Abstract
In addition to causing regression of the Mullerian duct in the male embryo, Mullerian Inhibiting Substance (MIS) inhibits the growth of epithelial ovarian cancer cells, which are known to be of Mullerian origin. Because the uterine cervix is derived from the same Mullerian duct precursor as the epithelium of the ovary, we tested the hypothesis that cervical cancer cells might also respond to MIS. A number of cervical cancer cell lines express the MIS type II receptor, and MIS inhibits the growth of both human papilloma virus-transformed and non-human papilloma virus-transformed cervical cell lines, with a more dramatic effect seen in the latter. As in the ovarian cancer cell line OVCAR8, suppression of growth of the C33A cervical cancer cell line by MIS is associated with induction of the p16 tumor suppressor protein. However, in contrast to OVCAR8 cells, induction of p130 and p107 appears to play an important role in the inhibition of growth of C33A cells by MIS. Finally, normal cervical tissue expresses the MIS type II receptor in vivo, supporting the idea that MIS could be a targeted therapy for cervical cancer.
Mullerian Inhibiting Substance (MIS), also known as anti-Mullerian hormone, has long been known for its canonical activity of causing the regression of the embryonic Mullerian duct, the anlagen of the fallopian tubes, the surface epithelium of the ovaries, the uterus, the cervix, and the upper third of the vagina. Given the sensitivity of these tissues to MIS, we proposed that this 140-kDa hormone could inhibit the growth of Mullerian duct-derived tumors and demonstrated that partially purified preparations of bovine MIS inhibited growth of an ovarian cancer cell line (1, 2) and of primary ovarian and endometrial cells in vitro (3, 4). Using more highly purified recombinant human MIS, we demonstrated growth inhibition of both human ovarian cancer cell lines and primary tumors in vitro and in vivo (5–8). Recently, we showed that the epithelial ovarian cancer cell line OVCAR8 expresses the MIS type II receptor and responds to MIS by growth inhibition mediated through a retinoblastoma protein (pRB)-independent mechanism involving the up-regulation of p16 (6). As a member of the INK4 family of cyclin-dependent kinase (CDK) inhibitors, p16 regulates the cell cycle by inhibiting the kinase activity of cyclin/CDK (CDK 4/6) complexes (9), and induction of p16 is known to disrupt cell cycle progression and to regulate apoptosis (10–14).
To study whether other Mullerian duct-derived tumors might be sensitive to MIS, we investigated its effect on human cervical cancer cell lines. The third most common neoplasm of the female genital tract, cervical cancer accounts for ≈10% of all cancers in women and resulted in 4,800 deaths in 1999 in the United States (15). A significant proportion of these cancers are due to infection with high risk subtypes of the human papilloma virus (HPV) (16). Despite a significant reduction in the annual cervical cancer death rate in the United States since the introduction of the Papanicolaou test (17), cervical cancer remains a major health threat in third world countries (18). Moreover, treatment of advanced disease with chemotherapy and radiation carries with it significant toxicity and often results in infertility among premenopausal women.
In support of the idea that cervical cancer cells might be a target for MIS, Wang et al. (19, 20) demonstrated by electron microscopy that several cervical cancer cell lines (CaSki and HeLa) bind and internalize gold-labeled MIS ligand purified from avian testes. In addition, the spectrum of cell cycle defects in these cell lines is similar to that observed in OVCAR8 cells, which are sensitive to MIS (6). Most commonly studied cervical cancer cell lines retain functional p16 while pRB is inactivated by mutation (C33A) or by HPV, mediated by the oncoprotein E7 (CaSki, HeLa) (21, 22). Furthermore, transfection of p16 can inhibit growth of HeLa cells (10). Therefore, we hypothesized that, similar to OVCAR8 cells, cervical cancer cells might also be growth inhibited by MIS treatment through a mechanism involving the up-regulation of p16.
In the current study, we evaluated both HPV-transformed and non-HPV-transformed human cervical cancer cell lines for the expression of the MIS type II receptor and for response to MIS. Although cervical cancer cells bind MIS (19, 20), receptor expression has not been formally demonstrated, and the functional consequences of this interaction remain unknown. Therefore, we examined effects of MIS on cell cycle regulatory proteins, comparing findings in cervical cancer cells to those previously reported for ovarian cancer cells (6). Finally, we analyzed MIS type II receptor expression in rat cervical tissue to determine whether these findings might be physiologically significant in vivo.
Materials and Methods
Cell Lines and Cultures. Cervical cancer cell lines without HPV (C33A) or transformed with HPV-16 (CaSki and SiHa) were gifts from Karl Munger (Department of Pathology and Center for Cancer Biology, Harvard Medical School). OVCAR8 cells were described (6), and the green monkey kidney cell line COS cells served as a negative control. All cell lines were maintained in DMEM and 10% female FBS at 37°C in an atmosphere of 5% CO2. Cells were grown to 80% confluency before passage, and experiments were restricted to passages 5–15.
