Abstract
One of the hallmarks of human pancreatic ductal adenocarcinoma (PDAC) is its pronounced type I collagen-rich fibrotic reaction. Although recent reports have shown that the fibrotic reaction can limit the efficacy of gemcitabine chemotherapy, the underlying mechanisms remain poorly understood. In this report we show that the type I collagen allows PDAC cells to override checkpoint arrest induced by gemcitabine. Relative to cells grown on tissue culture plastic, PDAC cells grown in 3D collagen microenvironment have minimal Chk1 phosphorylation and continue to proliferate in the presence of gemcitabine. Collagen increases membrane type 1-matrix metalloproteinase (MT1-MMP)-dependent ERK1/2 phosphorylation to limit the effect of gemcitabine. Collagen also increases MT1-MMP-dependent HMGA2 expression, a non-histone DNA-binding nuclear protein involved in chromatin remodeling and gene transcription, to attenuate the effect of gemcitabine. Over-expression of MT1-MMP in the collagen microenvironment increases ERK1/2 phosphorylation and HMGA2 expression, and thereby further attenuates gemcitabine-induced checkpoint arrest. MT1-MMP also allows PDAC cells to continue to proliferate in the presence of gemcitabine in a xenograft mouse model. Clinically, human tumors with increased MT1-MMP demonstrate increased HMGA2 expression. Overall, our data demonstrate that collagen upregulation of MT1-MMP contributes to gemcitabine resistance in vitro and in a xenograft mouse model, and suggest that targeting MT1-MMP could be a novel approach to sensitize pancreatic tumors to gemcitabine.
Keywords: HMGA2, ERK1/2, MT1-MMP, fibrosis
INTRODUCTION
Very little progress has been made in the treatment of pancreatic ductal adenocarcinoma (PDAC), the fourth leading cause of cancer-related deaths in the U.S. (1). Since pancreatic cancer causes minimal early signs or symptoms, most patients present with locally advanced or metastatic disease at the time of diagnosis (2). The primary treatment option for patients with advanced disease includes chemotherapy, with gemcitabine as the preferred therapy. Despite being the first line treatment for patients with PDAC, majority of the patients do not benefit from gemcitabine (3). Molecular targeted therapies have failed to show a clinically significant improvement over gemcitabine alone (4), raising an urgent need to understand the reasons for the poor response of PDAC tumors to current therapeutic agents.
Gemcitabine, a nucleoside analog that competes with cytidine during DNA replication, has been shown to activate cell cycle checkpoints that allow cells time for repair and determine whether to progress through the cell cycle or to undergo apoptosis. Gemcitabine activates Chk1 and Chk2 kinases, key effectors of the checkpoint response (5). Activation of Chk1 during DNA replication stalls cell cycle progression, prevents premature mitotic entry and allows time to repair the damaged DNA. Cells resistant to arrest continue replicating despite the accumulated double-strand breaks, resulting in cells with mutations progressing through the cell cycle, causing genomic instability. Although Chk1 and Chk2 were initially thought to have specific roles, Chk2 may be redundant in checkpoint activation and may primarily modulate Chk1 responses (6).
Human pancreatic cancers are associated with an intense fibrotic reaction, the encasing tissue composed of interstitial extracellular matrix (ECM) and proliferating stromal cells. The fibrotic reaction was recently shown to limit the delivery and efficacy of gemcitabine in a mouse model of pancreatic cancer (7, 8). It also contributes to the malignant phenotype of PDAC (9), in part by increasing expression of the proteinase membrane type-1 matrix metalloproteinase (MT1-MMP) (10). PDAC is also associated with increased expression of high mobility group A2 (HMGA2), a non-histone DNA-binding protein involved in chromatin remodeling and gene transcription (11). HMGA2 is increased in high-grade pancreatic tumors with lymph node metastases (12), and consistent with it’s role in pancreatic cancer invasion, HMGA2 is involved in maintaining Ras-induced epithelial-mesenchymal transition (13). HMGA2 also modulates expression of genes that are important for cell proliferation, DNA repair and apoptosis (14, 15).
In this report we examine the mechanism by which collagen promotes gemcitabine resistance. We show that the 3D collagen microenvironment protects PDAC cells from gemcitabine-induced proliferation- and cell cycle-arrest. The effect of collagen is mediated through increased MT1-MMP-dependent ERK1/2 phosphorylation and HMGA2 expression. Blocking MT1-MMP induction sensitizes pancreatic cancer cells in collagen to gemcitabine-induced checkpoint activation, while overexpressing MT1-MMP further attenuates gemcitabine-induced checkpoint arrest. Clinically, human tumors with increased MT1-MMP demonstrate increased HMGA2 expression. Overall, we show that collagen-mediated upregulation of MT1-MMP contributes to gemcitabine resistance in vitro and in a xenograft mouse model, and targeting MT1-MMP could be a novel approach to sensitize pancreatic tumors to gemcitabine.
MATERIALS AND METHODS
Chemicals/Reagents
MT1-MMP antibody was purchased from Abcam (Cambridge, MA), ppERK1/2 and PARP antibodies from Cell Signaling (Danvers, MA), HMGA2 antibody from Biocheck Inc. (Foster City, CA) and α-tubulin antibody from Santa Cruz Biotechnology (Santa Cruz, CA). Secondary antibodies were purchased from Sigma (St. Louis, MO). Type I collagen was purchased from BD Biosciences (Franklin Lakes, NJ), Matrigel from R&D Systems (Minneapolis, MN) and MEK inhibitor U0126 from Cell Signaling. Gemcitabine was obtained from Eli Lilly (Indianapolis, IN). Nucleofector electroporation kit was purchased from Lonza (Walkersville, MD) and Lipofectamine RNAiMax from Invitrogen (Carlsbad, CA). An HMGA2 siRNA and pre-miRs let-7a, let-7d, let-7g and negative control #1 were purchased from Applied Biosystems (Foster City, CA). An siRNA for MT1-MMP targeted against nucleotides 228–248 was purchased from Invitrogen (16).
Cell culture
Panc1, CD18/HPAF-II and AsPC1 cells were obtained from ATCC (Manassas, VA). Cells were maintained in DMEM containing 10% FBS and antibiotics (100 U/ml Penicillin and 100 µg/ml Streptomycin). The cells were tested in December 2009 and June 2010 by STR profiling at the Johns Hopkins Genetic Resources Core Facility and showed a similar profile to that on the ATCC website.
Generation of PDAC cells inducibly expressing MT1-MMP
Full-length MT1-MMP and the ΔC mutant were subcloned into pRetroX-Tight-Pur vector (Clontech, Mountain View, CA). Panc1 and CD18 cells inducibly expressing MT1-MMP were generated as previously described (17).
Transfection
Cells were transfected with siRNA against MT1-MMP, HMGA2 or control siRNA (50 nmoles) or the pre-miR to let-7 or pre-miR negative control (at 50 nmoles) using Nucleofector Kit R (Lonza), allowed to recover overnight and then plated either on plastic or in 3D collagen gels (2 mg/ml). Alternatively, cells were transfected using RNAimax (Invitrogen) according to manufacturer’s instructions before plating into collagen.
Proliferation assay
Proliferation by 3H-thymidine incorporation was measured as previously described (18).
Quantitative Real Time-PCR analysis
Quantitative gene expression was performed for MT1-MMP, HMGA2 and GAPDH as previously published (17). Similarly, expression of let-7a/d/g and RNU48 was analyzed as previously published (19).
Immunoblotting
Immunoblotting was done as previously described (10). For cells grown in collagen, the matrix was first dissolved in collagenase (Worthington Biologicals, Lakewood, NJ) and then lysed as previously described (10).
Zymography
MT1-MMP activity analysis was done by SDS-PAGE gelatin zymography as described previously (17).
