Abstract
Objective
The effects of cognitive-behavioral relapse prevention (RP), contingency management (CM), and their combination (CM+RP) were evaluated in a randomized trial with 100 cocaine dependent patients (58% female, 89% African American) who were engaged in treatment for at least two weeks and had an average of 44 days of abstinence at baseline.
Method
The participants were from intensive outpatient programs (IOPs), which provide 10 hours per week of group counseling. The CM protocol provided gift certificates (maximum value of $1,150; mean received= $740) for cocaine-free urines over 12 weeks using an escalating reinforcement schedule, and weekly individual RP sessions were offered for up to 20 weeks. Average number of RP sessions attended was 3 in RP and 13 in CM+RP.
Results
GEE analyses over 18 months post-randomization showed significant effects for CM (but not RP) on urine toxicology and self-reported cocaine use (p=.05), with no significant CM × RP interactions. Secondary analyses indicated CM+RP produced better cocaine urine toxicology outcomes at 6 months than TAU [OR=3.96 (1.33,11.80), p< .01] and RP [OR=4.89 (1.51,15.86), p< .01), and better cocaine urine toxicology outcomes at 9 months than TAU [OR=4.21 (1.37,12.88), p< .01] and RP [OR=4.24 (1.32,13.65), p< .01). Trends also favored CM+RP over CM at 6 [OR=2.93 (0.94,9.07), p= .06] and 9 [OR=2.93 (0.94,9.10), p= .06) months. Differences between the conditions were not significant after 9 months.
Conclusions
These results suggest CM can improve outcomes in cocaine dependent IOP patients who have achieved initial engagement, particularly when combined with relapse prevention.
Keywords: cocaine dependence, treatment, contingency management, relapse prevention
One of the pressing needs in the treatment of substance use disorders is the identification of adjunctive services or interventions that can be added to “treatment as usual” to improve outcomes. At this point, there is evidence that the provision of services to address psychiatric, family, employment, and housing problems can bring about improvements in these areas, and in some cases, in substance use outcomes (Friedmann, Hendrickson, Gerstein, & Zhang, 2004; McLellan et al., 1997, 1998; O'Farrell, Choquette, & Cutter, 1998). Continuing care, which serves to extend the duration of treatment, has also been shown to improve substance use outcomes (McKay, 2005). However, it can be difficult to deliver adjunctive interventions to patients in standard outpatient treatment due to the high drop out rates in these programs. For example, recent data from a national survey of addictions treatment programs indicated that the median length of stay in intensive outpatient programs was 46 days, with only 36% of admissions completing treatment (SAMHSA, 2008).
One intervention that has shown considerable promise as a means to both increase retention and improve substance use outcomes is contingency management (CM), which provides incentives linked to performance. CM interventions, most notably in the form of voucher-based reinforcement therapy (VBRT) for abstinence, have demonstrated efficacy in the treatment of substance use disorders, as documented in several recent meta-analyses (Dutra, Stathopoulou, Basden, Leyro, Powers, & Otto, 2008; Lussier, Heil, Mongeon, Badger, & Higgines, 2006, Prendergast, Podus, Finney, Greenwell, & Roll, 2006). Notably, CM interventions have also proved effective in community settings (Petry, Alessi, Marx, Austin, & Tardif, 2005; Petry, Alessi, & Hanson, 2007). The majority of trials utilizing CM for drug abstinence have focused on initiating abstinence early in treatment. What has been much less frequently addressed is whether CM works as an adjunctive relapse prevention intervention in individuals who have achieved initial engagement in treatment and already sharply reduced or stopped using drugs.
Another intervention that, at least theoretically, should promote better outcomes in initially stabilized patients with substance use disorders is relapse prevention, or RP (Marlatt & Gordon, 1985). Most RP interventions make use of techniques from cognitive-behavioral and social skills therapies to increase awareness of situations in which the individual has been particularly likely to use drugs (i.e., “high risk situations”) and improve coping responses for these situations through rehearsal and homework assignments (Annis & Davis, 1989; Carroll, 1998; Marlatt & Gordon, 1985). Improvements in these areas are thought to increase self-efficacy, which in term promotes further improvements in coping abilities.
Although CBT-based interventions are widely seen as evidence-based (Carroll, 1996), recent research has suggested they are not more effective than other bona fide treatments for substance use disorders, whether delivered as a primary treatment or as a form of continuing care (Dutra et al., 2008; Irvin, Bowers, Dunn, & Wang, 1999; Longabaugh & Morgenstern, 1999; McKay et al., 1999; McKay, Lynch, Shepard, & Pettinati, 2005; Morgenstern & McKay, 2007; Rawson et al., 2006). Therefore, it is not clear that supplementing standard addiction treatment with RP for patients who have achieved initial engagement will in fact lead to better outcomes.
There is also mixed evidence regarding possible additive effects for interventions that combine RP/CBT and CM over RP/CBT or CM alone. Epstein, Hawkins, Covi, Umbricht, and Preston (2003) reported that CM alone was more effective in decreasing cocaine use in methadone maintenance patients than CM+CBT during treatment, but additive effects favoring the CM+CBT condition over CM alone emerged at 12 months. Shoptaw et al. (2005) found that methamphetamine dependent participants had better drug use outcomes in CM+CBT than in CBT alone, but the combination condition was not more effective than CM alone. Finally, in studies with methadone maintenance and methamphetamine dependent patients, Rawson and colleagues found that while shorter term outcomes favored CM over CBT or control conditions, longer term outcomes in CBT conditions were not different from those in CM conditions. Moreover, there were no additive effects for CM+CBT over CM or CBT (Rawson et al., 2002; Rawson et al., 2006). It should be noted that all of these studies were done as abstinence-initiation projects, rather than as continuing care or relapse prevention projects.
Another important consideration in the selection of adjunctive interventions to reduce relapse in stabilized patients is whether the effects of the intervention persist into the post-treatment period. There is some evidence that RP/CBT interventions can exhibit sleeper effects, where the positive effects of the intervention in relation to comparison conditions does not become apparent until after the interventions are over (Carroll et al., 1994; McKay et al., 1999; Rawson et al., 2002; Rawson et al., 2006), although this is not the case in all studies. Until recently, most studies of CM did not have post-treatment follow-ups. However, a number of newer studies have included post-treatment follow-ups, usually out to 12 months. The results have been mixed, with some studies showing evidence of sustained voucher effects (Alessi, Hanson, Wieners, & Petry, 2007; Epstein et al., 2003; Higgins, Wong, Badger, Ogden, & Dantona, 2000; Higgins et al., 2003; Higgins et al., 2006), and other studies showing no sustained effects (Milby et al., 2003; Petry et al., 2005; Petry et al., 2006; Rawson et al., 2002; Rawson et al., 2006; Shoptaw et al., 2005).