Methylthiazoletetrazolium (MTT) Growth Inhibition Assay. Plating of cells was preceded by five passages to ensure log phase growth and active transit through the cell cycle. Caski, C33A, SiHa, and COS cells were harvested at 80% confluency and plated on 96-well plates at 2,000 cells per well. Each lane (consisting of 10 wells) was either treated with MIS (10 μg/ml) or an equivalent volume of PBS 36 h after plating. After day 4 of treatment, viable cells were quantified by the MTT (methylthiazoletetrazolium) assay, in which reduction of the yellow tetrazolium salt to a purple formazan salt detects active mitochondria in living cells (23). Cells were washed in PBS before adding 60 μl of MTT solution (1:2 MTT in PBS to 100 mM succinic acid in ddH20). After incubation for 3–5 h, DMSO was added and the formazan precipitate dissolved, and the plates were read on an ELISA plate reader and the absorbance at 550 nm was recorded. MIS-treated and -untreated cell lines were compared, and statistical analysis was performed by using the ANOVA paired t test, with P ≤ 0.05 considered to be statistically significant.
Colony Assays. Colonies were generated by stably transfecting C33A cells at 80% confluency on 10-cm plates with 1.0 μg of a hygromycin resistance plasmid and 7.5 μg of expression constructs consisting of empty vector, full-length MIS, leaderless MIS (24), p16, antisense p16, p130, p107, or E2F1 by using the calcium phosphate DNA precipitation technique. Cells were then maintained in medium containing 50 μg/ml hygromycin for 3–4 wk to select for colony formation. Cells were stained with crystal violet, and the number of colonies >50 cells in size were counted for each transfection and normalized to the empty vector control. Each experiment was done in triplicate, and quantitation of colonies was performed in a blinded fashion. SDs were calculated, and results were tested for statistical significance by comparing each sample to vector control.
Abs and Western Blot Analyses. The MIS type II receptor rabbit polyclonal Ab (designated 153P) was generated by injecting animals with the kinase domain of the receptor expressed in Escherichia coli cells and purified by histidine affinity chromatography (M. Lorenzen, T. Lorenzen, P.K.D., and D.T.M., unpublished data). Ab was then ligand affinity purified before use. Mouse mAb against p16 (JC6) in conditioned media was a kind gift from Ed Harlow (Cancer Center, Massachusetts General Hospital). The rabbit anti-p27, p130, p107, E2F1, E2F2, E2F3, E2F4, and E2F5 Abs were purchased from Santa Cruz Biotechnology.
Proteins from cell lines were harvested in RIPA buffer (50 mM Hepes, pH 7.0/150 mM NaCl/0.1% Triton X-100/0.1% sodium deoxycholate/0.1% SDS), and protein concentration was determined by Bradford analysis. Proteins were then reduced with 2-mercaptoethanol, separated on SDS-polyacrylamide gels (100 μg per lane), and transferred to an Immobilon-P membrane. The blots were blocked in TBST (Tris-buffered saline and Tween 20) (25 mM Tris, pH 7.4/136 mM NaCl/5 mM KCl/0.1% Tween) containing 10% powdered milk for 1 h and then incubated in 1% milk/TBST with the appropriate Ab for 2 h at room temperature. The rabbit anti-MIS type II receptor Ab and preimmune Ab were used at a 1:1,000 dilution, the mouse anti-p16 Ab was used at a 1:10 dilution, and all other Abs were used at a 1:2,000 dilution. Blots were then washed three times with 1% milk/TBST and incubated with the corresponding horseradish peroxidase-conjugated Abs. Bound Abs were detected with enhanced chemiluminescence. Experiments were performed in duplicate or triplicate with representative blots shown.
Specificity of Rabbit Anti-Human MIS Type II Receptor Ab. COS cells were plated at equal densities to achieve 50% confluency after 2 d. Four plates were then transfected with the human MIS type II receptor construct by using FuGENE 6 (Roche Molecular Biochemicals); 5 μg of construct was used with 15 μl of FuGENE 6 per plate. After 48 h, proteins from transfected and untransfected cells were then harvested for Western blot analysis. Bradford analysis was performed to determine protein concentration. The lysates were then analyzed by gel electrophoreses with equal protein loading at 100 μg per lane in triplicate. The rabbit anti-human MIS type II receptor Ab was incubated overnight at 4°Cata1:10dilution with 300 μg of lysate from COS cells transfected with the MIS type II receptor. In parallel, the rabbit Ab was also incubated with 300 μg of lysate from untransfected COS cells. A standard mixture of protease inhibitors was added to the Ab/lysate mixtures. The triplicate Western blots containing COS-transfected and -untransfected proteins were then divided, and each blot was probed with (i) Ab alone, (ii) Ab preincubated with COS-untransfected lysate, and (iii) Ab preincubated with COS-transfected lysate. OVCAR8 and OVCAR8 transfected with the MIS type II receptor cells were also harvested for protein and analyzed in the same manner.
Analysis of MIS Type II Receptor Expression in Vivo. For analysis of receptor expression in vivo, lysates from adult rat cervix, ovaries, testes, lower vagina, and small intestine were harvested by first pulverizing the fresh tissues over dry ice with mortar/pestle followed by addition of electrophoresis sample buffer with 5% 2-mercaptoethanol at five times the weight of the tissue, and tituration by using a 21-gauge needle and syringe. The resultant extract was spun at 12,000 rpm for 3 min at room temperature. Of the 500 μl of lysate, 50 μl was loaded per lane for each tissue with the exception of the testes, for which 10 μl of lysate was loaded because of high levels of MIS receptor expression in this tissue. Western analysis was then performed by using the 153P Ab as described for the cell lines. Amido black staining of the blot confirmed the presence of similar amounts of total protein in each lane, except for the testes, which contained reduced amounts of protein (data not shown).
