Background
Aging and Angiogenesis
Aging is associated with an increased risk for development of coronary and peripheral artery diseases [1]. The extent of ischemic damage and functional recovery in case of extensive impairment of vascularization due to arterial obliteration in either circulatory system largely depends on the development of new collateral blood vessels. Aging is also accompanied by a steady decline in immune functions that include but are not limited to defects in signaling pathways and altered cytokine (IFNγ, VEGF) expression [2–4]. Age-related impairment of angiogenesis has been documented in many previous studies [5–9]. Deficiencies in multiple components of ischemia-induced neovascularization including but not limited to inhibition of EC proliferation and function [7, 10–12], impaired expression of angiogenic growth factors, such as VEGF, bFGF, TGF-β, and PDGF [7, 13–15], were implied in delayed or failed angiogenesis in adult tissue. In addition, some recent studies have also demonstrated significant contribution of ischemia-induced inflammatory responses to delayed cutaneous wound healing with age [16, 17].
TNF, TNF Receptors, and Angiogenesis
Angiogenesis is accompanied by perivascular inflammation and monocyte/macrophage accumulation [18]. Tumor necrosis factor alpha (TNF-α), a macrophage/monocyte-derived pluripotent mediator, can function as an angiogenic factor in one system and as an anti-angiogenic factor in another system [19–22]. These mutually exclusive TNF-α effects were attributed to TNF-α concentration and duration of the exposure, that is, low concentrations and short exposure is angiogenic, whereas high concentrations and prolonged exposure is anti-angiogenic [23]. Interestingly, impaired TNF-α and other cytokine signaling in EC were correlated with enhanced apoptotic response in cutaneous microvasculature in adult tissue [24]. TNF-α has been reported to induce the expression of many immunologically relevant and angiogenesis-related genes [25, 26], through two different TNF-α receptors, TNF-αR1 (p55) and TNF-αR2 (p75) [27–29]. In ECs, TNF-α increases the expression of well-known angiogenic factors VEGF, bFGF, IL-8 [26]; however, the role of two distinct TNF-α receptors in mediating these responses is still unclear. Although the distribution of p55 is more widespread, p75 is present in greater amounts on endothelial and hematopoietic lineage cells [30]. Because p55 is largely known to mediate cytotoxic effects of TNF-α, whereas signaling through p75 is mostly implied in protective effects of TNF-α [31, 32], and because aging is associated with increased expression of p55 and decreased expression of p75 in lymphocytes from aged humans [33] we hypothesize that TNF signaling via its receptors may play significant and, perhaps, divergent effects in neovascularization, repair, and regeneration in adult tissue after acute myocardial infarction (AMI).
Aging, TNF Signaling, and Post-ischemic Recovery
Numerous reports suggest that VEGF and bFGF are critical growth factors in therapeutic angiogenesis [34–37]. Because treatment with TNF activates transcription factor NFκB and NFκB is known to regulate, both, VEGF and bFGF expression [26, 38], it is conceivable that impairment in TNF signaling may affect adversely post-ischemic recovery. Hence, it is possible that age-associated changes in TNF signaling may influence post-ischemic recovery processes in several ways. First, decreases with age expression of TNFR2/p75 coupled with post-ischemic increases in the systemic levels of TNF may favor apoptosis in adult cardiomyocytes and resident ECs [39] through impaired p75-mediated anti-apoptotic signaling via NFκB, subsequently leading to inhibition of angiogenesis. It is also conceivable that in adult tissue with decreased p75 receptor expression in general [40] unopposed signaling through p55 receptor increases predominantly pro-apoptotic cascade via increase in FADD, TRADD, and FASDD [33, 40]. Furthermore, it is also likely that previously reported decrease in VEGF expression in adult cells [2–4] may be a direct consequence of age-associated decrease in the expression/signaling via p75. In this case, not apoptosis, but rather direct inhibition of TNF-mediated angiogenic signaling (TNF→NFκB→VEGF, bFGF) could be responsible for failure of post-ischemic recovery and regeneration in adult tissue. Potential scenario could be as follows: in young tissue intact signaling through both p55 and p75 receptors assures proper NFκB activation, followed by transcriptional activation of VEGF and bFGF and other TNF-induced NFκB-mediated pro-angiogenic genes (i.e., IL-8), whereas in cells from adult donors with decreased p75 receptor expression [33, 40] signaling through p55 receptor alone is not sufficient for proper NFκB activation [41] and regulation of transcription of pro-angiogenesis genes which, at least in part, may be responsible for impaired angiogenic response in adult tissue [5–9]. Finally, VEGF, which is an early angiogenic growth factor response to ischemia [42], has been shown to mobilize BM-derived EPCs in murine models and in humans [43, 44]. Hence, age-associated decrease in TNF-induced NFκB-mediated VEGF expression may be due to, in part, decreased p75 receptor expression, which may lead to subsequent decrease in mobilization and/or recruitment of BM-derived EPCs to the ischemic areas [41], thereby representing an additional mechanism of impaired repair, angiogenesis, and regeneration in adult coronary tissue.