This paper presents results from a program of research on the effectiveness of enhancements to improve continuing care in outpatient specialty care for drug and alcohol dependence. Participants were recruited from cocaine-dependent patients attending a publicly funded intensive outpatient program (IOP) in Philadelphia, who had successfully engaged in IOP for at least two weeks and met other study inclusion criteria. They also reported that they had been abstinent for an average of 44 consecutive days at entrance into the study, although abstinence was not a requirement for participation.
The study featured a 2 × 2 design, which crossed contingency management (CM; yes/no) with relapse prevention (RP; yes/no). The CM protocol used in the study was modeled after the protocol developed by Silverman et al. (1996), which in turn was adapted from work by Higgins and colleagues (1993). Participants earned vouchers for cocaine-free urines on an escalating schedule over a 12 week period, with a total maximal value of $1,150 if all urines were cocaine-free. The vouchers could be redeemed for goods and services consistent with recovery. The cognitive-behavioral RP protocol (Annis & Davis, 1989) was delivered in up to 20 weekly individual sessions. Participants in the condition that provided both CM and RP were required to be attending their RP sessions for the first 12 weeks in order to be eligible to participate in the CM procedures during that time.
The primary goal of the study was to determine whether providing CM, RP, or the combination of CM and RP to participants who had achieved initial engagement in intensive outpatient treatment would produce better cocaine use outcomes than treatment as usual (TAU) over an 18 month follow-up period. We predicted that significant main effects for CM and RP would be obtained, and the best outcomes would be found in the CM+RP condition. These predictions were tested through an examination of main and interaction effects in the 2 × 2 design and post hoc contrast analyses that compared the four treatment conditions.
Method
Participants
The participants were 100 adults with current DSM-IV diagnoses of cocaine dependence at the time of entrance to treatment. The other criteria for eligibility were achievement of initial engagement in IOP, as indicated by at least two weeks of regular attendance; no psychiatric or medical condition that precluded outpatient treatment (i.e., severe dementia, current audio or visual hallucinations); being between the ages of 18 and 70; no IV heroin use within the past 12 months; ability to read at approximately the 4th grade level; at least a minimum degree of stability in living situation (e.g., not living on the street); and willingness to participate in research and be randomly assigned to one of the four treatment conditions. To facilitate follow-up, participants had to be able to provide the names, addresses, and telephone numbers of at least three contacts. Patients who were currently taking medication for psychiatric conditions were also required to have a follow-up appointment with a provider of psychiatric services to ensure continuity of medication.
The participants averaged 41.0 years of age, 11.5 years of education, 13.8 years of regular cocaine use, 14.8 years of regular alcohol use, 3.8 prior drug abuse treatments, and 2.8 prior alcohol treatments. The majority of participants were female (58%), African American (89%), and not currently married (82%). Participants averaged 39.09% days abstinence (sd= 35.97) during the 4-month period that preceded IOP. On average, participants reported having been abstinent for 44.23 consecutive days at study intake (range 3 to 79 days, sd= 20.19), with 70% reporting between 31 and 70 consecutive days of abstinence. The conditions did not differ on length of abstinence [F(3,96)= 1.23, p= .29].
Intensive Outpatient Treatment
All participants were patients at one of two publicly funded, community-based IOPs. One program was located in a community hospital affiliated with the University of Pennsylvania (N=84), the other was a free-standing program without university affiliation (N=16). In both programs, patients attended treatment three days per week (9–10 hours total per week), for up to four months. Treatment was provided through a combination of didactic and counseling groups, with only sporadic individual sessions. The orientation of the programs was 12-step and traditional addictions counseling, with a focus on overcoming denial, fostering participation in self-help groups, and providing information about the process of addiction and cues to relapse. Therefore, these IOPs were highly representative of the vast majority of publicly funded addictions treatment programs (McLellan & Meyers, 2004).
Study Treatment Conditions
Treatment as usual (TAU, N= 25)
Participants in this condition received IOP followed by standard outpatient continuing care (i.e., one group session per week), up until the point at which they either dropped out or completed the program.
Contingency management (CM, N= 26)
Our contingency management intervention was based on protocols developed by Silverman et al. (1996) and Higgins et al. (1993). Patients were asked to provide three urine samples per week at specified times, over a 12 week period. These samples were analyzed on site for cocaine metabolites using rapid test kits. Failure to provide a urine sample was counted as a cocaine-positive sample on that day, unless the absence was excused. For every cocaine-free urine sample provided, patients received vouchers that could be redeemed for goods and services consistent with recovery. The voucher program was implemented by study research technicians who did not provide any clinical care.
The value of the vouchers started at $2.50, and escalated by $1.25 for each consecutive cocaine-free urine provided. Patients also earned a bonus of $10.00 for three consecutive cocaine-free urines in one week. Positive urine/breath tests or the failure to provide a sample on schedule reset the value of the vouchers back to the initial $2.50. However, five consecutive negative urines/breath tests after submission of a positive or missed specimen returned the value of the vouchers to their level prior to reset. Patients could earn a maximum of $1150 worth of vouchers if all urines were cocaine free. It should be noted that we also required that patients have alcohol free breath tests at the time that they provided urine samples to receive vouchers. However, there were only a few occasions when a patient had a cocaine-free urine but had alcohol in his/her system.
Due to staffing limitations, we did not purchase specified goods when participants wanted to redeem their vouchers, as has been done in some CM studies (Higgins et al., 1993). Rather, we provided gift certificates to a range of retail businesses that had policies that did not permit the exchange of gift certificates for cash. We also paid rent, utility bills, and other such bills, up to the amounts earned by the participants, if they wished to use their gift certificates for that purpose.
Relapse Prevention (RP, N= 24)
Participants were offered one individual CBT-RP session per week, for up to 20 weeks. This protocol, which was developed by Annis and Davis (1989), is based on self-efficacy theory (Bandura, 1977) and makes use of standardized assessment instruments to specify appropriate cognitive-behavioral interventions for each patient. A treatment manual (McKay, Feeley, & Annis, 1993) and a series of structured modules were used to guide within-session activities, as well as between-session homework assignments. These modules dealt with identifying risky situations in the past, self-monitoring current risky situations, learning to anticipate future risky situations, and improving coping responses in these situations.
Contingency management plus CBT-RP (CM+RP, N= 25)
Participants received both the 12 week CM protocol and the 20 session CBT-RP protocol. In order to be eligible for CM vouchers, participants were required to attend CBT sessions, unless they had an excused absence.
Therapists and Adherence to Relapse Prevention Protocol
Therapists
The three therapists in the study were experienced, masters-level substance abuse counselors, who had worked on prior continuing care studies at the Penn Center for Studies of Addiction (McKay et al., 1997).