Subcutaneous tumor studies
Mice were treated in accordance with guidelines approved by the Northwestern University IACUC. Eight-week old athymic nu/nu animals were injected subcutaneously with 10 × 106 tet-on Panc1-tet-V in the left flank while Panc1-tet-ΔC cells were injected into the right flank of the same animal. For CD18-tet-V and CD18-tet-ΔC, 5×105 cells were injected. Twenty-four hours later, doxycycline (200mg/L) was added to the drinking water to induce protein expression. After 1 week, mice were intraperitonially injected with two doses of gemcitabine (15mg/kg) two days apart (20). Mice were then euthanized and tumors processed for mRNA and histologic studies. The experiment to study the effect of inhibiting MT1-MMP expression in CD18 cells was conducted similarly. After maintaining the mice (n=10) on doxycycline water for 2 weeks, 5 mice were switched to regular water for 1 week to inhibit MT1-MMP expression. 4 mice in each group were then administered gemcitabine as described earlier. The tumor samples were stained for trichrome, H&E and immunostained for PCNA (1:1000, Santa Cruz Biotechnology). Images were taken using a Carl Zeiss Axiovert 200 imaging microscope at 80X (Trichrome and H&E) or 320X (PCNA) magnification, maintaining similar settings between vector and MT1-MMP samples. The PCNA quantification for percentage of cells with nuclear staining was done using Image J software.
Human pancreatic tumor samples
Pancreatic tissue was obtained from patients with PDAC on an IRB-approved protocol. Cancerous and adjacent normal tissue samples were dissected and processed for RNA extraction using Trizol. RNA quality was checked using Bioanalyzer-309 and samples with RNA Integrity Number (RIN) greater than 6 were used for gene expression studies.
Statistical analysis
All statistical analyses were done using GraphPad Instat 3 (San Diego, CA) and Microsoft Excel (student’s t-test).
RESULTS
3D type I collagen protects PDAC cells from gemcitabine-induced cell cycle arrest
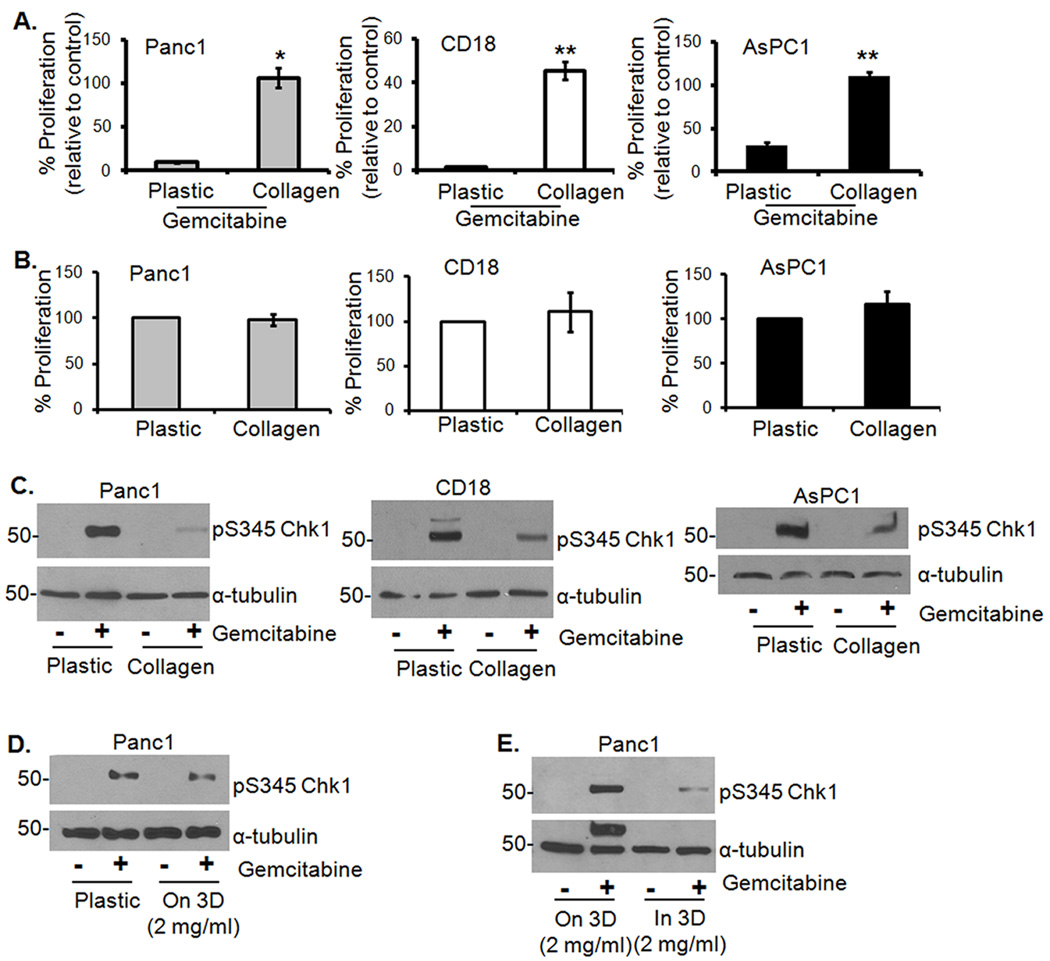
Recently it was demonstrated that the effect of gemcitabine was reduced by the fibrotic reaction in a mouse model of pancreatic cancer (7). To identify whether type I collagen, the most common ECM present in the fibrotic reaction, contributes to chemoresistance, we treated PDAC cells (Panc1, CD18 and AsPC1) grown on plastic or in 3D collagen with low levels of gemcitabine (100 µM) for 24 hours and assessed proliferation by 3H-thymidine incorporation. As seen in Fig. 1A, while only a minority of cells on plastic proliferate following gemcitabine treatment, most cells in 3D collagen continue to proliferate in the presence of gemcitabine. In the absence of gemcitabine, 3D collagen had no effect on proliferation (Fig. 1B). We also examined the effect of gemcitabine on checkpoint activation by immunoblotting for Chk1 phosphorylation on Ser345. Cells growing in collagen displayed minimal activation of Chk1 compared to the robust Chk1 activation in cells grown on plastic (Fig. 1C), indicating that collagen helps cells evade arrest and continue to proliferate. Note that there was minimal to no PARP cleavage following treatment with 100 µM gemcitabine (Supplemental Fig. S1). To examine whether the effect on checkpoint arrest was due to collagen or the 3D microenvironment, Panc1 cells were grown on plastic, atop 3D collagen gels or within 3D collagen gels and then treated with gemcitabine. Chk1 phosphorylation was similar between cells grown on plastic and on 3D collagen (Fig. 1D). However, compared to cells grown on 3D collagen, there was a significant reduction in Chk1 phosphorylation in cells grown in 3D (Fig. 1E). To determine whether the effect of the 3D microenvironment on checkpoint activation was unique to collagen or applicable to other matrices, we also examined the effect of 3D matrigel on checkpoint activation. As shown in Supplemental Fig. S2, cells grown in 3D matrigel showed similar response as in 3D collagen, indicating that the effect is mediated by the 3D microenvironment. Since collagen is the predominant ECM in human PDAC tumors, we conducted subsequent experiments in 3D collagen gels.
Figure 1. Growth in 3D type I collagen protects PDAC cells from gemcitabine-induced cell cycle arrest.
A. Panc1, CD18 and AsPC1 cells growing on plastic or in 3D type I collagen gels (2 mg/ml) were treated with gemcitabine (100 µM) for 24 hours. The effect on proliferation was quantified by determining the ability of cells to incorporate 3H-thymidine and normalized to control untreated samples. *, p < 0.05 relative to cells grown on plastic; **, p < 0.01 relative to cells grown on plastic. B. PDAC cells were plated on tissue culture plastic or in 3D I collagen gels for 24 hours. The effect on proliferation was quantified by 3H-thymidine incorporation. C. To examine the effect on checkpoint activation, cells were extracted out of collagen with collagenase treatment and the lysates immunoblotted for pS345Chk1 and α-tubulin. D, E. Panc1 cells were grown on plastic versus on 3D collagen gels (D), or on 3D collagen versus in 3D collagen gels (E), treated with gemcitabine for 24 hours and immunoblotted for pS345Chk1 and α-tubulin.