Results
Constitutive Expression of p75 Is Decreased in Human PB EPCs from Donors of Increasing Age
We and others have previously reported that there is an inverse relationship between increased age and decreased angiogenesis [5, 7, 8]. Interestingly, similar inverse relationship between increased age and decreased expression of TNFR2/p75 has been reported, in contrast to increased levels of TNFR1/p55 in lymphocytes from adult donors [33]. To evaluate age-associated changes in TNFR1 and 2 levels in human ECs we measured p55 and p75 mRNA expression levels (using ribonuclease protection assay) in human PB EPCs isolated from donors of increasing age. There was no significant change in the levels of p55 expression in EPCs from elderly donors compared to more than 20 year younger adults (Fig. 45.1a). In contrast, there was more than 55% (p<0.01) decrease in p75 mRNA levels in EPCs from elderly donors vs. younger adults (Fig. 45.1b).
Fig. 45.1.
p75 TNFR2 expression is decreased in peripheral blood EPCs from elderly donors. Human adult PB EPCs were collected and processed for evaluation of constitutive mRNA expression of TNF receptors p55 and p75 by ribonuclease protection assay. We divided (arbitrary) our donors into two groups: younger than 55 and older than 79 (n=5/age group). (a) Densitometric analysis of p55 TNFR1 mRNA expression in human adult PB EPCs. RDU –relative densitometric units. (b) Densitometric analysis of p75 TNFR2 mRNA expression in human adult PB EPCs
Post-AMI Survival and Functional Myocardial Recovery Is Impaired in Old p75KO Mice
It was reported previously that TNF receptor knockout (KO) mice reveal exacerbated damage and altered NFκB activation after ischemic injury [45]. We hypothesized that signaling through p75 may be essential in pro-survival signaling in adult tissue. To examine whether age-associated decreases in p75 expression may contribute to the failure of post-AMI recovery in adults we evaluated survival, cardiac function, angiogenesis, and recovery in a series of experiments in murine model of acute myocardial infarction (AMI) [ligation of the left anterior descending (LAD) coronary artery] in young and old p75KO and p55KO mice and age-matched wild-type (WT) controls. Overall surgical mortality death (within 24 h post-surgery) was <4% and these animals were excluded from the study, because surgical and anesthesia errors could not be ruled out as the cause of death. The rest of all the deaths occurred within 1 week post-AMI, and although not all mice hearts that died within 7 days were examined, of those that were examined (~75%), most showed evidence of cardiac rapture in peri-infarct myocardium and some revealed pericarditis (data not shown). There was significant age-associated increase in post-AMI mortality in old WT mice (12% vs. 45% in young vs. old WTs) (Fig. 45.2a). Not surprisingly, post-AMI mortality in young p75KO mice was approaching the mortality in old WT mice (35% vs. 45% in young p75KO vs. old WTs) (Fig. 45.2a), suggesting development of an aging phenotype in young p75KO mice. Interestingly, more than half of the mice in old p75KO group (60%) died within 7 days post-AMI (Fig. 45.2a). In contrast, no post-AMI mortality was observed in young p55 mice and post-AMI mortality in old p55KO was only 10%, approximately the same as in young WTs (Fig. 45.2a). These latter data strongly suggest that lack of signaling through p55 appears to improve significantly post-AMI survival (better than in WT mice) in young p55KO, as well as in old p55KO mice (recovery in old p55KOs was as good as in young WTs).
Fig. 45.2.
