To the Editor
In a recent study of modeling HCV kinetics under therapy with peginterferon-α±ribavirin, Snoeck et al.1 assumed the same maximum hepatocyte proliferation/regeneration rate, i.e., r=0.00562 day-1 for all the subjects (corresponding to hepatocyte doubling time t2=ln(2)/r=123 days), based on the increase of remnant liver volume observed in a study of healthy live-liver donors by Pomfret et al.2 Such a large t2 is striking compared with estimates in other studies of hepatic resection in animals and humans.3 To further examine this issue, we fitted the liver re-growth equation to the liver-regeneration data (Figure 1) presented by Pomfret et al. and another cohort by Nadalin et al.2, 4 Estimates of r from model fits, Figure 1A and 1B, are r=0.24 day-1 (t2=3 days) and r=0.052 day-1 (t2=13 days), respectively, which are one to two orders of magnitude higher than the estimate assumed by Snoeck et al1 (dashed lines, Figure 1).
Figure 1.
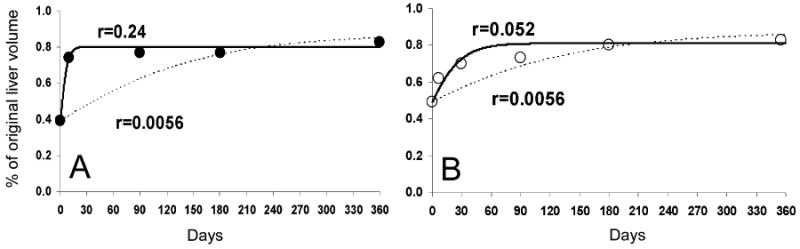
We fitted the liver-re-growth model, i.e., equation (3) described in the Supplementary Note 3 by Snoeck et al1, to the mean kinetic data of regeneration of liver volume that was digitized from Fig. 1 of Pomfret et al2 (open circles) and from Fig. 1A of Nadalin et al.4 (filled circles). Solid curves represent the best fits (using Berkeley-Madonna, Version 8.3.9 (http://www.berkeleymadonna.com) of the liver cell number divided by the original cell count, i.e., Tmax as defined by Snoeck et al. Except for the maximum liver proliferation rate constant, r, all the other parameters of the liver-re-growth model were held constant as described in the Supplementary Note 3 by Snoeck et al1. Dashed lines represent model curves with r=0.0056 day-1 as suggested by Snoeck et al1. We assume that the remnant livers reach ∼80% of the original volume (i.e., 0.8×Tmax) as observed by Pomfret et al. and Nadalin et al.
In a recent study by Rong et al.5, rapid emergence of drug-resistant HCV variants against a protease inhibitor, telaprevir, was explained by a model that includes hepatocyte proliferation, which suggests that a replication space (availability of uninfected hepatocytes) is needed for the growth of resistant variants. Estimates of the hepatocyte proliferation rate constant (from 0.3 to 2.5 day-1) were also considerably higher than that assumed by Snoeck et al1.
To our best knowledge, late (several weeks after therapy initiation) viral rebounds (termed viral breakthrough (VB) and partial-virologic response (PVR); Figure 1 in 1) under peginterferon-α±ribavirin have not been characterized. Interestingly, however, the model by Snoeck et al1 predicts these patterns when r is small (<∼0.01 day-1). A larger r reduces the time at which viral rebound occurs. Thus, if late VB and PVR patterns were not related to other factors such as dose reduction or suboptimal drug adherence, the results indicate that the development of a late VB or PVR might be associated, in part, with a low hepatocyte proliferation rate.
In conclusion, Snoeck et al. made an important step towards the development of a comprehensive HCV kinetic model. Although the value of r used was substantially lower than estimated by viral kinetic studies5 and/or liver re-growth (Figure 1), it might be true for some patients. We suggest that Snoeck and colleagues describe the proportion and characteristics of subjects who had a late VB and PVR to provide a better understanding of factors associated with nonresponse in their large cohort.
Footnotes
Conflict of interest: The authors have nothing to disclose.
References
- 1.Snoeck E, et al. A comprehensive hepatitis C viral kinetic model explaining cure. Clin Pharmacol Ther. 87:706–13. doi: 10.1038/clpt.2010.35. [DOI] [PubMed] [Google Scholar]
- 2.Pomfret EA, et al. Liver regeneration and surgical outcome in donors of right-lobe liver grafts. Transplantation. 2003;76:5–10. doi: 10.1097/01.TP.0000079064.08263.8E. [DOI] [PubMed] [Google Scholar]
- 3.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 4.Nadalin S, et al. Volumetric and functional recovery of the liver after right hepatectomy for living donation. Liver Transpl. 2004;10:1024–9. doi: 10.1002/lt.20182. [DOI] [PubMed] [Google Scholar]
- 5.Rong L, Dahari H, Ribeiro RM, Perelson AS. Rapid emergence of protease inhibitor resistance in hepatitis C virus. Sci Transl Med. 2010;2:30ra32. doi: 10.1126/scitranslmed.3000544. [DOI] [PMC free article] [PubMed] [Google Scholar]


