconstruction (Heming 2007; Abebe 2009; Kopf 2010; Scanlan 2010). Specifically, some transtibial techniques, in which the femoral tunnel is placed through the tibial tunnel, might be prone to anterior and proximal placement of the graft on the femur (Kohn 1998; Arnold 2001; Gavriilidis 2008; Harner 2008; Kaseta 2008; Abebe 2009; Dargel 2009). Given the critical role the ACL plays in stabilizing tibiofemoral motion (Zhang 2003; Andriacchi 2006; DeFrate 2006; Gao 2010), understanding the effect of femoral graft placement on in vivo knee kinematics is critical to improving surgical treatments following ACL injury.
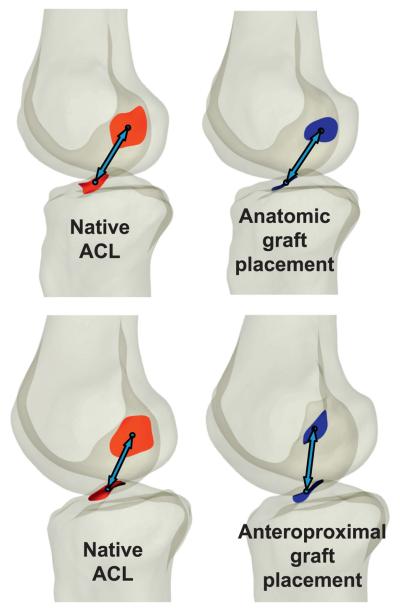
Hence, the purpose of this study was to quantify the effect of femoral graft placement on the ability of ACL reconstruction to restore normal knee kinematics under in vivo loading conditions. Two different patient populations were studied (Figure 1): one in which the femoral tunnel was placed near the anterior and proximal border of the ACL using a transtibial technique (anteroproximal group), and another where the femoral tunnel was placed near the center of the ACL using a tibial tunnel independent technique (anatomic group) (Abebe 2009). In the anatomic group, grafts were placed within an average of 3mm of the center of the ACL, while in the anteroproximal group, grafts were placed an average of 9mm from the center of the femoral attachment of the ACL, near its anteroproximal border (Abebe 2009). Our hypothesis was that anatomically placed grafts more closely mimic native ACL function and thus, more closely reproduce native tibiofemoral kinematics.
Figure 1.
In the anatomic placement group (top), the graft was placed near the center of the native ACL footprint on the femur, while in the anteroproximal placement group (bottom), the graft was centered near the anteroproximal border of the femoral footprint (Abebe 2009), as demonstrated in two subjects.
2. Materials and Methods
Subject Recruitment
Twenty two subjects (16 men and 6 women, mean age: 31 years, age range: 19 to 49 years) between 6 and 36 months after unilateral ACL reconstruction participated in this study. Chart reviews were performed to identify potential candidates for this study. Patients were sorted by operative date, and invited in chronological order to participate. Those with osteoarthritis, articular cartilage defects, major tears of the meniscus (requiring removal of more than 10% of the medial or lateral meniscus), or any other history of injury or surgery to either knee were excluded. All patients had stable knees under Lachman and pivot shift examinations. At the time of the study, all patients were doing well and had returned to sports activity without restriction. Those who agreed to participate signed an IRB-approved consent form prior to participation in the study.
Subjects were recruited from the clinics of two surgeons, both of whom had at least fifteen years of experience in sports medicine. The surgeons practiced two different single bundle arthroscopic procedures. Previous analysis indicated that one procedure resulted in anatomic femoral graft placement (within an average of 3mm of the center of the ACL footprint) and the other resulted in anteroproximal femoral graft placement (within an average of 9mm of the center of the ACL footprint, near its anteroproximal border) (Abebe 2009). Twelve subjects (9 men, 3 women, mean age: 32 years) were in the anteroproximal placement group, and the other ten subjects (7 men, 3 women; mean age: 30 years) were in the anatomic placement group.
Surgical Protocol
Anteroproximal graft placement
After diagnostic arthroscopy was performed to confirm ACL injury, the tibial tunnel was placed using a Concept Precision guide pin (ConMed Linvatec, Largo, FL) aligned at 57° in the sagittal plane and 65° in the coronal plane (Abebe 2009). Each tibial tunnel was reamed with a reamer equal in size to the graft diameter used in the procedure. The tibial tunnel location was aimed to allow placement of a 7mm offset guide at the 1:30 position or the 10:30 position. A graft size appropriate cannulated reamer was then passed through the tibial tunnel and over the guide pin to create the femoral socket. Limited notchplasty that avoided the articular surface was performed whenever intraoperative assessment showed a risk of impingement. Graft diameter sizes varied from 7 to 9mm. Five patients had intact menisci, and the remaining seven had tears requiring removal of less than 10% of the meniscus (five lateral tears and two medial tears). Using this technique, the grafts were placed near the anteroproximal border of the ACL footprint, an average of 9mm from the center of the femoral attachment of the ACL (Abebe 2009).