Results
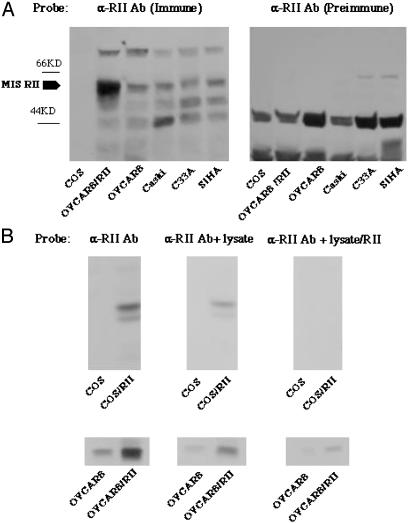
Expression of MIS Type II Receptor Protein in Cervical Cancer Cells. MIS type II receptor expression in cervical cancer cells was demonstrated by Western analysis by using a rabbit Ab raised against the kinase domain of the human MIS type II receptor. This Ab detected a band at 63 kDa, the deduced molecular mass of the MIS type II receptor, in WT OVCAR8 cells and in OVCAR8 cells transfected with a full-length human MIS type II receptor construct (Fig. 1A). Western analysis performed simultaneously detected the 63-kDa MIS type II receptor in three cervical cancer cell lines, CaSki, SiHa, and C33A (Fig. 1A). COS cells, used as a negative control, do not contain this band, and receptor was not detected by preimmune rabbit serum (Fig. 1B). Ab specificity was tested by Western analysis of extracts of COS and OVCAR8 cells transfected with the MIS RII gene. The receptor was detected in the transfected COS cell extracts and in OVCAR 8 cells but not in control COS cell extracts (Fig. 1C). The band is significantly diminished when the Ab is adsorbed by MIS type II receptor by incubation with extracts of cells transfected with the receptor gene before use in Western analysis. Control cell extracts were without effect (Fig. 1C).
Fig. 1.
Cervical cancer cell lines express the MIS type II receptor [α-RII Ab (immune)]. (A) Cell lysates prepared from COS cells, OVCAR8 cells, and the cervical cell lines Caski, C33A, and SiHa were probed with Ab against the MIS type II receptor. The 63-kDa receptor is present in all three cervical cell lines (lanes 4–6), as well as in WT OVCAR8 cells (lane 3). Overexpression of the receptor in OVCAR8 cells results in enhancement of this band (lane 2), and this band is absent in COS cells, which were used as a negative control (lane 1). Analogous lanes probed with preimmune serum do not show receptor [α-RII Ab (preimmune)]. (B) Cell lysates were prepared from untransfected COS cells and COS cells transfected with the MIS type II receptor (Upper), as well as untransfected OVCAR8 cells and OVCAR8 cells transfected with MIS RII (Lower). Blots probed MIS RII Ab alone recognize the 63-kDa receptor (lanes 1 and 2). Similar results are seen when the Ab is preincubated with COS lysate (lanes 3 and 4). In contrast, receptor recognition is significantly reduced when Ab is preincubated with lysate from COS cells expressing the MIS type II receptor, reflecting its retention in the lysate and confirming Ab specificity (lanes 5 and 6).
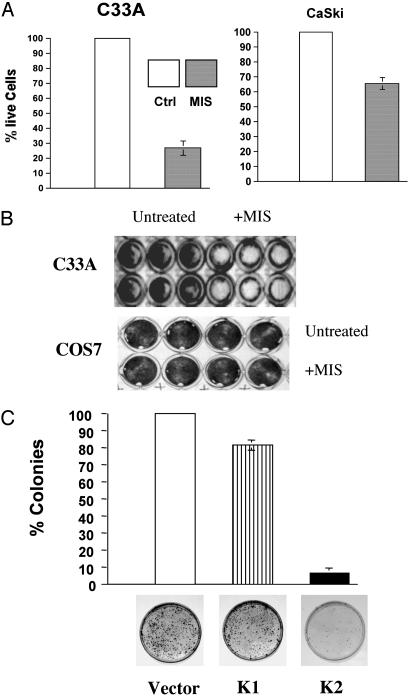
MIS Inhibits the Growth of Cervical Cancer Cells. Given that the cervical cancer cell lines tested express the MIS type II receptor, we treated the HPV-transformed cell lines CaSki and SiHa and a non-HPV-transformed cell line, C33A, with MIS (10 μg/ml). A difference in cell number became evident after 3–4 d of MIS treatment. The MTT assay was used to quantify the degree of growth suppression. Whereas CaSki cells exhibited moderate growth inhibition across multiple experiments (30–40%) and SiHa cells demonstrated minimal response (data not shown), the growth of C33A cells was more strongly inhibited by MIS treatment (70–80%) (Fig. 2 A and B). As predicted, MIS had no effect on COS cells, which do not express the MIS type II receptor (Fig. 2B). Given the marked sensitivity of C33A cells to exogenous MIS treatment, these cells were also stably transfected with constructs containing functional MIS (K2), a leaderless inactive form of MIS (K1), or vector alone. We previously demonstrated by ELISA that transfection of cells with MIS results in its secretion into the conditioned media (6). C33A cells transfected with the functional MIS construct showed a dramatic inhibition of colony growth compared to the vector control, ranging from 90% to 95% across experiments (Fig. 2C). In contrast, transfection of cells with the inactive MIS construct had a minimal effect on colony number (Fig. 2C). Thus, in addition to expressing the MIS type II receptor, growth of cervical cancer cell lines is inhibited by MIS.