Adherence to RP protocol
The RP sessions were audio-taped to facilitate supervision and monitor adherence to the protocol as described in the manuals. The study clinical coordinator reviewed the majority of these tapes, and determined whether the content of the sessions matched the material that was supposed to be covered, using a content checklist derived from the treatment manual. Supervision was provided weekly by the study clinical coordinator, and one group supervision session was also was also held per week in which therapeutic issues were discussed with the senior clinical research staff on the project. Any deviations from the treatment protocol that were identified by the clinical coordinator were immediately addressed in the weekly supervision meetings.
Procedures
Recruitment of participants
New patients were given information about the research study during their intake appointment at the IOPs, and told to talk to research technicians who visited the programs on a weekly basis if they were interested in participating. Patients were screened by our research staff, and those who met inclusion criteria were told that they would become eligible for the study after they had completed at least two weeks of attendance in the program. Informed consent procedures and the baseline assessments were completed when patients formally entered the study after two weeks of IOP. Participants entered the study between March 2003 and August 2004, and the final follow-ups were concluded in February 2006. The size of the study sample was selected to provide power to find a medium size effect in the main effect analyses. The study was conducted in compliance with the policies of the Institutional Review Board of the University of Pennsylvania.
Representativeness of the study samples
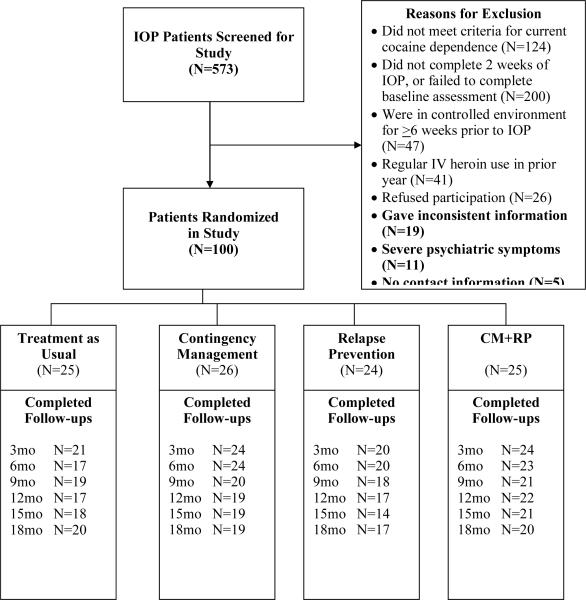
During the course of the study, 573 patients entering IOP were screened for participation in the study (see Figure 1). Of these patients, 473 were not eligible for the study. The number who were excluded for each primary reason were as follows: 200 did not achieve at least 2 weeks of participation in IOP or failed to follow through with baseline interviews (42.3%), 124 did not meet criteria for current cocaine dependence at entrance to IOP (26.2%), 47 were in a controlled environment for more than 6 weeks immediately prior to IOP (9.9%), 41 engaged in regular IV heroin use within the past year (8.7%), 26 declined to participate (5.5%), 19 gave inconsistent information (4.0%), 5 failed to provide contact information (1.1%), and 11 had other problems such as severe psychiatric symptoms (2.3%).
Figure 1.
Consort Diagram
Randomization procedures
In each of the study sites, participants were randomized to each of the four groups with equal probabilities. The randomizations were blocked in sets of 16 subjects, to ensure approximately equal numbers in the four groups at any given point in the study. The randomizations were generated by one of the authors (K.G. Lynch) and stored in envelopes, and the sequence was concealed until the treatment assignment was provided to the participant. Participants were notified of their treatment assignment by one of the research staff at the end of the baseline assessments.
Baseline and follow-up assessments
Baseline assessments were administered in 1 or 2 sessions that were started after the second week of IOP, prior to randomization. All patients who completed the baseline assessment and were randomized were considered to be in the study. The follow-up assessments were conducted at 3, 6, 9, 12, 15, and 18 months post baseline. In most cases, these assessments were done in person, at the research offices. A minority of follow-ups were conducted at other sites (i.e., prison), or over the telephone. All study interviews were conducted by research personnel who had received extensive training in the use of the assessment instruments and were closely monitored during the course of the study. These interviewers were blind to the study hypotheses but not to treatment condition.
Follow-up rates
The follow-up rates for self-report and urine toxicology data were, respectively, as follows: 3 months, 95% and 89%; 6 months, 94% and 83%; 9 months, 88% and 78%; 12 months, 84% and 72%; 15 months, 81% and 72%; and 18 months, 76% and 76%.
Measures
Psychiatric diagnoses
The Structured Clinical Interview for DSM-IV (SCID) (First et al., 1996) was used to to assess DSM-IV Axis I disorders. In the most rigorous evaluation to date of the reliability of the SCID, which was done with the DSM-III-R version of the instrument, kappas of .61 for current diagnoses and .68 for lifetime diagnoses were obtained when patients were interviewed on two separate occasions by different interviewers (Williams et al., 1992).
Problem severity
The Addiction Severity Index (ASI; McLellan, Luborsky, Woody, & O'Brien, 1980) was used to gather information on problem severity levels in six areas of functioning: employment, drug use, alcohol use, legal, family/social, and psychiatric. Composite scores provide an indication of overall problem severity in each area during the prior 30 days (range of 0.00 – 1.00, with higher scores indicating greater problem severity). The ASI has demonstrated good internal consistency, test-retest, and interrater reliabilities in different groups of substance abusers (Alterman, Brown, Zaballero, & McKay, 1994; McLellan et al., 1985).
Self-reported substance use
Time-line follow-back (TLFB) (Sobell, Maisto, Sobell, & Cooper, 1979) techniques were used to gather self-reports of alcohol and cocaine use during the 6 months preceding entrance into continuing care and the 18 month follow-up period. Studies with alcoholics (Sobell, Sobell, Leo, & Cancilla, 1988) and drug users (Ehrman & Robbins, 1994) have consistently demonstrated test-retest reliability of .80 or greater. In validity studies, TLFB reports of percent days abstinent have generally correlated .80 or better with collateral reports (Maisto, Sobell, & Sobell, 1979; Stout, Beattie, Longabaugh, & Noel, 1989). In drug abusers, TLFB reports of days of cocaine use were highly correlated with urine toxicology results (Ehrman & Robbins, 1994; Fals-Stewart et al., 2000).
The primary self-report outcome measure that was derived from the time-line data was a dichotomous measure of abstinence from any cocaine use within each 3 month segment of the follow-up. Total abstinence is a clinically relevant outcome measure because it is the expressed goal of most intensive outpatient program, including the two involved in this study. In addition, continuous measures of use were very badly skewed, to the point that data transformations still did not produce measures with distributions appropriate for analyses.