(a) Post-AMI mortality is increased in WT old and TNFR2/p75 young and old mice and decreased in young and old TNFR1/p55 mice. Compared to WT young (6–8 weeks) mice within 7 days there was approximately threefold increase in post-MI mortality in old (12–14 months) WT and young p75KO mice. Post-AMI mortality in old p75KO mice was fivefold higher compared to young WT mice. No post-AMI mortality was observed in young p55 mice and post-AMI mortality in old p55KO was only 10%, approximately the same as in young WTs. (b) Impaired signaling through TNFR2/p75 worsens age-related decrease in LV FS in old p75KO mice, whereas signaling through TNFR1/p55 may be harmful for post-AMI recovery in murine model of AMI. No significant difference in LV FS was observed between mice of different genotypes before surgery. LV FS was significantly (p<0.05 day 7, and p=NS day 28) decreased in old WT and young p75KO mice. The worst recovery was observed in old p75KO mice. LV FS was significantly (p<0.05 day 7, and p=NS day 28) decreased in old p55KO vs. young p55KO on day 28 post-AMI, suggesting some age-associated impairment in post-MI recovery in old p55KO mice, although this degree of recovery was as good as in young WT mice
For evaluation of infarct size and left ventricular (LV) dimensions transthoracic echocardiography was performed before surgery (LAD ligation), 7 and 28 days after surgery. We measured LV circumference in systole (LVs) and diastole (LVd) and these values were used to calculate change in fractional area using the following formula: [% LV FS=(LVd − LVs/LVd) × 100]. We also calculated infarct size in the same view by measuring infarct segment length and dividing it by diastolic circumference (× 100). For both, change in fractional area and infarct size, we measured all parameters three times and mean of these values were used to make comparisons. Compared to young WT mice, between days 7 and 28 post-AMI functional recovery, as measured by percent decrease in left ventricular fractional shortening (%LV FS), was considerably worse in mice of old WT and young p75KO mice (Fig. 45.2b). LV FS (%) was significantly worse on days 7 and 28 post-AMI in old p75KO mice compared to WT young (more than 20% decrease compared (p<0.05) and WT old and p75KO young (more than 10% decrease, p<0.05) (Fig. 45.2b). Our results strongly suggest that signaling through p75 may be a critical factor for the processes of post-ischemic recovery in adult tissue. Moreover, although lack of signaling through p55 reveals age-related decrease in LV FS in old vs. young p55KO mice (Fig. 45.2b), post-MI recovery of, both, young and old p55KO mice was significantly better statistically than in, both, young and old WT and p75KO age-matched counterparts, suggesting that signaling through p55 may be harmful for post-AMI recovery, as others have also shown previously [46, 47]. Please note that because of severe age-associated deficiency in old p75KO mice compared to very mild age-associated change in p55KOs in post-AMI recovery from here on all our data will give comparisons between young and old WT and p75KO mice only. Ischemia-induced inflammatory infiltrate is increased and longer-lasting in p75KO and decreased in p55KO mice post-AMI. To quantify post-AMI inflammatory infiltrate we evaluated the expression of myeloperoxidase-1 (MPO-1), a neutrophil marker, and CD-68, a glycoprotein normally expressed on macrophages, also known in mice as macrosialin. Compared to WT mice, quantification of MPO-1 (+) cells showed ~ 313% (p75KO) and 49% (p55KO) increases on day 7, and ~ 31% (p75KO) increase and 32% (p55KO) decrease on day 14 post-AMI (p < 0.03 for all, data not shown). Compared to WT mice, quantification of CD-68 (+) cells showed ~ 5% (p75KO) and 24% (p55KO) decreases on day 7, and ~ 85% (p75KO) increase and 39% (p55KO) decrease on day 14 post-AMI (p < 0.05 for all, data not shown), suggesting that in the absence of p75 signaling post-AMI inflammatory responses are increased and longer lasting, whereas in the absence on p55 signaling inflammatory responses decreased and are of short duration.
Cardiac Troponin I (cTnI) Expression Is Increased in Infarct Border Zone in p75KO Mice
To evaluate the magnitude of myocardial infarction and ongoing myocardial injury, we immunostained sections of infarct border-zone area with cardiac troponin I (cTnI), a marker that has proven to correlate well with infarct size and ongoing myocardial injury when measured in the serum [47] as well as in immunostained tissue [48]. Compared to normal non-infarct tissue (Fig. 45.3a, upper panel), myocardial tissue expression level of cTnI on day 7 post-AMI was significantly increased in infarct border zone of young p75KO and old WT and p75KO mice (Fig. 45.3b, upper panel and lower left panel). cTnI expression levels were the highest in old p75KO mice (Fig. 45.3b, lower right panel), suggesting that extent of myocardial damage and ongoing myocardial injury is still in dynamics 7 days post-AMI in both young and old p75KO mice.
Fig. 45.3.
Cardiac troponin I (cTnI) expression is increased in infarct border zone in p75KO mice. Representative confocal images of infarct border-zone myocardium of young and old WT and p75KO mice 7 days after AMI were stained with anti-cTnI antibodies (red fluorescence). cTnI expression was low in pre-surgery samples of all genotypes (p75KO old tissue is shown) and was consistently and substantially higher in the post-AMI myocardium of old WT and young and old p75KOs on day 7 post-AMI. Post-AMI hearts of at least three animals per genotype/time point were examined and similar cTnI expression pattern, as shown in these representative images, was observed in the tissues of young and old WT and p75KOs. White dotted line divides border zone from infarct area and blue staining is Topro-3 to identify nuclei
Capillary Density Is Decreased in Infarct and Infarct Border Zone in Old WT and Young and Old p75KO Mice
As a measure of collateral blood flow recovery after ischemia we evaluated capillary density in myocardium of young and old WT and p75KO mice 28 days after AMI surgery. Compared to young WT mice there was significant decrease in capillary density (first column – green staining) and the number of functional vessels (second column – red staining) in old WT mice in infarct and infarct border zone (Fig. 45.4a, b). Compared to both young and old WT mice post-AMI capillary density (green staining) and number of functional vessels (red staining) was further decreased in young as well as old p75KO mice (Fig. 45.4c, d). These data suggest age-associated decrease in post-AMI capillary density and patent/functional vessels in old WT mice and significantly greater deficiency in collateral vessel development and decrease in functional capillary network in young and old p75KOs.
Fig. 45.4.
