Abstract
Cancer growth and metastasis are regulated in part by stromal cells such as fibroblasts and immune cells within the tumor microenvironment. Endothelial cells (ECs) are also ubiquitous within tumors because tumors are vascular, and yet, the impact of tumor-resident ECs is less well understood. Through paracrine regulation, ECs modulate a diverse spectrum of pathophysiologic processes in normal and hyperplastic tissues. We hypothesized that ECs offer similar paracrine regulatory control of cancer biology. Indeed, secretions from quiescent ECs muted the proliferative and invasive phenotype of lung and breast cancer cells in vitro and reduced cancer cell protumorigenic and pro-inflammatory signaling. EC perlecan silencing significantly changed this regulatory relationship, eliminating the ability of ECs to inhibit cancer cell invasiveness via increased interleukin-6 secretion. Moreover, implanting ECs embedded within porous matrices slowed adjacent xenograft tumor growth and prevented architectural degeneration, with a concomitant reduction in proliferative and tumorigenic markers. Finally, lung carcinoma cells pretreated with intact EC-conditioned media, but not media conditioned with perlecan-silenced ECs, exhibited reduced micrometastatic burden after tail vein injection. These findings add to an emerging appreciation of EC-regulatory effects that transcend their structural roles and pave the way for improved characterization and control of EC-cancer cross-talk interactions for diagnosis, prognosis, and treatment of cancer.
INTRODUCTION
Tumor growth and metastasis depend critically on cellular and vascular elements. Indeed, Folkman seized on the vascular nature of tumors to propose that angiogenesis was rate-limiting for tumors and suggested antiangiogenesis therapies for cancer treatment (1). Tumor vessels were originally thought to control tumor growth through perfusion of metabolically active cancer cells (2). Tumor growth and dissemination was envisioned to arise in part from an imbalance in proangiogenic and antiangiogenic growth factors (2). More recently, the leakiness of tumor blood vessels has been indicted as contributing directly to tumor growth and metastasis by increasing tumor interstitial pressure (for example, facilitating efflux of cancer cells) and by creating foci of hypoxia and acidosis (3). Clinical trials of antiangiogenesis cancer therapies, however, have shown mixed results, with initial reduction in tumor burden (4, 5), but no significant extension of long-term patient survival (6, 7) and even a potential increase in cancer invasion and metastasis (8, 9).
The contemporary view of cancer envisions tumors as “ecosystems” (10, 11) consisting not simply of proliferating cells alone but of diverse collections of recruited stromal cells that regulate cancer behavior (12–17). The endothelial cells (ECs) that line blood vessels are the first cells in contact with any blood-borne element and are especially prevalent in tumors (18). ECs are also critical to the biology of normal tissues; tissue health is often synonymous with endothelial integrity (19–23). This is especially true in the vascular system, where ECs promote homeostasis when quiescent by suppressing local hyperplasia, angiogenesis, and inflammation, and enhance injury by stimulating these processes when they are diseased or “dysfunctional.” We hypothesize that ECs serve a similar role in tumors. In this paradigm, ECs, like other stromal cell types, regulate cancer cell behavior, promoting homeostasis when healthy and stimulating cancer when dysfunctional. ECs then function not simply as static structural cells of perfusing vessels but as active stromal regulatory cells with privileged access to the deepest recesses of tumors. Subtle changes in EC phenotype could be easily transmitted to the tumors with profound effects on cancer fate.
We now show that ECs can regulate diverse aspects of cancer cell function, including proliferation, invasiveness, and response to and elaboration of inflammatory mediators in vitro, as well as tumor growth and metastasis in vivo. Moreover, we demonstrate that altering the EC secretome can have a profound impact on these cancer-regulatory phenomena. These findings add to an emerging appreciation of potential EC cancer–regulatory effects that transcend the role these cells play as lining of a tumor-perfusing vascular network and offer new modes of cancer diagnosis, prognostication, and therapy.
RESULTS
Secretions from quiescent ECs reduce cancer cell proliferation and invasiveness
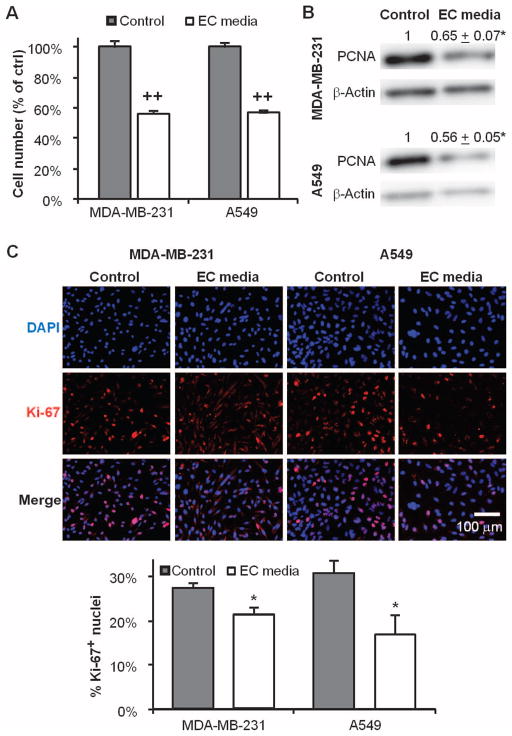
We assayed how culture in EC-conditioned media affects cancer cell proliferation. Media conditioned by confluent ECs reduced growth of MDA-MB-231 breast and A549 lung carcinoma cells by ~40% (P < 0.001 for both; fig. S1 and Fig. 1A). The reduction in cell number correlated with a 35 ± 12% (P < 0.05) and 44 ± 9% (P < 0.05) decrease in proliferating cell nuclear antigen (PCNA) expression (Fig. 1B) and with a 23 ± 5% (P < 0.05) and 45 ± 25% (P < 0.05) reduction in the fraction of cancer cells with Ki-67–positive nuclei (Fig. 1C).
Fig. 1.
Quiescent endothelial cells (ECs) secrete factors that suppress cancer cell proliferation. (A) Growth of MDA-MB-231 breast and A549 lung carcinoma cells for 4 days in unconditioned (control) or EC-conditioned media. (B) Expression of proliferating cell nuclear antigen (PCNA) protein in cancer cells by Western blot. (C) Ki-67 nuclear expression via immunofluorescence staining in the same groups. *P < 0.05 versus control by t test. Error bars show SEM.
