Abstract
Telomeres play a critical role in maintaining genome integrity. Telomere shortening is associated with the risk of many aging-related diseases. Classic twin studies have shown that genetic components may contribute up to 80% of the heritability of telomere length. In the study we report here, we used a multi-stage genome-wide association study (GWAS) to identify genetic determinants of telomere length. The mean telomere length in peripheral blood leukocytes was measured by quantitative real-time polymerase chain reaction. We first analyzed 300,000 single-nucleotide polymorphisms (SNPs) in 459 healthy controls, finding 15,120 SNPs associated with telomere length at P < 0.05. We then validated these SNPs in two independent populations comprising 890 and 270 healthy controls, respectively. Four SNPs, including rs398652 on 14q21, were associated with telomere length across all three populations (pooled P-values of < 10−5). The variant alleles of these SNPs were associated with longer telomere length. We then analyzed the association of these SNPs with the risk of bladder cancer in a large case-control study. The variant allele of rs398652 was associated with a significantly reduced risk of bladder cancer (odds ratio = 0.81; 95% confidence interval, 0.67–0.97; P = 0.025), consistent with the correlation of this variant allele with longer telomeres. We then conducted a mediation analysis to examine whether the association between rs398652 and reduced bladder cancer risk is mediated by telomere length, finding that telomere length was a significant mediator of the relationship between rs398652 and bladder cancer (P = 0.013), explaining 14% of the effect. In conclusion, we found that the SNP rs398652 on 14q21 was associated with longer telomere length and a reduced risk of bladder cancer and that a portion of the effect of this SNP on bladder cancer risk was mediated by telomere length.
Keywords: SNP, telomere length, GWAS, bladder cancer risk
Introduction
Telomeres are nucleoprotein complexes at the ends of chromosomes and consist of tandem arrays of short repetitive sequences (TTAGGG in humans) and a set of specialized proteins (1–3). As caps at the ends of chromosomes, telomeres play a critical role in protecting chromosomes from degradation, end-to-end fusion, abnormal recombination, and other detrimental chromosomal events (4, 5). In somatic cells under normal physiological conditions, telomeres are progressively eroded by ~30 to ~200 bp because of the end-replication problem during each mitotic cell division (6). The erosion rate of telomeres is modified by many exogenous and endogenous factors; for example, life stress and oxidative stress may accelerate telomere shortening (7, 8). When telomere lengths become critically short, the process of cell senescence is initiated, resulting in cell-cycle arrest or apoptosis in normal cells (9). For cancer cells, however, the majority gain unlimited proliferation through the overexpression of telomerase that compensates telomere erosion (1). Telomere shortening has been associated with aging and many aging-related diseases including cancer (6, 10–12). A number of epidemiologic studies have shown that short telomere length in peripheral blood leucocytes is associated with increased cancer risks (13–20).
Although telomere length is inversely correlated with age, there is considerable inter-individual variation of telomere length among people of the same age (21). Previous studies have shown that overall telomere length and the length of each specific chromosome’s telomere are under strong genetic control. Genetic heritability of telomere length in leukocytes has been estimated at approximately 80% (22, 23). The relative length of each specific telomere is defined in the zygote, determined by inherited factors, and maintained throughout life, but average telomere length shortens with increasing age (24–26). Because of the high genetic heritability of telomere length, there have been several attempts to identify genetic loci that may determine telomere length. Putative loci were mapped to chromosome 3p26.1, 10q26.13, 12q12.22 (BICD1 rs2630778), and 14q23.2 in a family-based linkage analysis (27–29). Three recent genome-wide association studies (GWAS) identified associations between single-nucleotide polymorphisms (SNPs) in 18q12.2 (VPS34/PIKC3C rs2162440 and rs7235755; ref. (30), 3q26 (TERC rs12696304; ref. (31), and 10q24.33 (OBFC1 rs4387287; ref. (32) and leukocyte telomere length. The identified variants only explain a small fraction of the genetic heritability of telomere length, however, and more loci remain to be identified. Moreover, none of the identified SNPs have been associated with altered cancer risk. In this study, we used a GWAS design to identify SNPs associated with telomere length in leukocytes and then tested the associations of the top SNPs with bladder cancer risk. We then conducted a mediation analysis to examine whether telomere length mediated the association between SNPs and bladder cancer risk (33, 34).