Fig. 2.
Inhibition of cervical cancer cell growth by MIS. (A) The MTT assay was used to quantify cell growth in response to treatment with MIS. C33A cells treated with MIS exhibit a 70–80% decline in cell number after 4 d compared to untreated controls, whereas CaSki cells exhibit 30–40% inhibition of cell growth after MIS treatment. All P values are statistically significant (<0.001). (B) Representative wells containing C33A cells and COS7 cells analyzed by the MTT assay after treatment with MIS. (C) C33A cells were stably transfected with empty vector, the leaderless inactive form of MIS (K1), and active MIS (K2). Minimal reduction in colony number is seen after transfection with inactive MIS, compared to 90–95% inhibition of colony growth in cells transfected with active MIS.
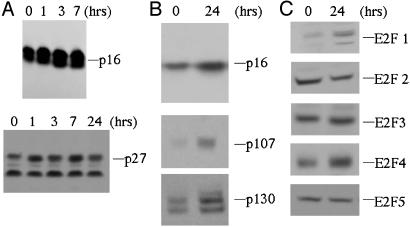
Cell Cycle Protein Expression in C33A Cells Treated with MIS. As cyclin-dependent kinase inhibitors play a key role in proliferation, differentiation, and apoptosis by inhibiting the kinase activity of cyclin/CDK complexes (9), we analyzed the effects of MIS on the expression of CDK inhibitors. We previously showed that MIS-mediated growth inhibition of the ovarian cancer cell line OVCAR8 required the up-regulation of p16 (6). Given that both OVCAR8 and C33A cells express functional p16 and defective pRB (21, 22), we hypothesized that the mechanism of MIS-mediated growth inhibition of C33A cells would be similar to that of OVCAR8 cells. In fact, treatment of C33A cells with MIS (10 μg/ml) resulted in induction of p16 as early as 1 h after treatment with persistent elevation at 24 h, whereas p27 levels remained at baseline (Fig. 3 A and B). Because C33A cells contain a mutation at a splice junction affecting exon 20 of Rb resulting in an in-frame deletion and inactive protein (21), we determined the levels of the other pocket proteins p107 and p130 in MIS-treated C33A cells, finding both proteins to be induced (Fig. 3B).
Fig. 3.
Induction of cell cycle proteins in response to MIS treatment. (A) C33A cells were harvested after treatment with MIS for various lengths of time. Induction of p16 is seen after only several hours of treatment, in contrast to p27, the levels of which remain constant. (B) Levels of p16 remain elevated after 24 h of MIS treatment. In addition, the pRB-related proteins p107 and p130 are induced after treatment of C33A cells with MIS for 24 h. (C) Examination of E2F family members in MIS-treated C33A cells reveals that E2Fs 1 and 4 are induced after 24 h of treatment, whereas the levels of E2Fs 2, 3 and 5 remain constant. All experiments were performed in duplicate or triplicate, and representative blots are shown.
The ability of pRB, p107, and p130 to regulate the cell cycle depends on their capacity to associate with and regulate the activity of cellular partners, notably the E2F family of transcription factors. In accordance with our previous findings with OVCAR8 cells, MIS treatment of C33A cells induces E2F1 and E2F4 at 24 h, whereas the levels of E2F2, E2F3, and E2F5 did not change appreciably (Fig. 3C).
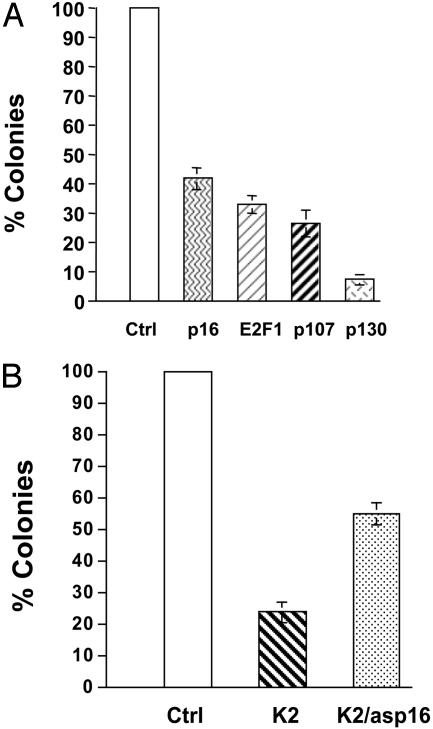
To determine which cell cycle proteins affect C33A growth inhibition when overexpressed, C33A cells were stably transfected with p16, p107, p130, or E2F1 constructs (Fig. 4A). Transfection with p16 produced 60% inhibition of colony formation in C33A cells. Similar strong inhibition of growth occurred after overexpression of E2F1 and p107. The greatest colony suppression was seen after overexpression of p130. Furthermore, the expression of antisense p16 dramatically rescues the MIS growth inhibitory effect in C33A cells (Fig. 4B). These results suggest that up-regulation of p16 is important in the MIS-mediated growth inhibition of C33A cells and that other cell cycle regulators may also play key roles.