HMGA2 contributes to gemcitabine resistance of PDAC cells in 3D collagen
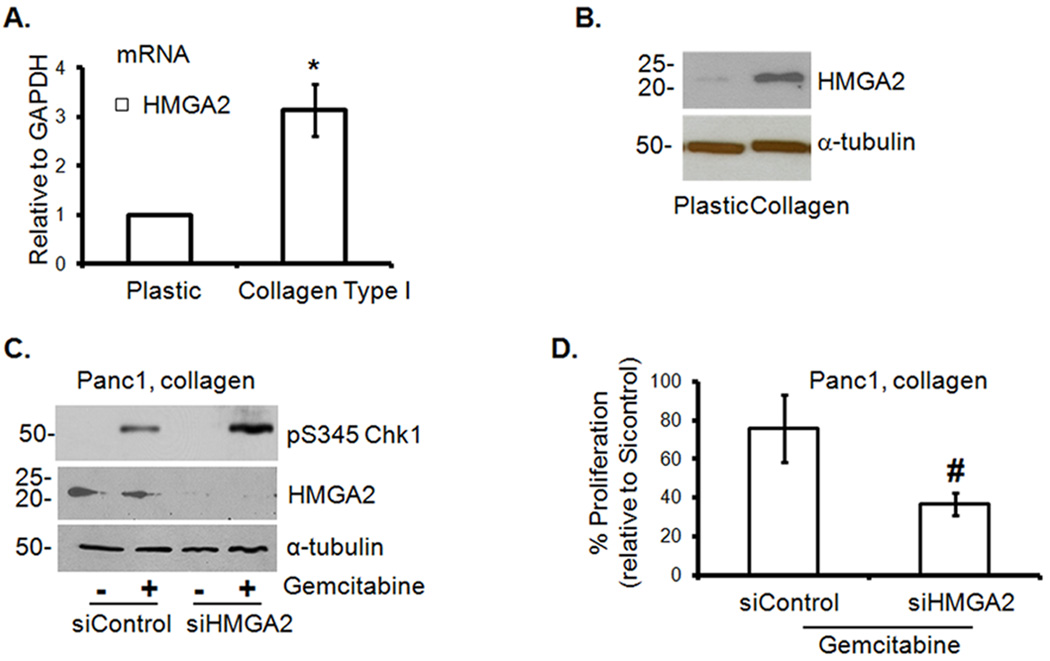
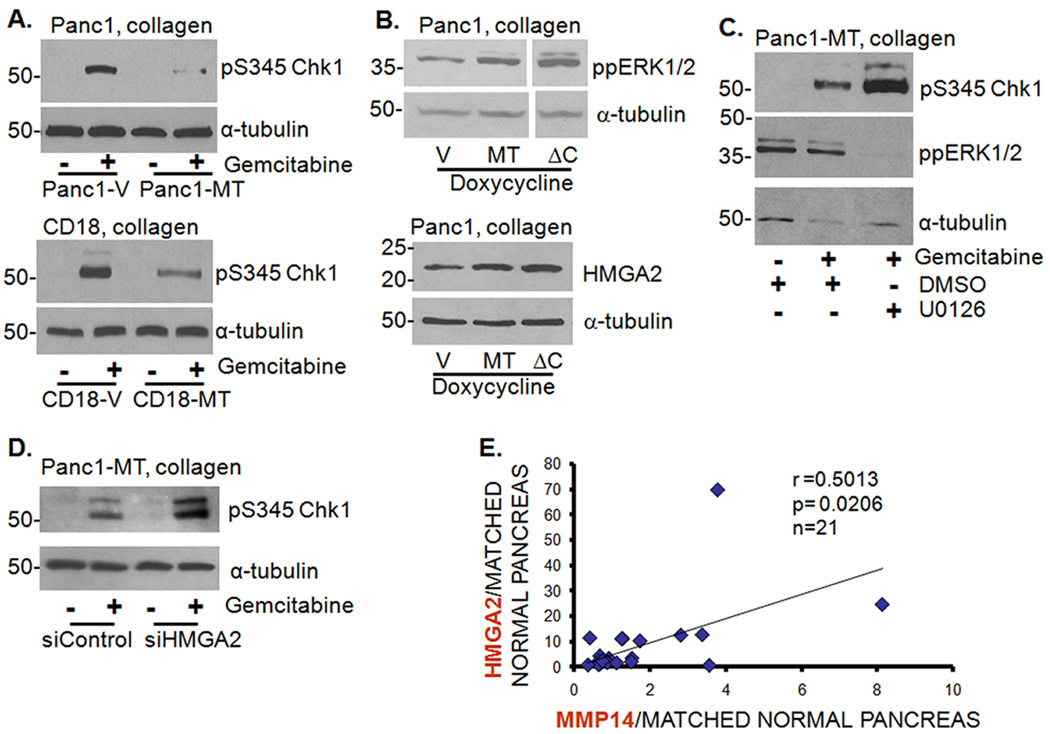
HMGA2 was recently shown to protect cancer cells from DNA-damage-induced apoptosis (21). We therefore examined the role of HMGA2 in mediating the effect of collagen on gemcitabine-induced Chk1 phosphorylation. We initially found that Panc1 cells grown in collagen show increased expression of HMGA2 mRNA (Fig. 2A) and protein (Fig. 2B) relative to cells grown on plastic. To demonstrate that HMGA2 mediates the effect of collagen on gemcitabine-induced checkpoint arrest, Panc1 cells depleted of HMGA2 protein were grown in collagen in the presence or absence of gemcitabine. As shown in Fig. 2C, knocking down HMGA2 sensitized Panc1 cells to gemcitabine-mediated cell cycle arrest as seen by the relative increase in Chk1 phosphorylation when compared to the control siRNA transfected cells. Increased Chk1 phosphorylation in HMGA2-depleted cells was associated with a trend towards further reduction in proliferation in 3D collagen (Fig. 2D). Since the microRNA let-7 is a known inhibitor of HMGA2 (22), we examined the effect of collagen on let-7 levels and the contribution of let-7 to collagen-mediated attenuation of Chk1 phosphorylation. Panc1 cells in collagen show reduced levels of let-7 relative to cells on plastic (Supplemental Fig. S3A). Although pre-miR let-7 repressed HMGA2 expression (Supplemental Figs. S3B and S3C), it did not reverse the effect of collagen on gemcitabine-induced Chk1 phosphorylation (Supplemental Fig. S3D), indicating that let-7 and HMGA2 differentially affect Chk1 phosphorylation in collagen.
Figure 2. HMGA2 contributes to gemcitabine resistance of PDAC cells in 3D collagen.
A, B. Panc1 cells were grown on plastic or in 3D collagen gels for 24 hours and analyzed for HMGA2 by qRT-PCR (A). *, p < 0.05 relative to cells grown on plastic. The effect on HMGA2 protein was determined by immunoblotting using α-tubulin as loading control (B). C. Panc1 cells were transfected with control siRNA or HMGA2 siRNA, allowed to recover and then grown in 3D collagen before treatment with gemcitabine for 24 hours. Cell lysates were immunoblotted for pS345Chk1 and α-tubulin. D. Panc1 cells were transfected with control siRNA or HMGA2 siRNA, allowed to recover and then grown in collagen before treatment with gemcitabine and the effect on proliferation quantified by 3H-thymidine incorporation and normalized to untreated siControl cells. #, p = 0.1356 relative to siControl cells.