Materials and Methods
Study population and data collection
For the associations of SNPs with telomere length, we used the data of healthy controls from three independent case-control studies in lung, bladder, and kidney cancer. Detailed information from these three studies was reported previously (35–39). Controls were healthy individuals without a prior history of cancer (except non-melanoma skin cancer). Controls for the lung and bladder studies were recruited from Kelsey-Seybold, the largest multispecialty physician practice group in the Houston metropolitan area, and controls for the renal cell carcinoma cases were recruited via random digit dialing (RDD) in Texas. For the bladder cancer case-control study, all cases were newly diagnosed, histologically confirmed, incident urothelial cell carcinoma patients recruited at MD Anderson Cancer Center and Baylor College of Medicine. Cases and controls were frequency-matched on age (±5 years), sex, and ethnicity. Informed consent was obtained from all participants before the collection of epidemiological data and blood samples. Epidemiologic and demographic data were collected through in-person interviews by trained M. D. Anderson interviewers. “Ever smokers,” including current and former smokers, were defined as having smoked > 100 cigarettes in their lifetime; “never smokers” were defined as having smoked < 100 cigarettes lifetime. Individuals who had quit smoking at least 1 year before diagnosis (for cases) or prior to the interview (for controls) were categorized as former smokers. A 40 ml blood sample was extracted immediately after the interview and sent to the lab for DNA extraction. All studies were approved by the Institutional Review boards of MD Anderson, Baylor College of Medicine, and Kelsey-Seybold.
Overall telomere length assessment
Genomic DNA was isolated from peripheral blood using the QIAamp DNA blood Maxi Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. The relative overall telomere length was measured by quantitative real-time polymerase chain reaction (PCR), as described by Cawthon (40). Briefly, the relative overall telomere lengths was proportional to the ratio of the telomere repeat copy number and the single (human globulin) copy number. The ratio of each sample was normalized to a calibrator DNA to standardize between different runs. The PCR reaction mixture for telomere amplification consists of 5 ng genomic DNA, 1 × SYBR Green Mastermix (Applied Biosystem), 200 nmol/L Tel-1 primer, and 200 nmol/L Tel-2 primer. The PCR reaction mixture for human globulin consists of 5 ng genomic DNA, 1 × SYBR Green Mastermix, 200 nmol/L Hgb-1 primer, and 200 nmol/L Hgb-2 primer. The thermal reaction conditions were 95° C for 10 minutes followed by 40 cycles of 95° C for 15 seconds and 56° C (for telomere amplification) or 58° C (for Hgb amplification) for 1 minute. In each plate, negative and positive controls and a calibrator DNA were included. Each plate contained twofold serially diluted reference DNA (from 20 to 0.625 ng) to generate a six-point standard curve.
Genotyping
Genotyping for the lung study controls was completed using Illumina’s HumanHap300 v1.1 BeadChips with 317K tagging SNPs (37). After removing SNPs that deviated from Hardy-Weinberg equilibrium (P < 0.001), that had a call rate < 95%, or with minor allele frequency < 0.01, there were 312,531 SNPs that passed quality control measures. Genotyping analyses for the bladder cancer (38) and renal cancer studies were performed using Illumina’s HumanHap610 chip and HumanHap660 chip, respectively. After quality control and merging with telomere-length data, we were able to analyze SNPs with telomere length in 459 lung-study controls, 890 bladder-study controls, and 270 kidney-study controls.
Statistical analysis
We applied a two-stage design to identify SNPs that determine telomere length. In the first stage, we performed whole-genome screening and analyzed the association of 312,531 SNPs with telomere length in 459 controls from the lung study. In the second stage (validation), we used 890 bladder-study controls and 270 kidney-study controls to validate the top 15,120 SNPs (P < 0.05) from the first stage. Telomere length was analyzed as a continuous variable. Analysis of SNP and telomere-length associations was performed in PLINK software by multivariate linear regression. We assumed an additive model for each SNP. The covariates included in the analysis were age and gender. The associations of SNPs with bladder cancer risk were assessed in a large case-control study. Unconditional logistic regression was used to calculate the odds ratio (OR) and 95% confidence interval (CI), adjusting for age, gender, and smoking using STATA software. A Q-Q-plot was used to compare the distribution of observed P-values with the expected distribution. The inflation factor was assessed by the ratio of the median of observed and expected values. This analysis was done using R software. We used IMPUTE2 (41) to impute genotypes for SNPs that were not genotyped on arrays. Phased haplotypes from combined 1000 Genome and HapMap3 CEU samples were provided as the reference haplotypes to impute the unobserved genotypes based on the local haplotype information. Haploview software (v4.1) was used to obtain the linkage disequilibrium (LD) structure within 100 kb of the significant SNP. The screen shot of all known genes within 1 Mb of the SNP was obtained from the University of California Santa Cruz genome browser (http://genome.ucsc.edu).
We conducted a mediation (or indirect effect) analysis involving telomere length and SNP associations with bladder cancer risk. Mediation occurs when the effect of an independent variable (e.g., SNP) on a dependent variable (e.g., cancer risk) is transmitted through a mediator (e.g., telomere length). If the effect of the independent variable vanishes after controlling for the mediator, then a complete mediation has occurred. If the effect of the independent variable is significant even after controlling for the mediator, however, then a partial mediation is indicated.