Capillary density and the number of functional vessels are decreased in infarct and infarct border zone in old WT and mice of p75KO genotype. Five minutes before harvesting hearts mice were perfused with fluorescently labeled BS1-lectin (red) to identify functional/perfused vessels. After harvesting and fixation heart sections were again stained with fluorescently labeled BS1-lectin (green) to identify all vessels. (a and b) Representative confocal images of infarct border-zone myocardium of young and old WT mice. Compared to young WT mice capillary density and the number of functional vessels were consistently and substantially lower in the myocardium of old WT 28 days post-AMI. (c and d) Representative confocal images of infarct border-zone myocardium of young and old p75KO mice. Compared to young and old WT mice capillary density and the number of functional vessels were consistently and substantially lower in the myocardium of young and old p75KO mice 28 days post-AMI. White dotted line divides border zone from infarct area and blue staining is Topro-3 to identify nuclei. Post-AMI hearts of at least there animals per genotype/time point were examined and similar pattern, as shown in these representative images of randomly chosen areas of 0.09 mm2 of myocardial tissue, was observed in the tissues of young and old WT and p75KO mice
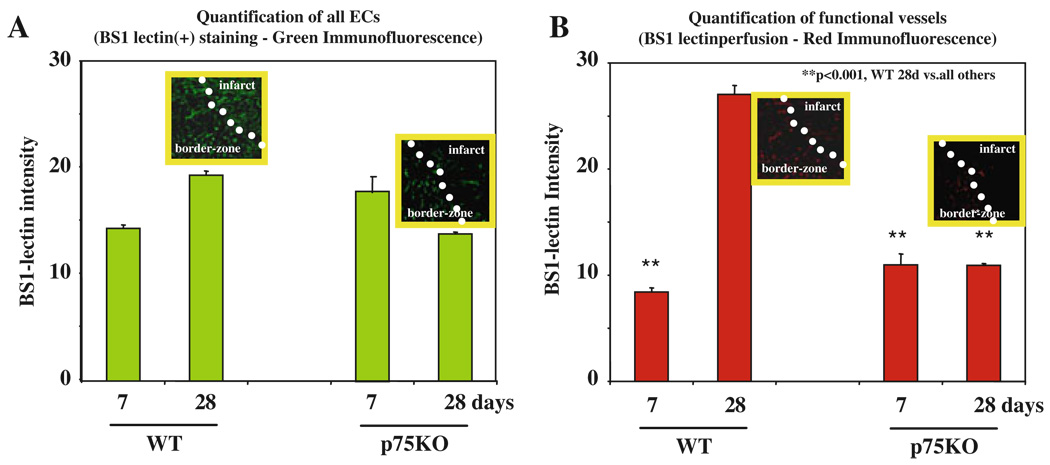
Compared to Old WT Mice Post-AMI Functional Capillary Density Is Decreased in Old p75KOs in Infarct Border Zone
We quantified the capillary density and the number of functional vessels in old WT vs. old p75KO mice. To make sure that each and every endothelial cell is counted we used a Z-stack mode of confocal microscope and took at least 10 focal images each 0.5 µm thick (data not shown). We then used computer-assisted ImageJ (NIH software) to calculate the intensity of green (all ECs) and red (functional capillaries) in infarct myocardium 7 and 28 days post-AMI. When we compared capillary density in BS1-lectin-stained samples there was no significant difference between old WT and old p75KO mice (Fig. 45.5a). Between days 7 and 28 in old WT mice there was a significant increase in the functional capillary network (8.49±1.2 vs. 27.3±3.4, days 7 vs. day 28, respectively, p<0.001) (Fig. 45.5b), suggesting development of collateral vessels in old WT mice. Compared to old WT mice, the network of functional vessels in old p75KO mice was also not significantly different between old WT and old p75KO mice on day 7 post-AMI (8.49±1.2 vs. 11.2±3.4, old WT vs. old p75KO, respectively, p=NS) (Fig. 45.5b). Interestingly, by day 28 there was approximately threefold decrease in the functional capillary network in old p75KO mice compared to old WT mice (10.9±0.7 vs. 27.3±3.4, respectively, p<0.001) (Fig. 45.5b), strongly suggesting significant deficiency in development of collateral vessels in old p75KO mice post-AMI.
Fig. 45.5.