Cocaine urine toxicology
Urine samples were obtained at baseline and at each follow-up point to serve as the primary cocaine use outcome measure and to validate self-report data on abstinence. The urine samples were tested for the cocaine metabolite benzoylecgonine using either the Emit assay system or FPIA analysis (with quantitative output converted to a dichotomous variable). Agreement between self-reports of cocaine use obtained with the TLFB and urine toxicology results was good. Of participants who denied all cocaine use in the prior month, the rate of cocaine positive urines was lower than 15% at each of the follow-up points.
Treatment services received
Attendance data from the IOP were obtained through a review of chart records. The measure of attendance used in the study was total number of IOP groups attended. Data on RP sessions provided were obtained directly from the study therapists, using a standard form that included dates of scheduled sessions, whether the sessions were attended, and reasons for absences.
Data Analyses
Differences between the four continuing care conditions at baseline were evaluated with one-way ANOVAs (continuous measures) and chi-square tests (categorical measures). Treatment differences in weeks retained in continuing care and in RP sessions received during the follow-up were also evaluated with one-way ANOVAs or T-tests.
The primary outcomes considered were repeated binary indicators of cocaine use, with one set based on toxicology tests, and another based on self-reports. The toxicology tests were available at three-month intervals, and the self-report TLFB data were aggregated into three month segments. Generalized estimating equations (GEE) models (SAS PROC GENMOD) were used to compare the continuing care groups on these binary outcomes. The outcome analyses were intent-to-treat, and made use of all data obtained from each randomized participant.
Each model contained a factor indicating treatment site and a TLFB self-report of percent days cocaine use during the pre-continuing care baseline period (6 months, including IOP), as covariates. The models also included terms for the effects of treatment group and time trends, interactions between the two treatment conditions, and interactions between group and time. These models were used to test for main effects of incentives and relapse prevention and for interaction effects. Non-significant interaction terms were removed from the final models for the main effect analyses. Where indicated, post-hoc contrast analyses were also performed to more fully explain significant main or interaction effects by testing for differences between treatment conditions at each follow-up point, with interactions involving treatment conditions and time included in the models.
Missing data occurred in the outcomes, either because of permanent dropout or because of intermittent missing time points. Our basic model imputed intermittently missing data as “use” but did not impute a value for missed responses after dropout. As further sensitivity analyses, we also reran the GEE models with all missing data ignored, and with all missing data imputed as use. Finally, we also used nonlinear mixed effects models to rerun our basic models, as these models have less stringent assumptions on the ignorability of dropout, but require more accurate modeling of the covariance structure of the responses. If these different models yield concordant results, it would provide increased confidence in the validity of the results generated by the basic model.
Results
Comparison of Treatment Conditions at Baseline
Participants in the four treatment conditions were compared on 14 demographic, diagnostic, treatment, and problem severity level variables assessed at baseline. These results are presented in Table 1. The groups did not differ significantly on any of these variables. Patients reported having been abstinent an average of 44.9 consecutive days (sd=19.9, range 3 to 79) at baseline [means in the four conditions ranged from 38 to 47 days; F(3, 96)= 1.26, p= .29].
Table 1.
Characteristics of Sample at Baseline
| TAU | CM | RP | CM+RP | |
|---|---|---|---|---|
| N=25 | N=26 | N=24 | N=25 | |
| Demographic Measures | ||||
| Age (m, sd) | 42.71 (5.77) | 40.30 (6.88) | 41.53 (6.17) | 39.34 (7.05) |
| Education (m, sd)* | 11.32 (2.04) | 12.12 (1.63) | 10.79 (1.82) | 11.72 (1.59) |
| Gender | ||||
| Female (%) | 56.00 | 61.54 | 50.00 | 64.00 |
| Race | ||||
| White (%) | 16.00 | 7.69 | 4.17 | 4.00 |
| Black (%) | 80.00 | 88.46 | 91.67 | 96.00 |
| Other (%) | 4.00 | 3.85 | 4.17 | 0.00 |
| Marital Status* | ||||
| Married/remarried (%) | 0.00 | 23.08 | 29.17 | 20.00 |
| Widowed/separated/divorced (%) | 56.00 | 42.31 | 25.00 | 52.00 |
| Never married (%) | 44.00 | 34.62 | 45.83 | 28.00 |
| Substance Use Measures | ||||
| Days of consecutive abstinence at intake | 46.80 (17.69) | 47.19 (19.04) | 38.08 (20.18) | 47.24 (22.15) |
| Days of cocaine use in past 30 (m, sd) | 1.56 (5.03) | 1.12 (2.41) | 1.00 (2.17) | 0.92 (2.52) |
| Years of regular cocaine use (m, sd) | 13.52 (6.25) | 14.00 (6.15) | 16.04 (5.72) | 11.80 (4.96) |
| Alcohol dependence (past month) (%) | 58.33 | 60.00 | 65.22 | 66.67 |
| ASI Composite Scores | ||||
| ASI alcohol composite (m, sd) | 0.11 (0.14) | 0.09 (0.15) | 0.13 (0.16) | 0.10 (0.13) |
| ASI drug composite (m, sd) | 0.10 (0.08) | 0.11 (0.08) | 0.10 (0.06) | 0.10 (0.07) |
| ASI employment composite (m, sd) | .90 (.17) | .83 (.23) | .91 (.14) | .90 (.15) |
| ASI legal composite (m, sd) | .09 (.18) | .05 (.13) | .03 (.07) | .07 (.14) |
| ASI family/social composite (m, sd) | .19 (.24) | .25 (.23) | .21 (.24) | .18 (.19) |
| ASI psychiatric composite (m, sd) | .22 (.23) | .25 (.21) | .18 (.24) | .20 (.21) |
p < .10
Treatment Services and Incentives Received
Average number of IOP session attended was 38 in TAU and CM+RP, 37 in CM, and 25 in RP [CM × RP interaction F(1, 92) = 5.36, p= .023], which represented an average length of stay in IOP of around 3 months. Participants in the CM+RP condition attended a mean of 13.16 (sd=7.39) RP sessions, whereas those in the RP condition attended an average of only 3.33 (sd=5.04) RP sessions [t(42.5)= −5.15, < .001]. Mean number of cocaine-free urines provided was 28.23 (sd=10.78) in the CM condition, and 29.84 (sd= 10.42) in the CM+RP condition [t(49)= −.54, p=.59]. Most of the urines samples that were not cocaine free were missing (m=6.40) rather than cocaine positive (m= .57). The mean longest number of consecutive cocaine free urine samples was 24.31 (sd= 13.34) in the CM condition and 28.24 (sd= 11.94) in the CM+RP condition [t(49)= −1.11, p= .27]. Mean value of incentives earned was $740 (sd=466) in the CM condition and $856 (sd= 443) in the CM+RP condition [t(49)= −.91, p= .37].