For the mediation analysis of rs398652, the dependent variable was case-control status coded as 0 for controls and 1 for cases. The independent variable, rs398652, was coded as an additive variable, as follows: 0 for GG genotype, 1 for AG, and 2 for AA. The mediator variable, telomere length, is continuous but not normally distributed. Therefore, we dichotomized the variable at the median value (1.31) among the controls. Individuals with telomere length higher and lower than 1.31 were coded as 0 and 1, respectively. Although, the mediation analysis was originally developed for linear regression models, it has been shown to be valid for logistic regression as well (42, 43). The coefficients obtained from the logistic regression were transformed to make them comparable before the Sobel test was applied for the mediation analysis (42–44). All the analyses were adjusted for age, gender, and smoking status.
Results
In the discovery stage, we evaluated the association of 312,531 genetic variations with telomere length in 459 controls from a lung-cancer case-control study. The Q-Q-plot showed no evidence of a systematic inflation of P-values (genomic inflation factor λ = 1.004; Supplementary Fig. 1). We identified 15,120 SNPs significantly associated with telomere length at P < 0.05. We validated 14,409 of these 15,120 SNPs in two independent populations including 890 controls from a bladder and 270 controls from a kidney study. Thirteen SNPs were validated and significantly associated with telomere length in the same direction, with Ps < 0.05 in each population (Supplementary Table 1). We then performed a pooled analysis of these 13 SNPs in all controls. The 4 SNPs rs6028466 on 20q11.22, rs621559 on 1p34.2, rs398652 on 14q21, and rs654128 on 6q22.1 reached P < 10−5 (Table 1). The variant alleles of all 4 of these SNPs were associated with increased telomere length.
Table 1.
SNPs significantly associated with telomere length in three control populations and with a pooled P<10−5
| Group | Lung Controls | Bladder Controls | Kidney Controls | Pooled Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | Locus | N | β. | P value | N | β | P value | N | β | P value | N | β | P value |
| rs6028466 | 20q11.22 | 459 | 0.261 | 0.003109 | 890 | 0.078 | 0.024833 | 270 | 0.361 | 0.001396 | 1619 | 0.192 | 3.02E-07 |
| rs621559 | 1p34.2 | 459 | 0.201 | 0.008673 | 890 | 0.102 | 0.000364 | 270 | 0.307 | 0.015791 | 1619 | 0.16 | 1.65E-06 |
| rs398652 | 14q21 | 458 | 0.206 | 0.000352 | 890 | 0.049 | 0.028771 | 270 | 0.204 | 0.0215 | 1618 | 0.12 | 2.05E-06 |
| rs654128 | 6q22.1 | 459 | 0.147 | 0.012535 | 890 | 0.055 | 0.017219 | 270 | 0.305 | 0.001103 | 1619 | 0.122 | 3.01E-06 |
We next analyzed the associations of these four SNPs with bladder cancer risk in a large case-control study of 969 bladder cancer cases and 946 controls (who include the 890 validation controls in the previous paragraph). The variant allele of rs398652 on 14q21 was associated with a significantly reduced risk of bladder cancer, with an OR of 0.81 (95% CI, 0.67–0.97; P = 0.025), adjusting for age, gender, and smoking status, in an additive model (Table 2). This risk-reduction association was only evident in never smokers (OR = 0.63, 95% CI, 0.45–0.88; P = 0.007); the OR in ever smokers was 0.91 (95% CI, 0.73–1.14; P = 0.423). In addition, there was a joint effect between the risk genotype (GG, the major genotype) and smoking in elevating bladder cancer risk. Compared with never smokers with the protective genotypes (GA, AA), the ORs were 1.65 (95% CI, 1.14–2.39) for never smokers with the risk genotype, 2.86 (95% CI, 1.93–4.23) for smokers with the protective genotypes, and 3.13 (95% CI, 2.21–4.45) for smokers with the risk genotype, (P for trend < 0.001). The other three SNPs were not significantly associated with bladder cancer risk (Table 3).
Table 2.
Association of rs398652 on 14q21 with bladder cancer risk
| Genotype | Case | Control | OR (95% CI) | P |
|---|---|---|---|---|
| Overall | ||||
| GG | 736 | 685 | Ref. | |
| GA | 213 | 246 | 0.78 (0.63–0.97) | 0.025 |
| AA | 20 | 25 | 0.77 (0.41–1.43) | 0.406 |
| Additive | 0.81 (0.67–0.97) | 0.025 | ||
| Dominant | 0.78 (0.63–0.96) | 0.02 | ||
| Recessive | 0.82 (0.44–1.51) | 0.521 | ||
| Never smoker | ||||
| GG | 203 | 285 | Ref. | |
| GA | 50 | 111 | 0.63 (0.43–0.92) | 0.016 |
| AA | 3 | 11 | 0.41 (0.11–1.50) | 0.178 |
| Additive | 0.63 (0.45–0.88) | 0.007 | ||
| Dominant | 0.61 (0.42–0.88) | 0.008 | ||
| Recessive | 0.46 (0.13–1.66) | 0.234 | ||
| Ever smoker | ||||
| GG | 532 | 400 | Ref. | |
| GA | 163 | 135 | 0.88 (0.67–1.15) | 0.335 |
| AA | 17 | 14 | 0.97 (0.46–2.04) | 0.941 |
| Additive | 0.91 (0.73–1.14) | 0.423 | ||
| Dominant | 0.88 (0.68–1.15) | 0.355 | ||
| Recessive | 1.00 (0.48–2.10) | 0.991 | ||
Table 3.