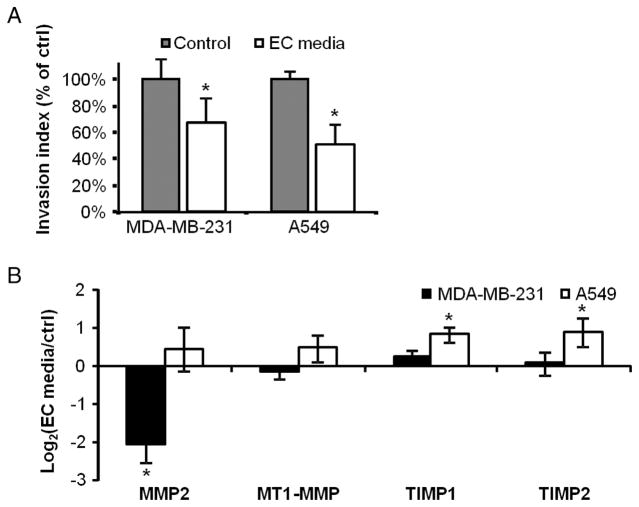
Cancer cell invasiveness is a key trait in determining the aggressiveness and metastatic potential of tumors. Migration and invasion were measured in a dual-chamber culture system. Migration was measured by passage of cancer cells through 8-μm porous membrane inserts into a chemokine-filled chamber, and invasion by cancer cells passing through pores coated with Matrigel (24). Four days of culture in EC-conditioned media significantly reduced in vitro invasiveness of both cancer cell lines (Fig. 2A). Migration was unchanged in both cancer cell types (126 ± 27 versus 133 ± 42 cells per field for MDA-MB-231, 336 ± 28 versus 331 ± 85 for A549), and all of the effect seen in the invasion index was from changes in invasion (33 ± 6 versus 24 ± 7 for MDA-MB-231, 50 ± 3 versus 25 ± 12 for A549). Intriguingly, gene expression associated with reduced invasiveness was different in the two cancer lines. Inhibition of invasion in MDA-MB-231 cells was accompanied by a 4.2 ± 0.9–fold reduction (P < 0.01) in extracellular matrix (ECM) pro-remodeling enzyme matrix metalloproteinase 2 (MMP2) expression, whereas the effects on A549 cells were associated with an increase in MMP inhibitors, including a 1.7 ± 0.4–fold increase (P < 0.05) in expression of TIMP1 (tissue inhibitor of MMP1) and a 1.8 ± 0.7–fold (P < 0.05) increase in TIMP2 (Fig. 2B). However, even the activities of these individual proteins do not account for the entire observed effect. Indeed, although MMP2 can enhance cancer cell invasiveness (25), and ECs secrete MMP2 (26) and deposit this enzyme on cancer cells, the EC secretome successfully inhibited cancer invasiveness despite the presence of deposited MMP2 or exogenously administered activated MMP2 (fig. S2).
Fig. 2.
Quiescent ECs secrete factors that suppress cancer cell invasiveness. (A) Invasiveness of MDA-MB-231 breast and A549 lung carcinoma cells after 4 days of culture in unconditioned (control) or EC-conditioned media. (B) Selected matrix-regulating gene expression (qRT-PCR) of both lines under the same treatment conditions. *P < 0.05 versus control by t test. Error bars show SEM.
Conditioned media from confluent fibroblasts served as a control and had no effect on cancer cell proliferation or invasiveness (fig. S3). These findings suggest a specific regulatory role for quiescent ECs in promoting homeostasis by suppression of both aberrant cancer cell proliferation and invasiveness.
EC secretions affect multiple tumorigenic pathways in cancer cells
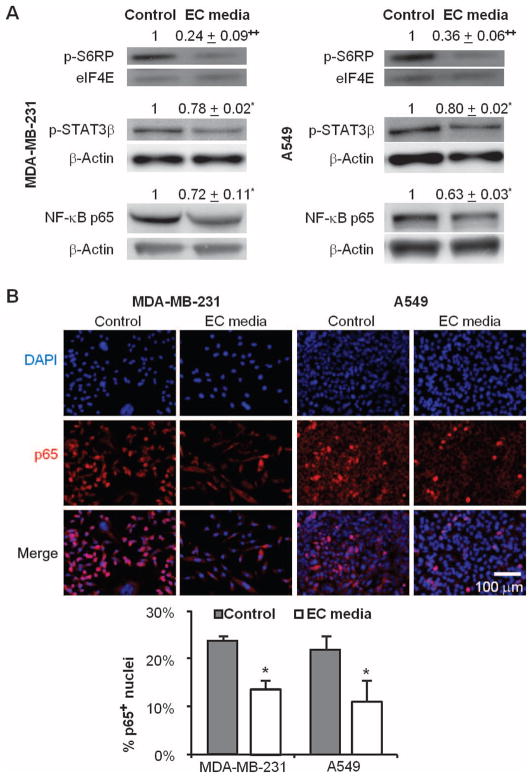
We examined a subset of signaling pathways that contribute to cancer cell biology, including those signals that govern cell growth and proliferation, like the mammalian target of rapamycin (mTOR) pathway (27), and prometastatic nuclear factor κB (NF-κB) and signal transducer and activator of transcription 3 (STAT3) pathways critical for inflammatory signaling (28). Each of these signals is regulated by ECs in vascular repair and disease (29, 30). Four days of culture in EC-conditioned media significantly reduced phosphorylation of S6RP (S6 ribosomal protein) and STAT3β, and decreased the total NF-κB p65 in both cell lines similarly (P < 0.05; Fig. 3A). Phosphorylation of S6RP fell 76 ± 9% for MDA-MB-231 and 64 ± 6% for A549, and STAT3β phosphorylation decreased by 22 ± 2% for MDA-MB-231 and 20 ± 2% for A549. Total NF-κB was decreased by 28 ±11% for MDA-MB-231 and by 37 ±3% for A549 cells relative to culture in control media. Additionally, the nuclear localization of NF-κB p65 was reduced by 41 ± 7% for MDA-MB-231 and by 50 ± 15% for A549 after culture in EC-conditioned media (P < 0.05; Fig. 3B).
Fig. 3.
Signaling through protumorigenic and proinflammatory pathways is attenuated when cancer cells are cultured with media conditioned by quiescent ECs. (A) Phosphorylation of S6RP and STAT3β and total expression of NF-κB p65 in MDA-MB-231 and A549 cells after 4 days of culture in EC-conditioned media, with β-actin as a loading control. (B) Nuclear localization of NF-κB p65 by immunofluorescence staining of both cell types. *P < 0.05 versus control by t test. Error bars show SEM.