The interplay between ERK1/2 and HMGA2 contributes to gemcitabine resistance of PDAC cells in collagen
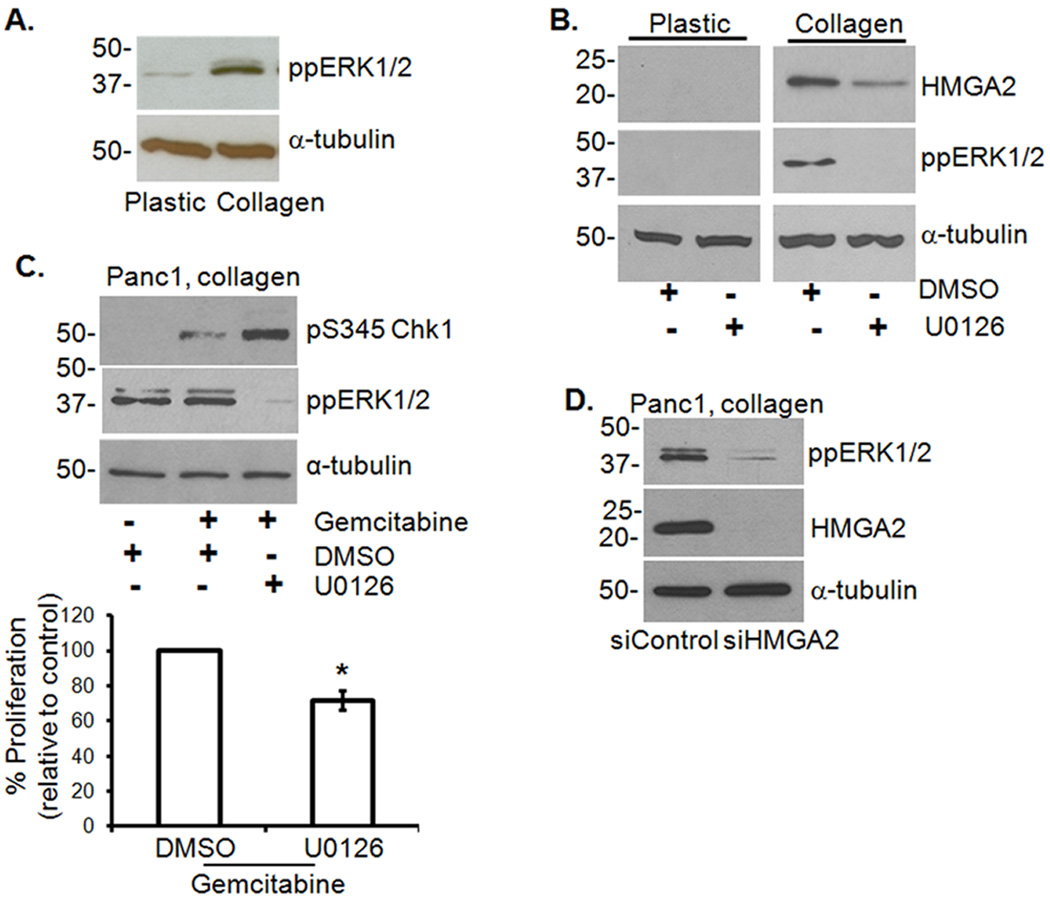
The MEK/ERK1/2 signaling pathway is known to be responsible for promoting survival signals, and blocking ERK1/2 phosphorylation with U0126 increases gemcitabine sensitivity (23, 24). Since Panc1 cells grown in collagen showed increased ERK1/2 phosphorylation compared to cells grown on plastic (Fig. 3A), we examined the effect of U0126 on collagen regulation of Chk1 phosphorylation. Treatment with U0126 inhibited collagen-induced ERK1/2 activation and HMGA2 expression (Fig. 3B), enhanced gemcitabine-induced Chk1 phosphorylation in Panc1 cells (Fig. 3C, top) and reduced proliferation (Fig. 3C, bottom). Interestingly, knocking down HMGA2 in Panc1 cells grown in collagen also decreased ERK1/2 phosphorylation (Fig. 3D), suggesting that a feed-back loop exists between HMGA2 and ERK1/2 to help sustain ERK1/2 phosphorylation in the collagen microenvironment.
Figure 3. ERK1/2 regulation of HMGA2 contributes to gemcitabine resistance of PDAC cells in 3D collagen.
A. Panc1 cells grown on plastic or in collagen were analyzed for ppERK1/2 and α-tubulin. B. Panc1 cells grown on plastic or in collagen were treated with U0126 (10 µM) or DMSO for 24 hours and lysates immunoblotted for HMGA2, ppERK1/2 and α-tubulin. C. Panc1 cells grown in 3D collagen were treated with gemcitabine and co-treated with U0126 or DMSO for 24 hours. Lysates were immunoblotted for pS345Chk1, ppERK1/2 and α-tubulin (upper panel). The effect of U0126 on proliferation was quantified by 3H-thymidine incorporation (lower panel) and normalized to cells treated with DMSO alone. *, p < 0.05 relative to cells treated with DMSO. D. Panc1 cells were transfected with either control or HMGA2 siRNA (50 nM), allowed to recover, grown in 3D collagen and immunoblotted for ppERK1/2, HMGA2 and α-tubulin.
MT1-MMP siRNA potentiates gemcitabine-induced checkpoint arrest and decreases HMGA2 and phospho-ERK1/2
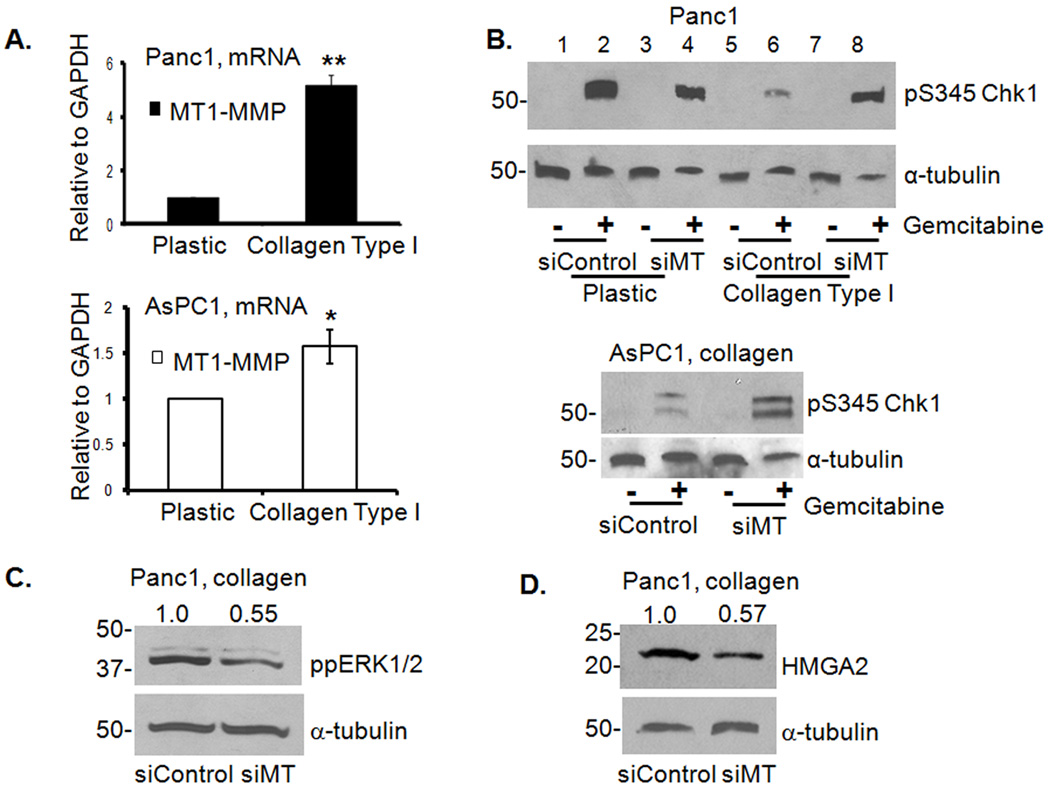
As we had previously shown that collagen induces MT1-MMP expression in PDAC cells [(10) and Fig. 4A], we examined the effect of MT1-MMP siRNA (Supplemental Fig. S4) on collagen regulation of Chk1 phosphorylation. Treatment with gemcitabine robustly induced pS345Chk1 in control siRNA-transfected cells plated on plastic and collagen significantly attenuated the effect (Fig. 4B, upper panel, compare lanes 2 and 6). Although MT1-MMP siRNA-transfected cells also demonstrated robust pS345Chk1 on plastic, there was minimal attenuation of Chk1 phosphorylation in collagen (Fig. 4B, upper panel, compare lanes 4 and 8). Similarly, MT1-MMP siRNA attenuated the effect of collagen on gemcitabine-induced pS345Chk1 in AsPC1 cells (Fig. 4B lower panel). These results demonstrate that collagen contributes to gemcitabine resistance of PDAC cells by increasing MT1-MMP. Knocking-down MT1-MMP also decreased collagen-induced ERK1/2 phosphorylation (Fig. 4C) and HMGA2 expression (Fig. 4D).
