Abstract
Cancers originating from organs in the peritoneal cavity (e.g., ovarian, pancreatic, colorectal, gastric and liver) account for approximately 250,000 new cancer cases annually in the USA. Peritoneal metastases are common owing to locoregional spread and distant metastases of extraperitoneal cancers. A logical treatment is intraperitoneal therapy, as multiple studies have shown significant targeting advantage for this treatment, including significant survival benefits in stage III, surgically debulked ovarian cancer patients. However, the clinical use of intraperitoneal therapy has been limited, in part, by toxicity, owing to the use of indwelling catheters or high drug exposure, by inadequate drug penetration into bulky tumors (>1 cm) and by the lack of products specifically designed and approved for intraperitoneal treatments. This article provides an overview on the background of peritoneal metastasis, clinical research on intraperitoneal therapy, the pharmacokinetic basis of drug delivery in intraperitoneal therapy and our development of drug-loaded tumor-penetrating microparticles.
Keywords: intraperitoneal therapy, microparticle, peritoneal metastasis, solid tumor, tumor penetration, tumor priming
The peritoneal cavity is a common site for metastases. In general, presence of peritoneal metastasis is a poor prognoisis indicator. Peritoneal carcinomatosis or widespread peritoneal metastasis throughout the peritoneal cavity is present in end-stage disease. For advanced ovarian cancer, aggressive surgical tumor debulking combined with intraperitoneal (IP)/intravenous chemotherapy has yielded some benefits. However, for peritoneal carcinomatosis from nongynecologic malignancies, including gastric, colorectal and pancreatic cancer, the median survival time is less than 6 months [1].
Inadequate drug delivery to solid tumors is a major cause of treatment failure [2]. Following a systemic administration, drug delivery to cells in solid tumors involves three processes (i.e., transport within a vessel, such as blood circulation, transport across vasculature walls into surrounding tissues and transport through the interstitial space within a tumor). These processes are determined by the physicochemical properties of a drug or particle (e.g., molecular or particle size, diffusivity and drug binding to cellular macromolecules) and the biologic properties of a tumor (e.g., tumor vasculature, extracellular matrix components, interstitial fluid pressure, tumor cell density and tissue structure and composition). Extravasation and interstitial transport (via diffusion and convection) are diminished by high interstitial pressure, hypovascularity, high tumor cell density and/or large fraction of stroma; these problems are more serious in larger, bulky tumors [3,4]. Among the peritoneal tumors, drug delivery to pancreatic cancer is particularly problematic, owing to the high stromal fraction (>80%) and the cross-talk between tumor and stromal cells (hedgehog signaling), resulting in the sparse vasculature that is only partially functional and physically separated from cancer cells by the stroma [5,6].
Intraperitoneal therapy represents a logical alternative means of delivering high drug concentrations to tumors located in the peritoneal cavity. IP therapy has been under development for several decades. Multiple clinical studies have demonstrated that adding IP therapy to intravenous therapy produces survival benefits [7–9,201]. IP chemotherapy in patients typically uses the formulations developed for intravenous use. To date, there are no products specifically designed or approved by the US FDA for IP treatments, and IP therapy has not become a standard of care.
This article comprises five parts. Part I provides the background of peritoneal metastasis. Part II focuses on the pharmacokinetic (PK) rationale of IP therapy. Part III summarizes the clinical research on IP therapy. Part IV discusses the PK and pharmacodynamic (PD) considerations for designing drug-loaded carriers for IP therapy. Part V outlines the features and properties of tumor-penetrating microparticles (TPMs) tailored to the unique anatomical features of the peritoneal cavity.
Peritoneal metastasis
Cancer originating from organs in the peritoneal cavity (e.g., ovarian, pancreatic, colorectal, gastric, liver and peritoneal mesothelioma) account for approximately 250,000 new cases of cancer annually in the USA [10]. Peritoneal metastases are common owing to the locoregional spread (e.g., incidences of 90, 50 and 32% in ovarian, pancreatic and colon cancer, respectively). In the peritoneal cavity, movement of cells tends to follow the circulation of peritoneal fluid from the right pericolic gutter cephalad to the right hemidiaphragm. Peritoneal metastasis can also be formed due to distant metastases of extra-peritoneal cancers (e.g., pleural mesothelioma, breast and lung). Lodging of tumor cells in diaphragmatic or abdominal lymphatic ducts causes obstruction of lymphatic drainage and decreased outflow of peritoneal fluid, leading to formation of carcinomatosis or ascites [11,12]. Patients with carcinomatosis suffer from abdominal distention, loss of appetite, shortness of breath, abdominal pain, low blood pressure, weakness, fatigue and intestinal obstruction due to adhesions formed between intestinal loops [13]. The current treatment objectives for these patients are primarily palliative (e.g., pain control and repeated drainage of peritoneal fluid), and there are no meaningful therapeutic options.
Metastasis of an ovarian cancer can occur before its capsule is ruptured. Exfoliation and spreading of tumor cells can be IP and transperitoneal, and tend to follow the circulatory path of the peritoneal fluid [14–16]. Spreading through the lymphatics also occurs. In patients with advanced disease, peritoneal metastases are found in approximately 70% of cases and lymphatic dissemination to the pelvic and para-aortic lymph nodes in approximately 40% cases [17]. The most common extra-abdominal site of metastasis is the pleural space [15,18].
Peritoneal metastasis of gastric and colorectal cancer involves infiltration of the serosal layer and peritoneal implantation [19,20]. Approximately 50% of patients with serosa-invasive gastric carcinoma develop peritoneal recurrence and die of this disease during the first 2 years. In colon cancer, peritoneal metastases are also frequent in patients with recurrent disease (~40% cases).
A part of the pancreas (tail) is located in the IP space. For the remaining parts of the pancreas (head, body and neck) that are located in the retroperitoneal space, there are multiple structures (e.g., reflections and ligaments) that enable transport and metastasis between the retroperitoneal space and IP space [21–23]. Approximately 20–30% of advanced pancreatic cancer is stage III locally advanced disease, where tumors have invaded nearby organs, such as the stomach, spleen, large bowel and nearby large blood vessels or major nerves. At the later stage (stage IV), tumors invade the peritoneum and form transperitoneal metastases [24]. Peritoneal metastases are common and found in approximately 70–80% of nonresectable patients. For example, autopsy of 974 pancreatic cancer patients has established that approximately 50% of patients had peritoneal metastases at time of death, and another 20–30% patients who otherwise did not have liver or peritoneal metastases showed malignant cells in the peritoneal cavity [13].
Since patients with peritoneal metastases often also have distant metastases in systemic organs, intravenous therapy is usually the standard of care. Multiple clinical studies have shown that adding IP therapy to intravenous therapy provides additional disease control and prolongs patient survival in ovarian cancer patients with small tumors (<1 cm diameter). However, IP therapy does not appear to produce benefits in patients with larger tumors or carcinomatosis. As discussed in later sections, the lack of efficacy of IP therapy in these situations may be owing to the off-label use of intravenous drug formulations that have suboptimal PK/PD properties.
PK rationale of IP therapy
The goal of regional therapy, such as IP therapy, is to achieve high drug exposure in tumors while sparing the systemic host tissues from drug toxicity. Examples of successful regional chemotherapy include intravesical treatment of nonmuscle-invading bladder cancer, topical treatment of skin cancer, IP treatment of advanced ovarian cancer and metastatic gastrointestinal cancer, intrahepatic infusion for liver cancer and intrathecal therapy for brain cancer [25,26].
Dedrick and colleagues presented the distributed model to describe drug penetration in the peritoneum, which is perfused by capillaries [27–30]. This model assumes drug transfer across capillary-perfused tissue is determined by diffusion (Fick’s second law) and removal of drugs by capillary drainage. At steady-state conditions:
where ‘p’ is the capillary permeability and ‘a’ is the capillary surface area per unit tissue volume. The intrinsic capillary permeability of various types of mammalian muscle is similar [27–30]. This model predicts that the tissue concentration (Cx) at distance (x) from the peritoneum declines exponentially from the concentration at the surface (C0) to the averaged free-blood concentration (Cb). Half-width (w1/2) is the thickness of tissue over which the drug concentration declines by 50%. ‘Cb’ is the drug concentration in the blood perfusing the tissue, and is assumed to be equal throughout the tissue. At depths much greater than the w1/2, Cx approaches Cb. Note that a smaller w1/2 indicates a steeper concentration decline across the tissue. w1/2 is relatively insensitive to changes in molecular weight for hydrophilic compounds [27–30]. For example, w1/2 of urea (molecular weight [MW]: 60; w1/2= 95 μm) and inulin (MW: 5500; w1/2 = 143 μm) differed by a factor of only 1.5.
Following a systemic injection into a site distal to the tumor, the drug is distributed in the systemic circulation, and the tumor and normal tissues are exposed to the same AUC. Following IP treatment, the tissues in the peritoneal cavity receive relatively high drug concentrations until the drug is absorbed and distributed into the systemic circulation. The advantage of regional therapy versus systemic therapy (R) is defined as the ratio of total drug delivery to the target site. With the assumption that the only route of drug removal during IP therapy is due to drug absorption from the peritoneal cavity via passive diffusion (i.e., no clearance by lymphatic drainage), R is described by the following equation [31]:
where ‘AUCt’ and ‘AUCs’ are the areas under the curve (AUC) of the drug in the tumor compartment and in the systemic circulation. ‘P’ is the permeability coefficient of the peritoneum. ‘A’ is the surface area of the peritoneum. The product P × A equals the clearance of drug from the peritoneal cavity by absorption into the systemic circulation. This has been shown to be equal to the product of the volume of instilled fluid multiplied by the logarithmic slope of concentration decline [31]. The equation above states that the PK advantage of IP therapy is greatest for a drug with high total body clearance and/or slow absorption from the cavity. IP therapy offers little advantage and will not reduce the systemic toxicity of a compound, which is extensively and rapidly absorbed. As discussed later, lymphatic drainage is an additional clearance mechanism for large molecules or particles; this process can be incorporated in the equation above by adding a lymphatic flow-based clearance term.
Multiple studies have demonstrated significant PK advantages for IP chemotherapy in patients. The ratios of drug AUC in the peritoneal cavity and AUC in systemic blood are 12 for cisplatin, 10 for carboplatin, 65 for melphalan, 65 for etoposide, 75 for mitomycin C, 367 for 5-fluorouracil, 500 for doxorubicin, 915 for mitoxantrone and 1000 for paclitaxel dissolved in Cremophor EL® (BASF Corp., Germany)/ethanol [32–38]. However, the ratios between drug concentrations in tumors and plasma are much lower; IP treatments of cis-platin in animals yielded two- to three-times higher concentrations in tumor periphery, but no improvement in tumor center, compared with intravenous treatments [39,40]. Nonetheless, this difference appears sufficient to improve treatment efficacy, as IP cisplatin produced histologically proven complete remission in 30% of patients who failed on intravenous cisplatin [41].