Anatomic graft placement
A diagnostic arthroscopy was first performed to confirm ACL injury. The location and shape of the ACL footprint was visualized through the anteromedial and anterolateral portals. A guidepin was placed through the center of the visible tibial footprint of the ACL. A graft-size-appropriate cannulated reamer was used to create the tibial tunnel. Using the anteromedial portal, the femoral tunnel was placed by centering a guide (Retro-Drill, Arthrex, Naples, FL) on the ACL stump. A guide pin was placed from outside the joint through a small incision over the lateral femoral cortex just anterior to the iliotibial tract. The guide pin was drilled through the femur to the tip of the aiming guide. The pin was threaded to allow placement of a graft size appropriate cutter on the guide pin as it entered the joint through the femoral ACL footprint. The cutter then cut a socket into the femur to the desired depth. Tunnel sizes varied between 7.5-8.5mm depending on harvested graft, and no notchplasty was performed. Four patients had intact menisci, and the remaining six had tears requiring removal of less than 10% of the meniscus (three lateral tears and three medial tears). Using this technique, the grafts were placed an average of 3mm from the center of the ACL (Abebe 2009).
Modeling and testing protocol
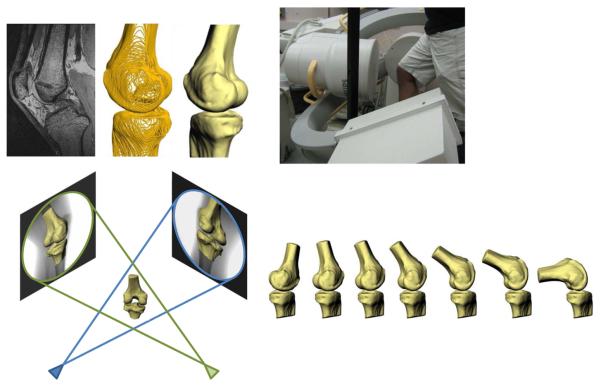
Both the operative and contralateral intact knee were imaged using a 3T scanner (Trio Tim, Siemens) with the patient in a supine, relaxed position. Sagittal plane images (512×512 pixels) with a field of view of 16×16cm, and spacing of 1mm were generated using a double-echo steady state sequence (DESS, Flip angle: 25°, TR: 17ms, TE: 6ms). From these images, three-dimensional computer models of each subject's femur and tibia were created (Figure 2) (Abebe 2009).
Figure 2.
High resolution MR images were segmented to create 3D models of the knee (top left). Next, the patients were imaged using biplanar fluoroscopy while performing a quasi-static lunge (top right). The fluoroscopic images and 3D models were then used to reproduce the motion of each subject's knee during the lunge (bottom).
Next, each patient was asked to stand on a level platform and perform a quasi-static, single leg lunge from 0° to 90° of flexion within the beams of two orthogonally positioned fluoroscopes (Pulsera, Philips, The Netherlands) (Figure 2). Anteromedial and anterolateral image sets (resolution 1024 × 1024 pixels) were obtained of each knee as the subject flexed in increments of 15°.
The orthogonal image sets were imported into solid modeling software (Rhinoceros 4.0, Robert McNeel and Associates, Seattle, WA) to reproduce the relative position and orientation of the fluoroscopes at the time of testing (Figure 2). Next, edge detection software written in Mathematica 6.0 (Wolfram Research, Champaign, IL) was used to outline the bone contours of the fluoroscopic images. Each subject's 3D models of the tibia and femur were then imported into the software, allowing for the models to be viewed from the two orthogonal directions corresponding to the views of the fluoroscopes during imaging. Finally, the models were manually manipulated in 6 degrees of freedom until the projections matched the edge-detected outlines on the fluoroscopic images, as described previously (DeFrate 2006; Caputo 2009). In this fashion, the 3D models of each subject were used to reproduce the motion of each subject's knee during the quasi-static lunge. This modeling approach allows for the measurement of tibiofemoral kinematics non-invasively.
Data Analysis
In order to measure the kinematics on both the operative and intact knees using the same coordinate system, all right knee models were mirrored into left knee models and aligned to the contralateral side using an iterative closest point technique (Caputo 2009). The registration of both the reconstructed and contralateral sides allowed identical coordinate systems to be created on both knees simultaneously (DeFrate 2006). First, the long axis of the tibia was created by fitting a cylinder to the shaft of the tibia. Next, a mediolateral axis was drawn perpendicular to the long axis of the tibia, and tangent to the posterior extremes of the tibia. Finally, the anteroposterior axis was drawn perpendicular to the other two axes. On the femur, axes were created along the long axis of the femur and through the transepicondylar line.