Figure 4. MT1-MMP siRNA inhibits ppERK1/2 and HMGA2 in collagen to potentiate gemcitabine-induced checkpoint.
A. Panc1 and AsPC1 cells grown on plastic or in collagen were analyzed for MT1-MMP mRNA expression by qRT-PCR. *, p < 0.05 relative to cells grown on plastic; **, p < 0.01 relative to cells grown on plastic. B. Panc1 and AsPC1 cells were transfected with control or MT1-MMP siRNA (siMT, 50 nM), allowed to recover, plated on plastic or in collagen before treatment with gemcitabine for 24 hours. Lysates were immunoblotted for pS345Chk1 and α-tubulin. C, D. Panc1 cells transfected with control or MT1-MMP siRNA (siMT) were immunoblotted for ppERK1/2 (C) and HMGA2 (D). Protein expression was quantified by densitometry and normalized to siControl transfected cells.
MT1-MMP expression attenuates gemcitabine-induced checkpoint arrest by increasing HMGA2 and phospho-ERK1/2
To further examine the role of MT1-MMP in mediating gemcitabine resistance in collagen, we generated Panc1 cells with inducible expression of wild-type or tail-less (ΔC) MT1-MMP. Doxycycline treatment increased MT1-MMP expression (Supplemental Fig. S5A) and promoted MMP-2 activation, with the ΔC mutant showing a more pronounced effect (Supplemental Fig. S5B). As shown in Fig. 5A, overexpression of MT1-MMP in collagen attenuated the effect of gemcitabine on Chk1 phosphorylation in Panc1 (upper panel) and CD18 cells (lower panel). Expression of both wild-type and tail-less MT1-MMP protein also increased ERK1/2 phosphorylation and HMGA2 expression (Fig. 5B). To demonstrate that the effect of MT1-MMP on Chk1 phosphorylation was mediated by ERK1/2 and HMGA2, Panc1-MT1-MMP cells were treated with U0126 or transfected with siRNA against HMGA2. Blocking ERK1/2 phosphorylation by U0126 (Fig. 5C) as well as knocking down HMGA2 expression (Fig. 5D) partly reversed the effect of MT1-MMP and sensitized the cells to gemcitabine as indicated by increased pS345Chk1 levels. We also examined the relationship between MT1-MMP and HMGA2 in human PDAC tumor samples. As shown in Fig. 5E, tumor samples with increased MT1-MMP demonstrated increased HMGA2 levels (r= 0.5013, p= 0.0206).
Figure 5. MT1-MMP expression attenuates gemcitabine-induced checkpoint arrest by inducing ERK1/2 phosphorylation and HMGA2 expression.
A. Panc1 and CD18 cells inducibly expressing vector (V) or full-length MT1-MMP were grown in collagen, treated with gemcitabine for 24 hours and immunoblotted for pS345Chk1. B. Panc1 cells expressing V, full-length MT1-MMP or tail-less mutant of MT1-MMP (ΔC) were grown in collagen and immunoblotted for ppERK1/2 and HMGA2. C. Panc1-MT cells grown in collagen were treated with gemcitabine and co-treated with DMSO or U0126 and analyzed for pS345Chk1 and ppERK1/2 expression. D. Panc1-MT cells transfected with control or HMGA2 siRNA (50 nM) were plated in collagen before treating with gemcitabine for 24 hours and immunoblotted for pS345Chk1. E. De-identified PDAC samples (n=21) were procured on an IRB-approved protocol. The cancerous and adjacent normal tissue samples were processed for MT1-MMP and HMGA2 mRNA by qRT-PCR and the relationship between MT1-MMP and HMGA2 was analyzed using Spearman’s rank correlation coefficient.
MT1-MMP attenuates gemcitabine-induced proliferation arrest in vivo
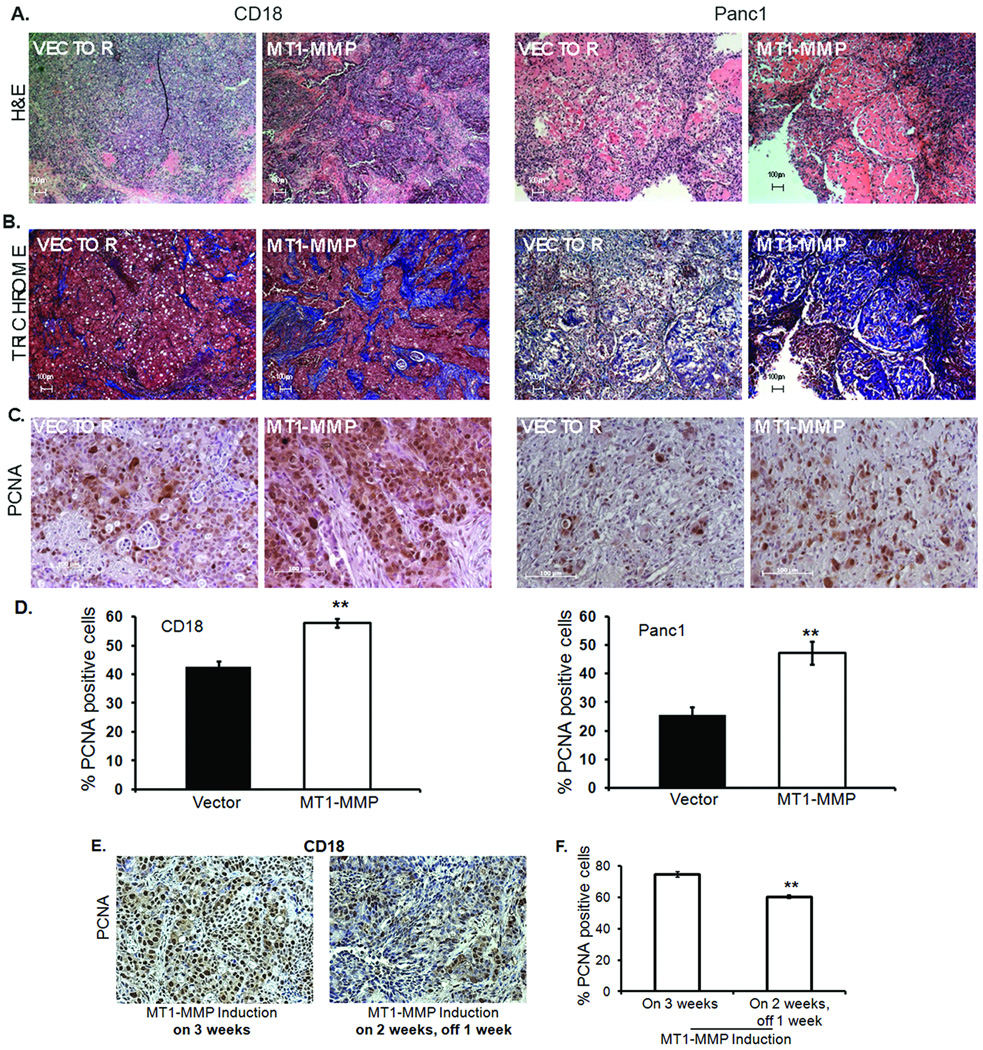
To examine the role of MT1-MMP in attenuating the effect of gemcitabine in vivo, nude mice were subcutaneously injected with Panc1 or CD18 cells expressing vector in the left flank and tail-less ΔC mutant of MT1-MMP in the right flank, allowed to develop tumors for a week, and then treated with gemcitabine. Tumors were excised and processed for MT1-MMP mRNA expression, and stained with H&E, with trichrome to assess fibrosis, and for PCNA to examine proliferation. There was robust MT1-MMP expression in CD18-MT1-MMP and Panc1-MT1-MMP tumors relative to the corresponding control tumors (data not shown). Consistent with previous reports that MT1-MMP-expressing tumors demonstrate increased stromal reaction (17, 25), there was also increased stromal reaction in the MT1-MMP-expressing PDAC tumors (Fig. 6A). Additionally, there was suggestion of increased collagen in the MT1-MMP-expressing tumors as indicated by the increased blue staining in the trichrome images (Fig. 6B). Although there was no difference in the size of these tumors or their proliferation in the absence of gemcitabine treatment, MT1-MMP-overexpressing PDAC tumors displayed greater proliferation relative to control PDAC tumors following gemcitabine treatment (Figs. 6C and 6D). The gemcitabine treatment regimen used for these animal studies did not induce apoptosis, and given the short duration of these animal studies no difference in tumor size was detected (data not shown).