Associations of rs6028466, rs621559 and rs654128 with bladder cancer risk
| Overall |
Never Smoker |
Ever Smoker |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | Case | Control | OR (95% CI) | P | Case | Control | OR (95% CI) | P | Case | Control | OR (95% CI) | P |
| rs6028466 | ||||||||||||
| GG | 867 | 848 | Ref. | 230 | 357 | Ref. | 636 | 491 | Ref. | |||
| GA | 102 | 107 | 0.93 (0.69–1.25) | 0.61 | 26 | 49 | 0.82 (0.49–1.36) | 0.44 | 76 | 58 | 0.93 (0.69–1.25) | 0.62 |
| AA | 0 | 2 | N/A* | 0 | 1 | N/A | 0 | 1 | N/A | |||
| Additive | N/A | N/A | N/A | |||||||||
| Dominant (GA+AA vs. GG) | 0.91 (0.68–1.22) | 0.53 | 0.80 (0.49–1.33) | 0.39 | 0.98 (0.67–1.42) | 0.91 | ||||||
| Recessive (AA vs. GG+GA) | N/A | N/A | N/A | |||||||||
| rs621559 | ||||||||||||
| GG | 837 | 822 | Ref. | 218 | 352 | Ref. | 618 | 470 | Ref. | |||
| GA | 127 | 123 | 0.98 (0.74–1.29) | 0.87 | 35 | 48 | 1.17 (0.73–1.87) | 0.51 | 92 | 75 | 0.89 (0.63–1.25) | 0.49 |
| AA | 4 | 12 | 0.39 (0.12–1.23) | 0.11 | 3 | 7 | 0.65 (0.17–2.57) | 0.54 | 1 | 5 | N/A | |
| Additive | 0.89 (0.70–1.14) | 0.37 | 1.03 (0.70–1.53) | 0.86 | N/A | |||||||
| Dominant (GA+AA vs. GG)) | 0.93 (0.71–1.21) | 0.59 | 1.10 (0.70–1.73) | 0.67 | 0.84 (0.60–1.18) | 0.32 | ||||||
| Recessive (AA vs. GG+GA) | 0.39 (0.12–1.23) | 0.11 | 0.64 (0.16–2.51) | 0.52 | N/A | |||||||
| rs654128 | ||||||||||||
| GG | 695 | 701 | Ref. | 178 | 298 | Ref. | 517 | 403 | Ref. | |||
| GT | 243 | 235 | 1.06 (0.86–1.32) | 0.57 | 68 | 100 | 1.14 (0.80–1.64) | 0.47 | 174 | 135 | 1.02 (0.78–1.34) | 0.86 |
| TT | 31 | 21 | 1.62 (0.91–2.89) | 0.10 | 10 | 9 | 1.80 (0.71–4.52) | 0.21 | 21 | 12 | 1.55 (0.74–3.20) | 0.25 |
| Additive | 1.13 (0.95–1.36) | 0.17 | 1.21 (0.90–1.63) | 0.21 | 1.09 (0.87–1.37) | 0.43 | ||||||
| Dominant (GT+TT vs. GG) | 1.11 (0.90–1.36) | 0.33 | 1.20 (0.85–1.69) | 0.31 | 1.07 (0.82–1.38) | 0.63 | ||||||
| Recessive (TT vs. GG+GT) | 1.60 (0.90–2.84) | 0.11 | 1.74 (0.69–4.35) | 0.24 | 1.54 (0.74–3.20) | 0.25 | ||||||
The number of homozygous variant genotype was ≤ in both cases and controls, only dominant model was applied.
We also analyzed the effect of telomere length on bladder cancer risk by smoking status. Shorter telomere length significantly increased risk in never smokers (OR = 1.64, 95% CI, 1.19–2.26; P = 0.0027) and in ever smokers (OR = 1.31, 95% CI, 1.03–1.66; P = 0.027). Although shorter telomeres increased risk more in never (OR 1.64) than ever (OR 1.31) smokers, the difference was not statistically significant in this stratified analysis, which had relatively small sample sizes.