The number of functional vessels is decreased in infarct border zone in old p75KO mice. (a) Quantification of all ECs. Insets are green BS1-lectin-positive cells; (b) quantification of functional capillary network. Insets are red BS1-lectin perfused, hence patent vessels. Compared to old WT mice capillary density and the number of functional vessels are significantly threefold lower in old p75KO mice 28 days post-AMI. Post-AMI hearts of at least three animals per genotype/time point were used to quantify the differences in capillary network between old WT and old p75KO mice
Ischemia-Induced Apoptosis Is Increased in the Myocardium of Old p75KO Mice 28 Days Post-AMI
To evaluate the apoptotic processes in infarct myocardium in young and old WT and p75KO mice we immunostained sections of infarct border-zone area for TUNEL, a marker of apoptosis, for BS1-lectin, a marker of ECs, and for TopRo3 to visualize nuclei. No apoptosis was detectable in hearts of WT or p75KO mice before AMI surgery (Fig. 45.6a, representative image of old p75KO heart). There were comparable increases in TUNEL (+) apoptotic cells (Fig. 45.6b, c, left column, green staining), as well as comparable decreases in BS1-lectin (+) cells (Fig. 45.6b, c, second column – red staining) on day 7 after AMI in ischemic myocardium of WT and p75KO old mice. By day 28, compared to old WT mice, p75KO old mice revealed extensive apoptosis in infarct area (Fig. 45.6d, e, left column – green staining) and significant decrease in BS1-lectin staining (Fig. 45.6d, e, second column – red staining), suggesting first that ischemia-induced myocardial apoptosis was augmented and long-lasting in old p75KO mice and second that there was a significant deficiency in functional collateral vessel development in old p75KO mice.
Fig. 45.6.
Ischemia-induced apoptosis is increased in the myocardium of old p75KO mice after AMI. Representative confocal images of triple-stained heart muscle of old WT and old p75KO mice to visualize TUNEL (+) cells. TUNEL (green) identifies apoptotic cells, BS1-lectin (red) identifies functional endothelial cells, Topro3 (blue) visualizes nuclei. Representative images show significant increase in the number of TUNEL (+) cells in old p75KO mice in infarct area 28 days post-AMI. Post-AMI hearts of at least three animals per genotype/time point were examined and similar pattern, as shown in these representative images of randomly chosen areas of 0.09 mm2 of myocardial tissue, was observed in the hearts of old WT and p75KO mice. White dotted line divides border zone from infarct area
Expression of Several Angiogenic Growth and Stem Cell-Derived Factors and Their Receptors Is Decreased in Peripheral Blood EPCs from Adult Donors
Finally, to determine if, in addition to decrease in expression of p75 in adult EPCs (Fig. 45.1b), there may be alterations in the expression of other genes that are involved in the processes of post-ischemic recovery, such as post-natal angiogenesis and regulation of stem and progenitor cell mobilization and recruitment to ischemic areas, we performed a series of qRT-PCR studies. We collected peripheral blood (PB) from healthy young donors (age 32±14) and from older patients (age 67±7) of our Catheterization Laboratory that came for a procedure. Please note that we did not need IRB approval for these studies because no identification of patients was required (except the age) and blood would otherwise be discarded. PB MNCs were isolated, then EPCs were grown on selective medium [41] and after being 5 days in the culture PB EPCs were harvested and processed for qRT-PCR. The expression of several potent angiogenic and pro-survival growth factors and cytokines such as VEGF-A, ANG-2, TGF-β, HIF-1α, ET-1 (Fig. 45.7a-e), as well as expression of -stromal cell-derived factors and their receptors, such as SDF-1α, CXCR4, and GCSFR (Fig. 45.7f-h) was significantly decreased (2–5-fold) in EPCs from adult patients when compared to the levels of these factors in the PB of normal volunteers. The significance of these finding may or may not be directly related or dependent on signaling through TNFR2/p75 pathway; we strongly believe that additional studies will be needed to elucidate the underlying molecular mechanisms through TNFR2/p75 that may affect expression of these genes.
Fig. 45.7.
Expression of several angiogenic growth and stem cell-derived factors and their receptors is decreased in peripheral blood EPCs from adult donors. qRT-PCR of PB EPCs from young normal volunteers vs. adult CAT lab patients. (a–h) Expression of several angiogenic growth and stromal cell factors and their receptors was decreased in PB EPCs obtained from adult patients. All experiments were conducted in triplicate. The differences were considered significant when p<0.05
Several major conclusions arise as a result of our studies: (1) expression of p75/TNFR2 is decreased in EPC from elderly donors; (2) loss of p75 expression impairs post-ischemic recovery in infarct myocardium and increases post-AMI mortality; (3) signaling via p55 TNFR1 is harmful for ischemia-induced repair and regeneration processes and absence of p55 receptor averts post-AMI mortality; (4) development of ischemia-induced functional collateral vessel network is mediated, at least in part, via p75 TNFR2; (5) with advanced age, signaling through p75 is required for collateral vessel development; (6) post-ischemic apoptotic responses in infarct myocardium are augmented in the absence of p75 TNFR2; (7) expression of several angiogenic growth and stromal cell-derived factors and their receptors is decreased in PB EPCs from adult donors.