The major processes for clearing drugs from the peritoneal cavity are diffusion through the peritoneal membrane and drainage via the blood and lymphatic systems. The peritoneum is a thin membrane (75 and 90 μm thick in rats and humans) [42]. Human peritoneum is highly permeable to molecules with molecular weights of less than 20 kD [43]. For absorption into blood vessels, the rate-limiting parameter is transfer across the capillary membrane for hydrophilic drugs and blood flow for lipophilic drugs. For lymphatic transport, the two most important determinants are lipophilicity and molecular/particle size; compounds with MWs greater than 500 and high lipophilicity (logPoctanol:water > 4) and particulates (e.g., liposomes and micelles) are absorbed through the lymphatic system [44]. The main lymphatic drainage from the peritoneal cavity is through the stomata on the subdiaphragmic surface, which connects to the lymphatic vessels located in deep diaphragmic tissues [45,46].
Clinical research on IP therapy
Intraperitoneal therapy has been under development for several decades; most clinical studies were conducted in patients with ovarian or gastrointestinal cancer [32–38]. IP therapy has been administered in various settings. Preoperative IP treatments are used to downstage the disease in order to facilitate surgical debulking [47,48]. Intraoperative and postoperative IP treatments are used to treat the residual small and microscopic tumors remaining after surgery, to reduce disease recurrence and to improve survival. Intraoperative treatments include hyperthermic intraoperative IP chemotherapy (HIPEC), where a solution of drugs, such as cisplatin and mitomycin C, is heated to 41–43°C and instilled into the peritoneal cavity; the drug solution is maintained for 30 min–2 h, followed by drainage [49–52]. The application of hyperthermia is to enhance drug uptake into tumors and drug efficacy [53,54]. HIPEC, owing to the poor patient tolerability, is limited to the intraoperative setting in anesthetized patients [55]. A third approach is to administer the IP therapy immediately after surgery, referred to as early postoperative IP chemotherapy (EPIC), where multiple consecutive daily doses of IP therapy are instilled into the peritoneal cavity, maintained for 4–24 h and followed by drainage [56–58]. The advantage of HIPEC and EPIC is the opportunity of attaining even drug distribution in the cavity prior to the formation of tissue adhesions due to surgery [59]. Currently, HIPEC or EPIC is used with cyto-reductive surgery for the treatment of peritoneal dissemination of gastric, colorectal and appendicle cancer [60–62]. It is unknown whether one is better than the other, since a direct comparison of HIPEC and EPIC has not been conducted yet, although retrospective analysis suggests greater effectiveness for HIPEC [63]. Another form of postoperative IP therapy, commonly used for the management of ovarian cancer patients, is to administer the therapy several weeks after surgery, after patients have recovered from the postoperative ileus or other complications. In this setting, a drug solution (usually 2 l in volume) is instilled over 30 min through an 18-G peritoneal catheter and allowed to remain in the peritoneal cavity. The drug solution is typically cleared from the cavity (e.g., for taxol, the half-life is 73.4 h), via absorption through the peritoneum and/or drainage into the lymphatics [36]. In theory, postoperative adhesions may result in uneven drug distribution, but this has not been demonstrated [64].
The survival advantage of IP therapy was first demonstrated 15 years ago, and has since been confirmed in multiple additional trials. For the treatment of peritoneal carcinomatosis of colorectal cancer origin, a combination of aggressive cytoreductive surgery with HIPEC shows substantial survival benefits compared with the standard treatment of systemic chemotherapy of 5-fluorouracil plus leucovorin with or without palliative surgery (22.3 vs 12.6 months), albeit the benefit was lessened in patients with extensive residual disease [65]. In ovarian cancer, adding IP chemotherapy to intravenous chemotherapy produces significantly longer progression-free and overall survival [7–9,201]; the most recent National Cancer Institute (NCI) Cooperative Group trial (Gynecologic Oncology Group [GOG] 172) in stage III patients with less than 1-cm tumors showed a 16-month longer overall survival. However, toxicities and other issues, discussed later, have prevented concomitant intravenous plus postoperative IP therapy becoming a standard of care [64,66].
The toxicities of IP therapy are generally related to procedures for administration and/or are drug-related, as in the case of postoperative therapy [36,67,68]. The use of an IP catheter is associated with a higher risk of infection and fever, and occasionally physical damages to peritoneal tissues (e.g., perforation). While hematologic toxicity is a major toxicity for drugs rapidly absorbed into the systemic circulation (e.g., cisplatin, carboplatin, melphalan and etoposide), local toxicity is dose-limiting for drugs that are slowly absorbed (e.g., paclitaxel, mitoxantrone and doxorubicin) or drugs that induce chemical peritonitis (e.g., mitomycin, 5-fluorouracil and oxaliplatin) or ileus (e.g., docetaxel) [33–36,67,69–74]. The GOG 172 trial showed that three-times more patients on the IP plus intravenous arm did not complete the assigned six-treatment cycle compared with the intravenous arm (58 vs 17%). For the former, 20% terminated early owing to catheter-related complications (e.g., infection, blocked or leaky catheter or port access problems), 22% owing to other toxicities (gastrointestinal, including abdominal pain or stomach cramp, dehydration, renal/metabolic or catheter-unrelated infection) and 9% due to patient refusal. The IP arm showed worse quality of life shortly after treatment (3–6 weeks), in part owing to receiving higher total drug dose from both intravenous plus IP therapy, but the difference diminished over time (e.g., after 1 year) [9].
Another major limitation of IP therapy is the lack of efficacy in larger, bulky tumors. In ovarian cancer, the postsurgical residual tumor size is the most significant prognostic indicator for IP therapy (e.g., platinum compounds, mitoxantrone, cytarabine, bleomycin, etopo-side or paclitaxel), with a better prognosis and longer survival interval in patients with smaller tumors (≤ 0.5 cm) compared with larger tumors (≥2 cm) [75–80]. These findings have led to the recommendation of using IP therapy in optimally surgically debulked stage III patients with tumors of less than 1 cm [9]. Several studies have shown that the tumor-size restriction is probably due to the inability of a drug to penetrate and/or accumulate in the tumor mass [81,82]. This notion is supported by the observations that, while cisplatin and carboplatin were approximately equally effective in ovarian cancer patients presenting with only positive margins (<0.5 cm), the analog that shows inferior penetration and seven-times lower drug levels in rodent tumors (i.e., carboplatin) also shows inferior activity in patients with larger tumors (1–3 cm) [83]. Hence, improving tumor penetration will probably improve the efficacy of IP therapy.
For patients with carcinomatosis, the recent introduction of a monoclonal antibody, catumaxomab, offers an interesting possibility. Catumaxomab, via its two binding arms specific for epithelial cell adhesion molecule (EpCAM) and CD3 (T lymphocytes) and its Fc region, binds simultaneously to tumor cells, T cells and antigen- presenting cells, and causes cell death [84,85]. Based on the statistically significant improvement of the primary end point of puncture-free survival (44 days in the catumaxomab-treated group vs 11 days in the control group treated with only paracentesis), catumaxomab received approval from the European Commission for treatment of malignant ascites [86]. Puncture-free survival is the duration over which paracentesis is not required for patient management. Whether catumaxomab can improve the management of patients with solid tumor nodules or without floating cells in the ascites fluid is not known. However, since the antitumor activity of catumaxomab relies on its ability to reach the cell surface binding sites, the well-known barriers to drug transport and delivery in solid tumors may present considerable challenges.
To date, there are no products specifically designed or approved by the FDA for IP therapy (except for catumaxomab for malignant ascites). The current practice is off-label use of drugs approved for intravenous administration. These are typically drug solutions and, as we have shown through a series of PK/PD studies described later, do not have the optimal properties for IP therapy. For example, the rapid clearance of solution from the peritoneal cavity limits the drug exposure and tumor-targeting advantage and creates the need for repeated administration (and use of an indwelling catheter), and the bolus presentation of high drug concentrations causes local toxicity.
In summary, in spite of the impressive survival advantage of IP therapy in optimally debulked stage III ovarian cancer patients, considerable debates continue on whether IP therapy should become the standard of care [64,66]. The controversies are, in large part, owing to treatment-related complications, clinicians’ lack of familiarity with catheter placement and administration techniques, the relatively demanding schedule that requires a patient to be treated on 3 separate days for each 3-week cycle and the lack of efficacy in bulky disease due to inability to penetrate large tumors. Hence, overcoming these problems is critical to gaining acceptance among patients and the medical community. These considerations motivated the development of drug-loaded TPMs that are designed to:
Have long retention in the peritoneal cavity;
Selectively adhere to tumors;
Have deep tumor penetration;
Provide instantaneous and sustained drug release to obtain optimal PD (see later section).
PK & PD considerations for designing drug-loaded carriers for IP therapy
Our laboratory has a long-standing interest in regional therapy. We have worked on intravesical therapy of nonmuscle-invading bladder cancer and have successfully translated the laboratory findings into clinical practice [87–98,202]. Although there are substantial anatomical and physiological differences between the urinary bladder and the peritoneal cavity, IP and intravesical therapy share similar PK/PD principles, and some of the lessons learned from intravesical therapy can be applied in IP therapy. The PK/PD basis for improving the efficacy of intravesical therapy has been detailed in a recent review [99]. Briefly, drug disposition in the bladder during intravesical therapy is affected by several attributes (i.e., physicochemical properties of the drug [molecular weight, hydrophilicity or lipophilicity and partition coefficient], urine volume and pH, patient hydration status and integrity of urothelium). Our laboratory developed several PK models to describe drug disposition in urine and bladder tissues in order to enable the prediction of changes in drug concentration in different parts of the bladder wall as a function of physiological, pathological or pharmacological parameters. The first set of equations describes the urine PK as functions of changes in physiological parameters that can vary from patient to patient (e.g., residual urine volume, urine pH and urine production rate) and changes in drug-related parameters (e.g., dose, dosing volume and degradation in acidic or basic environment). The second set of equations describes the drug transport in bladder tissues as a function of time and distance from the urine compartment. For this purpose, the bladder wall is divided into two sections: the urothelium (mucosa) that is not blood perfused and the submucosal and muscle layers of the bladder that contain blood vessels and lymphatics. Drug transport from the urine compartment across the urothelium is depicted by diffusion across a single homogeneous diffusion barrier – described by Fick’s first law – and drug transport across the submucosa and superficial muscle – described by the distributed model. These urine and tissue PK models jointly provide the basis for computing drug delivery to the targeted, tumor-residing sites in the bladder as a function of treatment conditions (e.g., dose, drug concentration, volume of dosing solution, patient hydration status and treatment duration) during intravesical therapy. The computed PK data were then compared with PD data, such as the effective drug concentrations in fragments of patient tumors, to predict the clinical outcome for specific treatment conditions (e.g., dose size, urine volume and pH). Computer simulations were used to compare the outcomes of seven possible changes, separately or individually. The simulation results indicated that changing one parameter at a time would yield small incremental improvements, whereas simultaneous changes in five treatment parameters would produce an improvement that is large enough to be detected with a relatively small number of patients (230 patients). The simulation results further showed that two additional changes in treatment parameters would not produce additional benefits. We next used the computer simulation results to synthesize an optimized mitomycin C treatment protocol, which was subsequently tested in a multicenter, two-arm Phase III clinical trial. The results show significant improvement in the median time to recurrence (29.2 vs 12.6 months in the standard arm with no changes; p < 0.001) and in the 5-year recurrence-free rate (44.3 vs 28.6%) [100].