Using these coordinate systems, we calculated the translation of the midpoint of the transepicondylar line relative to the coordinate system of the tibia (DeFrate 2006). Flexion was defined as the angle between the long axis of the femur and tibia, projected on the sagittal plane of the tibial coordinate system. Internal-external rotation was measured as rotation of the transepicondylar line relative to the medial-lateral axis of the tibia, projected onto the axial plane of the tibial coordinate system.
These coordinate systems were used to measure the in vivo anteroposterior and mediolateral translation, and internal-external rotation of the tibia relative to the femur between 0° and 90° of flexion. In order to directly compare the ability of a reconstruction to restore each patient's normal knee function, the relative differences between the reconstructed and intact contralateral knees were calculated. A two-tailed t-test was used to determine whether the motion of the reconstructed knees relative to the contralateral knees in both the anatomic and anteroproximal graft placement groups was significantly different from zero. Differences were considered statistically significant where p < 0.05.
Results
Anteroposterior tibial translation
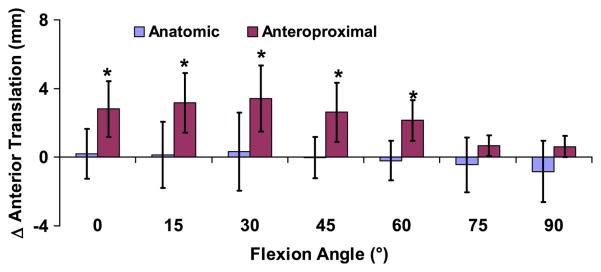
Patients with anteroproximal graft placement on the femur had increased anterior tibial translation in the reconstructed knee relative to the contralateral side between 0 and 60° (p<0.03, Figure 3). No differences were detected between the reconstructed and contralateral sides in the anatomic placement group at any flexion angle (p>0.32). At 30° of flexion, in the anteroproximal placement group there was a maximum of 3.4±1.9mm (mean and 95% confidence interval) more anterior tibial translation in the reconstructed knee relative to the contralateral knee (p=0.003), while in the anatomic group, anterior translation was 0.3±2.3mm (p=0.75). In the anatomic group, the mean difference in anterior translation across all flexion angles was −0.1±0.5mm, with no differences detected between the intact and reconstructed sides (p=0.66). These data have more than 80% power in detecting differences of 0.8mm in anterior translation relative to the contralateral knee with 95% confidence.
Figure 3.
The increase in anterior tibial translation of the reconstructed knee relative to the contralateral intact knee was measured as a function of flexion (mean and 95% confidence intervals). Zero denotes a knee that exactly mimics the motion of the contralateral side. Patients with grafts placed anteroproximally on the femur had increased anterior tibial translation relative to the contralateral side between 0 and 60° of flexion, while the anatomically placed grafts more closely restored normal knee motion. (*p < 0.05)
Mediolateral tibial translation
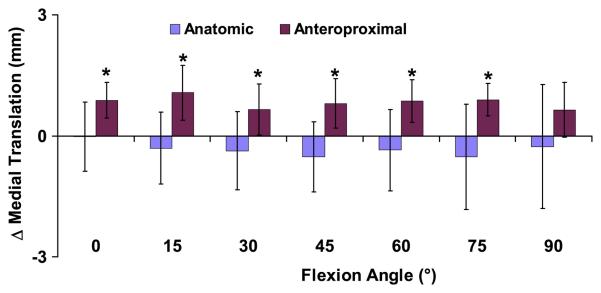
In the anteroproximal placement group, there was increased medial tibial translation in the reconstructed knee relative to the intact knee between 0 and 75° of flexion (p<0.05, Figure 4). No differences were detected between the reconstructed and contralateral knees in the anatomic group at any flexion angle (p>0.21). At 15° of flexion in the anteroproximal placement group, there was a maximum increase in medial tibial translation of 1.1±0.7mm in the reconstructed knees relative to the contralateral knees (p=0.005). In the anatomic group at 15° of flexion, there was a slight lateral shift of 0.3±0.9mm (p=0.46). In the anatomic group across all flexion angles, there was a mean difference in medial translation of −0.3±0.3mm, with no differences detected between the intact and reconstructed sides (p=0.06). These data have more than 80% power in detecting differences of greater than 0.5mm with 95% confidence.
Figure 4.