Summary
Our data strongly suggest a critical role of TNF signaling via its receptors p55 and p75 in the processes of post-ischemic recovery in adult tissue after myocardial infarction, that is, signaling via TNFR1/p55 is deleterious and signaling via TNFR2/p75 is protective in repair and regeneration processes in adult infarct myocardium. As illustrated in Diagram 45.1a and b if occlusion of coronary artery is not resolved fast enough to prevent development of myocardial infarction a cascade of pathological events and morphological changes in the myocardium will develop beginning with necrosis, acute inflammation (heavy neutrophilic infiltrate), apoptosis, followed by macrophage and mononuclear infiltration to initiate fibrovascular response and granulation processes, which then may culminate in fibrosis and scar formation. As depicted in our hypothetical Diagram 45.1c we propose that therapeutic measures aimed toward either inhibition of signaling via TNFR1/p55 or overexpression of TNFR2/p75 in infarct myocardium may decrease ischemia-induced inflammatory responses, enhance neovascularization, and improve tissue regeneration, thereby minimizing myocardial fibrosis and improving the poor recovery and development of severe ischemia-induced damage in adult coronary diseases. Indeed, in support of our hypothesis several other research groups, independently of our group, had reported that myocardial injection of mesenchymal stem cells, genetically modified to overexpress TNFR2/p75, attenuates inflammation and cardiac dysfunction following myocardial infarction [49]. Another group had recently reported that signaling via both p55 and p75 is necessary to prevent reperfusion injury after AMI during late preconditioning; however, they found that only signaling through p75 is protective in non-preconditioned myocardium [50]. Because post-AMI recovery is an “evolutionary” process and inflammatory cytokine levels, specifically TNF, are significantly increased in infarct myocardium we strongly believe that further studies aimed toward modulation of TNF receptor p55 and/or p75 expression at different evolutionary stages of myocardial infarction may have important overarching implications for (1) prevention of AMI-mediated myocardial injury, (2) augmentation of myocardial repair and regeneration, and (3) improvement of therapeutic outcome of stem and progenitor cell therapy for cardiovascular diseases.
Diagram 45.1.
(a and b) Microscopic morphologic changes that evolve over time after AMI. Evolution of microscopic morphologic changes after AMI: within first 24 h – myocardial fibers become wavy, edema, hemorrhage, coagulation necrosis, and early neutrophilic infiltrate develops; 2–4 days – loss of nuclei, microscopic necrosis, heavy neutrophilic infiltrate, and dilation of vessels develops; 5–14 days – macrophage and mononuclear infiltration and fibrovascular responses begin followed by prominent granulation; 2–10 days – fibrosis with scarring develops. (c) Hypothetical effect of modulation of TNF receptor expression on AMI recovery outcome. Inhibition of p55 receptor in first hours and days after AMI may decrease ischemia-induced acute inflammation and myocardial tissue damage, whereas p75 receptor overexpression within days and weeks after AMI may enhance collateral vessel development and myocardial regeneration
Acknowledgments
This work was supported in part by grant from NIH/NIA 5R21AG026777-02 (DAG) and American Heart Association SDG Award 0630223 N (DAG) and NIH/NHBLI grant 1RO1 HL 091983 (RK).
References
- 1.Kannel WB, Gordon T. Cardiovascular risk factors in the aged: the Framingham study. In: Haynes SG, Feinleib M, editors. Epidemiology of aging. Bethesda: NIH; 1980. pp. 65–98. [Google Scholar]
- 2.Ernst DN, Weigle WO, Noonan DJ, McQuitty DN, Hobbs MV. The age-associated increase in IFN-gamma synthesis by mouse CD8+ T cells correlates with shifts in the frequencies of cell subsets defined by membrane CD44, CD45RB, 3G11, and MEL-14 expression. J Immunol. 1993;151(2):575–587. [PubMed] [Google Scholar]
- 3.Rivard A, Asahara T, Takahashi T, Chen D, Isner JM. Contribution of endothelial progenitor cells to neovascularization (vasculogenesis) is impaired with aging. Circulation. 1998;98 I–39. [Google Scholar]
- 4.Saini A, Sei Y. Age-related impairment of early and late events of signal transduction in mouse immune cells. Life Sci. 1993;52(22):1759–1765. doi: 10.1016/0024-3205(93)90464-e. [DOI] [PubMed] [Google Scholar]
- 5.Edelberg JM, Reed MJ. Aging and angiogenesis. Front Biosci. 2003;8:s1199–s1209. doi: 10.2741/1166. [DOI] [PubMed] [Google Scholar]
- 6.Marinho A, Soares R, Ferro J, Lacerda M, Schmitt FC. Angiogenesis in breast cancer is related to age but not to other prognostic parameters. Pathol Res Pract. 1997;193(4):267–273. doi: 10.1016/S0344-0338(97)80003-9. [DOI] [PubMed] [Google Scholar]