Fig. 4.
Effects of cell cycle protein expression on C33A cell growth. (A) C33A cells were stably transfected with empty vector, p16, E2F1, p107, or p130, and results are presented from three separate experiments. Overexpression of p16 results in a moderate suppression of colony growth, comparable to that seen after transfection with E2F1 or p107. In contrast, a greater degree of growth inhibition is observed when p130 is overexpressed in C33A cells. All results are statistically significant. (B) C33A cells were stably transfected with empty vector (Ctrl), active MIS (K2), or K2 and antisense p16 (K2/asp16). Expression of MIS results in a significant inhibition of colony growth, which is partially rescued by antisense p16. Results are presented from three separate experiments and are statistically significant.
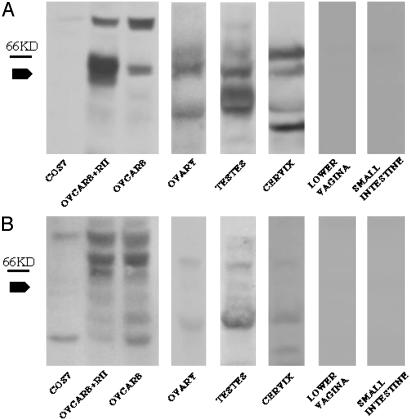
Expression of MIS Type II Receptor in Normal Rat Cervical Tissue. Because a number of cervical cancer cell lines express the MIS type II receptor and are growth inhibited by MIS, we examined whether the MIS type II receptor is expressed in normal adult cervical tissue to explore the possibility that MIS is involved in normal cervical function and that MIS treatment might be an effective therapeutic in vivo. Western analysis was performed on fresh rat tissue extracts prepared from the cervix, testes, ovary, small bowel, and lower vagina. The 63-kDa MIS type II receptor found in the ovary and testes was also present in cervical tissue (Fig. 5A). As expected, tissues isolated from the small intestine and lower vagina do not contain this 63-kDa band (Fig. 5A) despite the presence of significant amounts of protein in these lanes by amido black staining (data not shown). Western analysis by using preimmune rabbit serum did not detect the 63-kDa band in any of the tissues (Fig. 5B). These findings demonstrate that the MIS type II receptor is expressed in vivo in adult cervical tissue.
Fig. 5.
The MIS type II receptor is expressed in normal rat cervical tissue. (A) Lysates prepared from rat ovary, testes, cervix, lower vagina, and small intestine were probed with Ab against the MIS type II receptor. The 63-kDa receptor is present in OVCAR8 cells at endogenous levels and overexpressed in OVCAR8 cells transfected with the MIS type II receptor construct. Analogous bands are present in rat ovarian, testicular, and cervical tissue but not in the tissue prepared from the non-Mullerian tissues from the lower vagina or small intestine. (B) The 63-kDa receptor band is not seen when the blot is probed with preimmune serum, confirming specificity of the Ab.
Discussion
MIS inhibits the growth of ovarian cancer cells in vitro and in vivo, as shown in a number of previous studies (1–8). Mullerian-derived cervical cancer cells can bind and internalize gold-labeled MIS (19, 20), suggesting that cervical cancer cells might express MIS receptor; however, a functional consequence of this interaction has not been formally shown until now. In this study, we demonstrate that three human cervical cancer cell lines and normal rat cervix express the MIS type II receptor and that MIS can inhibit the growth of the cancer cell lines tested. The tumor suppressor protein p16 is induced by MIS treatment and appears to play a significant role in growth arrest, as was seen with the ovarian cancer cell lines. Moreover, because the MIS type II receptor is expressed in the cervix in vivo it may be physiologically relevant in normal tissue function. These data also lend support to the use of MIS in the treatment of cervical cancer.
Cervical cancer cells have been grouped into two categories: HPV-transformed and non-HPV-transformed cell lines. HPV-transformed cell lines including CaSki, HeLa, SiHa, and HT-3 (21, 22, 25) contain the oncoproteins E6, which targets p53, and E7, which inactivates all three pRB family members (26). In contrast the non-HPV cell line C33A is immortalized via a specific mutation in pRB. We reported earlier that the growth of the HOSE 6-3 human ovarian surface epithelial cell line, immortalized by the HPV oncoproteins E6 and E7 (27), can be inhibited by MIS but to a lesser degree than that observed for OVCAR8 cells (6). Similarly, we show here that CaSki cells demonstrate significant but less dramatic MIS-mediated inhibition of cell growth than C33A cells, whereas SiHa cells are minimally responsive to MIS. This varied response to MIS is similar to that found with transforming growth factor type β, where significant growth inhibition was seen in the cervical cell line HT-3, but minimal response was seen in CaSki cells and no response was observed in HeLa and ME-180 cells (28). These findings may relate to the importance of p107 or p130 in mediating growth inhibition in the absence of pRB (29, 30). Indeed, it has been shown that p130 can mediate transforming growth factor type β-induced growth arrest in the pRB mutant HT-3 cervical cell line (28), which others have shown to carry integrated HPV 30 DNA (25). In the present study, the more MIS-responsive C33A cells contain defective pRB but retain functional p107 and p130. In contrast, CaSki and SiHa cells, which are less responsive to MIS, express the E7 oncoprotein, which binds and inhibits all three pRB family members, including p107 and p130 (26). These results imply that growth inhibition by MIS may depend in part on p107 and p130 because treatment is less effective in cells where their function has been abrogated. Nevertheless, it is important to note that CaSki cells still exhibit a significant degree of growth inhibition (40%). Thus, response to MIS may not be fully predicted by HPV status. Therefore, it is important to survey a large number of cervical cancer cell lines for sensitivity to MIS and to anticipate alternative mechanistic pathways of MIS response. Such is the case observed in human breast and prostate cancer cell lines in which the NFκB pathway mediates MIS responsiveness (31–33).