The increase in medial tibial translation of the reconstructed knee relative to the contralateral intact knee was measured as a function of flexion (mean and 95% confidence intervals). Zero denotes a knee that exactly mimics the motion of the contralateral side. Patients with grafts placed anteroproximally on the femur had significantly increased medial tibial translation relative to the contralateral side between 0 and 75° of flexion, while the anatomically placed grafts more closely restored normal knee motion. (*p < 0.05)
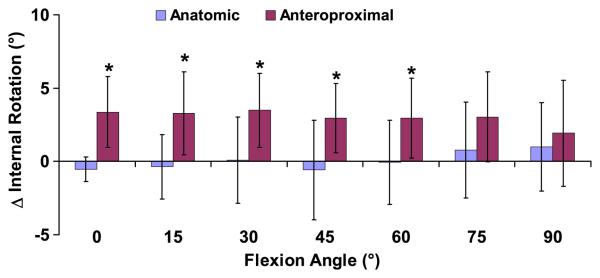
Internal-external tibial rotation
Patients with anteroproximal graft placement had increased internal tibial rotation in the reconstructed knee relative to the contralateral side between 0 and 60° (p<0.04, Figure 5). No differences were detected between the reconstructed side and contralateral side in the anatomic group at any flexion angle (p>0.19). At 30° of flexion in the anteroproximal group, there was a maximum of 3.5±2.5° more internal rotation in the reconstructed knee relative to the contralateral knee (p=0.01), while in the anatomic group internal rotation was within 0.1±2.9° (p=0.95). In the anatomic group across all flexion angles, there was an average difference in internal rotation of 0.1±1.0°, with no difference detected between the intact and reconstructed sides (p=0.93). These data have more than 80% power in detecting differences of greater than 1.5° with 95% confidence.
Figure 5.
The increase in internal tibial rotation of the reconstructed knee relative to the contralateral intact knee was measured as a function of flexion (mean and 95% confidence intervals). Zero denotes a knee that exactly mimics the motion of the contralateral side. Patients with grafts placed anteroproximally on the femur had increased internal tibial rotation relative to the contralateral side between 0 and 60° of flexion, while the anatomically placed grafts more closely restored normal knee motion. (*p < 0.05)
Discussion
Achieving anatomic graft placement remains a concern in ACL reconstruction (Bedi 2009; Scanlan 2009; Steiner 2009; Kopf 2010; Scanlan 2010), with studies often citing anterior and proximal placement on the femur as a problem (Kohn 1998; Arnold 2001; Garofalo 2007; Harner 2008; Kaseta 2008; Abebe 2009). This study used biplanar fluoroscopy and MR imaging to compare the effect of graft placement on the ability of ACL reconstruction to restore normal knee motion during a quasi-static lunge. Two different placement groups were compared: one in which a tibial tunnel independent technique was used to place the graft an average of 3mm from the center of the ACL, and another in which grafts were placed transtibially near the anteroproximal border of the ACL, an average of 9mm from the center of the ACL (Abebe 2009).
This study indicates that patients with the anteroproximal placement of the graft on the femur had increased anterior tibial translation, medial tibial translation, and internal tibial rotation compared to the contralateral native knee during a quasi-static lunge. Similar increases in these motions were observed in the ACL deficient knee (DeFrate 2006). In contrast, knees with anatomic graft placement more closely restored native knee motion under the same loading conditions. These findings suggest that the grafts placed anteroproximally on the femur provide insufficient constraint under these loading conditions, while the anatomically placed grafts more closely replicate native ACL function.
Our findings on the anteroposterior motion of the knee are consistent with previous in vivo studies. Under similar loading conditions, increases in anterior tibial translation as high as 3.5 mm were reported in ACL deficient patients (DeFrate 2006). Others have also documented increased anterior tibial translations in ACL deficient patients during stair climbing (Brandsson 2001) and walking (Georgoulis 2003; Gao 2010). After ACL reconstruction, several studies have reported a decrease in anterior translation with current surgical approaches (Logan 2004; Yoo 2005). In the present study, patients with the anteroproximal graft placement had increases of up to 3.4mm, while the more anatomically placed grafts restored anterior translation to within 1mm.
Similarly, our data on medial tibial translation is consistent with previous studies. Other studies have documented increased medial translation, with an increase of up to 1.2mm in ACL deficient patients under similar in vivo loading conditions (DeFrate 2006), and an increase of up to 1.3mm in a cadaver model of ACL deficiency (Li 2007). In the current study, patients with anteroproximal graft placement had increases in medial translation as high as 1.1mm, while the anatomically placed graft restored medial translation to within 0.5mm.