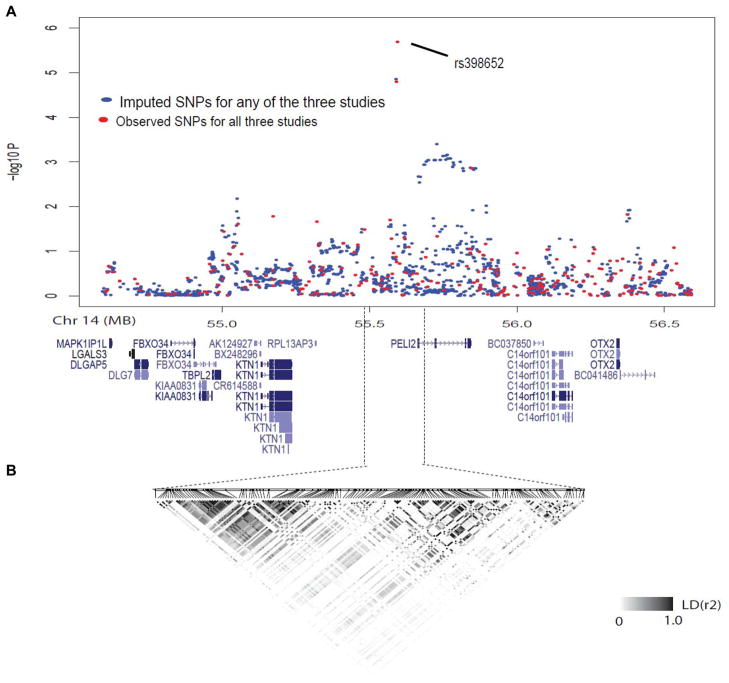
The SNP rs398652 is intergenic, with the closest gene being the pellino homolog 2 (Drosophila) gene (PELI2), about ~60 kb from rs398652 (Fig.1). SNPs in this region show weak patterns of LD. Using data from the 1000 Genome and HapMap database, we imputed genotypes within 1 Mb of rs398652 for SNPs not on any of the three chips we used. Rs398652 had the most significant association with telomere length (Fig.1).
Fig 1.
The locus of 14q21 containing rs398652. A, imputation of SNP association with telomere length from genome-wide screen: observed results from genotyped SNPs are in red and imputed results are in blue. All known genes in this region are also shown. B, linkage disequilibrium (LD) structure of this region. Boxes with darker shading indicate greater extent of LD between two SNPs. The triangles represent LD blocks identified by Haploview software.
Since the variant allele of this SNP was associated with long telomeres and reduced bladder cancer risk, the effect of this SNP on bladder cancer may be mediated by telomere length, a hypothesis tested in a mediation analysis. Following the steps of Baron and Kenny (33), we found that the total effect of rs398652 on bladder cancer risk was significant (OR = 0.81, 95 % CI, 0.67 0.97; P = 0.025; Table 2). Both the effects of the SNP on telomere length (OR = 0.72, 95% CI, 0.60–0.87; P =0.0006) and of short telomere length on bladder cancer (OR = 1.42, 95% CI, 1.17–1.71; P =0.0004) were statistically significant. Therefore, the conditions for the mediation analysis were satisfied. The Sobel test showed that telomere length was a significant mediator of the relationship between rs398652 and bladder cancer (P = 0.013). However, the direct effect of the SNP on bladder cancer, controlling for the mediator, was still statistically significant (P = 0.048), suggesting that telomere length provided a partial (14%) explanation of the effect. Telomere length was a significant mediator of the relationship between rs398652 and bladder cancer risk even when it was used as a continuous variable in the mediation analyses (P = 0.018).
Discussion
The SNP rs398652 on 14q21 and several other promising genetic variants were associated in our GWAS with telomere length in leukocytes. We then found that rs398652 was also associated with reduced bladder cancer risk. This SNP is located within the region of 14q that has a high linkage with leukocyte telomere length and is fewer than 450 kb from a microsatellite marker (D14S285) shown to have a very high logarithm (base 10) of odds (LOD) of approximately 3.5 for linkage with telomere length in a previous genetic linkage analysis (27). Furthermore, our mediation analysis to dissect the relationship between rs398652, telomere length, and bladder cancer risk suggests that the association of this SNP with bladder cancer is partially mediated by telomere length.
