Abstract
Using a solid-phase synthetic approach, a bioactive reverse turn heterocyclic was incorporated into a cyclic peptide template to probe melanocortin receptor potency and ligand structural conformations. The five melanocortin receptor isoforms (MC1R-MC5R) are G-protein coupled receptors (GPCRs) that are regulated by endogenous agonists and antagonists. This pathway is involved in pigmentation, weight, and energy homeostasis. Herein, we report novel analogues of the chimeric AGRP-melanocortin peptide template integrated with a small molecule moiety to probe the structural and functional consequences of the core His-Phe-Arg-Trp peptide domain using a reverse-turn heterocycle. A series of six compounds are reported that result in inactive to full agonists with nM potency. Biophysical structural analysis [2D 1H NMR and computer-assisted molecular modeling (CAMM)] were performed on selected analogues, resulting in the identification that these peptide-small molecule hybrids possessed increased flexibility and fewer discrete conformational families as compared to the reference peptide and result in a novel template for further structure-function studies.
Keywords: melanocortins, Melanotropin, obesity, agouti related protein, AGRP, solid phase synthesis, small molecule, MC3R, MC4R, MC1R, GPCR, peptide, reverse turn mimetic
Introduction
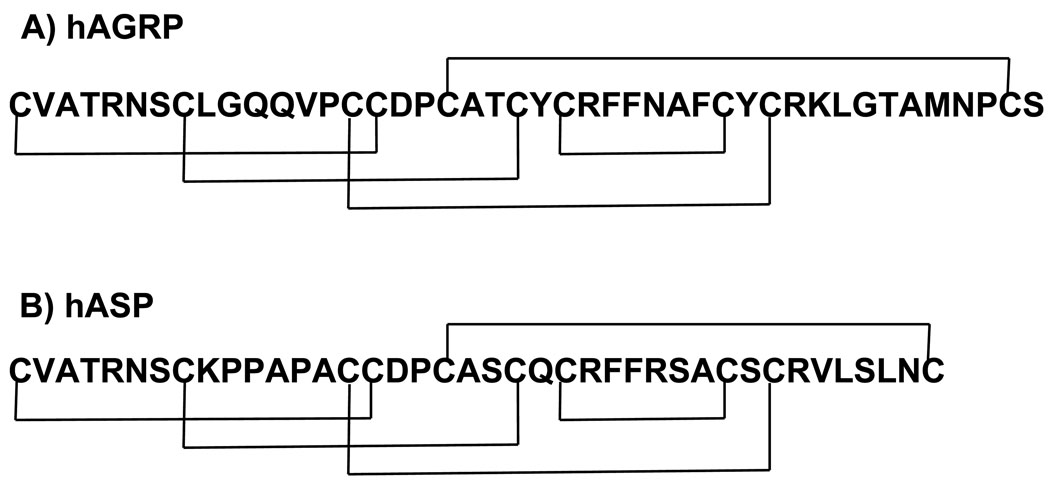
The melanocortin pathway has been implicated in a variety of physiological functions including the regulation of pigmentation,1 blood pressure and heart rate,2,3 erectile function,4,5 food intake6 and obesity.7 The endogenous agonists for the five melanocortin receptors (MC1-5R),8–14 include α -, β -, γ -melanocyte stimulating hormones (MSH) and adrenocorticotropin (ACTH) that are derived from the post-translational modification of proopiomelanocortin (POMC).15,16 Endogenous antagonists for the melanocortin receptors have also been identified as agouti17 and agouti-related protein (AGRP).18 The α-MSH peptide is 13 amino acids in length with an acetylated N-terminus and amidated C-terminus. All of the melanocortin hormones have a common core domain consisting of the His-Phe-Arg-Trp residues postulated to be important for receptor molecular recognition and stimulation (Table 1).19–21 The melanocortin receptors (MCRs) belong to the family of seven transmembrane (TM) spanning G-protein coupled receptors (GPCRs) that stimulate the cAMP signal transduction pathway. The MC1R is expressed in skin and is involved in tanning and pigmentation.8,9 The MC2R only responds to stimulation by ACTH, is expressed in the adrenal gland, and is involved in steroidogenesis.9 The MC3R is expressed in a variety of tissues including the brain, placenta, heart, and gut and participates in energy balance via a mechanism that remains to be identified.10–12,22,23 The MC4R is expressed primarily in the brain and regulates obesity, feeding behavior, and satiety,6,7 making it an attractive target for anti-obesity drugs. The MC5R is expressed in a wide variety of tissues, and has been identified to be important for exocrine gland function in mice,24 with its other physiological roles remaining to be identified. The endogenous melanocortin receptor antagonists Agouti-signaling protein (ASP)17 and Agouti-related protein (AGRP)18 are competitive antagonists at distinct melanocortin receptors (Figure 1). AGRP also functions as an MC4R inverse agonist.25,26
Table 1.