Signaling changes in one pathway might cause lateral signaling changes in other pathways. Because we observed the largest reduction in expression of phosphorylated S6RP (p-S6RP) after culture in EC media, we examined whether inhibition of mTOR signaling alone could reproduce our effects. Rapamycin (0.13 μg/ml) completely inhibited phosphorylation of S6RP but only slightly reduced the number of cancer cells after 4 days (P < 0.05; fig. S4A) and had no significant effect on STAT3β phosphorylation or the total amounts of NF-κB p65 (fig. S4B). These data suggest that the changes in cancer cell proliferation and invasion are affected by modulating multiple regulatory pathways, and possibly via the actions of multiple EC-secreted molecules.
Perlecan knockdown increases EC inflammatory secretions and eliminates EC ability to suppress cancer invasiveness
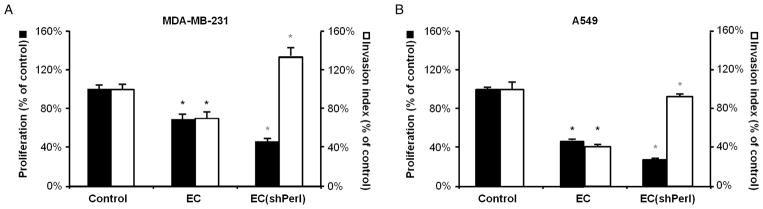
Perlecan, the major extracellular heparan sulfate proteoglycan expressed by ECs, is a complex regulator of vascular biology and tumor angiogenesis (20, 31). ECs were stably transduced with a lentiviral plasmid containing short hairpin RNA (shRNA) targeting perlecan (fig. S5A). Such ECs (ECshPerl) expressed 55 ± 11% less perlecan messenger RNA (mRNA) than did control ECs [quantitative reverse transcription polymerase chain reaction (qRT-PCR); P < 0.01; fig. S5B]. Perlecan silencing did not change EC morphology (fig. S5C) or growth kinetics (fig. S5D) significantly, yet it did reduce EC tube-forming capabilities on Matrigel (P < 0.001; fig. S5E). Media conditioned by ECshPerl had a slightly increased inhibitory effect on cancer cell proliferation compared to media conditioned by control-transduced ECs but could no longer suppress invasiveness of either MDA-MB-231 (Fig. 4A; P = not significant) or A549 cells (Fig. 4B; P = not significant).
Fig. 4.
EC perlecan expression is required for EC-mediated suppression of cancer cell invasiveness. (A and B) Proliferation (black bars) and invasiveness (white bars) of MDA-MB-231 (A) and A549 (B) cells after 4 days of culture in unconditioned (control) media, media conditioned by normal ECs, and media conditioned by perlecan-silenced ECs (ECshPerl). *P < 0.05 (black versus control, gray versus EC) by t test. Error bars show SEM.
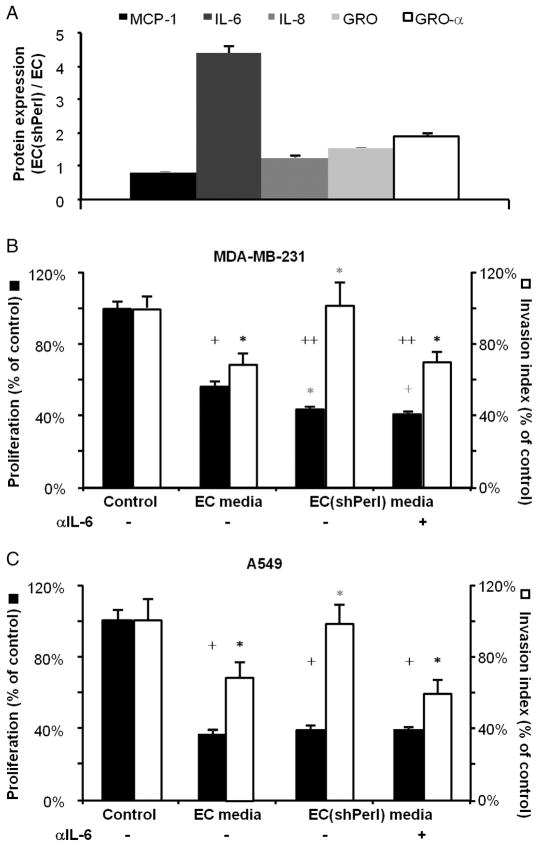
Because perlecan can bind many growth factors and cytokines (31), we assayed the effects of silencing this proteoglycan on EC cytokine release. Perlecan-silenced ECs (ECshPerl) released 4.5 times more interleukin-6 (IL-6) into medium compared with EC transduced with a control plasmid (P < 0.001; Fig. 5A); release of IL-8, GRO, and GRO-α also increased but more modestly (P < 0.001; Fig. 5A). To determine whether the increased IL-6 release from ECshPerl was responsible for the differential effects on cancer phenotype, we preincubated ECshPerl-conditioned media with IL-6–neutralizing antibody (50 μg/ml) or isotype control immunoglobulin G (IgG) antibody before use in cancer cell cultures. IL-6 neutralization had no effect on the inhibition of cancer cell proliferation by ECshPerl, but completely restored the ability of media conditioned by ECshPerl to inhibit cancer cell invasiveness (Fig. 5, B and C). Recombinant IL-6 (10 ng/ml) completely abrogated EC suppression of cancer cell invasiveness (fig. S6A). These findings are consistent with the established role of IL-6 in promoting cancer cell invasive/metastatic behavior (32, 33) and imply that the increased IL-6 secretion with perlecan silencing induced differential effects of EC-secreted factors on cancer cell invasiveness.
Fig. 5.
Perlecan knockdown abrogates EC suppression of cancer cell invasiveness via increased IL-6 release. (A) Quantification of cytokine arrays showing ratios of different cytokines in perlecan-silenced ECs (ECshPerl) versus control ECs. (B and C) Effects of IL-6 neutralization (neutralizing anti-body, 50 μg/ml) in media conditioned by ECs and ECshPerl on the regulation of proliferation (black bars) and invasiveness (white bars) of MDA-MB-231 (B) and A549 (C). *P < 0.05, +P < 0.005, ++P < 0.001 by t test (black versus control, gray versus EC media). Error bars show SEM.
To reduce the likelihood that the perlecan–IL-6 EC coregulation arose from off-target effects, we assayed IL-6 gene expression after perlecan silencing using three different perlecan-silencing shRNAs. IL-6 gene expression by qRT-PCR increased directly with perlecan silencing (fig. S6B; P < 0.05). We examined further the response to pharmacologic inhibition of pathways relevant to IL-6 expression in ECs transduced with (shPerl) and without (pLKO.1) perlecan-silencing plasmids. Whereas inhibition of p38 mitogen-activated protein kinase (MAPK) almost completely inhibited IL-6 secretion for perlecan-silenced and control ECs, inhibition of MEK (mitogen-activated or extracellular signal–regulated protein kinase kinase)/ERK (extracellular signal–regulated kinase) signaling increased IL-6 secretion by a factor of >2 (fig. S6C). Inhibition of STAT3, cyclooxygenase (COX), and NF-κB signaling was not as important in this regard.