- 7.Rivard A, Fabre JE, Silver M, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99(1):111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 8.Sadoun E, Reed MJ. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J Histochem Cytochem. 2003;51(9):1119–1130. doi: 10.1177/002215540305100902. [DOI] [PubMed] [Google Scholar]
- 9.Yamaura H, Matsuzawa T. Decrease in capillary growth during aging. Exp Gerontol. 1980;15(2):145–150. doi: 10.1016/0531-5565(80)90086-8. [DOI] [PubMed] [Google Scholar]
- 10.Mogford JE, Tawil N, Chen A, Gies D, Xia Y, Mustoe TA. Effect of age and hypoxia on TGFbeta1 receptor expression and signal transduction in human dermal fibroblasts: impact on cell migration. J Cell Physiol. 2002;190(2):259–265. doi: 10.1002/jcp.10060. [DOI] [PubMed] [Google Scholar]
- 11.Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152(6):1445–1452. [PMC free article] [PubMed] [Google Scholar]
- 12.Reed MJ, Corsa AC, Kudravi SA, McCormick RS, Arthur WT. A deficit in collagenase activity contributes to impaired migration of aged microvascular endothelial cells. J Cell Biochem. 2000;77(1):116–126. [PubMed] [Google Scholar]
- 13.Augustin-Voss HG, Voss AK, Pauli BU. Senescence of aortic endothelial cells in culture: effects of basic fibroblast growth factor expression on cell phenotype, migration, and proliferation. J Cell Physiol. 1993;157(2):279–288. doi: 10.1002/jcp.1041570210. [DOI] [PubMed] [Google Scholar]
- 14.Beck LS, DeGuzman L, Lee WP, Xu Y, Siegel MW, Amento EP. One systemic administration of transforming growth factor-beta 1 reverses age- or glucocorticoid-impaired wound healing. J Clin Invest. 1993;92(6):2841–2849. doi: 10.1172/JCI116904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarzani R, Arnaldi G, Chobanian AV. Hypertension-induced changes of platelet-derived growth factor receptor expression in rat aorta and heart. Hypertension. 1991;17(6 Pt 2):888–895. doi: 10.1161/01.hyp.17.6.888. [DOI] [PubMed] [Google Scholar]
- 16.Ashcroft GS, Horan MA, Ferguson MW. Aging alters the inflammatory and endothelial cell adhesion molecule profiles during human cutaneous wound healing. Lab Invest. 1998;78(1):47–58. [PubMed] [Google Scholar]
- 17.Swift ME, Burns AL, Gray KL, DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermat. 2001;117(5):1027–1035. doi: 10.1046/j.0022-202x.2001.01539.x. [DOI] [PubMed] [Google Scholar]
- 18.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.BenEzra D, Hemo I, Maftzir G. In vivo angiogenic activity of interleukins. Arch Ophthalmol. 1990;108(4):573–576. doi: 10.1001/archopht.1990.01070060121061. [DOI] [PubMed] [Google Scholar]
- 20.Frater-Schroder M, Risau W, Hallmann R, Gautschi P, Bohlen P. Tumor necrosis factor α, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci U S A. 1987;84:5277–5281. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leibovich SJ, Polverini PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987;329(6140):630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- 22.Sato N, Fukuda K, Nariuchi H, Sagara N. Tumor necrosis factor inhibiting angiogenesis in vitro. J Natl Cancer Inst. 1987;79(6):1383–1391. [PubMed] [Google Scholar]
- 23.Fajardo LF, Kwan HH, Kowalski J, Prionas SD, Allison AC. Dual role of tumor necrosis factor-alpha in angiogenesis. Am J Pathol. 1992;140(3):539–544. [PMC free article] [PubMed] [Google Scholar]
- 24.Chang E, Yang J, Nagavarapu U, Herron GS. Aging and survival of cutaneous microvasculature. J Invest Dermatol. 2002;118(5):752–758. doi: 10.1046/j.1523-1747.2002.01714.x. [DOI] [PubMed] [Google Scholar]
- 25.Kronke M, Schutze S, Scheurich P, Pfizenmaier K. TNF signal transduction and TNF-responsive genes. Immunol Ser. 1992;56:189–216. [PubMed] [Google Scholar]
- 26.Yoshida S, Ono M, Shono T, et al. Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol Cell Biol. 1997;17(7):4015–4023. doi: 10.1128/mcb.17.7.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Botchkina GI, Meistrell ME, 3rd, Botchkina IL, Tracey KJ. Expression of TNF and TNF receptors (p55 and p75) in the rat brain after focal cerebral ischemia. Mol Med. 1997;3(11):765–781. [PMC free article] [PubMed] [Google Scholar]
- 28.Hoefer IE, van Royen N, Rectenwald JE, et al. Direct evidence for tumor necrosis factor-alpha signaling in arteriogenesis. Circulation. 2002;105(14):1639–1641. doi: 10.1161/01.cir.0000014987.32865.8e. [DOI] [PubMed] [Google Scholar]
- 29.Leeuwenberg JF, van Tits LJ, Jeunhomme TM, Buurman WA. Evidence for exclusive role in signalling of tumour necrosis factor p55 receptor and a potentiating function of p75 receptor on human endothelial cells. Cytokine. 1995;7(5):457–462. doi: 10.1006/cyto.1995.0062. [DOI] [PubMed] [Google Scholar]