Outcome Analyses with Urine Toxicology Data
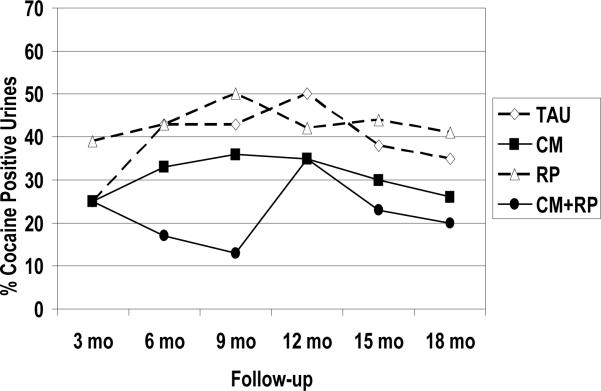
Data on the percentage of participants in each condition with cocaine-positive urines are presented in Figure 2. With regard to the first study hypothesis, analyses of main effects using the GEE model showed a significant effect for incentives (CM and CM+RP vs. RP and TAU) (χ2(1)=3.77, p=0.05). Rates of cocaine positive urines in the two conditions that did not receive incentives averaged 10 to 20 percentage points higher than the rates of cocaine positive urines in the two conditions that did receive incentives. Conversely, there was no main effect for RP (CM+RP and RP vs. CM and TAU) (χ2(1)= .39, p=0.53), and the interaction of CM and RP was not significant (χ2(1)= .80, p=0.37). There were also no significant interactions between the treatment conditions and time (ps> .30).
Figure 2.
Cocaine urine toxicology
Percent of urine samples obtained at each follow-up that were positive for cocaine. Missing samples prior to dropout from follow-up were imputed as positive for use, whereas those missing after final follow-up obtained were considered missing.
Although the CM × RP interaction was not significant, an examination of the data in Figure 2 clearly indicates that much of the significant main effect favoring CM was accounted for by the CM+RP condition, which had the lowest rates of cocaine positive urine samples for much of the follow-up. To examine these effects, we refit the model, this time including the CM by RP interactions, and a CM by RP by Time interaction. Exploratory post hoc contrasts from this model indicated that the CM and RP conditions did not differ significantly from TAU on rates of cocaine positive urines at any of the follow-up points. The odds ratios from these analyses, adjusted for all other terms in the model including baseline covariates, are presented in Table 2. Although rates of cocaine positive urines were higher in RP than in CM (odds ratios of 1.34 – 2.03), none of these differences reached the p< .05 level of significance. However, the CM+RP condition produced lower rates of cocaine positive urines than TAU and RP at both 6 and 9 months (p< .01), and trends favoring CM+RP over CM were also evident at the same two time points (p= .06). All but three of the 18 odds ratios comparing the other three treatment conditions to CM+RP on rates of cocaine positive urine samples at each follow-up were 1.7 or greater, and 11 were greater than 2.
Table 2.
Odds ratios for post hoc comparisons of treatment conditions on cocaine urine toxicology outcomes
| Contrast | 3 mo | 6 mo | 9 mo | 12 mo | 15 mo | 18 mo |
|---|---|---|---|---|---|---|
| RP vs. TAU | 2.08 (0.57, 7.69) | 1.23 (0.39, 3.87) | 1.01 (0.35, 2.93) | 1.00 (0.33, 3.05) | 1.06 (0.33, 3.53) | 1.07 (0.29, 4.01) |
| CM vs. TAU | 1.03 (0.26, 4.01) | 0.74 (0.24, 2.23) | 0.70 (0.24, 1.98) | 0.75 (0.27, 2.08) | 0.79 (0.25, 2.49) | 0.73 (0.20, 2.61) |
| RP vs. CM | 2.03 (0.56, 7.42) | 1.67 (0.53, 5.29) | 1.45 (0.52, 4.06) | 1.34 (0.47, 3.85) | 1.34 (0.41, 4.34) | 1.47 (0.39, 5.55) |
| TAU vs. CM+RP | 1.06 (0.28, 4.06) | 3.96** (1.33, 11.80) | 4.21** (1.37, 12.88) | 2.53 (0.84, 7.66) | 1.74 (0.54, 5.62) | 2.73 (0.67, 11.16) |
| RP vs. CM+RP | 2.20 (0.61, 8.00) | 4.89** (1.51, 15.86) | 4.24** (1.32, 13.65) | 2.53 (0.76, 8.47) | 1.85 (0.52, 6.54) | 2.92 (0.67, 12.66) |
| CM vs. CM+RP | 1.08 (0.28, 4.18) | 2.93* (0.94, 9.07) | 2.93* (0.94, 9.10) | 1.89 (0.62, 5.77) | 1.38 (0.41, 4.61) | 1.99 (0.48, 8.32) |
Odds of a cocaine positive urine result; higher ratios indicate greater likelihood of cocaine positive urine result. Odds ratios adjusted for other terms in the models
p= .06;
p< .01
Outcome Analyses with Self-Report Data
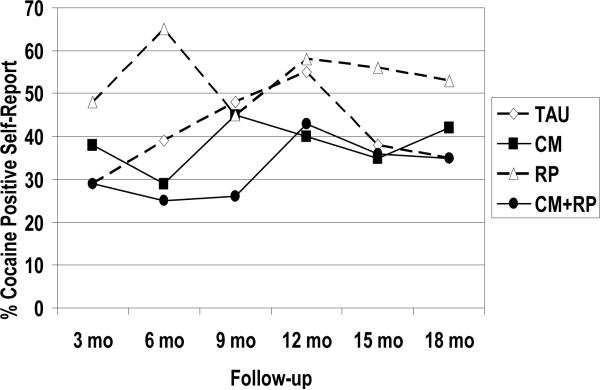
Data on the percentage of participants within each condition who reported any cocaine use in the 3-month follow-up segments are presented in Figure 3. The results of the analyses were similar to those obtained with the urine toxicology data, although in some cases less robust. With regard to main effects, a significant effect favoring incentives over no incentives was obtained (χ2(1)=3.76, p=0.05). Rates of reported cocaine use in the two conditions that did not receive incentives averaged 10 to 20 percentage points higher than in the two conditions that did receive incentives. Once again, the main effect for RP did not approach significance (χ2(1)= .44, p=0.51), nor did the CM × RP interaction (χ2(1)= 1.57, p=0.21). Once gain, we fit a further interaction model, to examine CM × RP interaction effects across time, as described above. There were no significant interactions between the treatment conditions and time (ps> .30). In the post-hoc contrast analyses, the only significant difference obtained was between the CM+RP condition and the RP condition at six months (p= .03), with trends for differences between CM+RP and RP (p=0.07) and between CM+RP and TAU (p=0.10) at nine months.
Figure 3.
TLFB reports of cocaine use
Percent of TLFB self-reports obtained at a given follow-up that were indicative of any cocaine use over the prior 3 months. Missing samples prior to dropout from follow-up were imputed as positive for use, whereas those missing after final follow-up obtained were considered missing.