Matrix-embedded ECs suppress xenograft tumor growth
To understand whether the effects observed with cultured cancer cells could be recapitulated in controlling cancer cells in vivo, we examined the role of endothelial cell implants in modulating primary tumor growth. ECs embedded within three-dimensional (3D) porous gelatin matrices preserve their phenotype and enable controlled cell implantation in a wide range of models without eliciting an immune response (34–36). Such matrix-embedded ECs (MEECs) have a similar morphology to ECs cultured on gelatin-coated tissue culture polystyrene (TCPS) (fig. S7A), and provide similar regulation of in vitro cancer cell proliferation (fig. S7B) and invasiveness (fig. S7C). Thus, MEECs function as stable, implantable EC constructs useful for studying EC paracrine functions in a wide variety of culture and animal systems.
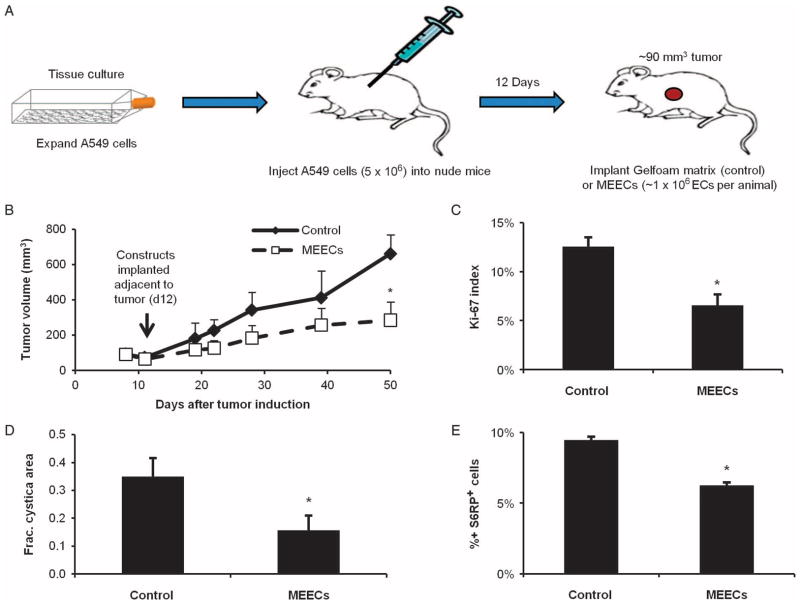
MEECs implanted adjacent to established subcutaneous A549 xenograft tumors in nude mice (Fig. 6A) reduced tumor growth (P < 0.05; Fig. 6B). Tumor growth inhibition correlated with a 46 ± 15% decrease in the fraction of Ki-67+ cancer cell nuclei within the tumor (P < 0.05; Fig. 6C and fig. S8A) and with a 55 ± 21% decrease in the fraction of the tumor filled with cysts (P < 0.05; Fig. 6D and fig. S8). In addition, p-S6RP levels were reduced by 34 ± 2% in the A549 cancer cells of xenograft tumors as in cell culture (P < 0.001; Fig. 6E and fig. S7B). By the end of the experiment, implanted MEEC constructs had almost completely degraded. There was no evidence that MEECs invaded any of the tumor or that tumor cells occupied any of the microscopic remnants of the implanted matrices.
Fig. 6.
Implantation of matrix-embedded ECs (MEECs) adjacent to xenograft tumors reduces tumor growth and aggressiveness. (A) Schematic of xenograft tumor model with adjacent MEEC implantation. (B) Kinetic growth curves for A549 xenograft tumors in nude mice with control (acellular matrix) or MEEC implants. (C to E) Ki-67 percent nuclear staining (C), cystic mass fraction (D), and p-S6RP percent staining (E) of tumor parenchyma in the above groups. *P < 0.05 versus control group by t test. Error bars show SEM.
Cancer cells preconditioned with EC media are less metastatic in vivo
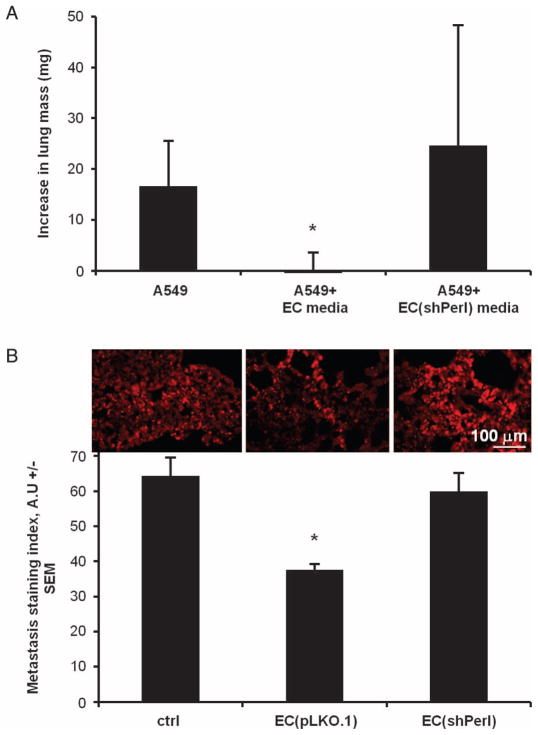
Because perlecan was important for regulation of cancer cell invasiveness in vitro, we examined the role of EC perlecan expression in controlling experimental metastasis. Exponentially growing A549 lung carcinoma cells were injected into the tail veins of nude mice after culture for 4 days in unconditioned media or in media conditioned by either unmodified ECs or perlecan-silenced ECs. Lung mass increased significantly in mice injected with A549 cancer cells cultured in unconditioned media compared with non–tumor-bearing mice 22 days after injection, but not when the A549 cells had been exposed to EC-conditioned media (Fig. 7A). This protection was lost when A549 cells were injected after exposure to ECs whose perlecan had been silenced. These findings correlated with immunofluorescence staining for the injected A549 cells (Fig. 7B): The degree of pulmonary metastasis of A549 cells as defined by a staining index was 41 ± 6% lower in mice injected with cancer cells exposed to intact ECs than in animals injected with cancer cells cultured in unconditioned media (P < 0.05). Similar to the lung mass increase, metastasis inhibition was lost in the ECshPerl media–precultured group. Thus, EC-regulatory effects in vivo are consistent with our in vitro findings.