We have taken similar PK/PD-based approaches in our work on IP therapy. We have completed a series of studies to address the limitations in IP therapy. As summarized later, we found that these limitations are, in part, owing to the off-label IP instillation of intravenous formulations, as these formulations are not designed for IP therapy (e.g., rapid clearance/absorption through lymphatics and the peritoneum membrane, no tumor selectivity and bolus injection of the entire dose all at once). We have since developed TPMs tailored to the unique anatomical properties in the peritoneal cavity.
PK model of disposition of IP therapy
Effects of the carrier
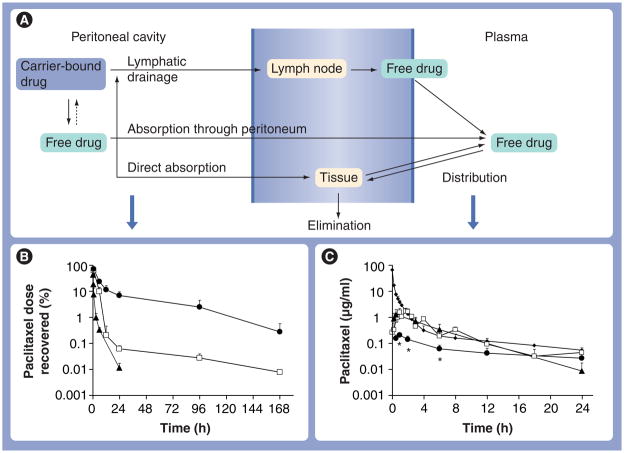
Figure 1A depicts a PK model describing the processes involved in drug disposition in the peritoneal cavity and systemic circulation after IP administration. The model accounts for clearance from the peritoneal cavity, absorption from the peritoneal cavity (through the peritoneum and lymphatics) to the systemic circulation (e.g., plasma) and elimination (first pass and systemic). The model further incorporates direct drug distribution/penetration into peritoneal organs and tissues, such as the intestines. For transport across the peritoneum, there are no known active processes; absorption (by diffusion or convection) through the peritoneum is a major pathway for small compounds with MWs of less than 20 kD [43]. Larger compounds or particulates are drained through the lymphatic ducts [101,102]. At the size range between 50 and 700 nm, the clearance of particulates from the peritoneal cavity is independent of size [102]. Within the lymphatic system, smaller particulates (<50 nm diameter) can pass through lymph nodes, while larger particulates (>500 nm) are mostly trapped in lymph nodes [102]. Hence, the model assumes that only the free drug or small nanoparticles (<50 nm) are absorbed into the systemic circulation and the surrounding organs in the peritoneal cavity. Conversely, larger particles cannot be transported across the peritoneum or through the lymphatics, and drug absorption occurs only after release from the particles.
Figure 1. Pharmacokinetic model of disposition of intraperitoneal therapy: effects of the carrier.
Three formulations of paclitaxel, paclitaxel solubilized in Cremophor EL®/ethanol (open squares), paclitaxel-loaded gelatin nanoparticles (triangles) and paclitaxel-loaded polymeric microparticles (circles) were administered by intraperitoneal injections at 10 mg/kg. For comparison, an additional group of mice received an intravenous dose of paclitaxel solubilized in Cremophor EL/ethanol (diamonds). (A) A model of kinetic processes during intraperitoneal treatment. (B & C) Paclitaxel concentration–time profiles in (B) peritoneal lavage samples and (C) plasma samples. Note the different time scales for (B) and (C). At least three mice were used for each time point. Symbols represent means ± standard deviation.
*p < 0.001 compared with other groups by one-way analysis of variance with Tukey post hoc test.
Reproduced with permission from [133].
Spatial drug distribution after IP administration
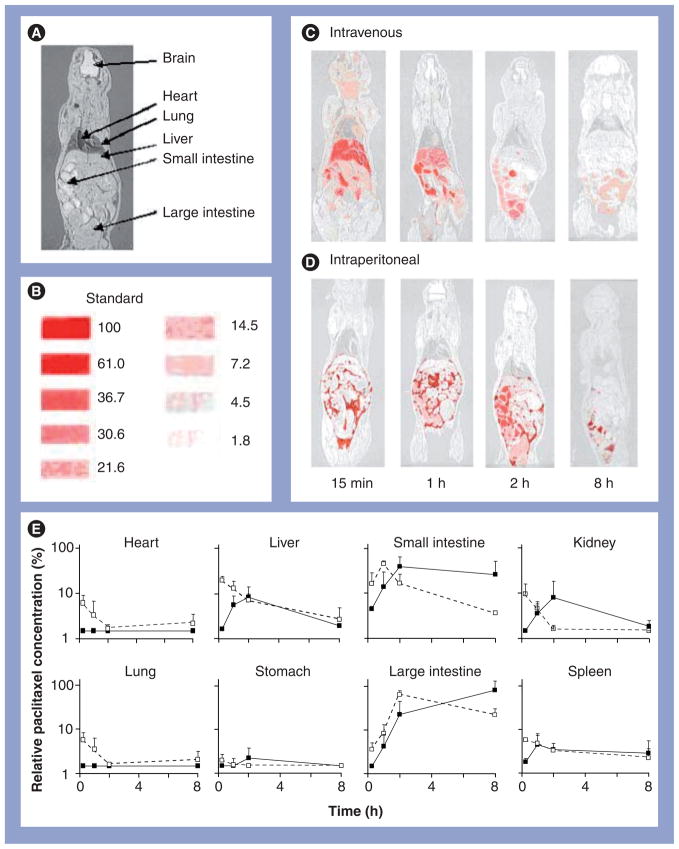
We compared the spatial distribution of 3H-paclitaxel in mice after IP or intravenous injection, using whole–body autoradiography [103]. Paclitaxel was dissolved in 50:50 Cremophor:ethanol. In Figure 2A–D, the results show a wide distribution of the intravenous dose throughout the body. By contrast, the IP dose was confined to the peritoneal cavity at all times, residing primarily in the space surrounding the visceral tissues initially, followed by appearance in the liver and intestines. These results confirm the targeting advantage of IP therapy relative to intravenous therapy. A comparison of the kinetics of the radioactivity in the liver and intestines after the two treatment routes suggests direct absorption of the IP dose into the intestines (Figure 2E), which would explain the greater gastrointestinal toxicity of IP therapy. The autoradiographic results further indicate a relatively rapid clearance of the IP dose, with most of the dose disappearing after 8 h. Based on these data, we concluded that fractionated dose presentation may reduce the intestinal toxicity, and enhancing drug retention in the peritoneal cavity may improve the efficacy of IP paclitaxel. A longer retention may also eliminate the need of using indwelling catheters. These features were captured in TPMs (see later).
Figure 2. Spatial and tissue distribution of intravenous and intraperitoneal injections of 3H-paclitaxel solubilized in Cremophor EL®/ethanol.
A mouse was administered an intraperitoneal or intravenous injection of the Cremophor formulation of paclitaxel (a mixture of radiolabeled and nonlabeled paclitaxel, equivalent to 10 mg/kg and 1 mCi/kg). (A) Whole-body section of a mouse. (B) Densitometric signals of microscale tritium standards. The numbers correspond to the relative concentrations, with the highest level set at 100%. (C) Whole-body autoradiographs at various time points after an intravenous dose. (D) Whole-body autoradiographs after an intraperitoneal dose. No radioactivity was detected in the brain following either administration route (limit of detection was 2 μg/g). (E) Relative tissue concentration–time profiles determined by digital videodensitometry after an intravenous dose (white symbols, dashed lines) or an intraperitoneal dose (black symbols, solid lines). No radioactivity was detected in the brain following either administration route. At least three mice were used for each time point. Symbols represent means ± standard deviation.
Reproduced with permission from [133].
Effects of carriers on PK/PD of IP therapy
Drug delivery systems can alter drug clearance from the peritoneal cavity and drug toxicity profiles. For example, IP injection of cisplatin-loaded polymeric microspheres (100–200 μm, which releases cisplatin over 3–4 weeks) resulted in localization of microspheres and significantly higher drug concentrations in omental tumors and improved the survival of tumor-bearing animals compared with cisplatin solution [104,105]. A study of cisplatin-loaded polylactic acid particles (50–150 μm) in 13 patients with malignant ascites derived from cancers of the digestive system suggests benefits for locoregional control of cancer [105]. Similarly, 5-fluorouracil-loaded poly(lactide-co-glycolide; PLG) microspheres, which release the drug over 3 weeks, yielded significantly higher drug concentrations in IP tissues (i.e., omentum and mesentery) compared with systemic tissues (i.e., blood, lungs and heart) [106,107]. IP paclitaxel delivered in liposomes is better tolerated compared with paclitaxel in Cremophor [108]. In addition, controlled-release formulations may reduce treatment frequency.
We extended the PK model to depict the effects of properties of drug-loaded delivery systems (i.e., kinetics of drug release and particle size) on the PK/PD of IP therapy. The model incorporates two types of drug release/reuptake mechanisms. For delivery systems that incorporate a drug through chemical manipulations, the drug will not re-enter the particles after release. For delivery systems in which a drug partitions into the particles (e.g., paclitaxel in Cremophor micelles), the uptake and release are reversible processes. To determine the effects of carriers on PK/PD of IP therapy, we used three paclitaxel formulations: gelatin nanoparticles, Cremophor micelles, and PLG microparticles. These formulations have different drug release rates and different particle sizes. The rank order of drug release was nanoparticles (100% release in 4 h under sink conditions), followed by Cremophor formulation (maintaining an equilibrium of ~10% free-drug fraction until the entire drug load is released or until depletion of Cremophor micelles), followed by microparticles (~70% in 24 h under sink conditions). The rank order of particle sizes was microparticles (~4 μm diameter), then nanoparticles (~600 nm) and then Cremophor micelles (13 nm).