Lastly, an increase in internal tibial rotation of 2.2° has been reported in ACL deficient patients during a similar activity to that performed in the current study (DeFrate 2006). Increased internal tibial rotation relative to the intact knee has also been observed with ACL deficiency during walking (Georgoulis 2003; Andriacchi 2004; Andriacchi 2006; Gao 2010). For example, Gao et al reported an increase of 2 to 4° in internal tibial rotation of the deficient knee relative to the intact knee throughout the gait cycle (Gao 2010). After ACL reconstruction, several studies have reported decreases in internal tibial rotation relative to the deficient knee during walking (Georgoulis 2003; Gao 2010), while other studies have reported an external tibial rotation relative to the intact knee during walking (Scanlan 2010) or running (Tashman 2004). In the present study, the patients reconstructed with anteroproximally placed grafts on the femur had up to 3.5° more internal rotation, while those with the anatomic femoral graft placement were within 1° of the contralateral side.
The results of this study can be explained in part by the orientation of the graft resulting from the placement of the femoral tunnel. Anterior and proximal graft placement is likely to produce a graft that is more vertical than the native ACL (Arnold 2001; Heming 2007; Harner 2008; Pearle 2008; Abebe 2009). This was confirmed in a study of graft orientaiton in this same patient population, where anteroproximal femoral graft placement resulted in a more vertically oriented graft in the coronal and sagittal planes than the native ACL during weight-bearing flexion (Abebe 2010).
From a biomechanical perspective, vertically oriented grafts are likely to be less effective at resisting motions in the transverse plane. Previous cadaver studies have indicated that vertical grafts in the sagittal plane require higher forces to resist the same anterior shear force (Li 2006). Furthermore, more vertical grafts in the coronal plane have been shown to not restore rotational stability as effectively as more horizontal grafts in cadaver models (Loh 2003; Scopp 2004; Yamamoto 2004). More vertical grafts are also likely to be inefficient in controlling the increased medial translation observed with ACL deficiency (DeFrate 2006; Li 2006). Thus, it is likely that patients reconstructed with a graft placed anteroproximally on the femur have increased anterior translation, medial translation, and internal rotation due to a graft that does not mimic the orientation of the native ACL. In contrast, those patients with anatomically placed grafts likely have grafts that more closely restore native ACL orientation, resulting in a reconstruction that more closely restores normal knee motion.
Many investigators have hypothesized that abnormal tibiofemoral knee motion following injury predisposes the knee to osteoarthritis (Tashman 2004; Andriacchi 2005; Andriacchi 2006; Tashman 2007). Specifically, recent studies have indicated that the abnormal motions observed with ACL deficiency (including increased internal rotation, anterior translation, and medial translation of the tibia) can alter normal cartilage loading (Andriacchi 2006; Li 2006; Van De Velde 2009). In addition, many recent studies have suggested that the inability to correct abnormal kinematics with ACL reconstruction is an important factor contributing to degenerative changes observed after reconstruction (Logan 2004; Tashman 2004; Papannagari 2006; Scanlan 2010). This study demonstrated that more anatomically placed grafts more closely restored normal knee motion, while the more vertically oriented grafts in the anteroproximal graft placement group (Abebe 2010) did restrain anterior translation, medial translation, or internal rotation. These findings suggest that achieving anatomic graft placement is an important factor in reproducing normal ACL function and knee motion. Therefore, graft placement might be an important variable in decreasing the incidence of joint degeneration after ACL reconstruction.
There are some limitations with the present study. First, the reconstructions were performed by two different surgeons. While this can potentially introduce bias, it is important to note that using one surgeon to perform both surgeries would bias the technique with which the surgeon was most familiar. For this reason, we chose to retrospectively evaluate patients from two experienced surgeons each using the technique with which they were most comfortable. In the patients with anteroproximal graft placement on the femur, 6 patients received hamstring grafts and 6 had bone-patellar-tendon-bone grafts, while in the anatomic group, hamstring grafts were used for all patients. This difference was a result of difficulties recruiting patients that met all of the inclusion criteria with the same graft types in both groups. Nevertheless, anterior translation, internal rotation, and medial translation were consistently increased in patients with anteroproximal graft placement. In this study, we used the contralateral knee as a control for the motion of the reconstructed knee. Although there may be a degree of asymmetry in the knee motions within subjects, recent studies have suggested that the contralateral knee is a reliable control for kinematic and anatomic studies of cruciate ligament injury and reconstruction (Kozanek 2008; Jamison 2010; Scanlan 2010). Finally, this study only examined one quasi-static activity. Future studies should consider the effect of graft placement on knee motion during other activities of daily living.
In conclusion, this study compared the effect of graft placement on the ability of ACL reconstruction to restore native knee motion in subjects during weight-bearing flexion. The data showed that the more anatomically placed grafts more closely restored native knee kinematics compared to grafts placed anteroproximally relative to the ACL attachment site on the femur. These findings suggest that, regardless of technique, achieving anatomic femoral placement of the graft is crucial to reproducing native knee kinematics and might help to decrease the incidence of joint degeneration after ACL reconstruction.