Fig. 7.
A549 cells cultured in media conditioned by intact ECs, but not perlecan-silenced ECs, were less metastatic than control cells. (A) Increase in lung weights, relative to tumor-free animals, of A549 cells cultured for 4 days in unconditioned (control) media, media conditioned by intact ECs, and media conditioned by perlecan-silenced ECs (ECshPerl). (B) Metastatic index (see Materials and Methods) of lung cryosections in the above groups. *P < 0.05 versus control group by t test. Error bars show SEM.
Together, these data suggest that ECs suppress cancer cell proliferation (tumor growth) and invasiveness (metastasis) in a manner highly dependent on the quiescent and intact endothelial phenotype. When this EC phenotype is disrupted, there is concomitant alteration of EC cancer–regulatory effects.
DISCUSSION
The vasculature is essential to cancer biology, ensuring perfusion of the tumor mass (2) and control of the biophysical microenvironment (3). ECs line all vessels and their integrity is critical to vascular health (19, 22, 23, 37–39). Quiescent ECs suppress every phase of vascular disease, including degree of injury, exposure to toxic products, local thrombosis, inflammation, proliferation, and matrix remodeling. Injured or dysfunctional ECs can promote these events (40). We now report that quiescent ECs release factors that suppress cancer proliferation and invasiveness in vitro. Moreover, perlecan silencing significantly altered EC-mediated regulation of cancer cell phenotype. In addition, the implantation of ECs supported within 3D porous gelatin matrices adjacent to murine xenograft tumors limited primary tumor growth, and preculturing cancer cells with EC-secreted factors reduced their metastatic capacity in an experimental metastasis model. Together, our studies support the concept of ECs as paracrine cancer regulators and add depth to the paradigm of tumor angiogenesis by showing how EC-derived signals can directly regulate tumor parenchyma.
The notion of bidirectional EC-tumor interactions can be found in the earliest work on tumor angiogenesis (1). Increasingly, it is postulated that the complex interplay between tumors and their vasculature depends on more than perfusion alone and that EC-controlled paracrine, or “angiocrine,” modes of regulation must be considered (41). Contact-dependent interactions between the EC surface receptor DARC and the carcinoma cell surface receptor KAI1 induce carcinoma cell senescence and thereby reduce metastasis (42). Subsets of brain vasculature ECs maintain the stem cell compartment of brain tumors through contact-dependent and -independent means (43). We now describe how quiescent ECs regulate cancer cell behavior in vitro and control tumor growth and metastatic potential of carcinoma cells in vivo. These endothelial effects were not recapitulated by individual EC-secreted factors or by other stromal cells. Fibroblasts and leukocytes, including myeloid and lymphoid cells, serve initially as tumor-suppressive or -permissive regulators, but can be converted into cancer-stimulatory cells (16). Indeed, ECs, but not fibroblasts, inhibited the proliferation and in vitro invasiveness of two distinct cancer cell lines, and the intact EC secretome was more physiologically relevant than a proven clinical cancer chemotherapeutic agent. Rapamycin completely inhibited S6RP phosphorylation, but only modestly curbed cancer cell growth and exhibited none of the anti-inflammatory effects of intact ECs. Moreover, unlike directed pharmacologic effects that target specific pathways, ECs reduced invasiveness of two different cancer cell lines by potentially different means: in MDA-MB-231 through down-regulation of metalloproteinases, and in A549 through up-regulation of metalloproteinase inhibitors. The limitations of rapamycin’s effects validate the coordinated involvement of multiple critical pathways in the regulation of cancer by ECs, and the divergent effects of ECs on matrix remodeling genes speak of the likelihood of diverse mechanisms controlling different cancer cells. Cell embedding within matrices enables implantation of intact ECs that have a broader regulatory potential than isolated pharmacological agents.
The results with EC perlecan silencing illustrate further the complex cellular cross talk involved in regulating cancer behavior. EC-conditioned media contain several distinct molecules that likely synergistically regulate cancer phenotype. The heparan sulfate proteoglycan perlecan binds to and mediates the biochemistry of many ECM components, growth factors, and cytokines (20, 31). Perlecan silencing entirely eliminates EC secretome–mediated inhibition of cancer invasiveness with a more modest effect on growth, remarkably reminiscent of the role of ECs in vascular repair. ECs lacking perlecan expression lose the ability to inhibit thrombosis with a more modestly reduced ability to inhibit hyperplasia (20). The effects of perlecan modulation speak to the complexity of EC control over cancer and vascular biology and validate the idea that intact cells can restore physiologic balance more readily than a single pharmacologic compound alone. Perlecan knockdown increases EC secretion of several cytokines, including IL-6, but as with perlecan, IL-6 alone cannot explain fully the effects of ECs on cancer cells. Antibody neutralization of IL-6 restored the ability of ECshPerl to inhibit cancer cell invasiveness but had no effect on cancer cell proliferation, and the addition of IL-6 to EC media had the same effect as EC perlecan silencing. Our results corroborate the reported prometastatic effect of IL-6 (33) but may indirectly contrast with work showing that perlecan depletion (albeit in cancer cells, not ECs) slows tumor growth and reduces metastasis (44–46).
The in vivo validation of the EC-regulatory effects brings together many of the cell culture and gene expression findings on isolated cancer cells. We generated MEECs by culturing ECs within compressed gelatin matrices. Implanted MEECs slowed the growth, reduced intratumoral cyst formation, and muted the pro-growth signaling within subcutaneous A549 lung carcinoma xenograft tumors in nude mice when implanted adjacent to tumors. Preconditioning of A549 lung carcinoma cells by culture in EC media substantially reduced their capacity to invade and colonize the lungs of experimental animals, but not if EC perlecan expression was silenced. ECs within the 3D structure of the porous matrices adopt a phenotype that remains stable for months and can be readily implanted within a range of animal models to regulate tissue repair (20, 23, 38, 47). Allogeneic and even xenogeneic MEEC implants are effective tissue regulators that do not engender an immune response because of the matrix substratum that supports the embedded ECs (34, 48, 49). Allogeneic EC constructs from a single host allow formulation of unit doses that have a prolonged shelf life, are immediately effective upon implantation without need for cell entraining, and present consistent biosecretory profiles from sample to sample within a lot (38, 50) and consistent results from patient to patient (49). Matrix-embedded allogeneic EC implants prolonged vascular access graft survival in animals and humans with minimal immune response and fewer adverse effects than acellular control matrix implants (49, 50). One could well envision that MEECs could be used to reduce tumor size before excision, sensitize tumors to chemotherapy and radiation, and limit tumor metastasis and recurrence after excision.