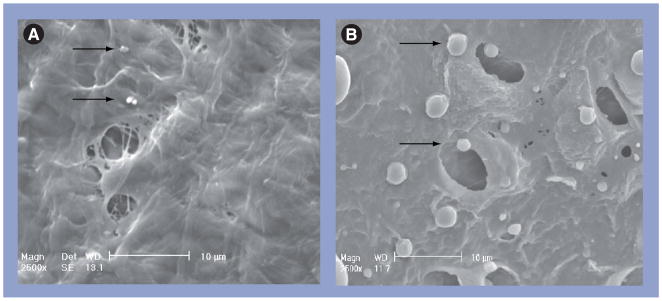
The results show rapid clearance of the Cremophor and nanoparticle formulations from the peritoneal cavity, with less than 0.1% of the dose remaining after 24 h, and a much longer retention and much higher peritoneal concentrations (17–700 times) for the microparticles (Figure 1B). Clearance of these three carriers, owing to their relatively large size, is primarily through the lymphatics. Lymphatic duct openings (stomata) on a mouse subdiaphragm surface show a diameter of between 3 and 4 μm (Figure 3), which explains the slower clearance of the larger microparticles (4 μm) compared with the two smaller nanosize formulations. The retention of microparticles was confirmed by comparing the clearance of IP doses of free rhodamine (dissolved in plant-based solvent [PBS]), free rhodamine plus unlabeled PLG microparticles or rhodamine-labeled microparticles; the first two groups showed undetectable fluorescence at 24 h, whereas the rhodamine-labeled microparticles showed strong fluorescence on the surface of the diaphragm, omentum and mesentery (Figure 4A).
Figure 3. Scanning electron micrographs of openings and particles on the diaphragm surface.
(A) Nanoparticles (660 nm diameter, arrows). (B) Microparticles (4 μm, arrows). Note that Cremophor® micelles (13 nm, not shown) would be approximately a fiftieth of the size of the nanoparticles.
Reproduced with permission from [133].
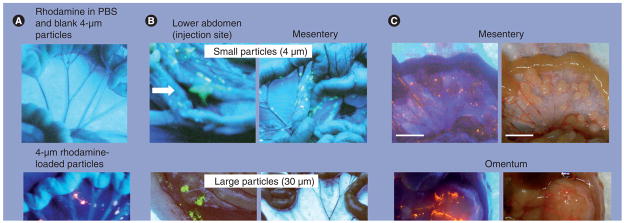
Figure 4. Intra-abdominal distribution of polymeric microparticles.
(A) Distribution. Tumor-free mice were given intraperitoneal (IP) injections of rhodamine dissolved in vehicle (0.01% Tween 80 in phosphate buffer solution) plus blank microparticles (top panel) or rhodamine-labeled microparticles (bottom panel). Rhodamine appears red under ultraviolet (UV) light. (B) Effect of particle size. Tumor-free mice were administered IP injections of acridine orange-labeled microparticles with average diameters of 4 or 30 μm. Acridine orange appears yellow under UV light. The smaller particles were dispersed throughout the cavity and on mesenteric membrane and omentum, which are common sites of local metastases of ovarian tumors. The larger particles were localized in the lower abdomen and were absent on mesenteric membrane and omentum. Arrows indicate the injection sites. (C) Localization of 4-μm particles on tumors. Mice were implanted with IP human ovarian SKOV3 xenograft tumors. After tumors were established (day 42), a mouse was administered an IP dose of rhodamine-labeled microparticles. At 3 days later, the animal was anesthetized and the abdominal cavity exposed. Photographs were taken in the region of the omentum and mesentery under UV light (left panels) and room light (right panels). Note the large tumor on the omentum (~13 mm [longest diameter]) and multiple small tumors on the mesenteric membrane (1–3 mm [longest diameter]). Red color under UV light indicated localization of rhodamine-labeled particles on the tumor surface. PBS: Plant-based solvent.
Reproduced with permission from [130].
The effect of drug release rates on peritoneal clearance is revealed by comparing the retention of the Cremophor and gelatin nanoparticles (Figure 1B). The more rapid clearance of gelatin nanoparticles with the approximate ten-times more rapid release, in spite of its 50-times larger size, indicates drug release is rate-limiting for the clearance of particles with a size smaller than the lymphatic openings.
Consistent with the more rapid clearance from the cavity, the Cremophor and nano-particle formulations yielded five-times more rapid/extensive absorption into the circulating blood (Figure 1C). The lower systemic absorption and higher peritoneal retention of the micro-particles resulted in 12–46-times greater peritoneal targeting advantage. These results indicate the choice of carriers affects the PK and, consequently, the PD of IP therapy.
Effects of particle size on spatial distribution in the peritoneal cavity
Next, we studied the effect of particle size on distribution (4 and 30 μm, labeled with acridine orange). The smaller particles were widely dispersed throughout the cavity, including the omentum, mesentery, diaphragm and lower abdomen, whereas the larger particles were primarily localized in the lower abdomen near the injection site (Figure 4B). The localization of the large particles in the lower abdomen may explain the observation that IP administration of a polymeric paclitaxel formulation (Paclimer®; 53 mm diameter) to ovarian cancer patients resulted in presence of inflammatory cells and polymer filaments in the lower part of the abdominal cavity [109].
Design & properties of tumor-penetrating microparticles
Based on the aforementioned PK/PD considerations, we elected a particle size of 4–6 μm to retard the lymphatic clearance and to promote distribution within the peritoneal cavity. TPMs have several additional features that enhance its tumor selectivity, promote its penetration into peritoneal tumors, reduce its host toxicity and provide rapid onset, as well as sustained anti-tumor activity. The preclinical results obtained thus far confirm that these features collectively result in greater therapeutic indices and eliminate the need for frequent treatments.
Tumor-penetrating microparticles use bio-compatible and biodegradable polymers. TPMs consist of PLG copolymers. Synthetic polymers, including polylactide, polyglycolide and PLG, have been used since the 1960s in a variety of biomedical devices, such as surgical sutures, implants, microspheres and nanoparticles [110–119]. PLG copolymers do not induce inflammation or toxicity, and break down to biocompatible and progressively smaller compounds (i.e., lactic acid and glycolic acid), which are further metabolized to carbon dioxide and water. Locoregional administration of PLG copolymer is generally well tolerated in humans; intramuscular administration of PLG microparticles elicited mild tissue responses followed by complete recovery [120,121]. Lupron Depot® (Abbott Laboratories, IL, USA) is an FDA-approved PLG-based drug delivery system for the treatment of advanced prostate cancer [203].
TPMs adhere to the tumor surface
Figure 4C shows that TPMs adhere to the tumor surface and are visibly absent on the surface of peritoneum and other IP organs, indicating preferential adherence of TPM to tumors. The selective tumor-adhering property of TPMs may be a result of interactions between PLG and tumor surface. Other carriers, such as activated carbon particles, also showed selective adherence to the surface of IP Yoshida sarcoma [122].
Tumor priming
During IP therapy, drug delivery to peritoneal tumors is from two sources. Recirculation of drug absorbed from the peritoneal cavity via the systemic circulation is a minor source, owing to the relatively low concentration in blood. The primary source is drug diffusion or convection through the interstitial space within a tumor mass. Hence, tumor priming, a technology that uses an apoptosis-inducing drug to expand the interstitial space, will promote the delivery and transport in the interstitium. We have shown that tumor priming with paclitaxel or doxorubicin reduces tumor cell density, expands interstitial space, decompresses tumor microvessels and enhances extravasation and convection-mediated transport, thereby improving drug and nanoparticle penetration and dispersion in solid tumors and, consequently, treatment efficacy [123–129]. Tumor priming is tumor selective owing to the greater susceptibility of tumor cells to apoptosis compared with normal cells [127].
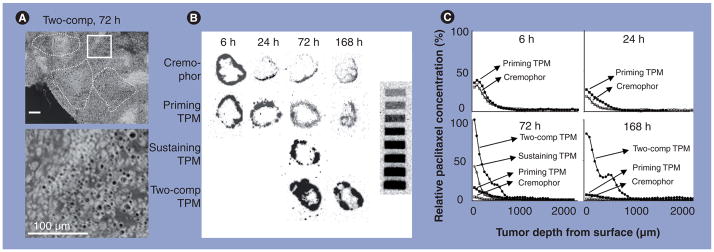
The design of TPMs is partly based on the tumor priming concept. TPMs consist of two components. One component releases paclitaxel rapidly (70% of the drug load in 1 day) to induce apoptosis (priming component), thereby promoting the penetration of the remaining particles. The second component releases paclitaxel slowly (1% per day), and thereby provides sustained drug levels to achieve an extended antitumor effect (sustaining component). These features offer several advantages over the Cremophor formulation that has been used in IP therapy to treat ovarian cancer patients. First, TPMs (priming component) produce significantly greater and more sustained priming, resulting in deeper penetration, wider dispersion and greater total uptake of micron-sized drug-free fluorescent latex beads (Figure 5A). Second, TPMs yielded higher and more sustained paclitaxel levels (fourfold higher Cmax and 16-fold higher AUC) (Figure 5B & C). As discussed later, these properties improve efficacy and reduce toxicity.
Figure 5. Effect of tumor priming on spatial drug distribution in tumors: autoradiographic results.
Mice bearing intraperitoneal SKOV3 tumors were administered intraperitoneal injections of either paclitaxel/Cremophor®, priming TPMs or sustaining TPMs (all at 20 mg/kg) or two-component TPMs (40 mg/kg, 1:1 priming:sustaining). (A) TPM penetration into tumor interior. An omental tumor was removed from a mouse at 72 h after treatment with two-component TPMs and sectioned and stained with hematoxylin and eosin. The image was converted using Photoshop®, and TPMs appeared as black dots. The top panel shows areas with clusters of TPMs (circumscribed with dotted lines). The bottom panel shows the enlarged picture of the boxed area. (B) Autoradiograms of tumor sections. (C) Concentration–depth profiles. Autoradiograms shown in (B) were processed to obtain measurements of total radioactivity using computer-assisted densitormetric analysis. Radioactivity was expressed as paclitaxel equivalents, with the highest level set at 100%. comp: Component; TPM: Tumor-penetrating microparticle.
Reproduced with permission from [130].