Telomere shortening and telomerase activation are critical early events of tumorigenesis. There have been a number of epidemiologic studies suggesting that shorter telomere length in leukocytes is associated with increased cancer risks for several cancers (13–20), with the strongest evidence for bladder cancer risk, as shown in three independent studies (one prospective and two retrospective; refs. (14, 16, 19). Given the critical role of telomere shortening in tumorigenesis, any factors (environmental or genetic) that are capable of accelerating telomere erosion are likely to promote tumorigenesis. Several studies have shown that some risk factors for cancer, such as smoking, obesity, unhealthy lifestyles, and oxidative stress, are associated with short telomeres (8, 14, 45–48), suggesting that telomeres may be a biologic mediator of certain environmental exposures and cancer risk. In our pesent study, shorter telomere length seemed to have a higher impact on bladder cancer risk in never smokers than in ever smokers, although the impact difference was not significant. Genetic determinants of telomere length are also good candidates for cancer predisposition. Previous studies have identified several SNPs that are associated with telomere length (30–32), but none of these SNPs have been evaluated for cancer associations. On the other hand, genetic variations in the 5p15.33 region containing the telomerase reverse transcriptase (TERT) gene (encoding the catalytic subunit of telomerase) have been associated with increased or decreased risks of multiple cancer types (49–54). Although it is hypothesized that SNPs in this region may act through TERT function and telomeres, no conclusive association between the cancer-predisposing SNPs in this region and telomere length has been found. These data indicate that the interplay between a SNP, telomere length, and cancer risk is complex. In indicating that telomere length accounted for 14% of the effect of the SNP variant rs398652 on bladder cancer risk, our mediation analysis and other data lend further support to the complexity of these inter-relationships.
Therefore, the major portion of the effect of this SNP on bladder cancer risk was apparently mediated by mechanisms other than telomere length, possibly through modulating innate immune response and inflammatory response. Rs398652 is ~60 kb from PELI2 (encoding pellino-2 protein). Pellino is a family of proteins that was initially identified as interaction proteins of interleukin-1 receptor (IL-1R)-associated kinases (IRAKs) and that mediated IL-1R signaling (55, 56). There are three highly homologous pellino proteins (pellino1, 2, and 3) in humans, and recent studies have found that a key function of pellino proteins is as E3 ubiquitin ligases that catalyze the polyubiquitylation of IRAK molecules in the Toll-like receptor (TLR) signaling pathways (57). TLRs are key players in the innate immune system and initiate inflammatory response to foreign pathogens. Stimulation of TLRs by pathogens triggers innate immune responses and the production of cytokines. Pellino proteins contain a C-terminal RING-like domain that confers E3 ubiquitin ligase activity to the protein and polyubiquitylates IRAK and other TLR signaling molecules, leading to the TLR- and IL-1R-dependent activation of nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways and downstream genes (57). The important function of PELI2 in innate immune response provides biological plausibility for the association of rs398652 with telomere length and cancer risk. Furthermore, chronic inflammation is associated with up to one-fourth of cancers and also may cause accelerated telomere erosion (8, 58, 59). PELI2 is involved in inflammatory response and production of cytokines. It is possible that chronic inflammation partially explains the association between rs398652 and telomere length and cancer risk.
Three other SNPs shown in our present study to modulate telomere length were not significantly associated with bladder cancer risk. Since SNPs only explain a small variation in telomere length and since telomere length only explains a small variation in bladder cancer risk, the indirect effect of SNPs on bladder cancer risk through telomere length is insignificant, suggesting that these three SNPs have no significant direct or indirect effect on bladder cancer risk, thus contrasting with the case of rs398652, whose major portion of association with bladder cancer risk is direct.
In conclusion, the major strength of the present study is its integration of analyses of SNPs, telomere length, and cancer risk in the same study and the critical application of a mediation analysis to dissect the relationship among these elements. To our knowledge, this is the first study that links a common human SNP with both telomere length and a reduced cancer risk in the same cohort. This approach has led to the important finding that SNP rs398652 reduced bladder cancer risk through the mediator of longer telomere length. We also show that rs398652 increases bladder cancer risk through multiple cellular pathways, dependent and independent of telomere length. Overall, these findings highlight the complexity of genetic regulation of telomere length in relation to the etiology of cancer of the bladder or other sites.
Supplementary Material
Acknowledgments
Grant Support
Supported by National Institutes of Health grants CA74880, CA91846, CA127615, CA131335, and CA121197.
Footnotes
Disclosure of Potential Conflicts of Interest
No conflicts of interest were disclosed.