Figure 6. MT1-MMP attenuates gemcitabine-induced proliferation arrest in vivo.
Nude mice (n=5) were injected with Panc1 or CD18 cells expressing vector in the left flank and ΔC mutant of MT1-MMP in the right flank as detailed in Materials and Methods, and maintained on doxycycline water to induce MT1-MMP expression. Mice were injected with two doses of gemcitabine (15mg/kg) intraperitonially 2 days apart and the tumors excised and fixed in 10% formalin. Shown here are representative H&E (A), trichrome (B) and immunostaining for PCNA (C). D. Quantitation of PCNA (+) cells for CD18 and Panc1 was performed using Image J software. **, p <0.01 relative to cells expressing vector. E, F. Nude mice (n=10) were subcutaneously injected with CD18 cells expressing vector in the left flank and ΔC mutant of MT1-MMP in the right flank and maintained on doxycycline water for 2 weeks to induce MT1-MMP expression. Five mice were then switched to regular water for 1 week to block MT1-MMP induction. Four animals in each group were then injected with 2 doses of gemcitabine (15mg/kg) intraperitonially, 2 days apart; the tumors were excised and analyzed for the percentage of proliferating cells using PCNA staining and Image J software. **, p <0.01 relative to cells expressing MT1-MMP.
We next examined whether switching off MT1-MMP in established tumors increases the sensitivity of PDAC cells to gemcitabine. CD18 cells expressing vector or ΔC were injected into nude mice and the mice were maintained on doxycycline for 2 weeks to induce MT1-MMP. Thereafter, half of the mice were switched to regular water for an additional week to stop MT1-MMP induction before treatment with gemcitabine. As shown in Supplemental Fig. S6, control mice maintained on doxycycline demonstrated a 4–10 fold MT1-MMP induction in the CD18-ΔC tumors, while mice switched to regular water demonstrated at most a 2-fold induction in MT1-MMP levels in the CD18-ΔC tumors. Significantly, switching off MT1-MMP expression in established tumors resulted in a statistically significant attenuation of proliferation (Figs. 6E and 6F). These experiments demonstrate that MT1-MMP can attenuate the effect of gemcitabine in the in vivo tumor microenvironment.
DISCUSSION
The collagen-rich tumor microenvironment plays an essential role in cancer progression. Type I collagen not only promotes tumor migration and invasion of cancer cells, but also protects cancer cells against chemotherapy. Work done in lung cancer demonstrated that collagen allows cells treated with chemotherapy to override checkpoints and continue through the cell cycle with damaged DNA (26). In our study we delineate the mechanism by which collagen reduces the effectiveness of gemcitabine in pancreatic cancer. The effect of collagen on checkpoint arrest was seen primarily in the 3D collagen microenvironment and was not detected atop 3D gels, suggesting the importance of 3D tumor microenvironment in regulating cellular response. Cells in 3D matrigel also showed attenuation of gemcitabine response, further demonstrating the importance of 3D microenvironment. The differential effect of on collagen versus in collagen could be because of decreased penetration of gemcitabine into 3D collagen gels; however, it is now well established that cells respond differently to 2D surfaces versus 3D environments, and activate distinct pathways on 2D tissue culture plastic versus in 3D gels (27, 28).
We show that 3D collagen increases HMGA2 and thereby attenuates the effect of gemcitabine. Expression of HMGA1 has been shown to be a determinant of chemoresistance in pancreatic adenocarcinoma (29, 30), but a role for HMGA2 is yet to be established. Although HMGA2 can sensitize breast cancer and salivary epithelial cells to doxorubicin (31), it can also protect cancer cells against DNA damage induced cytotoxicity. HMGA2 is part of the base end-join repair machinery that removes small damaged bases from the DNA, and has an apurinic/apyrimidinic lyase activity to protect against the effects of chemotherapy (21). HMGA2 contributes to genomic instability and tumor progression by inhibiting the DNA repair enzyme DNA-dependent protein kinase during the non-homologous DNA end joining repair process (32). HMGA2 has been shown to maintain cancer stem cells in their undifferentiated state (33) and since stem cells are resistant to most cytotoxic chemotherapy, HMGA2 may contribute to chemotherapy resistance by modulating stem cell function.
We have found that collagen-induced HMGA2 induction involves ERK1/2 signaling, which agrees with a previous report showing that Raf-1- and PMA-induced HMGA2 expression was blocked by the MEK1/2 inhibitor PD98059 (34). ERK1/2 was also shown to upregulate HMGA2 in metastatic breast cancer cells (19), while inhibition of ERK1/2 decreased HMGA2 levels in PDAC cells (13). The MEK/ERK signaling pathway promotes chemotherapy resistance in a number of different cancers including multiple myeloma (35), hepatocellular carcinoma (23) and pancreatic cancer (24). Inhibition of ERK1/2 signaling can also sensitize cancer cells to chemotherapy (36). Here we show that inhibiting ERK1/2 decreased collagen-induced HMGA2 induction and sensitized cells to gemcitabine-induced checkpoint and proliferation arrest. Interestingly, we found that HMGA2 may function to sustain ERK1/2 phosphorylation, as downregulation of HMGA2 decreased ERK1/2 activation by collagen. These findings demonstrate that the interplay between HMGA2 and ERK1/2 contributes to attenuating the effect of gemcitabine in the collagen microenvironment.
HMGA2 is also regulated by the let-7 family of microRNAs which can bind to the 3’UTR of HMGA2 and cause degradation of HMGA2 mRNA (22, 37). Although let-7 is a negative regulator of HMGA2 in PDAC cells, and we recently showed that MT1-MMP in turn inhibits let-7 in collagen (38), expression of pre-miR let-7 did not abrogate the effect of gemcitabine in the collagen microenvironment. Interestingly, a recent report showed that there was differential regulation of epithelial-mesenchymal transition and proliferation by HMGA2 and let-7 in pancreatic cancer cells (13). Knocking down HMGA2 with siRNA reversed the mesenchymal phenotype of pancreatic cancer cells and decreased proliferation; however, expression of let-7 did not affect EMT or proliferation even though HMGA2 levels were decreased (13).
The changes we observed in HMGA2-ERK1/2 signaling, and the downstream effect on gemcitabine sensitivity, were both mediated by collagen-induced MT1-MMP expression. Our studies show that knocking down MT1-MMP enhanced the effect of gemcitabine, while overexpressing MT1-MMP further attenuated the effect of gemcitabine in collagen. The effect of MT1-MMP on checkpoint arrest was mediated through HMGA2 and ERK1/2. Interestingly, the effect of MT1-MMP was only seen in the collagen microenvironment as modulation of MT1-MMP in cells on plastic had minimal effect on checkpoint arrest, suggesting that additional microevironment-dependent signaling is required. It is now well established that 3D focal adhesions of cells are very different in molecular composition when compared to 2D focal adhesions (28). Significantly, MT1-MMP can increase the functionality of the collagen-binding α2β1 integrin in breast cancer cells (39). Moreover, integrin-linked kinase, which can interact with the cytoplasmic tail of β1 integrin, has been shown to contribute to gemcitabine resistance in pancreatic cancer cells (40). Thus, it is possible that MT1-MMP potentiates integrin signaling only in the 3D microenvironment to enhance gemcitabine resistance.