Acknowledgements
This work was supported by grants from the NIH (AR055659) and the NFL Charities. The technical support of Holly A. Leddy, Ph.D., Elizabeth C. Pennington, and Richard R. Glisson is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Arthrex provided research support to Duke University Medical Center.
References
- 1.Abebe ES, Kim JP, Utturkar GM, Spritzer CE, Moorman CT, 3rd, Taylor DC, Garrett WE, Jr., DeFrate LE. In vivo graft deformation in transtibial and tibial tunnel independent ACL reconstruction techniques. Trans Orthop Res Soc. 2010 [Google Scholar]
- 2.Abebe ES, Moorman CT, 3rd, Dziedzic TS, Spritzer CE, Cothran RL, Taylor DC, Garrett WE, Jr., DeFrate LE. Femoral tunnel placement during anterior cruciate ligament reconstruction: an in vivo imaging analysis comparing transtibial and 2-incision tibial tunnel-independent techniques. Am J Sports Med. 2009;37(10):1904–11. doi: 10.1177/0363546509340768. [DOI] [PubMed] [Google Scholar]
- 3.Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after ACL injury cause cartilage thinning. Clin Orthop Relat Res. 2006;442:39–44. doi: 10.1097/01.blo.0000197079.26600.09. [DOI] [PubMed] [Google Scholar]
- 4.Andriacchi TP, Dyrby CO. Interactions between kinematics and loading during walking for the normal and ACL deficient knee. J Biomech. 2005;38(2):293–8. doi: 10.1016/j.jbiomech.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447–57. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 6.Arnold MP, Kooloos J, van Kampen A. Single-incision technique misses the anatomical femoral anterior cruciate ligament insertion: a cadaver study. Knee Surg Sports Traumatol Arthrosc. 2001;9(4):194–9. doi: 10.1007/s001670100198. [DOI] [PubMed] [Google Scholar]
- 7.Barrance PJ, Williams GN, Snyder-Mackler L, Buchanan TS. Altered knee kinematics in ACL-deficient non-copers: a comparison using dynamic MRI. J Orthop Res. 2006;24(2):132–40. doi: 10.1002/jor.20016. [DOI] [PubMed] [Google Scholar]
- 8.Bedi A, Altchek DW. The “footprint” anterior cruciate ligament technique: an anatomic approach to anterior cruciate ligament reconstruction. Arthroscopy. 2009;25(10):1128–38. doi: 10.1016/j.arthro.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Brandsson S, Karlsson J, Eriksson BI, Karrholm J. Kinematics after tear in the anterior cruciate ligament: dynamic bilateral radiostereometric studies in 11 patients. Acta Orthop Scand. 2001;72(4):372–8. doi: 10.1080/000164701753542032. [DOI] [PubMed] [Google Scholar]
- 10.Brophy RH, Pearle AD. Single-bundle anterior cruciate ligament reconstruction: a comparison of conventional, central, and horizontal single-bundle virtual graft positions. Am J Sports Med. 2009;37(7):1317–23. doi: 10.1177/0363546509333007. [DOI] [PubMed] [Google Scholar]
- 11.Caputo AM, Lee JY, Spritzer CE, Easley ME, DeOrio JK, Nunley JA, 2nd, DeFrate LE. In vivo kinematics of the tibiotalar joint after lateral ankle instability. Am J Sports Med. 2009;37(11):2241–8. doi: 10.1177/0363546509337578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dargel J, Schmidt-Wiethoff R, Fischer S, Mader K, Koebke J, Schneider T. Femoral bone tunnel placement using the transtibial tunnel or the anteromedial portal in ACL reconstruction: a radiographic evaluation. Knee Surg Sports Traumatol Arthrosc. 2009;17(3):220–7. doi: 10.1007/s00167-008-0639-2. [DOI] [PubMed] [Google Scholar]
- 13.DeFrate LE, Papannagari R, Gill TJ, Moses JM, Pathare NP, Li G. The 6 degrees of freedom kinematics of the knee after anterior cruciate ligament deficiency: an in vivo imaging analysis. Am J Sports Med. 2006;34(8):1240–6. doi: 10.1177/0363546506287299. [DOI] [PubMed] [Google Scholar]
- 14.Fink C, Hoser C, Hackl W, Navarro RA, Benedetto KP. Long-term outcome of operative or nonoperative treatment of anterior cruciate ligament rupture--is sports activity a determining variable? Int J Sports Med. 2001;22(4):304–9. doi: 10.1055/s-2001-13823. [DOI] [PubMed] [Google Scholar]
- 15.Fithian DC, Paxton EW, Stone ML, Luetzow WF, Csintalan RP, Phelan D, Daniel DM. Prospective trial of a treatment algorithm for the management of the anterior cruciate ligament-injured knee. Am J Sports Med. 2005;33(3):335–46. doi: 10.1177/0363546504269590. [DOI] [PubMed] [Google Scholar]