Secondary Analyses Addressing Effects of Missing Data
For all conditions, the rate of missing responses increased linearly from about 4% at the 3-month point. By the 18 month follow-up, rates of missing urines ranged from 20% in TAU and CM+RP, to 27% in CM, and 29% in RP. In the main analyses described above, we imputed any intermittent missing urine samples as “use” and left urines that were missing due to dropout as missing. We also considered GEE models where all missing urine samples were imputed as positive for cocaine use, and other analyses where all missing urine samples were left missing (i.e. ignored). The results from these analyses were virtually identical to those described above: significant effects favoring CM+RP over the other conditions were observed at months 6 and 9, and not at other time points.
To further investigate the effects of missing data on the analyses, we reran the same sets of analyses using mixed effects models, which have less stringent assumptions on the nature of the missing data than the GEE models. Again, we saw similar effects at months 6 and 9 favoring CM+RP over the other conditions, with no differences at other time points. The concordance between the results of the main analyses described above and these other sensitivity analyses indicate that the missing responses did not have an appreciable influence on the conclusions of the study.
Discussion
The present study is among the first to evaluate the effectiveness of contingency management (CM) as a mechanism to prevent relapse, as opposed to initiate abstinence. The study participants had all achieved initial engagement and stabilization in IOP, and had been abstinent for an average of 44 consecutive days when they began in the study. The effects of individual cognitive-behavioral relapse prevention therapy and the combination of RP and CM were also examined. The results, based on self-report data and cocaine urine toxicology tests obtained at three-month intervals, indicated that the availability of RP to patients already receiving standard IOP services did not increase abstinence rates at any point in the follow-up. However, main effects analyses with both urine toxicology and self-report data over the 18 month follow-up indicated that participants who received incentives had better cocaine use outcomes than those who did not. Moreover, the provision of both CM and RP yielded higher cocaine urine toxicology abstinence rates at the 6 and 9 month follow-ups than either TAU or RP, with a trend evident in comparisons with CM. It should be noted that the bulk of the effect favoring incentives was carried by the CM+RP condition.
These results indicate that patients who have already achieved stabilization and abstinence in IOP can benefit from the addition of CM, particularly if it is combined with RP sessions, as long as eligibility to receive the incentives is linked to consistent participation in RP. Our results suggest that without such an incentive, IOP participants are not likely to attend additional treatment sessions. The significant positive effect of the combination of CM and RP extended for a full six months after the CM protocol ended, and for at least four months after the RP protocol ended. Therefore, these results are in keeping with a growing list of studies demonstrating sustained effects for CM beyond the point at which the provision of incentives for abstinence ceases (e.g., Alessi et al., 2007; Epstein et al., 2003; Higgins et al., 2000; Higgins et al., 2003; Higgins et al., 2006).
Interestingly, the beneficial effects of the CM+RP condition were found after these interventions had ended, not while they were being provided. There are at least two possible explanations for the delayed emergence of these effects. The positive effects of CM procedures may be extended beyond the provision of incentives if the windows of abstinence created by the incentives increase the likelihood that patients will be exposed to other abstinence-related “natural” incentives. For example, abstinent substance abusers might take up a new hobby, reconnect with friends who support continued abstinence, or do other things that are rewarding and are incompatible with a return to use. The within-treatment urine toxicology results were indicative of substantial periods of cocaine abstinence in the CM+RP condition, which may have opened up opportunities for this kind of participation in other rewarding activities. However, there was also considerable cocaine abstinence in the CM condition, in which there was less evidence of post-treatment positive effects.
The second possibility is that the RP sessions provided in this condition accounted for the “sleeper” effects that were observed. For example, the RP sessions may have equipped the patients with better coping skills or increased other factors that contributed to sustained recovery (e.g., self-efficacy, commitment to abstinence, and so forth). Moreover, this condition did provide patients with an opportunity to develop a relationship with a helpful and concerned therapist. There is considerable evidence that a strong therapeutic alliance and other general factors in psychotherapy and counseling predict better outcomes in the treatment of addiction (Connors, Carroll, DiClemente, Longabaugh, & Donovan, 2007) and other disorders (Baskin, Tierney, Minami, & Wampold, 2003; Kazdin, 2005; Orlinsky, Ronnestad, & Willutzki, 2003; Wampold, 2001, 2005). Possible mediation effects in this study will be examined in future publications.
One of the puzzling aspects of the results was the relatively poor outcomes in the RP condition. Although these outcomes were not significantly worse than those obtained in IOP only, rates of cocaine use in this condition were higher than those of the other three conditions, particularly when self-reports of cocaine use were considered. With a larger sample size, some of these differences might have been statistically significant. What might have accounted for these poor outcomes? We know that patients actually attended very few of the RP sessions—an average of three out of 20 possible—which likely explains why there were no positive effects in this condition. However, this really does not explain why results actually looked somewhat worse. It is notable that patients in the RP condition attended significantly fewer IOP groups than those in the other three conditions (25 vs. 38). The RP sessions were added onto the existing IOP without being fully integrated into that program and were also provided by different counselors. It is possible that this in some way confused the patients or undermined their motivation to continue in their regular IOP program. It is also possible that patients randomly assigned to RP may have entered treatment with poorer prognostic indicators on variables we did not measure, which could have contributed to earlier dropout and worse outcomes.
The relapse prevention design of the present study can be seen as a test of enhancements to continuing care, as all participants had attended IOP for at least two weeks and had an average of six weeks of reported abstinence from cocaine and other substances. On the other hand, these patients had not completed the IOP phase of treatment, which in these publicly funded programs lasted for as long as 4 months. The “betwixt and between” nature of our participants highlights a current trend in addictions treatment—the blurring of what were formally clear boundaries between the primary care and “aftercare” (i.e., continuing care) phases of treatment (McKay, 2009). Offering continuing care only to those patients who graduate from IOP can sharply limit the number of patients who are eligible for it, due to the relatively low completion rates in these programs. On the other hand, providing enhanced services too early in the treatment process may overwhelm some patients, who are either struggling to attend all their scheduled IOP sessions or dropping out due to ambivalence about treatment.
Limitations
The study had several limitations, which should be considered in interpreting the results. First, we did not obtain cocaine urine test results during treatment for patients who were not in the two CM conditions. Therefore, it is possible that a within-treatment effect might have been observed if these urines were collected in TAU. This possibility is buttressed by the very high rate of cocaine free urines obtained from the CM and CM+RP conditions during the 12 week CM procedure (M= 29 cocaine free urines out of 36 possible), along with the consistency of within treatment CM effect in prior studies (Dutra et al., 2008). Therefore, we cannot rule out the possibility that CM might have short-term beneficial effects as a relapse prevention treatment for patients in IOP.