TPMs provide fractionated dose presentation
From the PD standpoint, the rate of drug presentation should be optimized so that the drug level in tumors is:
High enough to provide adequate control of the disease but at the same time below the threshold for producing significant local toxicity;
Sufficient to control tumors with different growth characteristics as is often found in human tumors.
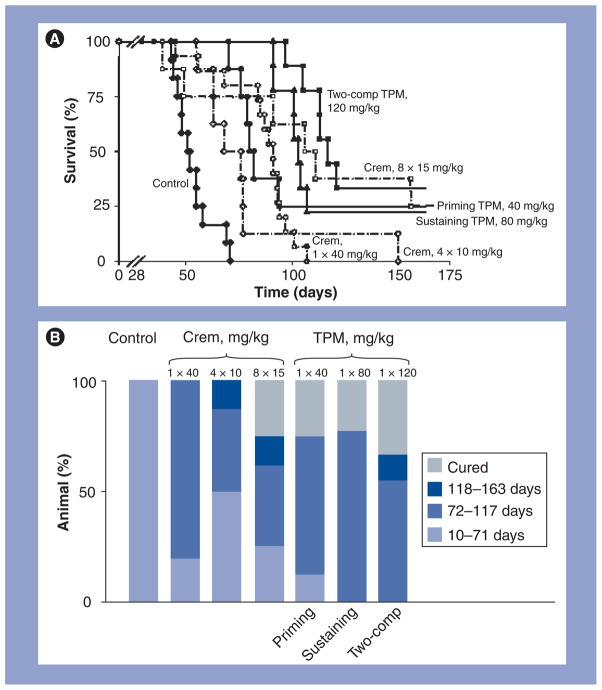
The two-component feature of TPMs provides fractionated drug delivery. The first component releases a small fraction of the dose rapidly to produce tumor priming. The second component releases the remainder of the dose slowly and provides sustained antitumor activity. The fractionated dose presentation is important to minimize the local toxicity that could result from bolus presentation of the entire dose all at once. The sustained release is important to eliminate the need of frequent treatments. These expectations have been confirmed by studies in tumor-bearing animals (Figure 6). Fractionated dose presentation has a further theoretical advantage of improving the control of rapidly and slowly growing tumors.
Figure 6. Antitumor activity of tumor-penetrating microparticles.
(A) Kaplan–Meier plot. (B) Distribution of time of deaths. Mice were implanted with 20 × 106 SKOV3 cells intraperitoneally on day 0. At 28 days later, mice were treated with physiologic saline (control: n = 12, solid diamonds, solid line), a single dose of 40 mg/kg paclitaxel/Crem (n = 15; open circles, broken line), four doses of 10 mg/kg paclitaxel/Crem twice weekly (n = 8; open diamonds, broken line), eight doses of 15 mg/kg paclitaxel/Crem twice weekly (n = 8; open squares, broken line), a single dose of priming TPMs (40 mg/kg paclitaxel; n = 8; solid circles, solid line), a single dose of sustaining TPMs (80 mg/kg paclitaxel; n = 9; solid triangles, solid line) or a single dose of two-comp TPMs (120 mg/kg paclitaxel, 1:2 priming:sustaining; n = 9; solid squares, solid line). Two animals in single-dose paclitaxel/Crem died within 10 days after treatments and were censored. Animals remaining at the end of experiments (between 163 and 174 days) were euthanized; these include two mice in the priming TPM group, two in the sustaining TPM group, three in the two-comp TPM group and two in the eight 15 mg/kg paclitaxel/Crem dose group. None of these animals showed visible tumors in the peritoneal cavity and were considered long-term cures.
Comp: Component; Crem: Cremophor; TPM: Tumor-penetrating microparticle.
Reproduced with permission from [130].
TPMs are less toxic compared with the intravenous solution formulation
Previous clinical studies of IP paclitaxel used the formulation approved for intravenous administration (i.e., paclitaxel dissolved in 50:50 Cremophor:ethanol). For the latter, the entire dose is administered all at once. As shown in Figure 2, peritoneal tissues are bathed in the drug solution, resulting in appreciable drug concentrations in the intestines. This problem is minimized by two features of TPMs: tumor-selective adherence and fractionated dose presentation. The equitoxic dose of TPMs (120 mg/kg paclitaxel-equivalent; 1:2 priming:sustaining) is three-times that of the Cremophor formulation (40 mg/kg), and the equieffective dose of TPMs (single dose of 120 mg/kg) produces less intestinal toxicity (measured as reduction of intestinal crypt-labeling index) compared with the Cremophor formulation (three daily doses of 40 mg/kg).
In mice, TPMs do not cause tissue adhesion (none observed in 26 mice) [130]. This may be because TPMs comprise low molecular mass polymers (8–40 kDa). A separate animal study evaluated the effect of PLG MW on tissue adhesion by comparing IP injections of different PLG microparticles (MW ranging from 7 to 90 kDa and diameter ranging from 5 to 250 μm); the results show higher frequency of adhesion for a high MW PLG microparticles (e.g., 90 kDa) [131]. Another possibility is that the paclitaxel in TPMs suppresses adhesion, as reported previously [132].
TPMs are more efficacious compared with the intravenous solution formulation
We compared the efficacy of TPMs and the Cremophor formulation at several doses and treatment schedules in IP human pancreatic and ovarian xenograft tumors [130,133,134]. In all circumstances, TPMs are more efficacious in reducing early death, prolonging overall survival time and increasing the cure rate. An example in the SKOV3 ovarian tumor model is shown in Figure 6. A single dose of TPMs is equally or more effective compared with multiple (four or eight) doses of the Cremophor formulation.
In summary, TPMs are designed to address the key challenges in IP therapy. Direct comparison of the paclitaxel-loaded TPMs with the Cremophor paclitaxel formulation in tumor-bearing animals demonstrated that, upon IP administration, TPMs show deeper tumor penetration, greater efficacy and lower toxicity and requires less frequent treatments.
Conclusion & future perspective
The unique anatomy of the peritoneal cavity and the nature of IP tumors (e.g., large size and widely disseminated), together with the inadequate drug delivery to IP tumors by the conventional intravenous administration route, indicate alternative treatment strategies, such as IP therapy, warrant additional research and evaluation. This article provides an overview of the history of IP therapy and the PK/PD basis for the development of TPMs. TPMs specifically tailored to the unique properties of the peritoneal cavity and IP tumors represent a potentially useful strategy for managing peritoneal tumors. TPMs have several features that offer advantages over the off-label use of drug solutions designed for intravenous administration. These features may help to eliminate the need of indwelling catheters, minimize the local toxicity and improve the compliance of patients and medical staff. The use of multiple components with different drug release rates presents an additional theoretical advantage, in that the combination of rapid and slow drug presentation enables the control of tumor cells with different growth rates. Finally, the good safety records of paclitaxel and PLG copolymers in humans support the clinical evaluation of two-component TPMs. TPMs are currently undergoing investigational new drug-enabling studies, and our plan is to evaluate TPMs in chemorefractory patients with peritoneal metastases or carcinomatosis arising from pancreatic and other gastrointestinal cancer and from ovarian cancer. As in the development of intravesical bladder cancer therapy, we are applying computation and simulations to identify the optimal treatment protocols. Demonstration of clinical efficacy of TPMs can potentially broaden the utility of IP therapy and render it as a standard of care.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This project was partly supported by grant R37CA49816, R43CA103133, R44CA103133, R43CA134047 and R0ICA123159 from National Cancer Institute, NIH, Department of Human Health and Services. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪ of considerable interest
- 1.Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88(2):358–363. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 2.Au JL, Jang SH, Wientjes MG. Clinical aspects of drug delivery to tumors. J Controlled Release. 2002;78(1–3):81–95. doi: 10.1016/s0168-3659(01)00488-6. [DOI] [PubMed] [Google Scholar]
- 3.Jain RK. Transport of molecules across tumor vasculature. Cancer Metastasis Rev. 1987;6(4):559–593. doi: 10.1007/BF00047468. [DOI] [PubMed] [Google Scholar]
- 4.Jang SH, Wientjes MG, Lu D, Au JL. Drug delivery and transport to solid tumors. Pharm Res. 2003;20(9):1337–1350. doi: 10.1023/a:1025785505977. [DOI] [PubMed] [Google Scholar]
- 5.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99(19):1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 7.Gadducci A, Carnino F, Chiara S, et al. Intraperitoneal versus intravenous cisplatin in combination with intravenous cyclophosphamide and epidoxorubicin in optimally cytoreduced advanced epithelial ovarian cancer a randomized trial of the Gruppo Oncologico Nord-Ovest. Gynecol Oncol. 2000;76(2):157–162. doi: 10.1006/gyno.1999.5677. [DOI] [PubMed] [Google Scholar]
- 8.Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19(4):1001–1007. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 9▪.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. doi: 10.1056/NEJMoa052985. Demonstrates the significant benefits of intraperitoneal chemotherapy in a randomized trial. [DOI] [PubMed] [Google Scholar]
- 10.American Cancer Society. Cancer Facts and Figures 2002. American Cancer Society; GA, USA: 2002. [Google Scholar]
- 11.Coates G, Bush RS, Aspin N. A study of ascites using lymphoscintigraphy with 99mTc-sulfur colloid. Radiology. 1973;107(3):577–583. doi: 10.1148/107.3.577. [DOI] [PubMed] [Google Scholar]
- 12.Feldman GB, Knapp RC, Order SE, Hellman S. The role of lymphatic obstruction in the formation of ascites in a murine ovarian carcinoma. Cancer Res. 1972;32(8):1663–1666. [PubMed] [Google Scholar]