Potential limitations in this study provide insight into avenues for future work. Our “experimental metastasis” model in immuno-compromised mice is widely used (51) and enabled examination of the metastatic potential of the same cell lines studied in vitro, but lacks the spontaneous detachment from a primary tumor and extravasation into the circulation seen in spontaneous metastasis. We must also consider that as with other stromal elements that regulate cancer cell proliferation and/or invasiveness, there may be the potential for a conversion to cancer stimulation (17) when cancer cells evolve to dominate the stroma. Quiescent ECs promote homeostasis, but ECs that are exposed to high concentrations of inflammatory mediators in the tumor milieu (12) may lose this regulatory ability or even promote tumor growth or metastasis. The elevations in IL-6 seen in the tumor micro-environment (28, 33, 52) may act on the ECs and other stromal elements as well as on the cancer cells themselves. This idea fits with the recent observation that chemotherapy can stimulate EC IL-6 secretion to create a pro-lymphoma niche (53). Such observations clearly require that, in the future, we distinguish between quiescent ECs and those harvested directly from tumors or cultured in vitro in a tumor-like environment (54–56).
Our results suggest the coordinate involvement of multiple EC-secreted factors in the regulation of cancer cell biology that requires a whole-cell perspective for a complete appreciation and treatment of neoplastic diseases. The potential of ECs to regulate cancer biology likely transcends their structural roles in tumor vascular conduits and begs further study. In addition, embedding ECs in porous 3D gelatin matrices enables us to examine the tumor-regulatory impact of ECs from a range of sites and spectrum of differentiation, and/or exposed to a series of pre-treatments and altered environments that include the tumor milieu. The role of proteoglycans, such as perlecan, and cytokines, such as IL-6, illustrates complexities in understanding the mechanisms of EC regulation of cancer cell phenotype. Future work will undoubtedly refine our observations, help delineate direct effects on tumor parenchyma from indirect effects on stroma, speak to the impact of specific signaling pathways, and determine how understanding of EC-cancer cross talk will aid in cancer diagnosis, prognosis, and treatment. The confluence of emerging elements in cancer biology and tissue engineering holds great promise for the future control of neoplastic diseases.
MATERIALS AND METHODS
Cell culture
Primary human umbilical vein ECs (HUVECs; Invitrogen) were cultured in EGM-2 (Lonza) with an additional 3% fetal bovine serum (FBS) on gelatin-coated TCPS plates and used between passages 2 and 6. Cells were passaged by detachment with trypsin and split one to five. EC-conditioned media were generated from confluent HUVEC monolayers by 48 hours of culture in MCDB131 (Invitrogen) supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml). Cells and debris were removed by centrifugation (5 min, 500×g), and media were aliquoted and stored at −80°C. Primary human lung fibroblasts (Lonza) were cultured in the same manner as ECs. A549 (lung carcinoma) and MDA-MB-231 (breast carcinoma) cells (American Type Culture Collection) were cultured on TCPS dishes. Cells were cultured under standard conditions (37°C, 5% CO2).
MEECs were generated by culturing ECs within sterile Gelfoam compressed matrices (Pfizer) (23). Matrices were cut into 1.25 × 1 × 0.3–cm blocks and hydrated in EC growth medium at 37°C for 2 to 48 hours. ECs were suspended in medium, seeded onto hydrated blocks, and allowed to attach for 1.5 hours, and then the contralateral side was seeded with an equal number of cells. After additional time for cell engraftment, two blocks were added to 30-ml polypropylene tubes containing 6 ml of EGM-2. MEECs were cultured for up to 3 weeks, with media changed every 48 to 72 hours, under standard culture conditions. Samples from each lot were digested with collagenase (type I, Worthington Biochemicals), and cell-seeding efficiency was determined with a Z1 Coulter particle counter (Beckman Coulter). Cell viability was assessed by trypan blue exclusion.
Chemoinvasion/chemomigration assay
Chemoinvasion kits (BioCoat, Becton-Dickinson) were used according to the manufacturer’s instructions. Invaded or migrated cells adherent to the bottom of the inserts were fixed, stained with 4′, 6-diamidino-2-phenylindole (DAPI) (1 μg/ml, 30 min), and imaged by epifluorescence microscopy. Images were analyzed by visual inspection and cytometric quantification of four random 20× fields at the microscope or with the “particle counter” feature of ImageJ for the central 10× field. Data are expressed as an invasion index (24), the average number of invaded cells, and the average number of migrated cells of a given condition, normalized to the control condition with at least three wells used per condition.
Xenograft tumor and tail vein metastasis in vivo models
All in vivo experiments were approved by the Massachusetts Institute of Technology Committee on Animal Care and comply with National Institutes of Health guidelines. Female nude mice, aged ~6 weeks, were purchased from Charles River Labs and housed in sterile cages with sterile bedding, food (ad libitum), and water. Mice were allowed at least 4 days to adjust to the animal facility and then were used in one of the following models.
For the primary xenograft tumor model, 5 × 106 human A549 lung carcinoma cells were injected [suspended in 100-μl Hanks’ balanced salt solution (HBSS) after harvesting and rinsing twice with HBSS to remove serum and trypsin] subcutaneously on the dorsal surface. After allowing 12 days for tumor engraftment, either acellular Gelfoam (control) or MEECs (~1 × 106 cells per animal) were rinsed twice with HBSS and implanted adjacent to the tumors; the surgical site was sealed with tissue clips. At the end of the experiment, animals were killed by CO2 inhalation. All surgeries were performed under anesthesia with 2% isoflurane delivered via nose cone and buprenorphine (0.1 mg/kg) administered perioperatively. Tumors were measured one to two times per week with vernier calipers, using two measurements to estimate the volume, assuming a prolate spheroid geometry.
Tail vein metastasis experiments followed established protocols (51). Briefly, exponentially growing A549 lung carcinoma cells were trypsinized, washed twice in phosphate-buffered saline (PBS), resuspended at a density of 5 × 106 cells/ml, and injected into the tail vein of mice under 2% isoflurane anesthesia. Twenty-two days after cell injection, the animals were euthanized (CO2 inhalation) and the lungs were explanted, fixed, weighed, and cryosectioned. To generate a “metastasis staining index, ” we examined three 20× fields per lung section, four sections per animal, for the presence of exogenously seeded A549 cells. The average fluorescent intensity was determined from eight 80 × 80–pixel boxes in each field of a given image. The early time point was selected to study invasion and colonization of the lungs—more in line with in vitro work—rather than subsequent secondary tumor growth.