References
- 1.Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12:1133–8. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 2.Brown WR. Molecular cloning of human telomeres in yeast. Nature. 1989;338:774–6. doi: 10.1038/338774a0. [DOI] [PubMed] [Google Scholar]
- 3.Shampay J, Szostak JW, Blackburn EH. DNA sequences of telomeres maintained in yeast. Nature. 1984;310:154–7. doi: 10.1038/310154a0. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579:859–62. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 5.Kurenova EV, Mason JM. Telomere functions. A review. Biochemistry (Mosc) 1997;62:1242–53. [PubMed] [Google Scholar]
- 6.Klapper W, Parwaresch R, Krupp G. Telomere biology in human aging and aging syndromes. Mech Ageing Dev. 2001;122:695–712. doi: 10.1016/s0047-6374(01)00223-8. [DOI] [PubMed] [Google Scholar]
- 7.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–44. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 9.Mathon NF, Lloyd AC. Cell senescence and cancer. Nat Rev Cancer. 2001;1:203–13. doi: 10.1038/35106045. [DOI] [PubMed] [Google Scholar]
- 10.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–22. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 11.Armanios M. Syndromes of telomere shortening. Annu Rev Genomics Hum Genet. 2009;10:45–61. doi: 10.1146/annurev-genom-082908-150046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shay JW, Wright WE. Telomeres and telomerase: implications for cancer and aging. Radiat Res. 2001;155:188–93. doi: 10.1667/0033-7587(2001)155[0188:tatifc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Shao L, Wood CG, Zhang D, et al. Telomere dysfunction in peripheral lymphocytes as a potential predisposition factor for renal cancer. J Urol. 2007;178:1492–6. doi: 10.1016/j.juro.2007.05.112. [DOI] [PubMed] [Google Scholar]
- 14.McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16:815–9. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- 15.Willeit P, Willeit J, Mayr A, et al. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304:69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- 16.Wu X, Amos CI, Zhu Y, et al. Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst. 2003;95:1211–8. doi: 10.1093/jnci/djg011. [DOI] [PubMed] [Google Scholar]
- 17.Zheng YL, Zhou X, Loffredo CA, Shields PG, Sun B. Telomere deficiencies on chromosomes 9p, 15p, 15q and Xp: potential biomarkers for breast cancer risk. Hum Mol Genet. 2011;20:378–86. doi: 10.1093/hmg/ddq461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirabello L, Garcia-Closas M, Cawthon R, et al. Leukocyte telomere length in a population-based case-control study of ovarian cancer: a pilot study. Cancer Causes Control. 2010;21:77–82. doi: 10.1007/s10552-009-9436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broberg K, Bjork J, Paulsson K, Hoglund M, Albin M. Constitutional short telomeres are strong genetic susceptibility markers for bladder cancer. Carcinogenesis. 2005;26:1263–71. doi: 10.1093/carcin/bgi063. [DOI] [PubMed] [Google Scholar]
- 20.Risques RA, Vaughan TL, Li X, et al. Leukocyte telomere length predicts cancer risk in Barrett's esophagus. Cancer Epidemiol Biomarkers Prev. 2007;16:2649–55. doi: 10.1158/1055-9965.EPI-07-0624. [DOI] [PubMed] [Google Scholar]
- 21.Lansdorp PM, Verwoerd NP, van de Rijke FM, et al. Heterogeneity in telomere length of human chromosomes. Hum Mol Genet. 1996;5:685–91. doi: 10.1093/hmg/5.5.685. [DOI] [PubMed] [Google Scholar]
- 22.Jeanclos E, Schork NJ, Kyvik KO, Kimura M, Skurnick JH, Aviv A. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000;36:195–200. doi: 10.1161/01.hyp.36.2.195. [DOI] [PubMed] [Google Scholar]
- 23.Slagboom PE, Droog S, Boomsma DI. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55:876–82. [PMC free article] [PubMed] [Google Scholar]
- 24.Graakjaer J, Londono-Vallejo JA, Christensen K, Kolvraa S. The pattern of chromosome-specific variations in telomere length in humans shows signs of heritability and is maintained through life. Ann N Y Acad Sci. 2006;1067:311–6. doi: 10.1196/annals.1354.042. [DOI] [PubMed] [Google Scholar]
- 25.Graakjaer J, Der-Sarkissian H, Schmitz A, et al. Allele-specific relative telomere lengths are inherited. Hum Genet. 2006;119:344–50. doi: 10.1007/s00439-006-0137-x. [DOI] [PubMed] [Google Scholar]
- 26.Graakjaer J, Bischoff C, Korsholm L, et al. The pattern of chromosome-specific variations in telomere length in humans is determined by inherited, telomere-near factors and is maintained throughout life. Mech Ageing Dev. 2003;124:629–40. doi: 10.1016/s0047-6374(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 27.Andrew T, Aviv A, Falchi M, et al. Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. Am J Hum Genet. 2006;78:480–6. doi: 10.1086/500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangino M, Brouilette S, Braund P, et al. A regulatory SNP of the BICD1 gene contributes to telomere length variation in humans. Hum Mol Genet. 2008;17:2518–23. doi: 10.1093/hmg/ddn152. [DOI] [PubMed] [Google Scholar]