Similar to our prior study in OVCAR 8 cells, we find that MIS treatment of C33A cells results in up-regulation of p16 with no change in the levels of p27 (ref. 6 and Fig. 3 A and B). Both OVCAR 8 cells and C33A cells lack pRB, which suggests that p16 is functioning in growth arrest in a pRB-independent fashion. Interestingly, it has recently been shown that cell cycle arrest induced by p16 depends not only on pRB, but also on p107 and p130 (34, 35). Mouse embryo fibroblasts deficient in both p107 and p130 do not respond to p16-mediated growth arrest, despite retaining functional pRB (34). Furthermore, mouse embryo fibroblasts lacking E2F4 and E2F5 are also insensitive to growth inhibition by p16, suggesting that p107-E2F and/or p130-E2F complexes are required for this arrest because p107 and p130 associate exclusively with E2F4 and E2F5, whereas pRB associates with E2Fs 1–4 (34, 35). Thus, induction of p16 by MIS in C33A cells and OVCAR8 cells could promote cell cycle arrest via mechanisms involving p107 and p130.
Although transforming growth factor type β has been shown to repress the levels of E2F1 (36, 37), our studies of OVCAR 8 cells demonstrate up-regulation of E2F1 by MIS (6). In accordance with our prior findings with OVCAR 8 cells, treatment of C33A cells with MIS resulted in induction of E2F1. These findings can be explained by the dual role of E2F1 as both oncogene and tumor suppressor gene (38–45). Furthermore, the induction of E2F4 in C33A cells treated with MIS may reflect the importance of p107-E2F4 or p130-E2F4 repressor complexes in mediating the cell cycle arrest observed in these cells. It is important to note that these studies were conducted by using actively cycling cells as opposed to quiescent cells stimulated to enter the cell cycle. Although there are minimal changes in E2F complexes in rapidly dividing cells, there are significant changes in these complexes in serum starved cells induced into S phase, which could differentially affect the ability of MIS to inhibit cell growth in actively cycling cells versus those entering the cell cycle from quiescence (46, 47).
There appear to be subtle differences in the mechanism of MIS mediated growth inhibition between C33A cells and OVCAR8 cells. The degree of p16 induction in response to MIS treatment in C33A cells is not as robust as that seen in OVCAR8 cells (ref. 6 and Fig. 3A). In addition, overexpression of p16 in C33A cells results in a lesser degree of growth inhibition than in OVCAR8 cells, and antisense p16 does not rescue MIS mediated growth inhibition to the same degree in C33A cells as in OVCAR8 cells (ref. 6 and Fig. 4). Although these differences could in part be due to variation in the effectiveness of transfection between the two cell lines, they suggest that there are other factors in addition to p16 that promote growth arrest of C33A cells in response to treatment by MIS. We reported previously that MIS treatment of OVCAR8 cells results in no change in the levels of p107 and suppression of p130 (6). In contrast, we find that MIS treatment of C33A cells results in up-regulation of both p107 and p130. Although induction of p130 is tightly linked with cell cycle exit, up-regulation of p107 is typically associated with progression from G1 into S phase (48). This discrepancy could be explained by difference in pocket protein regulation in the absence of pRB, but it will be important to examine p107 phosphorylation to clarify this issue further. Nonetheless, whereas p130 overexpression in OVCAR8 cells had a minimal effect on growth (data not shown), p130 significantly inhibited the growth of C33A cells, and moderate inhibition was induced by p107 as well (Fig. 4A). These findings suggest that in C33A cells, up-regulation of p107 and/or p130, in addition to induction of p16, is important for growth arrest by MIS. Thus, although mechanisms of growth inhibition by MIS appear to be conserved between the OVCAR8 and C33A cells, there are slight differences that could be due to either tissue specificity or differences in mutations between the two cell lines.
In summary, we have demonstrated that normal cervical tissue and cervical cancer cells express the type II MIS receptor. Like epithelial ovarian cancer cells, cervical cancer cells respond to MIS by growth inhibition. These studies further elucidate the down-stream molecular mechanism of MIS action and support the idea that MIS may also be an effective targeted therapy for cervical cancer.
Acknowledgments
We thank Dr. Karl Munger for cervical cancer cell lines and Dr. Ed Harlow for anti-p16 Ab. This work was funded by National Institutes of Health/National Cancer Institute Grant CA 17393 and National Institute of Child Health and Human Development Grant HD 32112 (to P.K.D. and D.T.M.).