Supplementary Material
www.sciencetranslationalmedicine.org/cgi/content/full/3/66/66ra5/DC1
Materials and Methods
Fig. S1. Long-term culture of cancer cells in endothelial cell–conditioned media slows cell growth.
Fig. S2. Although the secretome of ECs contains a large amount of latent MMP2, it inhibits significantly cancer cell invasiveness.
Fig. S3. Media conditioned by normal fibroblasts have no effect on cancer cell proliferation or invasiveness.
Fig. S4. Inhibition of one signaling pathway in cancer cells cannot recapitulate EC-mediated regulation of cancer cells.
Fig. S5. Description of perlecan silencing on EC phenotype.
Fig. S6. Further studies of the perlecan/IL-6 axis in endothelial cells and its role in the regulation of cancer cell invasiveness.
Fig. S7. MEECs are phenotypically similar to ECs.
Fig. S8. Representative Ki-67 and S6RP staining in control and MEEC-treated A549 xenograft tumors.
Fig. S9. H&E-stained sections from each tumor showing intratumoral cysts.
Table S1. List of primers used for qRT-PCR.
Acknowledgments
We thank E. Abraham for his experimental and technical advice and B. King for her help with the experimental metastasis model. Funding: Supported by NIH grant R01 GM49039 to E.R.E., NIH Medical Scientist Training Program funding for J.W.F., American Heart Association Scientist Development grant 2630129 to A.B.B., and NIH–National Institute of Diabetes and Digestive and Kidney Diseases (1K08DK080946) and National Kidney Foundation Young Investigator Grant Award to V.C.C.
Footnotes
Author contributions: J.W.F. helped conceive of and performed all experiments and data analysis and wrote the manuscript. A.B.B. aided in certain animal experiments and helped in editing the manuscript. V.C.C. aided in the perlecan silencing experiments and helped in editing the manuscript. E.R.E. conceived of and supervised all experiments and the writing of the manuscript. Competing interests: E.R.E. and J.W.F. are co-inventors on a patent application owned by Massachusetts Institute of Technology that describes the use of cell implants to modulate cancer behavior. E.R.E. is a founder of Pervasis Therapeutics, which has licensed the patent application. No other authors have competing interests to declare.
REFERENCES AND NOTES
- 1.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 3.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Antiangiogenesis in cancer therapy—endostatin and its mechanisms of action. Exp Cell Res. 2006;312:594–607. doi: 10.1016/j.yexcr.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: Targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74:72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain RK. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nat Rev Cancer. 2008;8:309–316. doi: 10.1038/nrc2346. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed F, Steele JC, Herbert JM, Steven NM, Bicknell R. Tumor stroma as a target in cancer. Curr Cancer Drug Targets. 2008;8:447–453. doi: 10.2174/156800908785699360. [DOI] [PubMed] [Google Scholar]
- 8.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 11.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Condeelis J, Pollard JW. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: Role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 16.Weinberg RA. Coevolution in the tumor microenvironment. Nat Genet. 2008;40:494–495. doi: 10.1038/ng0508-494. [DOI] [PubMed] [Google Scholar]
- 17.Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25:30–38. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 18.van Beijnum JR, Rousch M, Castermans K, van der Linden E, Griffioen AW. Isolation of endothelial cells from fresh tissues. Nat Protoc. 2008;3:1085–1091. doi: 10.1038/nprot.2008.71. [DOI] [PubMed] [Google Scholar]
- 19.Nugent MA, Karnovsky MJ, Edelman ER. Vascular cell–derived heparan sulfate shows coupled inhibition of basic fibroblast growth factor binding and mitogenesis in vascular smooth muscle cells. Circ Res. 1993;73:1051–1060. doi: 10.1161/01.res.73.6.1051. [DOI] [PubMed] [Google Scholar]
- 20.Nugent MA, Nugent HM, Iozzo RV, Sanchack K, Edelman ER. Perlecan is required to inhibit thrombosis after deep vascular injury and contributes to endothelial cell-mediated inhibition of intimal hyperplasia. Proc Natl Acad Sci USA. 2000;97:6722–6727. doi: 10.1073/pnas.97.12.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 22.Aird WC. Endothelium in health and disease. Pharmacol Rep. 2008;60:139–143. [PubMed] [Google Scholar]
- 23.Zani BG, Kojima K, Vacanti CA, Edelman ER. Tissue-engineered endothelial and epithelial implants differentially and synergistically regulate airway repair. Proc Natl Acad Sci USA. 2008;105:7046–7051. doi: 10.1073/pnas.0802463105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albini A, Benelli R. The chemoinvasion assay: A method to assess tumor and endothelial cell invasion and its modulation. Nat Protoc. 2007;2:504–511. doi: 10.1038/nprot.2006.466. [DOI] [PubMed] [Google Scholar]
- 25.Kähäri VM, Saarialho-Kere U. Matrix metalloproteinases and their inhibitors in tumour growth and invasion. Ann Med. 1999;31:34–45. doi: 10.3109/07853899909019260. [DOI] [PubMed] [Google Scholar]
- 26.Roomi MW, Monterrey JC, Kalinovsky T, Rath M, Niedzwiecki A. Distinct patterns of matrix metalloproteinase-2 and -9 expression in normal human cell lines. Oncol Rep. 2009;21:821–826. [PubMed] [Google Scholar]
- 27.Pal SK, Figlin RA, Reckamp KL. The role of targeting mammalian target of rapamycin in lung cancer. Clin Lung Cancer. 2008;9:340–345. doi: 10.3816/CLC.2008.n.049. [DOI] [PubMed] [Google Scholar]