- 13.del Castillo CF, Warshaw L. Peritoneal metastases in pancreatic carcinoma. Hepatogastroenterology. 1993;40(5):430–432. [PubMed] [Google Scholar]
- 14.Amadori D, Sansoni E, Amadori A. Ovarian cancer. natural history and metastatic pattern. Front Biosci. 1997;2:G8–G10. [PubMed] [Google Scholar]
- 15.Cannistra SA, Gershenson DM, Recht A. In: Ovarian Cancer, Fallopian Tube Carcinoma, And Peritoneal Carcinoma. DeVita VT, Hellman S, Rosenberg SA, editors. Lippincott Williams and Wilkins; DC, USA: 2008. pp. 1568–1594. [Google Scholar]
- 16.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351(24):2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 17.Amadori D, Sansoni E, Amadori A. Ovarian cancer. natural history and metastatic pattern. Front Biosci. 1997;2:G8–G10. [PubMed] [Google Scholar]
- 18.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351(24):2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 19.Marutsuka T, Shimada S, Shiomori K, et al. Mechanisms of peritoneal metastasis after operation for non-serosa-invasive gastric carcinoma: an ultrarapid detection system for intraperitoneal free cancer cells and a prophylactic strategy for peritoneal metastasis. Clin Cancer Res. 2003;9(2):678–685. [PubMed] [Google Scholar]
- 20.Cintron JR, Pearl RK. Colorectal cancer and peritoneal carcinomatosis. Semin Surg Oncol. 1996;12(4):267–278. doi: 10.1002/(SICI)1098-2388(199607/08)12:4<267::AID-SSU6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Raptopoulos V, Gourtsoyiannis N. Peritoneal carcinomatosis. Eur Radiol. 2001;11(11):2195–2206. doi: 10.1007/s003300100998. [DOI] [PubMed] [Google Scholar]
- 22.Van Hoe L, Claiken B. The pancreas: normal radiological anatomy and variants. In: Baert AL, editor. Radiology of the Pancreas. Springer; NY, USA: 1999. pp. 19–68. [Google Scholar]
- 23.Meyers MA. In: Dynamic Radiology of the Abdomen: Normal and Pathologic Anatomy. Meyers MA, editor. Springer; NY, USA: 2000. [Google Scholar]
- 24.Douglass HO, Jr, Penetrante RB. Pancreatic cancer. Why patients die. Int J Pancreatol. 1990;7(1–3):135–140. doi: 10.1007/BF02924230. [DOI] [PubMed] [Google Scholar]
- 25.Otto SE. Advanced concepts in chemotherapy drug delivery: regional therapy. J Intraven Nurs. 1995;18(4):170–176. [PubMed] [Google Scholar]
- 26.Collins JM. Pharmacologic rationale for regional drug delivery. J Clin Oncol. 1984;2(5):498–504. doi: 10.1200/JCO.1984.2.5.498. [DOI] [PubMed] [Google Scholar]
- 27.Dedrick RL, Flessner MF, Collins JM, Schultz JS. Is the peritoneum a membrane? Am Soc Artific Int Org J. 1982;5:1–8. [Google Scholar]
- 28.Flessner MF, Dedrick RL, Schultz JS. A distributed model of peritoneal–plasma transport: theoretical considerations. Am J Physiol. 1984;246(4 Pt 2):R597–R607. doi: 10.1152/ajpregu.1984.246.4.R597. [DOI] [PubMed] [Google Scholar]
- 29.Flessner MF, Fenstermacher JD, Dedrick RL, Blasberg RG. A distributed model of peritoneal–plasma transport: tissue concentration gradients. Am J Physiol. 1985;248(3 Pt 2):F425–F435. doi: 10.1152/ajprenal.1985.248.3.F425. [DOI] [PubMed] [Google Scholar]
- 30.Flessner MF, Dedrick RL, Schultz JS. A distributed model of peritoneal–plasma transport: analysis of experimental data in the rat. Am J Physiol. 1985;248(3 Pt 2):F413–F424. doi: 10.1152/ajprenal.1985.248.3.F413. [DOI] [PubMed] [Google Scholar]
- 31.Collins JM, Dedrick RL. Pharmacokinetics of anticancer drugs. In: Chabner B, editor. Pharmacological Principles of Cancer Treatment. WB Saunders; PA, USA: 1982. pp. 77–99. [Google Scholar]
- 32.Nagel JD, Varossieau FJ, Dubbelman R, Bokkel Huinink WW, McVie JG. Clinical pharmacokinetics of mitoxantrone after intraperitoneal administration. Cancer Chemother Pharmacol. 1992;29(6):480–484. doi: 10.1007/BF00684852. [DOI] [PubMed] [Google Scholar]
- 33.Elferink F, van der Vijgh WJ, Klein I, Bokkel Huinink WW, Dubbelman R, McVie JG. Pharmacokinetics of carboplatin after intraperitoneal administration. Cancer Chemother Pharmacol. 1988;21(1):57–60. doi: 10.1007/BF00262740. [DOI] [PubMed] [Google Scholar]
- 34.Zimm S, Cleary SM, Lucas WE, et al. Phase I/pharmacokinetic study of intraperitoneal cisplatin and etoposide. Cancer Res. 1987;47(6):1712–1716. [PubMed] [Google Scholar]
- 35.Speyer JL, Collins JM, Dedrick RL, et al. Phase I and pharmacological studies of 5-fluorouracil administered intraperitoneally. Cancer Res. 1980;40(3):567–572. [PubMed] [Google Scholar]
- 36.Markman M, Rowinsky E, Hakes T, et al. Phase I trial of intraperitoneal taxol: a Gynecoloic Oncology Group study. J Clin Oncol. 1992;10(9):1485–1491. doi: 10.1200/JCO.1992.10.9.1485. [DOI] [PubMed] [Google Scholar]
- 37.Markman M, Hakes T, Reichman B, et al. Intraperitoneal therapy in the management of ovarian carcinoma. Yale J Biol Med. 1989;62(4):393–403. [PMC free article] [PubMed] [Google Scholar]
- 38.Kerr DJ, Los G. Pharmacokinetic principles of locoregional chemotherapy. Cancer Surv. 1993;17:105–122. [PubMed] [Google Scholar]
- 39.Los G, Mutsaers PH, van der Vijgh WJ, Baldew GS, De Graaf PW, McVie JG. Direct diffusion of cis-diamminedichloroplatinum (II) in intraperitoneal rat tumors after intraperitoneal chemotherapy: a comparison with systemic chemotherapy. Cancer Res. 1989;49(12):3380–3384. [PubMed] [Google Scholar]
- 40.Los G, Mutsaers PH, Lenglet WJ, Baldew GS, McVie JG. Platinum distribution in intraperitoneal tumors after intraperitoneal cisplatin treatment. Cancer Chemother Pharmacol. 1990;25(6):389–394. doi: 10.1007/BF00686048. [DOI] [PubMed] [Google Scholar]
- 41.Bokkel Huinink WW, Dubbelman R, Aartsen E, Franklin H, McVie JG. Experimental and clinical results with intraperitoneal cisplatin. Semin Oncol. 1985;12(3 Suppl 4):43–46. [PubMed] [Google Scholar]
- 42.Baron MA. Structure of the intestinal peritoneum in man. Am J Anat. 1941;69:439–497. [Google Scholar]
- 43.Flessner MF, Fenstermacher JD, Blasberg RG, Dedrick RL. Peritoneal absorption of macromolecules studied by quantitative autoradiography. Am J Physiol. 1985;248(1 Pt 2):26–32. doi: 10.1152/ajpheart.1985.248.1.H26. [DOI] [PubMed] [Google Scholar]
- 44.Supersaxo A, Hein WR, Steffen H. Mixed micelles as a proliposomal, lymphotropic drug carrier. Pharm Res. 1991;8(10):1286–1291. doi: 10.1023/a:1015807913934. [DOI] [PubMed] [Google Scholar]
- 45.Negrini D, Mukenge S, Del Fabbro M, Gonano C, Miserocchi G. Distribution of diaphragmatic lymphatic stomata. J Appl Physiol. 1991;70(4):1544–1549. doi: 10.1152/jappl.1991.70.4.1544. [DOI] [PubMed] [Google Scholar]
- 46.Li JC, Yu SM. Study on the ultrastructure of the peritoneal stomata in humans. Acta Anat (Basel) 1991;141(1):26–30. [PubMed] [Google Scholar]
- 47.Ajani JA, Mansfield PF, Lynch PM, et al. Enhanced staging and all chemotherapy preoperatively in patients with potentially resectable gastric carcinoma. J Clin Oncol. 1999;17(8):2403–2411. doi: 10.1200/JCO.1999.17.8.2403. [DOI] [PubMed] [Google Scholar]
- 48.Yano M, Yasuda T, Fujiwara Y, Takiguchi S, Miyata H, Monden M. Preoperative intraperitoneal chemotherapy for patients with serosa-infiltrating gastric cancer. J Surg Oncol. 2004;88(1):39–43. doi: 10.1002/jso.20133. [DOI] [PubMed] [Google Scholar]
- 49.Esquivel J, Sticca R, Sugarbaker P, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement Society of Surgical Oncology. Ann Surg Oncol. 2007;14(1):128–133. doi: 10.1245/s10434-006-9185-7. [DOI] [PubMed] [Google Scholar]
- 50.Fernandez-Trigo V, Stuart OA, Stephens AD, Hoover LD, Sugarbaker PH. Surgically directed chemotherapy: heated intraperitoneal lavage with mitomycin C. Cancer Treat Res. 1996;81:51–61. doi: 10.1007/978-1-4613-1245-1_6. [DOI] [PubMed] [Google Scholar]
- 51.Stephens AD, Belliveau JF, Sugarbaker PH. Intraoperative hyperthermic lavage with cisplatin for peritoneal carcinomatosis and sarcomatosis. Cancer Treat Res. 1996;81:15–30. doi: 10.1007/978-1-4613-1245-1_3. [DOI] [PubMed] [Google Scholar]
- 52.Witkamp AJ, de Bree E, Van Goethem R, Zoetmulder FA. Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat Rev. 2001;27(6):365–374. doi: 10.1053/ctrv.2001.0232. [DOI] [PubMed] [Google Scholar]
- 53.Hahn GM, Shiu EC. Effect of pH and elevated temperatures on the cytotoxicity of some chemotherapeutic agents on Chinese hamster cells in vitro. Cancer Res. 1983;43(12 Pt 1):5789–5791. [PubMed] [Google Scholar]
- 54.Los G, van Vugt MJ, Pinedo HM. Response of peritoneal solid tumours after intraperitoneal chemohyperthermia treatment with cisplatin or carboplatin. Br J Cancer. 1994;69(2):235–241. doi: 10.1038/bjc.1994.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmidt C, Creutzenberg M, Piso P, Hobbhahn J, Bucher M. Peri-operative anaesthetic management of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Anaesthesia. 2008;63(4):389–395. doi: 10.1111/j.1365-2044.2007.05380.x. [DOI] [PubMed] [Google Scholar]
- 56.Sugarbaker PH, Graves T, DeBruijn EA, et al. Early postoperative intraperitoneal chemotherapy as an adjuvant therapy to surgery for peritoneal carcinomatosis from gastrointestinal cancer: pharmacological studies. Cancer Res. 1990;50(18):5790–5794. [PubMed] [Google Scholar]