We were also limited in our ability to identify significant effects by the relatively small sample size of the study, particularly interaction effects. Although prior CM studies have often had similar sample sizes, the fact that our study featured a relapse prevention design likely reduced the magnitude of potential effects, thereby resulting in reduced power compared to prior studies. Although lack of power did not seem to be an issue in the comparisons of CM and RP to TAU, as evidenced by the small magnitude of the differences in these comparisons, several other findings significant at the level of a trend may have reached the p< .05 level with a larger sample. In addition, many of the non-significant post hoc tests in Table 2 that compared CM+RP to the other treatment conditions had odds ratios of greater than 2, which implies that power to find effects in these comparisons was limited.
In evaluating therapist adherence in the RP conditions, we utilized audio taping of sessions and weekly supervision provided by the clinical coordinator, who had considerable experience in delivering and supervising CBT-based interventions. However, we did not implement the more rigorous and systematic procedures advocated by Carroll and other psychotherapy researchers for demonstrating adherence to treatment manuals (Carroll et al., 2000). This raises the possibility that the poor performance of the RP condition might have been due to a failure to deliver RP as outlined in the manual. However, the same therapists delivered RP in the CM+RP condition, in which the best outcomes were achieved. Therefore, the more likely explanation for the results in the RP condition is the fact that patients only came to an average of 3 sessions. Without the “carrot” represented by the pairing of CM with RP, the participants apparently had little interest in going to RP sessions in addition to the required groups in IOP.
Finally, restriction of the study sample to relatively good prognosis patients—those who had achieved initial engagement in IOP and in many cases had already been abstinent for several weeks or more—likely limited our ability to find treatment effects. Given this, the differences in rates of cocaine-positive urines between the CM+RP condition and the RP and TAU conditions at 6 and 9 months are all the more notable. However, it is not clear whether the results observed in the study would have been obtained with the large percentage of patients who did not remain in IOP for two weeks or failed to attend the baseline interview.
Clinical Implications
The findings presented in this article indicate that cocaine dependent patients who have achieved initial engagement in IOP and significant reductions in cocaine use can benefit from incentives for the provision of cocaine-free urines. These effects were sustained for up to 6 months after the incentives were discontinued, and were particularly strong if the incentives were combined with individual CBT-RP sessions. These improved outcomes come at a cost, however. In a future publication, we will report on the actual costs of the CM and RP enhancements, and their economic feasibility as indicated by the results of cost-effectiveness and benefit-cost analyses.
Acknowledgments
This research was supported by grants R01 DA14059 and K02 DA000361 from the National Institute on Drug Abuse.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/ccp
References
- Alessi SM, Hanson T, Wieners M, Petry NM. Low-cost contingency management in community clinics: delivering incentives partially in group therapy. Exp Clin Psychopharmacol. 2007;15:293–300. doi: 10.1037/1064-1297.15.3.293. [DOI] [PubMed] [Google Scholar]
- Alterman AI, Brown LS, Zaballero A, McKay JR. Interviewer severity ratings and the composite scores of the ASI: a further look. Drug and Alcohol Dependence. 1994;34:201–209. doi: 10.1016/0376-8716(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Annis HM, Davis CS. Relapse prevention. In: Hester RK, Miller WR, editors. Handbook of alcoholism treatment approaches. Pergamon Press; NY: 1989. pp. 170–182. [Google Scholar]
- Bandura A. Self efficacy: Toward a unifying theory of behavioral change. Psychological Review. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- Baskin TW, Tierney SC, Minami T, Wampold BE. Establishing specificity in psychotherapy: A meta-analysis of structural equivalence of placebo controls. Journal of Consulting and Clinical Psychology. 2003;71:973–979. doi: 10.1037/0022-006X.71.6.973. [DOI] [PubMed] [Google Scholar]
- Carroll KM. Relapse prevention as a psychosocial treatment: A review of controlled studies. Experimental and Clinical Psychopharmacology. 1996;4:46–54. [Google Scholar]
- Carroll KM. A cognitive-behavioral approach: Treating cocaine addiction. National Institute on Drug Abuse; Rockville, MD: 1998. NIH publication 98-4308. [Google Scholar]
- Carroll KM, Nich C, Sifry RL, Nuro KF, Frankforter TL, Ball SA, Fenton L, Rounsaville BJ. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug and Alcohol Dependence. 2000;57:225–238. doi: 10.1016/s0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin F. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence: Delayed emergence of psychotherapy effects. Archives of General Psychiatry. 1994;51:989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- Connors GJ, Carroll KM, DiClemente CC, Longabaugh R, Donovan DM. The therapeutic alliance and its relationship to alcoholism treatment participation and outcome. Journal of Consulting & Clinical Psychology. 2007;65:588–598. doi: 10.1037//0022-006x.65.4.588. [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry. 2008 doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ. Reliability and validity of six-month timeline reports of cocaine and heroin use in a methadone population. Journal of Consulting and Clinical Psychology. 1994;62:843–850. doi: 10.1037//0022-006x.62.4.843. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Hawkins WE, Covi L, Umbricht A, Preston KL. Cognitive-behavioral therapy plus contingency management for cocaine use: findings during treatment and across 12-month follow-up. Psychology of Addictive Behavior. 2003;17:73–82. doi: 10.1037/0893-164X.17.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fals-Stewart W, O'Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: Psychometric properties. Journal of Consulting and Clinical Psychology. 2000;68:134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders—patient edition. SCID-I/P, version 2.0 Biometrics Research Department, New York State Psychiatric Institute; NY: 1996. [Google Scholar]
- Friedmann PD, Hendrickson JC, Gerstein DR, Zhang Z. The effect of matching comprehensive services to patients' needs on drug use improvement in addiction treatment. Addiction. 2004;99:962–972. doi: 10.1111/j.1360-0443.2004.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Hughes JR, Feorg F, Gadger GB. Achieving cocaine abstinence with a behavioral approach. American Journal of Psychiatry. 1993;150:763–769. doi: 10.1176/ajp.150.5.763. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dantona R, Donham R, Matthews M, Badger GJ. Effects of varying the monetary value of voucher-based incentives on abstinence achieved during and following treatment among cocaine-dependent outpatients. Addiction. 2006;102:271–81. doi: 10.1111/j.1360-0443.2006.01664.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Sigmon SC, Wong CJ, Heil SH, Badger GJ, Donham R, et al. Community reinforcement therapy for cocaine-dependent outpatients. Archives of General Psychiatry. 2003;60:1043–1052. doi: 10.1001/archpsyc.60.9.1043. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Wong CJ, Badger GJ, Haug Ogden DE, Dantona RL. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. Journal of Consulting and Clinical Psychology. 2000;68:64–72. doi: 10.1037//0022-006x.68.1.64. [DOI] [PubMed] [Google Scholar]