Clinically, it was recently shown that pancreatic cancer cells are not inherently resistant to gemcitabine, but the pronounced desmoplastic reaction causes acquired resistance (7). Our results demonstrate that blocking MT1-MMP can be an effective way to increase the response to gemcitabine in collagen and in the in vivo microenvironment. Previously, broad spectrum MMP inhibitors in combination with gemcitabine in animal models were shown to decrease pancreatic tumor growth (41). However, a large phase III clinical trial in patients with pancreatic cancer showed no difference in response with broad-spectrum MMP inhibitors in combination with gemcitabine (42). The lack of efficacy may be because these inhibitors non-selectively targeted all MMPs (43, 44). It has now been clearly demonstrated that some MMPs possess tumor-inhibiting functions making it imperative to selectively target only the tumor-promoting MMPs in vivo (45–48). Recently, a highly selective anti-MT1-MMP antibody was shown to inhibit growth, angiogenesis and metastasis in a mouse model of breast cancer (49). Thus, evaluating this antibody in clinical trials may help address whether selective targeting of MT1-MMP blocks PDAC tumor growth and increases response to chemotherapy.
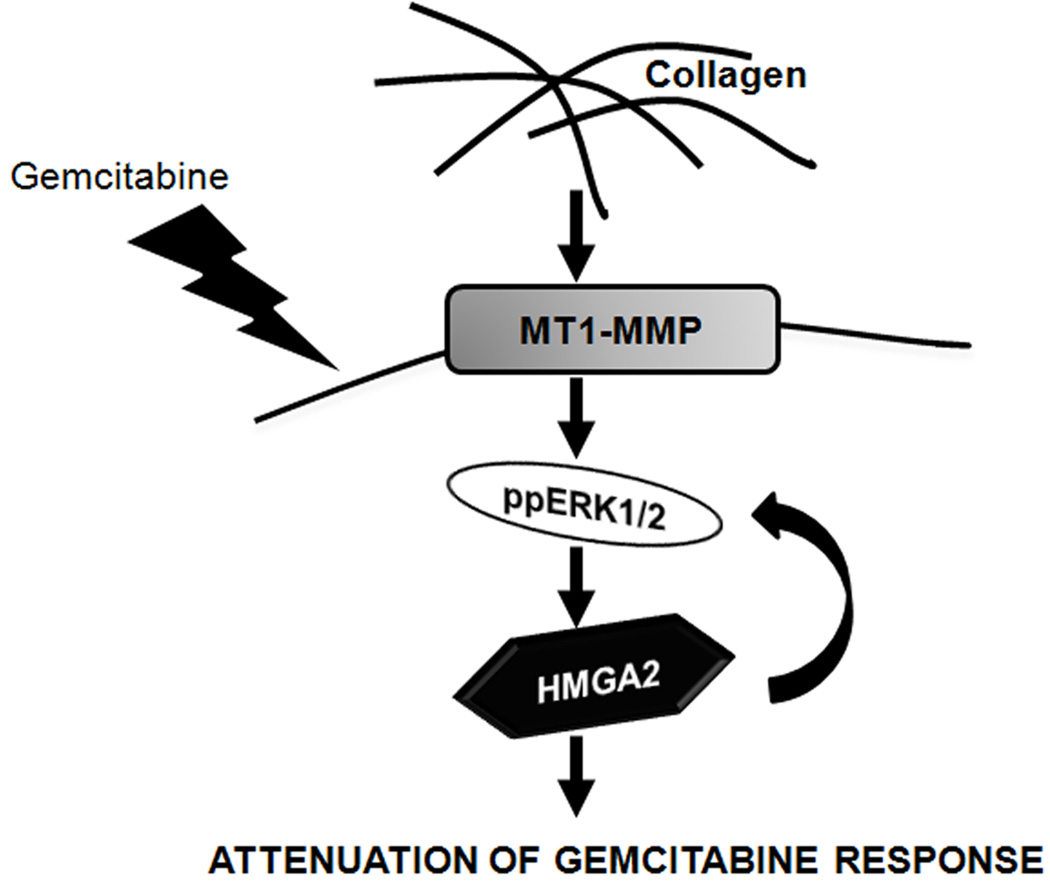
Overall, we demonstrate that the collagen-rich tumor microenvironment limits the effectiveness of gemcitabine through MT1-MMP-mediated HMGA2 expression as depicted by our model (Fig. 7). MT1-MMP is overexpressed in human PDAC tumors, particularly in areas of fibrosis. We now show that MT1-MMP expression is associated with increased HMGA2 expression in human PDAC tumors, suggesting that the pronounced fibrotic reaction may contribute to gemcitabine resistance through increased MT1-MMP-HMGA2 signaling. Given that very little progress has been made in the treatment of pancreatic cancer, targeting MT1-MMP could be a novel approach to sensitize pancreatic tumors to gemcitabine.
Figure 7. Enhancement of gemcitabine resistance by MT1-MMP in PDAC.
Our data demonstrate that PDAC cells in the collagen microenvironment express MT1-MMP, causing increased ERK1/2-dependent HMGA2 expression. This signaling pathway allows PDAC cells to resist gemcitabine-induced cell cycle-and proliferation-arrest, both in vitro and in vivo.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by grant R01CA126888 (H.G.M.) from the NCI, and funding from the Elsa U. Pardee Foundation (H.G.M.) and the Rosenberg Family Fund (H.G.M.).
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Keleg S, Buchler P, Ludwig R, Buchler MW, Friess H. Invasion and metastasis in pancreatic cancer. Mol Cancer. 2003;2:14. doi: 10.1186/1476-4598-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akada M, Crnogorac-Jurcevic T, Lattimore S, et al. Intrinsic chemoresistance to gemcitabine is associated with decreased expression of BNIP3 in pancreatic cancer. Clin Cancer Res. 2005;11:3094–3101. doi: 10.1158/1078-0432.CCR-04-1785. [DOI] [PubMed] [Google Scholar]
- 4.Vulfovich M, Rocha-Lima C. Novel advances in pancreatic cancer treatment. Expert Rev Anticancer Ther. 2008;8:993–1002. doi: 10.1586/14737140.8.6.993. [DOI] [PubMed] [Google Scholar]
- 5.Morgan MA, Parsels LA, Parsels JD, Mesiwala AK, Maybaum J, Lawrence TS. Role of checkpoint kinase 1 in preventing premature mitosis in response to gemcitabine. Cancer Res. 2005;65:6835–6842. doi: 10.1158/0008-5472.CAN-04-2246. [DOI] [PubMed] [Google Scholar]
- 6.Xiao Z, Xue J, Sowin TJ, Zhang H. Differential roles of checkpoint kinase 1, checkpoint kinase 2, and mitogen-activated protein kinase-activated protein kinase 2 in mediating DNA damage-induced cell cycle arrest: implications for cancer therapy. Mol Cancer Ther. 2006;5:1935–1943. doi: 10.1158/1535-7163.MCT-06-0077. [DOI] [PubMed] [Google Scholar]
- 7.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science (New York, NY. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson P, Hanahan D. Cancer. Breaching the cancer fortress. Science (New York, NY. 2009;324:1400–1401. doi: 10.1126/science.1175940. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong T, Packham G, Murphy LB, et al. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:7427–7437. doi: 10.1158/1078-0432.CCR-03-0825. [DOI] [PubMed] [Google Scholar]
- 10.Ottaviano AJ, Sun L, Ananthanarayanan V, Munshi HG. Extracellular matrix-mediated membrane-type 1 matrix metalloproteinase expression in pancreatic ductal cells is regulated by transforming growth factor-beta1. Cancer Res. 2006;66:7032–7040. doi: 10.1158/0008-5472.CAN-05-4421. [DOI] [PubMed] [Google Scholar]
- 11.Abe N, Watanabe T, Suzuki Y, et al. An increased high-mobility group A2 expression level is associated with malignant phenotype in pancreatic exocrine tissue. Br J Cancer. 2003;89:2104–2109. doi: 10.1038/sj.bjc.6601391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hristov AC, Cope L, Reyes MD, et al. HMGA2 protein expression correlates with lymph node metastasis and increased tumor grade in pancreatic ductal adenocarcinoma. Mod Pathol. 2009;22:43–49. doi: 10.1038/modpathol.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe S, Ueda Y, Akaboshi S, Hino Y, Sekita Y, Nakao M. HMGA2 maintains oncogenic RAS-induced epithelial-mesenchymal transition in human pancreatic cancer cells. Am J Pathol. 2009;174:854–868. doi: 10.2353/ajpath.2009.080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borrmann L, Schwanbeck R, Heyduk T, et al. High mobility group A2 protein and its derivatives bind a specific region of the promoter of DNA repair gene ERCC1 and modulate its activity. Nucleic Acids Res. 2003;31:6841–6851. doi: 10.1093/nar/gkg884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tessari MA, Gostissa M, Altamura S, et al. Transcriptional activation of the cyclin A gene by the architectural transcription factor HMGA2. Mol Cell Biol. 2003;23:9104–9116. doi: 10.1128/MCB.23.24.9104-9116.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li XY, Ota I, Yana I, Sabeh F, Weiss SJ. Molecular dissection of the structural machinery underlying the tissue-invasive activity of membrane type-1 matrix metalloproteinase. Mol Biol Cell. 2008;19:3221–3233. doi: 10.1091/mbc.E08-01-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dangi-Garimella S, Redig AJ, Shields MA, Siddiqui MA, Munshi HG. Rho-ROCK-myosin signaling mediates membrane type 1 matrix metalloproteinase-induced cellular aggregation of keratinocytes. J Biol Chem. 285:28363–28372. doi: 10.1074/jbc.M110.146019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strouch MJ, Cheon EC, Salabat MR, et al. Crosstalk between mast cells and pancreatic cancer cells contributes to pancreatic tumor progression. Clin Cancer Res. 16:2257–2265. doi: 10.1158/1078-0432.CCR-09-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dangi-Garimella S, Yun J, Eves EM, et al. Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7. EMBO J. 2009;28:347–358. doi: 10.1038/emboj.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rejiba S, Bigand C, Parmentier C, Hajri A. Gemcitabine-based chemogene therapy for pancreatic cancer using Ad-dCK::UMK GDEPT and TS/RR siRNA strategies. Neoplasia. 2009;11:637–650. doi: 10.1593/neo.81686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Summer H, Li O, Bao Q, et al. HMGA2 exhibits dRP/AP site cleavage activity and protects cancer cells from DNA-damage-induced cytotoxicity during chemotherapy. Nucleic Acids Res. 2009;37:4371–4384. doi: 10.1093/nar/gkp375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YS, Dutta A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 2007;21:1025–1030. doi: 10.1101/gad.1540407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto K, Nagahara T, Okano J, Murawaki Y. The growth inhibition of hepatocellular and cholangiocellular carcinoma cells by gemcitabine and the roles of extracellular signal-regulated and checkpoint kinases. Oncol Rep. 2008;20:863–872. [PubMed] [Google Scholar]
- 24.Zhao Y, Shen S, Guo J, et al. Mitogen-activated protein kinases and chemoresistance in pancreatic cancer cells. J Surg Res. 2006;136:325–335. doi: 10.1016/j.jss.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 25.Soulie P, Carrozzino F, Pepper MS, Strongin AY, Poupon MF, Montesano R. Membrane-type-1 matrix metalloproteinase confers tumorigenicity on nonmalignant epithelial cells. Oncogene. 2005;24:1689–1697. doi: 10.1038/sj.onc.1208360. [DOI] [PubMed] [Google Scholar]
- 26.Hodkinson PS, Mackinnon AC, Sethi T. Extracellular matrix regulation of drug resistance in small-cell lung cancer. Int J Radiat Biol. 2007;83:733–741. doi: 10.1080/09553000701570204. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Lao J, Chen BP, et al. Genomic analysis of smooth muscle cells in 3-dimensional collagen matrix. Faseb J. 2003;17:97–99. doi: 10.1096/fj.02-0256fje. [DOI] [PubMed] [Google Scholar]
- 28.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 29.Liau SS, Rocha F, Matros E, Redston M, Whang E. High mobility group AT-hook 1 (HMGA1) is an independent prognostic factor and novel therapeutic target in pancreatic adenocarcinoma. Cancer. 2008;113:302–314. doi: 10.1002/cncr.23560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liau SS, Whang E. HMGA1 is a molecular determinant of chemoresistance to gemcitabine in pancreatic adenocarcinoma. Clin Cancer Res. 2008;14:1470–1477. doi: 10.1158/1078-0432.CCR-07-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boo LM, Lin HH, Chung V, et al. High mobility group A2 potentiates genotoxic stress in part through the modulation of basal and DNA damage-dependent phosphatidylinositol 3-kinase-related protein kinase activation. Cancer Res. 2005;65:6622–6630. doi: 10.1158/0008-5472.CAN-05-0086. [DOI] [PubMed] [Google Scholar]
- 32.Li AY, Boo LM, Wang SY, et al. Suppression of nonhomologous end joining repair by overexpression of HMGA2. Cancer Res. 2009;69:5699–5706. doi: 10.1158/0008-5472.CAN-08-4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu F, Yao H, Zhu P, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 34.Li D, Lin HH, McMahon M, Ma H, Ann DK. Oncogenic raf-1 induces the expression of non-histone chromosomal architectural protein HMGI-C via a p44/p42 mitogen-activated protein kinase-dependent pathway in salivary epithelial cells. J Biol Chem. 1997;272:25062–25070. doi: 10.1074/jbc.272.40.25062. [DOI] [PubMed] [Google Scholar]
- 35.Dai Y, Chen S, Pei XY, et al. Interruption of the Ras/MEK/ERK signaling cascade enhances Chk1 inhibitor-induced DNA damage in vitro and in vivo in human multiple myeloma cells. Blood. 2008;112:2439–2449. doi: 10.1182/blood-2008-05-159392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirmohammadsadegh A, Mota R, Gustrau A, et al. ERK1/2 is highly phosphorylated in melanoma metastases and protects melanoma cells from cisplatin-mediated apoptosis. J Invest Dermatol. 2007;127:2207–2215. doi: 10.1038/sj.jid.5700870. [DOI] [PubMed] [Google Scholar]
- 37.Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science. 2007;315:1576–1579. doi: 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dangi-Garimella S, Strouch MJ, Grippo PJ, Bentrem DJ, Munshi HG. Collagen regulation of let-7 in pancreatic cancer involves TGF-beta1-mediates membrane type 1-matrix metalloproteinase expression. Oncogene. 2010 doi: 10.1038/onc.2010.485. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baciu PC, Suleiman EA, Deryugina EI, Strongin AY. Membrane type-1 matrix metalloproteinase (MT1-MMP) processing of pro-alphav integrin regulates cross-talk between alphavbeta3 and alpha2beta1 integrins in breast carcinoma cells. Exp Cell Res. 2003;291:167–175. doi: 10.1016/s0014-4827(03)00387-2. [DOI] [PubMed] [Google Scholar]
- 40.Duxbury MS, Ito H, Benoit E, Waseem T, Ashley SW, Whang EE. RNA interference demonstrates a novel role for integrin-linked kinase as a determinant of pancreatic adenocarcinoma cell gemcitabine chemoresistance. Clin Cancer Res. 2005;11:3433–3438. doi: 10.1158/1078-0432.CCR-04-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haq M, Shafii A, Zervos EE, Rosemurgy AS. Addition of matrix metalloproteinase inhibition to conventional cytotoxic therapy reduces tumor implantation and prolongs survival in a murine model of human pancreatic cancer. Cancer Res. 2000;60:3207–3211. [PubMed] [Google Scholar]
- 42.Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87:161–167. doi: 10.1038/sj.bjc.6600446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 44.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 45.Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–808. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- 46.Lynch CC, Vargo-Gogola T, Martin MD, Fingleton B, Crawford HC, Matrisian LM. Matrix metalloproteinase 7 mediates mammary epithelial cell tumorigenesis through the ErbB4 receptor. Cancer Res. 2007;67:6760–6767. doi: 10.1158/0008-5472.CAN-07-0026. [DOI] [PubMed] [Google Scholar]
- 47.Overall CM, Kleifeld O. Tumour microenvironment - opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 48.Fingleton B. MMPs as therapeutic targets--still a viable option? Semin Cell Dev Biol. 2008;19:61–68. doi: 10.1016/j.semcdb.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Devy L, Huang L, Naa L, et al. Selective inhibition of matrix metalloproteinase-14 blocks tumor growth, invasion, and angiogenesis. Cancer Res. 2009;69:1517–1526. doi: 10.1158/0008-5472.CAN-08-3255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.