- 57.Yu W, Whang I, Suh I, Averbach A, Chang D, Sugarbaker PH. Prospective randomized trial of early postoperative intraperitoneal chemotherapy as an adjuvant to resectable gastric cancer. Ann Surg. 1998;228(3):347–354. doi: 10.1097/00000658-199809000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sugarbaker PH, Cunliffe WJ, Belliveau J, et al. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin Oncol. 1989;16(4 Suppl 6):83–97. [PubMed] [Google Scholar]
- 59.Shen P, Levine EA, Hall J, et al. Factors predicting survival after intraperitoneal hyperthermic chemotherapy with mitomycin C after cytoreductive surgery for patients with peritoneal carcinomatosis. Arch Surg. 2003;138(1):26–33. doi: 10.1001/archsurg.138.1.26. [DOI] [PubMed] [Google Scholar]
- 60.Stewart JH, Levine EA, Shen P. The current role of hyperthermic intraperitoneal chemotherapy for peritoneal dissemination of appendiceal tumors. Curr Probl Cancer. 2009;33(3):142–153. doi: 10.1016/j.currproblcancer.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 61.Jacquet P, Averbach A, Stephens AD, Stuart OA, Chang D, Sugarbaker PH. Heated intraoperative intraperitoneal mitomycin C and early postoperative intraperitoneal 5-fluorouracil: pharmacokinetic studies. Oncology. 1998;55(2):130–138. doi: 10.1159/000011847. [DOI] [PubMed] [Google Scholar]
- 62.de Bree E, Witkamp AJ, Zoetmulder FA. Peroperative hyperthermic intraperitoneal chemotherapy (HIPEC) for advanced gastric cancer. Eur J Surg Oncol. 2000;26(6):630–632. doi: 10.1053/ejso.2000.0964. [DOI] [PubMed] [Google Scholar]
- 63.Elias D, Benizri E, Di Pietrantonio D, Menegon P, Malka D, Raynard B. Comparison of two kinds of intraperitoneal chemotherapy following complete cytoreductive surgery of colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2007;14(2):509–514. doi: 10.1245/s10434-006-9167-9. [DOI] [PubMed] [Google Scholar]
- 64.Markman M, Walker JL. Intraperitoneal chemotherapy of ovarian cancer: a review, with a focus on practical aspects of treatment. J Clin Oncol. 2006;24(6):988–994. doi: 10.1200/JCO.2005.05.2456. [DOI] [PubMed] [Google Scholar]
- 65.Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 66.Ozols RF, Bookman MA, Young RC. Intraperitoneal chemotherapy for ovarian cancer. N Engl J Med. 2006;354(15):1641–1643. doi: 10.1056/NEJMc060264. [DOI] [PubMed] [Google Scholar]
- 67.Elias DM, Sideris L. Pharmacokinetics of heated intraoperative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis. Surg Oncol Clin N Am. 2003;12(3):755–769. xiv. doi: 10.1016/s1055-3207(03)00051-6. [DOI] [PubMed] [Google Scholar]
- 68.Wenzel LB, Huang HQ, Armstrong DK, Walker JL, Cella D. Health-related quality of life during and after intraperitoneal versus intravenous chemotherapy for optimally debulked ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2007;25(4):437–443. doi: 10.1200/JCO.2006.07.3494. [DOI] [PubMed] [Google Scholar]
- 69.Alberts DS, Surwit EA, Peng YM, et al. Phase I clinical and pharmacokinetic study of mitoxantrone given to patients by intraperitoneal administration. Cancer Res. 1988;48(20):5874–5877. [PubMed] [Google Scholar]
- 70.Demicheli R, Bonciarelli G, Jirillo A, et al. Pharmacologic data and technical feasibility of intraperitoneal doxorubicin administration. Tumori. 1985;71(1):63–68. doi: 10.1177/030089168507100112. [DOI] [PubMed] [Google Scholar]
- 71.Howell SB, Pfeifle CE, Olshen RA. Intraperitoneal chemotherapy with melphalan. Ann Intern Med. 1984;101(1):14–18. doi: 10.7326/0003-4819-101-1-14. [DOI] [PubMed] [Google Scholar]
- 72.Monk BJ, Surwit EA, Alberts DS, Graham V. Intraperitoneal mitomycin C in the treatment of peritoneal carcinomatosis following second-look surgery. Semin Oncol. 1988;15(3 Suppl 4):27–31. [PubMed] [Google Scholar]
- 73.Morgan RJ, Jr, Doroshow JH, Synold T, et al. Phase I trial of intraperitoneal docetaxel in the treatment of advanced malignancies primarily confined to the peritoneal cavity: dose-limiting toxicity and pharmacokinetics. Clin Cancer Res. 2003;9(16 Pt 1):5896–5901. [PubMed] [Google Scholar]
- 74.O’Dwyer PJ, LaCreta FP, Daugherty JP, et al. Phase I pharmacokinetic study of intraperitoneal etoposide. Cancer Res. 1991;51(8):2041–2046. [PubMed] [Google Scholar]
- 75.Gitsch E, Sevelda P, Schmidl S, Salzer H. First experiences with intraperitoneal chemotherapy in ovarian cancer. Eur J Gynaecol Oncol. 1990;11(1):19–22. [PubMed] [Google Scholar]
- 76.Recio FO, Piver MS, Hempling RE, Driscoll DL. Five-year survival after second-line cisplatin-based intraperitoneal chemotherapy for advanced ovarian cancer. Gynecol Oncol. 1998;68(3):267–273. doi: 10.1006/gyno.1998.4940. [DOI] [PubMed] [Google Scholar]
- 77.Piver MS, Recio FO, Baker TR, Driscoll D. Evaluation of survival after second-line intraperitoneal cisplatin-based chemotherapy for advanced ovarian cancer. Cancer. 1994;73(6):1693–1698. doi: 10.1002/1097-0142(19940315)73:6<1693::aid-cncr2820730623>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 78.Barakat RR, Sabbatini P, Bhaskaran D, et al. Intraperitoneal chemotherapy for ovarian carcinoma: results of long-term follow-up. J Clin Oncol. 2002;20(3):694–698. doi: 10.1200/JCO.2002.20.3.694. [DOI] [PubMed] [Google Scholar]
- 79.Topuz E, Saip P, Aydmer A, Salihoglu Y, Berkman S, Bengisu E. Intraperitoneal cisplatin–mitoxantrone and intravenous ifosfamide combination as first-line treatment of ovarian cancer. Eur J Gynaecol Oncol. 1998;19(3):265–270. [PubMed] [Google Scholar]
- 80.Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335(26):1950–1955. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- 81.Markman M. Salvage intraperitoneal therapy of small-volume residual ovarian cancer: impact of pretreatment finding of peritoneal carcinomatosis on the surgical complete response rate. J Cancer Res Clin Oncol. 1992;118(3):235–237. doi: 10.1007/BF01410140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Markman M. Intraperitoneal chemotherapy. Crit Rev Oncol Hemat. 1999;31(3):239–246. doi: 10.1016/s1040-8428(99)00017-7. [DOI] [PubMed] [Google Scholar]
- 83.Markman M, Reichman B, Hakes T, et al. Evidence supporting the superiority of intraperitoneal cisplatin compared to intraperitoneal carboplatin for salvage therapy of small-volume residual ovarian cancer. Gynecol Oncol. 1993;50(1):100–104. doi: 10.1006/gyno.1993.1171. [DOI] [PubMed] [Google Scholar]
- 84.Sebastian M, Kuemmel A, Schmidt M, Schmittel A. Catumaxomab: a bispecific trifunctional antibody. Drugs Today (Barc) 2009;45(8):589–597. doi: 10.1358/dot.2009.45.8.1401103. [DOI] [PubMed] [Google Scholar]
- 85.Linke R, Klein A, Seimetz D. Catumaxomab: clinical development and future directions. MAbs. 2010;2:2. doi: 10.4161/mabs.2.2.11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Au JL, Kalns J, Gan Y, Wientjes MG. Pharmacologic effects of paclitaxel in human bladder tumors. Cancer Chemother Pharmacol. 1997;41(1):69–74. doi: 10.1007/s002800050709. [DOI] [PubMed] [Google Scholar]
- 87.Au JL, Badalament RA, Wientjes MG, et al. Methods to improve efficacy of intravesical mitomycin C: results of a randomized Phase III trial. J Natl Cancer Inst. 2001;93(8):597–604. doi: 10.1093/jnci/93.8.597. [DOI] [PubMed] [Google Scholar]
- 88.Au JLS, Kalns J, Gan Y, Wientjes MG. Pharmacologic effects of taxol in human bladder tumors. Cancer Chemother Pharmacol. 1997;41:69–74. doi: 10.1007/s002800050709. [DOI] [PubMed] [Google Scholar]
- 89.Chai M, Wientjes MG, Badalament RA, Burgers JK, Au JL. Pharmacokinetics of intravesical doxorubicin in superficial bladder cancer patients. J Urol. 1994;152(2 Pt 1):374–378. doi: 10.1016/s0022-5347(17)32742-8. [DOI] [PubMed] [Google Scholar]
- 90.Chen D, Song D, Wientjes MG, Au JL. Effect of dimethyl sulfoxide on bladder tissue penetration of intravesical paclitaxel. Clin Cancer Res. 2003;9(1):363–369. [PubMed] [Google Scholar]
- 91.Dalton JT, Wientjes MG, Badalament RA, Drago JR, Au JL. Pharmacokinetics of intravesical mitomycin C in superficial bladder cancer patients. Cancer Res. 1991;51(19):5144–5152. [PubMed] [Google Scholar]
- 92.Dalton JT, Weintjes MG, Au JL. Effects of bladder resorption on pharmacokinetic data analysis. J Pharmacokinet Biopharm. 1994;22(3):183–205. doi: 10.1007/BF02353328. [DOI] [PubMed] [Google Scholar]
- 93.Gao X, Au JL, Badalament RA, Wientjes MG. Bladder tissue uptake of mitomycin C during intravesical therapy is linear with drug concentration in urine. Clin Cancer Res. 1998;4(1):139–143. [PubMed] [Google Scholar]
- 94.Wientjes MG, Dalton JT, Badalament RA, Dasani BM, Drago JR, Au JL. A method to study drug concentration-depth profiles in tissues: mitomycin C in dog bladder wall. Pharm Res. 1991;8(2):168–173. doi: 10.1023/a:1015827700904. [DOI] [PubMed] [Google Scholar]
- 95.Wientjes MG, Dalton JT, Badalament RA, Drago JR, Au JL. Bladder wall penetration of intravesical mitomycin C in dogs. Cancer Res. 1991;51(16):4347–4354. [PubMed] [Google Scholar]
- 96.Wientjes MG, Badalament RA, Au JL. Use of pharmacologic data and computer simulations to design an efficacy trial of intravesical mitomycin C therapy for superficial bladder cancer. Cancer Chemother Pharmacol. 1993;32(4):255–262. doi: 10.1007/BF00686169. [DOI] [PubMed] [Google Scholar]