- Irvin JE, Bowers CA, Dunn M, Wang MC. Efficacy of relapse prevention: A meta-analytic review. Journal of Consulting and Clinical Psychology. 1999;67:563–570. doi: 10.1037//0022-006x.67.4.563. [DOI] [PubMed] [Google Scholar]
- Kazdin AE. Treatment outcomes, common factors, and continued neglect of mechanisms of change. Clinical Psychology: Science and Practice. 2005;12:184–188. [Google Scholar]
- Longabaugh R, Morgenstern J. Cognitive-behavioral coping skills therapy for alcohol dependence: Current status and future directions. Alcohol Research and Health. 1999;32:78–86. [PMC free article] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Maisto SA, Sobell LC, Sobell MB. Comparison of alcoholics' self-reports of drinking behavior with reports of collateral informants. Journal of Consulting and Clinical Psychology. 1979;47:106–122. [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR, editors. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. Guilford; New York: 1985. [Google Scholar]
- McKay JR. Is there a case for extended interventions for alcohol and drug use disorders? Addiction. 2005;100:1594–1610. doi: 10.1111/j.1360-0443.2005.01208.x. [DOI] [PubMed] [Google Scholar]
- McKay JR. Treating substance use disorders with adaptive continuing care. American Psychological Association; Washington DC: 2009. [Google Scholar]
- McKay JR, Alterman AI, Cacciola JS, O'Brien CP, Koppenhaver JM, Shepard DS. Continuing care for cocaine dependence: Comprehensive 2-year outcomes. Journal of Consulting and Clinical Psychology. 1999;67:420–427. doi: 10.1037//0022-006x.67.3.420. [DOI] [PubMed] [Google Scholar]
- McKay JR, Feeley M, Annis HM. Manual for individualized relapse prevention aftercare. University of Pennsylvania; 1993. [Google Scholar]
- McKay JR, Lynch KG, Shepard DS, Pettinati HM. The effectiveness of telephone based continuing care for alcohol and cocaine dependence: 24 month outcomes. Archives of General Psychiatry. 2005;62:199–207. doi: 10.1001/archpsyc.62.2.199. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Grissom GR, Zanis D, Randall M, Brill P, O'Brien CP. Problem-service `matching' in addiction treatment. Archives of General Psychiatry. 1997;54:730–735. doi: 10.1001/archpsyc.1997.01830200062008. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Hagan TA, Levine M, Gould F, Meyers K, Bencivengo M, et al. Supplemental social services improve outcomes in public addiction treatment. Addiction. 1998;93:1489–1499. doi: 10.1046/j.1360-0443.1998.931014895.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr H, O'Brien CP. New data from the Addiction Severity Index: Reliability and validity in three centers. Journal of Nervous and Mental Disease. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: The Addiction Severity Index. Journal of Nervous and Mental Disease. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Meyers K. Contemporary addiction treatment: A review of systems problems for adults and adolescents. Biological Psychiatry. 2004;56:764–770. doi: 10.1016/j.biopsych.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Milby JB, Schumacher JE, Wallace D, Frison J, McNamara C, Usdan S, Michael M. Day treatment with contingency management for cocaine abuse in homeless persons: 12-month follow-up. Journal of Consulting and Clinical Psychology. 2003;71:619–621. doi: 10.1037/0022-006x.71.3.619. [DOI] [PubMed] [Google Scholar]
- Morgenstern J, McKay JR. Rethinking the paradigms that inform behavioral treatment research for substance use disorders. Addiction. 2007;102:1377–1389. doi: 10.1111/j.1360-0443.2007.01882.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell TJ, Choquette KA, Cutter HSG. Couples relapse prevention sessions after behavioral marital therapy for male alcoholics: Outcomes during the three years after starting treatment. Journal of Studies on Alcohol. 1998;59:357–370. doi: 10.15288/jsa.1998.59.357. [DOI] [PubMed] [Google Scholar]
- Orlinsky , Ronnestad M, Willutzki U. In: Handbook of Psychotherapy and Behavior Change. 5th ed. Bergin AE, Garfield SL, editors. John Wiley & Sons; New York: 2003. [Google Scholar]
- Petry NM, Alessi SM, Carroll KM, Hanson T, MacKinnon S, Rounsaville B, Sierrra S. Contingency management treatments: Reinforcing abstinence versus adherence with goal-related activities. Journal of Consulting and Clinical Psychology. 2006;74:592–601. doi: 10.1037/0022-006X.74.3.592. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Hanson R. Contigency management improves abstinence and quality of life in cocaine users. Journal of Consulting and Clinical Psychology. 2007;75:307–315. doi: 10.1037/0022-006X.75.2.307. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Marx J, Austin M, Tardif M. Vouchers versus prizes: contingency management treatment of substance abusers in community settings. Journal of Consulting and Clinical Psychology. 2005;73:1005–14. doi: 10.1037/0022-006X.73.6.1005. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction. 2006;101:1546–1650. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Huber A, McCann M, Shoptaw S, Farabee D, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Archives of General Psychiatry. 2002;59:817–24. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101:267–74. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Reback CJ, Peck JA, Yang X, Rotheram-Fuller E, Larkins S, Veniegas RC, Freese TE, Hucks-Ortiz C. Behavioral treatment approaches for methamphetamine dependence and HIV-related sexual risk behaviors among urban gay and bisexual men. Drug and Alcohol Dependence. 2005;78:125–34. doi: 10.1016/j.drugalcdep.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Silverman K, Higgins ST, Brooner RK, Montoya ID, Cone EJ, Schuster CR, Preston KL. Sustained cocaine abstinence in methadone maintenance patients through voucher-based reinforcement therapy. Archives of General Psychiatry. 1996;53:409–415. doi: 10.1001/archpsyc.1996.01830050045007. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers' self-reports of drinking behavior. Behavior Research and Therapy. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: Assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. British Journal of Addictions. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Stout RL, Beattie MC, Longabaugh R, Noel N. Factors affecting correspondence between patient and significant other reports of drinking. Alcoholism: Clinical and Experimental Research. 1989;12:336. abstract. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies . Treatment Episode Data Set (TEDS): 2005. Discharges from Substance Abuse Treatment Services. Rockville, MD: 2008. (DASIS Series: S-41). DHHS Publication No. (SMA) 08-4314. [Google Scholar]
- Wampold B. The great psychotherapy debate: Models, methods, and findings. Lawrence Erlbaum Associates; Madison, WI: 2001. [Google Scholar]
- Wampold BE. Establishing specificity in psychotherapy scientifically: design and evidence issues. Clinical Psychology: Science and Practice. 2005;12:194–197. [Google Scholar]
- Williams JB, First MB, Spitzer RL, Davis M, Borus J, Howes MJ, Kane J, Pope HG, Rounsaville B, Wittchen HU. The Structured Clinical Interview for DSM-III-R (SCID): II. Multisite test-retest reliability. Archives of General Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]