Abbreviations: CDK, cyclin-dependent kinase; HPV, human papilloma virus; MIS, Mullerian Inhibiting Substance; MTT, methylthiazoletetrazolium; pRB, retinoblastoma protein.
References
- 1.Donahoe, P. K., Swann, D. A., Hayashi, A. & Sullivan, M. D. (1979) Science 205, 913–915. [DOI] [PubMed] [Google Scholar]
- 2.Fuller, A. F., Jr., Guy, S., Budzik, G. P. & Donahoe, P. K. (1982) J. Clin. Endocrinol. Metab. 54, 1051–1055. [DOI] [PubMed] [Google Scholar]
- 3.Donahoe, P. K., Fuller, A. F., Jr., Scully, R. E., Guy, S. R. & Budzik, G. P. (1981) Ann. Surg. 194, 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuller, A. F., Jr., Budzik, G. P., Krane, I. M. & Donahoe, P. K. (1984) Gynecol. Oncol. 17, 124–132. [DOI] [PubMed] [Google Scholar]
- 5.Masiakos, P. T., MacLaughlin, D. T., Maheswaran, S., Teixeira, J., Fuller, A. F., Jr., Shah, P. C., Kehas, D. J., Kenneally, M. K., Dombkowski, D. M., Ha, T. U., et al. (1999) Clin. Cancer Res. 5, 3488–3499. [PubMed] [Google Scholar]
- 6.Ha, T. U., Segev, D. L., Barbie, D., Masiakos, P. T., Tran, T. T., Dombkowski, D., Glander, M., Clarke, T. R., Lorenzo, H. K., Donahoe, P. K., et al. (2000) J. Biol. Chem. 275, 37101–37109. [DOI] [PubMed] [Google Scholar]
- 7.Stephen, A. E., Masiakos, P. T., Segev, D. L., Vacanti, J. P., Donahoe, P. K. & MacLaughlin, D. T. (2001) Proc. Natl. Acad. Sci. USA 98, 3214–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephen, A. E., Pearsall, L. A., Christian, B. P., Donahoe, P. K., Vacanti, J. P. & MacLaughlin, D. T. (2002) Clin. Cancer Res. 8, 2640–2646. [PubMed] [Google Scholar]
- 9.Sherr, C. J. & Roberts, J. M. (1995) Genes Dev. 9, 1149–1163. [DOI] [PubMed] [Google Scholar]
- 10.Schreiber, M., Muller, W. J., Singh, G. & Graham, F. L. (1999) Oncogene 18, 1663–1676. [DOI] [PubMed] [Google Scholar]
- 11.Wolf, J. K., Kim, T. E., Fightmaster, D., Bodurka, D., Gershenson, D. M., Mills, G. & Wharton, J. T. (1999) Gynecol. Oncol. 73, 27–34. [DOI] [PubMed] [Google Scholar]
- 12.Urashimi, M., DeCaprio, J. A., Chaunhan, D., Teoh, G., Ogata, A., Treon, S. P., Hoshi, Y. & Anderson, K. C. (1997) Blood 90, 4106–4115. [PubMed] [Google Scholar]
- 13.Naruse, I., Heike, Y., Hama, S., Mori, M. & Saijo, N. (1998) Anticancer Res. 18, 4275–4282. [PubMed] [Google Scholar]
- 14.Hama, S., Heike, Y., Naruse, I., Takahashi, M., Yoshioka, H., Arita, K., Kurisu, K., Goldman, C. K., Curiel, D. T. & Saijo, N. (1998) Int. J. Cancer 77, 47–54. [DOI] [PubMed] [Google Scholar]
- 15.Landis, S. H., Murray, T., Bolden, S. & Wingo, P. A. (1999) CA Cancer J. Clin. 49, 8–31. [DOI] [PubMed] [Google Scholar]
- 16.Alani, R. M. & Munger, K. (1998) J. Clin. Oncol. 16, 330–337. [DOI] [PubMed] [Google Scholar]
- 17.Beral, V., Hermon, C., Muñoz, N. & Devesa, S. S. (1994) in Trends in Cancer Incidence and Mortality, eds. Doll, R., Fraumeni, J. F., Jr., & Muir, C. S. (Cold Spring Harbor Lab. Press, Plainview, NY), pp. 265–285.
- 18.Parkin, D. M., Muir, C., Whelan, S. L., Gao, Y. T., Ferlay, J. & Powell, J. (1992) Cancer Incidence in Five Continents (Int. Agency for Research on Cancer, Lyon, France).