- 30.Schottelius AJ, Moldawer LL, Dinarello CA, Asadullah K, Sterry W, Edwards CK., 3rd Biology of tumor necrosis factor-alpha- implications for psoriasis. Exp Dermatol. 2004;13(4):193–222. doi: 10.1111/j.0906-6705.2004.00205.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76(6):959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 32.Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA, Jr, Goeddel DV. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci U S A. 1991;88(20):9292–9296. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aggarwal S, Gollapudi S, Gupta S. Increased TNF-alpha-induced apoptosis in lymphocytes from aged humans: changes in TNF-alpha receptor expression and activation of caspases. J Immunol. 1999;162(4):2154–2161. [PubMed] [Google Scholar]
- 34.Banai S, Jaklitsch MT, Shou M, et al. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation. 1994;89:2183–2189. doi: 10.1161/01.cir.89.5.2183. [DOI] [PubMed] [Google Scholar]
- 35.Isner JM, Pieczek A, Schainfeld R, et al. Clinical evidence of angiogenesis following arterial gene transfer of phVEGF165. Lancet. 1996;348:370–374. doi: 10.1016/s0140-6736(96)03361-2. [DOI] [PubMed] [Google Scholar]
- 36.Pearlman JD, Hibberd MG, Chuang ML, et al. Magnetic resonance mapping demonstrates benefits of VEGF-induced myocardial angiogenesis. Nat Med. 1995;1(10):1085–1089. doi: 10.1038/nm1095-1085. [DOI] [PubMed] [Google Scholar]
- 37.Takeshita S, Zheng LP, Brogi E, et al. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest. 1994;93(2):662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryuto M, Ono M, Izumi H, et al. Induction of vascular endothelial growth factor by tumor necrosis factor alpha in human glioma cells. Possible roles of SP-1. J Biol Chem. 1996;271(45):28220–28228. doi: 10.1074/jbc.271.45.28220. [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann J, Haendeler J, Aicher A, et al. Aging enhances the sensitivity of endothelial cells toward apoptotic stimuli: important role of nitric oxide. Circ Res. 2001;89(8):709–715. doi: 10.1161/hh2001.097796. [DOI] [PubMed] [Google Scholar]
- 40.Gupta S. Tumor necrosis factor-alpha-induced apoptosis in T cells from aged humans: a role of TNFR-I and downstream signaling molecules. Exp Gerontol. 2002;37(2–3):293–299. doi: 10.1016/s0531-5565(01)00195-4. [DOI] [PubMed] [Google Scholar]
- 41.Goukassian DA, Qin G, Dolan C, et al. Tumor necrosis factor-alpha receptor p75 is required in ischemia-induced neovascularization. Circulation. 2007;115(6):752–762. doi: 10.1161/CIRCULATIONAHA.106.647255. [DOI] [PubMed] [Google Scholar]
- 42.Lee SH, Wolf PL, Escudero R, Deutsch R, Jameson SW, Ghistlethwaite PA. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. New Eng J Med. 2000;342:626–633. doi: 10.1056/NEJM200003023420904. [DOI] [PubMed] [Google Scholar]
- 43.Asahara T, Takahashi T, Masuda H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18(14):3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalka C, Masuda H, Takahashi T, et al. Vascular endothelial growth factor(165) gene transfer augments circulating endothelial progenitor cells in human subjects. Circ Res. 2000;86(12):1198–1202. doi: 10.1161/01.res.86.12.1198. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan PG, Bruce-Keller AJ, Rabchevsky AG, et al. Exacerbation of damage and altered NF-kappaB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J Neurosci. 1999;19(15):6248–6256. doi: 10.1523/JNEUROSCI.19-15-06248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monden Y, Kubota T, Inoue T, et al. Tumor necrosis factor-alpha is toxic via receptor 1 and protective via receptor 2 in a murine model of myocardial infarction. Am J Physiol. 2007;293(1):H743–H753. doi: 10.1152/ajpheart.00166.2007. [DOI] [PubMed] [Google Scholar]
- 47.Ramani R, Mathier M, Wang P, et al. Inhibition of tumor necrosis factor receptor-1-mediated pathways has beneficial effects in a murine model of postischemic remodeling. Am J Physiol. 2004;287(3):H1369–H1377. doi: 10.1152/ajpheart.00641.2003. [DOI] [PubMed] [Google Scholar]
- 48.Lin NN, Kuo JS, Cheng CC, Tung KC, Cheng FC, Chiu YT. Early cardiac damage after subarachnoid hemorrhage in rats. Int J Cardiol. 2007 doi: 10.1016/j.ijcard.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 49.Bao C, Guo J, Lin G, Hu M, Hu Z. TNFR gene-modified mesenchymal stem cells attenuate inflammation and cardiac dysfunction following MI. Scand Cardiovasc J. 2008;42(1):56–62. doi: 10.1080/14017430701543556. [DOI] [PubMed] [Google Scholar]
- 50.Flaherty MP, Guo Y, Tiwari S, et al. The role of TNF-alpha receptors p55 and p75 in acute myocardial ischemia/reperfusion injury and late preconditioning. J Mol Cell Cardiol. 2008;45(6):735–741. doi: 10.1016/j.yjmcc.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]