- 29.Vasa-Nicotera M, Brouilette S, Mangino M, et al. Mapping of a major locus that determines telomere length in humans. Am J Hum Genet. 2005;76:147–51. doi: 10.1086/426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mangino M, Richards JB, Soranzo N, et al. A genome-wide association study identifies a novel locus on chromosome 18q12.2 influencing white cell telomere length. J Med Genet. 2009;46:451–4. doi: 10.1136/jmg.2008.064956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Codd V, Mangino M, van der Harst P, et al. Common variants near TERC are associated with mean telomere length. Nat Genet. 2010;42:197–9. doi: 10.1038/ng.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy D, Neuhausen SL, Hunt SC, et al. Genome-wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc Natl Acad Sci U S A. 2010;107:9293–8. doi: 10.1073/pnas.0911494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–82. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Spitz MR, Amos CI, Wilkinson AV, Wu X, Shete S. Mediating effects of smoking and chronic obstructive pulmonary disease on the relation between the CHRNA5-A3 genetic locus and lung cancer risk. Cancer. 2010;116:3458–62. doi: 10.1002/cncr.25085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clague J, Lin J, Cassidy A, et al. Family history and risk of renal cell carcinoma: results from a case-control study and systematic meta-analysis. Cancer Epidemiol Biomarkers Prev. 2009;18:801–7. doi: 10.1158/1055-9965.EPI-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purdue MP, Johansson M, Zelenika D, et al. Genome-wide association study of renal cell carcinoma identifies two susceptibility loci on 2p21 and 11q13.3. Nat Genet. 2011;43:60–5. doi: 10.1038/ng.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amos CI, Wu X, Broderick P, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40:616–22. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu X, Ye Y, Kiemeney LA, et al. Genetic variation in the prostate stem cell antigen gene PSCA confers susceptibility to urinary bladder cancer. Nat Genet. 2009;41:991–5. doi: 10.1038/ng.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horikawa Y, Wood CG, Yang H, et al. Single nucleotide polymorphisms of microRNA machinery genes modify the risk of renal cell carcinoma. Clin Cancer Res. 2008;14:7956–62. doi: 10.1158/1078-0432.CCR-08-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kenny DA. Mediation. Available at: http://davidakenny.net/cm/mediate.htm#SE.
- 43.Herr NR. Mediation with dichotomous outcomes. Available at: http://nrherr.bol.ucla.edu/Mediation/logmed.html.
- 44.Mackinnon DP, Dwyer JH. Estimating Mediated Effects in Prevention Studies. Evaluation Review. 1993;17:144–58. [Google Scholar]
- 45.Nordfjall K, Eliasson M, Stegmayr B, Melander O, Nilsson P, Roos G. Telomere length is associated with obesity parameters but with a gender difference. Obesity (Silver Spring) 2008;16:2682–9. doi: 10.1038/oby.2008.413. [DOI] [PubMed] [Google Scholar]
- 46.Pavanello S, Pesatori AC, Dioni L, et al. Shorter telomere length in peripheral blood lymphocytes of workers exposed to polycyclic aromatic hydrocarbons. Carcinogenesis. 2010;31:216–21. doi: 10.1093/carcin/bgp278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–4. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 48.Bekaert S, De Meyer T, Rietzschel ER, et al. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6:639–47. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 49.Baird DM. Variation at the TERT locus and predisposition for cancer. Expert Rev Mol Med. 2010;12:e16. doi: 10.1017/S146239941000147X. [DOI] [PubMed] [Google Scholar]
- 50.Petersen GM, Amundadottir L, Fuchs CS, et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet. 2010;42:224–8. doi: 10.1038/ng.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rafnar T, Sulem P, Stacey SN, et al. Sequence variants at the TERT-CLPTM1L locus associate with many cancer types. Nat Genet. 2009;41:221–7. doi: 10.1038/ng.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shete S, Hosking FJ, Robertson LB, et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet. 2009;41:899–904. doi: 10.1038/ng.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stacey SN, Sulem P, Masson G, et al. New common variants affecting susceptibility to basal cell carcinoma. Nat Genet. 2009;41:909–14. doi: 10.1038/ng.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y, Broderick P, Webb E, et al. Common 5p15.33 and 6p21.33 variants influence lung cancer risk. Nat Genet. 2008;40:1407–9. doi: 10.1038/ng.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Strelow A, Kollewe C, Wesche H. Characterization of Pellino2, a substrate of IRAK1 and IRAK4. FEBS Lett. 2003;547:157–61. doi: 10.1016/s0014-5793(03)00697-5. [DOI] [PubMed] [Google Scholar]
- 56.Yu KY, Kwon HJ, Norman DA, Vig E, Goebl MG, Harrington MA. Cutting edge: mouse pellino-2 modulates IL-1 and lipopolysaccharide signaling. J Immunol. 2002;169:4075–8. doi: 10.4049/jimmunol.169.8.4075. [DOI] [PubMed] [Google Scholar]
- 57.Moynagh PN. The Pellino family: IRAK E3 ligases with emerging roles in innate immune signalling. Trends Immunol. 2009;30:33–42. doi: 10.1016/j.it.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 58.Masi S, Salpea KD, Li K, et al. Oxidative stress, chronic inflammation, and telomere length in patients with periodontitis. Free Radic Biol Med. 2010 Dec 30; doi: 10.1016/j.freeradbiomed.2010.12.031. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 59.Houben JM, Mercken EM, Ketelslegers HB, et al. Telomere shortening in chronic obstructive pulmonary disease. Respir Med. 2009;103:230–6. doi: 10.1016/j.rmed.2008.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.