- 19.Wang, J. J. & Teng, C. S. (1989) Proc. Natl. Sci. Counc. Repub. China B 13, 267–275. [PubMed] [Google Scholar]
- 20.Wang, J. J., Roffler, S. R., Chou, H. H., Yin, F. Y. & Yin, C. S. (1994) Tissue Cell 26, 467–476. [DOI] [PubMed] [Google Scholar]
- 21.Scheffner, M., Munger, K., Byrne, J. C. & Howley, P. M. (1991) Proc. Natl. Acad. Sci. USA 88, 5523–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craig, C., Kim, M., Ohri, E., Wersto, R., Katayose, D., Li, Z., Choi, Y. H., Mudahar, B., Srivastava, S., Seth, P. & Cowan, K. (1998) Oncogene 16, 265–272. [DOI] [PubMed] [Google Scholar]
- 23.Denizot, F. & Lang, R. (1986) J. Immunol. Methods 89, 271–277. [DOI] [PubMed] [Google Scholar]
- 24.Kurian, M. S., de la Cuesta, R. S., Waneck, G. L., MacLaughlin, D. T., Manganaro, T. F. & Donahoe, P. K. (1995) Clin. Cancer Res. 1, 343–349. [PubMed] [Google Scholar]
- 25.Naeger, L. K., Goodwin, E. C., Hwang, E.-S., DeFilippis, R. A., Zhang, H. & DiMaio, D. (1999) Cell Growth Differ. 10, 413–422. [PubMed] [Google Scholar]
- 26.Munger, K. (2002) Front. Biosci. 7, 641–649. [DOI] [PubMed] [Google Scholar]
- 27.Tsao, S. W., Mok, S. C., Fey, E. G., Fletcher, J. A., Wan, T. S., Chew, E. C., Muto, M. G., Knapp, R. C. & Berkowitz, R. S. (1995) Exp. Cell Res. 218, 499–507. [DOI] [PubMed] [Google Scholar]
- 28.Choi, H. H., Jong, H.-S., Song, S. H., Kim, T. Y., Kim, N. K. & Bang, Y.-J. (2002) Gynecol. Oncol. 86, 184–189. [DOI] [PubMed] [Google Scholar]
- 29.Zhu, L., van den Heuvel, S., Helin, K., Fattaey, A., Ewen, M., Livingston, D., Dyson, N. & Harlow, E. (1993) Genes Dev. 7, 1111–1125. [DOI] [PubMed] [Google Scholar]
- 30.Zhu, L., Enders, G., Lees, J. A., Beijersbergen, R. L., Bernards, R. & Harlow, E. (1995) EMBO J. 14, 1904–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segev, D. L., Ha, T. U., Tran, T. T., Kenneally, M., Harkin, P., Jung, M., MacLaughlin, D. T., Donahoe, P. K. & Maheswaran, S. (2000) J. Biol. Chem. 275, 28371–28379. [DOI] [PubMed] [Google Scholar]
- 32.Segev, D. L., Hoshiya, Y., Hoshiya, M., Tran, T. T., Carey, J. L., Stephen, A. E., MacLaughlin, D. T., Donahoe, P. K. & Maheswaran, S. (2002) Proc. Natl. Acad. Sci. USA 99, 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segev, D. L., Hoshiya, Y., Stephen, A. E., Hoshiya, M., Tran, T. T., MacLaughlin, D. T., Donahoe, P. K. & Maheswaran, S. (2001) J. Biol. Chem. 276, 26799–26806. [DOI] [PubMed] [Google Scholar]
- 34.Bruce, J. B., Hurford, R. K., Jr., Classon, M., Koh, J. & Dyson, N. (2000) Mol. Cell 6, 737–742. [DOI] [PubMed] [Google Scholar]
- 35.Gaubatz, S., Lindeman, G. J., Ishida, S., Jakoi, L., Nevins, J. R., Livingston, D. M. & Rempel, R. E. (2000) Mol. Cell 6, 729–735. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz, J. K., Bassing, C. H., Kovesdi, I., Datto, M. B., Blazing, M., George, S., Wang, X. F. & Nevins, J. R. (1995) Proc. Natl. Acad. Sci. USA 92, 483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun, P., Dong, P., Dai, K., Hannon, G. J. & Beach, D. (1998) Science 282, 2270–2272. [DOI] [PubMed] [Google Scholar]
- 38.Dyson, N. (1998) Genes Dev. 12, 2245–2262. [DOI] [PubMed] [Google Scholar]
- 39.Stevens, C. & LaThangue, N. B. (2003) Arch. Biochem. Biophys. 412, 157–169. [DOI] [PubMed] [Google Scholar]
- 40.Johnson, D. G., Cress, W. D., Jakoi, L. & Nevins, J. R. (1994) Proc. Natl. Acad. Sci. USA 91, 12823–12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh, P., Wong, S. H. & Hong, W. (1994) EMBO J. 13, 3329–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu, G., Livingston, D. M. & Krek, W. (1995) Proc. Natl. Acad. Sci. USA 92, 1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu, X. & Levine, A. J. (1994)) Proc. Natl. Acad. Sci. USA 91, 3602–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamasaki, L., Jacks, T., Bronson, R., Goillot, E., Harlow, E. & Dyson, N. J. (1996) Cell 85, 537–548. [DOI] [PubMed] [Google Scholar]
- 45.Khleif, S. N., DeGregori, J., Yee, C. L., Otterson, G. A., Kaye, F. J., Nevins, J. R. & Howley, P. M. (1996) Proc. Natl. Acad. Sci. USA 93, 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith, E. J., Leone, G., DeGregori, J., Jakoi, L. & Nevins, J. R. (1996) Mol. Cell. Biol. 16, 6965–6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leone, G., DeGregori, J., Yan, Z., Jakoi, L., Williams, R. S. & Nevins, J. R. (1998) Genes Dev. 12, 2120–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lipinski, M. M. & Jacks, T. (1999) Oncogene 18, 7873–7882. [DOI] [PubMed] [Google Scholar]