- 97.Wientjes MG, Badalament RA, Wang RC, Hassan F, Au JL. Penetration of mitomycin C in human bladder. Cancer Res. 1993;53(14):3314–3320. [PubMed] [Google Scholar]
- 98.Wientjes MG, Badalament RA, Au JL. Penetration of intravesical doxorubicin in human bladders. Cancer Chemother Pharmacol. 1996;37(6):539–546. doi: 10.1007/s002800050426. [DOI] [PubMed] [Google Scholar]
- 99.Shen Z, Shen T, Wientjes MG, O’Donnell MA, Au JL. Intravesical treatments of bladder cancer: review. Pharm Res. 2008;25(7):1500–1510. doi: 10.1007/s11095-008-9566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Au JLS, Badalament RA, Wientjes MG, et al. Optimized intravesical mitomycin C treatment for superficial bladder cancer: long-term follow-up. J Urol. 2006;175:268. [Google Scholar]
- 101.Shih WJ, Coupal JJ, Chia HL. Communication between peritoneal cavity and mediastinal lymph nodes demonstrated by Tc99m albumin nanocolloid intraperitoneal injection. Proc Natl Sci Counc Repub China B. 1993;17(3):103–105. [PubMed] [Google Scholar]
- 102.Hirano K, Hunt CA. Lymphatic transport of liposome-encapsulated agents: effects of liposome size following intraperitoneal administration. J Pharm Sci. 1985;74(9):915–921. doi: 10.1002/jps.2600740902. [DOI] [PubMed] [Google Scholar]
- 103.Hornsey S, Howard A. Autoradiograph studies with mouse Ehrlich ascites tumor. Ann NY Acad Sci. 1956;63(5):915–928. doi: 10.1111/j.1749-6632.1956.tb50900.x. [DOI] [PubMed] [Google Scholar]
- 104.Natsugoe S, Tokuda K, Shimada M, et al. Morphology of the designed biodegradable cisplatin microsphere. Anticancer Res. 1999;19(6B):5163–5167. [PubMed] [Google Scholar]
- 105.Sugiyama T, Kumagai S, Nishida T, et al. Experimental and clinical evaluation of cisplatin-containing microspheres as intraperitoneal chemotherapy for ovarian cancer. Anticancer Res. 1998;18(4B):2837–2842. [PubMed] [Google Scholar]
- 106.Hagiwara A, Takahashi T, Sawai K, et al. Pharmacological effects of 5-fluorouracil microspheres on peritoneal carcinomatosis in animals. Br J Cancer. 1996;74:1392–1996. doi: 10.1038/bjc.1996.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hagiwara A, Sakakura C, Tsujimoto H, et al. Selective delivery of 5-fluorouracil (5-FU) to i.p tissues using 5-FU microspheres in rats. Anticancer Drugs. 1997;8:182–188. doi: 10.1097/00001813-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 108.Sharma A, Sharma US, Straubinger RM. Paclitaxel-liposomes for intracavitary therapy of intraperitoneal P388 leukemia. Cancer Lett. 1996;107(2):265–272. doi: 10.1016/0304-3835(96)04380-7. [DOI] [PubMed] [Google Scholar]
- 109.Armstrong DK, Fleming GF, Markman M, Bailey HH. A Phase I trial of intraperitoneal sustained-release paclitaxel microspheres (Paclimer®) in recurrent ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;103(2):391–396. doi: 10.1016/j.ygyno.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 110.Peppas NA, Langer R. New challenges in biomaterials. Science. 1994;263(5154):1715–1720. doi: 10.1126/science.8134835. [DOI] [PubMed] [Google Scholar]
- 111.Langer RS, Peppas NA. Present and future applications of biomaterials in controlled drug delivery systems. Biomaterials. 1981;2(4):201–214. doi: 10.1016/0142-9612(81)90059-4. [DOI] [PubMed] [Google Scholar]
- 112.Pillai O, Panchagnula R. Polymers in drug delivery. Curr Opin Chem Biol. 2001;5(4):447–451. doi: 10.1016/s1367-5931(00)00227-1. [DOI] [PubMed] [Google Scholar]
- 113.Okada H, Toguchi H. Biodegradable microspheres in drug delivery. Crit Rev Ther Drug Carrier Syst. 1995;12(1):1–99. doi: 10.1615/critrevtherdrugcarriersyst.v12.i1.10. [DOI] [PubMed] [Google Scholar]
- 114.Gombotz WR, Pettit DK. Biodegradable polymers for protein and peptide drug delivery. Bioconjug Chem. 1995;6(4):332–351. doi: 10.1021/bc00034a002. [DOI] [PubMed] [Google Scholar]
- 115.Piskin E. Biodegradable polymers as biomaterials. J Biomater Sci Polym Ed. 1995;6(9):775–795. doi: 10.1163/156856295x00175. [DOI] [PubMed] [Google Scholar]
- 116.Schacht EH. Using biodegradable polymers in advanced drug delivery systems. Med Device Technol. 1990;1(1):15–21. [PubMed] [Google Scholar]
- 117.Eldridge JH, Staas JK, Tice TR, Gilley RM. Biodegradable poly(DL-lactide-co-glycolide) microspheres. Res Immunol. 1992;143(5):557–563. doi: 10.1016/0923-2494(92)80069-w. [DOI] [PubMed] [Google Scholar]
- 118.Heller J. Biodegradable polymers in controlled drug delivery. Crit Rev Ther Drug Carrier Syst. 1984;1(1):39–90. [PubMed] [Google Scholar]
- 119.Gilding DK, Reed AM. Biodegradable polymers for use in surgery-polyglycolic/poly(lactic acid) homo- and copolymers. Polymer. 1979;20:1459–1484. [Google Scholar]
- 120.Visscher GE, Robison RL, Maulding HV, Fong JW, Pearson JE, Argentieri GJ. Biodegradation of and tissue reaction to 50:50 poly(DL-lactide-co-glycolide) microcapsules. J Biomed Mater Res. 1985;19(3):349–365. doi: 10.1002/jbm.820190315. [DOI] [PubMed] [Google Scholar]
- 121.Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28(1):5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 122.Hagiwara A, Takahashi T, Iwamoto A, Yoneyama C, Itoh M, Sasabe T. Affinity of intraperitoneally injected activated carbon particles adsorbing mitomycin C to tumor surface of Yoshida sarcoma. Anticancer Drug Dis. 1990;5(4):359–369. [PubMed] [Google Scholar]
- 123.Zheng JH, Chen CT, Au JL, Wientjes MG. Time- and concentration-dependent penetration of doxorubicin in prostate tumors. AAPS PharmSci. 2001;3(2):E15. doi: 10.1208/ps030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124▪▪.Kuh HJ, Jang SH, Wientjes MG, Weaver JR, Au JL. Determinants of paclitaxel penetration and accumulation in human solid tumor. J Pharmacol Exp Ther. 1999;290(2):871–880. Demonstrates paclitaxel penetration and accumulation into solid tumors and describes the limitations of drug penetration. [PubMed] [Google Scholar]
- 125▪▪.Jang SH, Wientjes MG, Au JL. Enhancement of paclitaxel delivery to solid tumors by apoptosis-inducing pretreatment: effect of treatment schedule. J Pharmacol Exp Ther. 2001;296(3):1035–1042. Demonstrates tumor priming enhances paclitaxel penetration into solid tumors. [PubMed] [Google Scholar]
- 126.Chen CT, Au JL, Wientjes MG. Pharmacodynamics of doxorubicin in human prostate tumors. Clin Cancer Res. 1998;4(2):277–282. [PubMed] [Google Scholar]
- 127▪▪.Lu D, Wientjes MG, Lu Z, Au JL. Tumor priming enhances delivery and efficacy of nanomedicines. J Pharmacol Exp Ther. 2007;322(1):80–88. doi: 10.1124/jpet.107.121632. Demonstrates tumor priming enhances nanoparticle penetration into solid tumors. [DOI] [PubMed] [Google Scholar]
- 128.Jang SH, Wientjes MG, Au JL. Determinants of paclitaxel uptake, accumulation and retention in solid tumors. Invest New Drugs. 2001;19(2):113–123. doi: 10.1023/a:1010662413174. [DOI] [PubMed] [Google Scholar]
- 129.Griffon-Etienne G, Boucher Y, Brekken C, Suit HD, Jain RK. Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: clinical implications. Cancer Res. 1999;59:3776–3782. [PubMed] [Google Scholar]
- 130▪▪.Lu Z, Tsai M, Lu D, Wang J, Wientjes MG, Au JL. Tumor-penetrating microparticles for intraperitoneal therapy of ovarian cancer. J Pharmacol Exp Ther. 2008;327(3):673–682. doi: 10.1124/jpet.108.140095. Describes the development of tumor penetrating microparticles for intraperitoneal therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kohane DS, Tse JY, Yeo Y, Padera R, Shubina M, Langer R. Biodegradable polymeric microspheres and nanospheres for drug delivery in the peritoneum. J Biomed Mater Res A. 2006;77(2):351–361. doi: 10.1002/jbm.a.30654. [DOI] [PubMed] [Google Scholar]
- 132.Jackson JK, Skinner KC, Burgess L, Sun T, Hunter WL, Burt HM. Paclitaxel-loaded crosslinked hyaluronic acid films for the prevention of postsurgical adhesions. Pharm Res. 2002;19(4):411–417. doi: 10.1023/a:1015175108183. [DOI] [PubMed] [Google Scholar]
- 133▪▪.Tsai M, Lu Z, Wang J, Yeh TK, Wientjes MG, Au JL. Effects of carrier on disposition and antitumor activity of intraperitoneal paclitaxel. Pharm Res. 2007;24(9):1691–1701. doi: 10.1007/s11095-007-9298-0. Describes the effects of the carrier on the intraperitoneal drug delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lu Z, Tsai M, Wang J, Wientjes MG, Au JLS. Tumor penetrating microparticles for intraperitoneal treatment of pancreatic cancer. Presented at: Annual Meeting of American Association of Pharmaceutical Scientists (AAPS); New Orleans, LA, USA. 8–12 November 2009. [Google Scholar]
Websites
- 201.National Cancer Institute. NCI issues clinical announcement for preferred method of treatment for advanced ovarian cancer. 2006 www.cancer.gov/newscenter/pressreleases/IPchemotherapyrelease.
- 202.European Medicines Agency. Removab: summary of product characteristics. www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000972/WC500051809.pdf.
- 203.US FDA access data on leuprolide acetate. www.accessdata.fda.gov/drugsatfda_docs/label/2009/019943s029,020011s036lbl.pdf.