- 16.Gao B, Zheng NN. Alterations in three-dimensional joint kinematics of anterior cruciate ligament-deficient and -reconstructed knees during walking. Clin Biomech (Bristol, Avon) 2010;25(3):222–9. doi: 10.1016/j.clinbiomech.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Garofalo R, Moretti B, Kombot C, Moretti L, Mouhsine E. Femoral tunnel placement in anterior cruciate ligament reconstruction: rationale of the two incision technique. J Orthop Surg. 2007;2:10. doi: 10.1186/1749-799X-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavriilidis I, Motsis EK, Pakos EE, Georgoulis AD, Mitsionis G, Xenakis TA. Transtibial versus anteromedial portal of the femoral tunnel in ACL reconstruction: a cadaveric study. Knee. 2008;15(5):364–7. doi: 10.1016/j.knee.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Georgoulis AD, Papadonikolakis A, Papageorgiou CD, Mitsou A, Stergiou N. Three-dimensional tibiofemoral kinematics of the anterior cruciate ligament-deficient and reconstructed knee during walking. Am J Sports Med. 2003;31(1):75–9. doi: 10.1177/03635465030310012401. [DOI] [PubMed] [Google Scholar]
- 20.Harner CD, Honkamp NJ, Ranawat AS. Anteromedial portal technique for creating the anterior cruciate ligament femoral tunnel. Arthroscopy. 2008;24(1):113–5. doi: 10.1016/j.arthro.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 21.Heming JF, Rand J, Steiner ME. Anatomical limitations of transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2007;35(10):1708–15. doi: 10.1177/0363546507304137. [DOI] [PubMed] [Google Scholar]
- 22.Jamison ST, Flanigan DC, Nagaraja HN, Chaudhari AM. Side-to-side differences in anterior cruciate ligament volume in healthy control subjects. J Biomech. 2010;43(3):576–8. doi: 10.1016/j.jbiomech.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaseta MK, Defrate LE, Charnock BL, Sullivan RT, Garrett WE., Jr. Reconstruction Technique Affects Femoral Tunnel Placement in ACL Reconstruction. Clin Orthop Relat Res. 2008 doi: 10.1007/s11999-008-0238-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohn D, Busche T, Carls J. Drill hole position in endoscopic anterior cruciate ligament reconstruction. Results of an advanced arthroscopy course. Knee Surg Sports Traumatol Arthrosc. 1998;6(Suppl 1):S13–5. doi: 10.1007/s001670050216. [DOI] [PubMed] [Google Scholar]
- 25.Kopf S, Forsythe B, Wong AK, Tashman S, Anderst W, Irrgang JJ, Fu FH. Nonanatomic tunnel position in traditional transtibial single-bundle anterior cruciate ligament reconstruction evaluated by three-dimensional computed tomography. J Bone Joint Surg Am. 2010;92(6):1427–31. doi: 10.2106/JBJS.I.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozanek M, Van de Velde SK, Gill TJ, Li G. The contralateral knee joint in cruciate ligament deficiency. Am J Sports Med. 2008;36(11):2151–7. doi: 10.1177/0363546508319051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg Am. 2006;88(8):1826–34. doi: 10.2106/JBJS.E.00539. [DOI] [PubMed] [Google Scholar]
- 28.Li G, Papannagari R, DeFrate LE, Yoo JD, Park SE, Gill TJ. Comparison of the ACL and ACL graft forces before and after ACL reconstruction: an in-vitro robotic investigation. Acta Orthop. 2006;77(2):267–74. doi: 10.1080/17453670610046019. [DOI] [PubMed] [Google Scholar]
- 29.Li G, Papannagari R, DeFrate LE, Yoo JD, Park SE, Gill TJ. The effects of ACL deficiency on mediolateral translation and varus-valgus rotation. Acta Orthop. 2007;78(3):355–60. doi: 10.1080/17453670710013924. [DOI] [PubMed] [Google Scholar]
- 30.Logan MC, Williams A, Lavelle J, Gedroyc W, Freeman M. Tibiofemoral kinematics following successful anterior cruciate ligament reconstruction using dynamic multiple resonance imaging. Am J Sports Med. 2004;32(4):984–92. doi: 10.1177/0363546503261702. [DOI] [PubMed] [Google Scholar]
- 31.Loh JC, Fukuda Y, Tsuda E, Steadman RJ, Fu FH, Woo SL. Knee stability and graft function following anterior cruciate ligament reconstruction: Comparison between 11 o'clock and 10 o'clock femoral tunnel placement. Arthroscopy. 2003;19(3):297–304. doi: 10.1053/jars.2003.50084. [DOI] [PubMed] [Google Scholar]