- 28.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 30.Kasza Z, Fetalvero KM, Ding M, Wagner RJ, Acs K, Guzman AK, Douville KL, Powell RJ, Hwa J, Martin KA. Novel signaling pathways promote a paracrine wave of prostacyclin-induced vascular smooth muscle differentiation. J Mol Cell Cardiol. 2009;46:682–694. doi: 10.1016/j.yjmcc.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitelock JM, Melrose J, Iozzo RV. Diverse cell signaling events modulated by perlecan. Biochemistry. 2008;47:11174–11183. doi: 10.1021/bi8013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B, Bromberg JF. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, Ceccarelli C, Santini D, Paterini P, Marcu KB, Chieco P, Bonafè M. IL-6 triggers malignant features in mammo-spheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Methe H, Nugent HM, Groothuis A, Seifert P, Sayegh MH, Edelman ER. Matrix embedding alters the immune response against endothelial cells in vitro and in vivo. Circulation. 2005;112:I89–I95. doi: 10.1161/01.CIRCULATIONAHA.105.524991. [DOI] [PubMed] [Google Scholar]
- 35.Methe H, Edelman ER. Tissue engineering of endothelial cells and the immune response. Transplant Proc. 2006;38:3293–3299. doi: 10.1016/j.transproceed.2006.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Methe H, Hess S, Edelman ER. Endothelial immunogenicity—a matter of matrix microarchitecture. Thromb Haemost. 2007;98:278–282. [PubMed] [Google Scholar]
- 37.Dodge AB, Lu X, D’Amore PA. Density-dependent endothelial cell production of an inhibitor of smooth muscle cell growth. J Cell Biochem. 1993;53:21–31. doi: 10.1002/jcb.240530104. [DOI] [PubMed] [Google Scholar]
- 38.Nathan A, Nugent MA, Edelman ER. Tissue engineered perivascular endothelial cell implants regulate vascular injury. Proc Natl Acad Sci USA. 1995;92:8130–8134. doi: 10.1073/pnas.92.18.8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 40.Rogers C, Parikh S, Seifert P, Edelman ER. Endogenous cell seeding. Remnant endothelium after stenting enhances vascular repair. Circulation. 1996;94:2909–2914. doi: 10.1161/01.cir.94.11.2909. [DOI] [PubMed] [Google Scholar]
- 41.Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bandyopadhyay S, Zhan R, Chaudhuri A, Watabe M, Pai SK, Hirota S, Hosobe S, Tsukada T, Miura K, Takano Y, Saito K, Pauza ME, Hayashi S, Wang Y, Mohinta S, Mashimo T, Iiizumi M, Furuta E, Watabe K. Interaction of KAI1 on tumor cells with DARC on vascular endothelium leads to metastasis suppression. Nat Med. 2006;12:933–938. doi: 10.1038/nm1444. [DOI] [PubMed] [Google Scholar]
- 43.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 44.Sharma B, Handler M, Eichstetter I, Whitelock JM, Nugent MA, Iozzo RV. Antisense targeting of perlecan blocks tumor growth and angiogenesis in vivo. J Clin Invest. 1998;102:1599–1608. doi: 10.1172/JCI3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasengaowa, Kodama J, Kusumoto T, Shinyo Y, Seki N, Nakamura K, Hongo A, Hiramatsu Y. Loss of basement membrane heparan sulfate expression is associated with tumor progression in endometrial cancer. Eur J Gynaecol Oncol. 2005;26:403–406. [PubMed] [Google Scholar]
- 46.Kodama J, Shinyo Y, Hasengaowa, Kusumoto T, Seki N, Nakamura K, Hongo A, Hiramatsu Y. Loss of basement membrane heparan sulfate expression is associated with pelvic lymph node metastasis in invasive cervical cancer. Oncol Rep. 2005;14:89–92. [PubMed] [Google Scholar]
- 47.Beck LH, Jr, Goodwin AM, D’Amore PA. Culture of large vessel endothelial cells on floating collagen gels promotes a phenotype characteristic of endothelium in vivo. Differentiation. 2004;72:162–170. doi: 10.1111/j.1432-0436.2004.07204004.x. [DOI] [PubMed] [Google Scholar]
- 48.Methe H, Hess S, Edelman ER. The effect of three-dimensional matrix-embedding of endothelial cells on the humoral and cellular immune response. Semin Immunol. 2008;20:117–122. doi: 10.1016/j.smim.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Conte MS, Nugent HM, Gaccione P, Guleria I, Roy-Chaudhury P, Lawson JH. Multicenter phase I/II trial of the safety of allogeneic endothelial cell implants after the creation of arteriovenous access for hemodialysis use: The V-HEALTH study. J Vasc Surg. 2009;50:1359–1368. e1. doi: 10.1016/j.jvs.2009.07.108. [DOI] [PubMed] [Google Scholar]
- 50.Nugent HM, Groothuis A, Seifert P, Guerraro JL, Nedelman M, Mohanakumar T, Edelman ER. Perivascular endothelial implants inhibit intimal hyperplasia in a model of arteriovenous fistulae: A safety and efficacy study in the pig. J Vasc Res. 2002;39:524–533. doi: 10.1159/000067207. [DOI] [PubMed] [Google Scholar]
- 51.Elkin M, Vlodavsky I. Curr Protoc Cell Biol. Unit 19.2. Chapter 19. 2001. Tail vein assay of cancer metastasis. [DOI] [PubMed] [Google Scholar]
- 52.Schafer ZT, Brugge JS. IL-6 involvement in epithelial cancers. J Clin Invest. 2007;117:3660–3663. doi: 10.1172/JCI34237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilbert LA, Hemann MT. DNA damage-mediated induction of a chemoresistant niche. Cell. 2010;143:355–366. doi: 10.1016/j.cell.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 55.van Beijnum JR, Dings RP, van der Linden E, Zwaans BM, Ramaekers FC, Mayo KH, Griffioen AW. Gene expression of tumor angiogenesis dissected: Specific targeting of colon cancer angiogenic vasculature. Blood. 2006;108:2339–2348. doi: 10.1182/blood-2006-02-004291. [DOI] [PubMed] [Google Scholar]
- 56.Aird WC. Molecular heterogeneity of tumor endothelium. Cell Tissue Res. 2009;335:271–281. doi: 10.1007/s00441-008-0672-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
www.sciencetranslationalmedicine.org/cgi/content/full/3/66/66ra5/DC1
Materials and Methods
Fig. S1. Long-term culture of cancer cells in endothelial cell–conditioned media slows cell growth.
Fig. S2. Although the secretome of ECs contains a large amount of latent MMP2, it inhibits significantly cancer cell invasiveness.
Fig. S3. Media conditioned by normal fibroblasts have no effect on cancer cell proliferation or invasiveness.
Fig. S4. Inhibition of one signaling pathway in cancer cells cannot recapitulate EC-mediated regulation of cancer cells.
Fig. S5. Description of perlecan silencing on EC phenotype.
Fig. S6. Further studies of the perlecan/IL-6 axis in endothelial cells and its role in the regulation of cancer cell invasiveness.
Fig. S7. MEECs are phenotypically similar to ECs.
Fig. S8. Representative Ki-67 and S6RP staining in control and MEEC-treated A549 xenograft tumors.
Fig. S9. H&E-stained sections from each tumor showing intratumoral cysts.
Table S1. List of primers used for qRT-PCR.