- 32.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–52. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 33.Oiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009;37(7):1434–43. doi: 10.1177/0363546509338827. [DOI] [PubMed] [Google Scholar]
- 34.Papannagari R, Gill TJ, Defrate LE, Moses JM, Petruska AJ, Li G. In vivo kinematics of the knee after anterior cruciate ligament reconstruction: a clinical and functional evaluation. Am J Sports Med. 2006;34(12):2006–12. doi: 10.1177/0363546506290403. [DOI] [PubMed] [Google Scholar]
- 35.Pearle AD, Shannon FJ, Granchi C, Wickiewicz TL, Warren RF. Comparison of 3-dimensional obliquity and anisometric characteristics of anterior cruciate ligament graft positions using surgical navigation. Am J Sports Med. 2008;36(8):1534–41. doi: 10.1177/0363546508315536. [DOI] [PubMed] [Google Scholar]
- 36.Salmon LJ, Russell VJ, Refshauge K, Kader D, Connolly C, Linklater J, Pinczewski LA. Long-term outcome of endoscopic anterior cruciate ligament reconstruction with patellar tendon autograft: minimum 13-year review. Am J Sports Med. 2006;34(5):721–32. doi: 10.1177/0363546505282626. [DOI] [PubMed] [Google Scholar]
- 37.Scanlan SF, Blazek K, Chaudhari AM, Safran MR, Andriacchi TP. Graft orientation influences the knee flexion moment during walking in patients with anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37(11):2173–8. doi: 10.1177/0363546509339574. [DOI] [PubMed] [Google Scholar]
- 38.Scanlan SF, Chaudhari AM, Dyrby CO, Andriacchi TP. Differences in tibial rotation during walking in ACL reconstructed and healthy contralateral knees. J Biomech. 2010;43(9):1817–22. doi: 10.1016/j.jbiomech.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scanlan SF, Lai J, Andriacchi TP. Side-to-Side Differences in ACL Insertion Anatomy in Healthy Subjects and ACL Reconstructed Patients. Trans Orthop Res Soc. 2010 [Google Scholar]
- 40.Scopp JM, Jasper LE, Belkoff SM, Moorman CT., 3rd The effect of oblique femoral tunnel placement on rotational constraint of the knee reconstructed using patellar tendon autografts. Arthroscopy. 2004;20(3):294–9. doi: 10.1016/j.arthro.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Steiner ME, Battaglia TC, Heming JF, Rand JD, Festa A, Baria M. Independent drilling outperforms conventional transtibial drilling in anterior cruciate ligament reconstruction. Am J Sports Med. 2009;37(10):1912–9. doi: 10.1177/0363546509340407. [DOI] [PubMed] [Google Scholar]
- 42.Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(4):975–83. doi: 10.1177/0363546503261709. [DOI] [PubMed] [Google Scholar]
- 43.Tashman S, Kolowich P, Collon D, Anderson K, Anderst W. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop Relat Res. 2007;454:66–73. doi: 10.1097/BLO.0b013e31802bab3e. [DOI] [PubMed] [Google Scholar]
- 44.Van De Velde SK, Bingham JT, Hosseini A, Kozanek M, DeFrate LE, Gill TJ, Li G. Increased tibiofemoral cartilage contact deformation in patients with anterior cruciate ligament deficiency. Arthritis Rheum. 2009;60(12):3693–702. doi: 10.1002/art.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63(3):269–73. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto Y, Hsu WH, Woo SL, Van Scyoc AH, Takakura Y, Debski RE. Knee stability and graft function after anterior cruciate ligament reconstruction: a comparison of a lateral and an anatomical femoral tunnel placement. Am J Sports Med. 2004;32(8):1825–32. doi: 10.1177/0363546504263947. [DOI] [PubMed] [Google Scholar]
- 47.Yoo JD, Papannagari R, Park SE, DeFrate LE, Gill TJ, Li G. The effect of anterior cruciate ligament reconstruction on knee joint kinematics under simulated muscle loads. Am J Sports Med. 2005;33(2):240–6. doi: 10.1177/0363546504267806. [DOI] [PubMed] [Google Scholar]
- 48.Zhang LQ, Shiavi RG, Limbird TJ, Minorik JM. Six degrees-of-freedom kinematics of ACL deficient knees during locomotion-compensatory mechanism. Gait Posture. 2003;17(1):34–42. doi: 10.1016/s0966-6362(02)00052-8. [DOI] [PubMed] [Google Scholar]