Amino acid sequence of endogenous melanocortin agonists.
| ACTH (1–24) | SYSMEHFRWGKPVGKKRRPVKVYP |
| α-MSH | Ac-SYSMEHFRWGKPV-NH2 |
| β-MSH | AEKKDEGPYRMEHFRWGSPPKD-OH |
| γ2-MSH | YVMGHFRWDRFG-OH |
Figure 1.
Sequence of the A) human agouti related protein (hAGRP) and B) human agouti signaling protein (hASP).
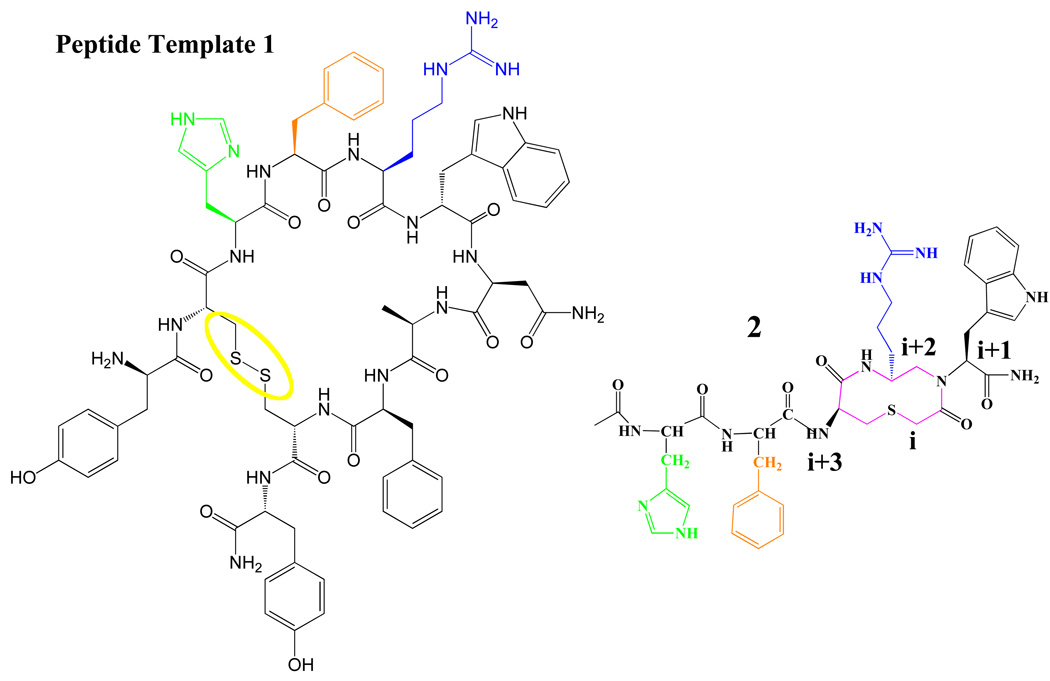
The rational design of peptide ligands towards the understanding of bioactive structures and ligand pharmacophores important for GPCR subtype potency and selectivity has been an on going theme for medicinal chemists for decades. These data are important towards understanding the molecular interactions of ligand-effector proteins as well as for the rational design of new ligands with desirable biological properties. These ligands can be used to probe the mechanism of human disease states and as potential therapeutic moieties. However, rational design of potent selective agonist and antagonist ligands for some of the melanocortin receptors is challenging. Synthetic peptide strategies used in recent years include introduction of cyclic constraints,27–31 N-methylation,32,33 D-amino acids,34 constrained amino acids,31,35,36 aza peptides,37 and peptoids38 in the bioactive peptides. These strategies can restrict peptide conformations, aid in conformational analysis, and often result in selective and potent ligands with the common underlying theme of stabilizing secondary structure(s). Herein, we have utilized a rational structure-based design approach that utilizes the unique and potent chimeric AGRP-melanocortin peptide template 1 (AMW3-130) Tyr-c[Cys-His-DPhe-Arg-Trp-Asn-Ala-Phe-Cys]-Tyr-NH239,40 incorporating a reverse-turn cyclic thioether based heterocyclic scaffold 241 (Figure 2). As an individual structural moiety, this 10-membered heterocyclic template 2 possessed 160 nM and 650 nM full agonist potency at the mMC1R and mMC4R, respectively.41 The strategy presented herein, is based upon the long-standing hypothesis that incorporation of semi-rigid or rigid moieties into a flexible peptide backbone should decrease the topographical space accessible to the molecule in this domain and, with the aid of biophysical studies such as 2D 1H NMR and computer assisted computational molecular modeling, identify putative bioactive peptide conformations and important pharmacophores. Our approach is hypothesized to bridge a conceptual gap between peptides and small molecules by incorporating a bioactive peptidomimetic scaffold into a highly potent cyclic peptide template. This experimental approach attempts to structurally identify the melanocortin agonist pharmacophore side chains important for receptor stimulation and potency as well as probe the side chain orientation of the core His-Phe-Arg-Trp residues. This design strategy incorporates the advantages of peptide derived potency and receptor targeting with the inclusion of a bioactive small molecule peptidomimetic moiety. Additionally, an objective of this study was to develop a synthetic strategy so that all the chemistry associated with the assembly of these modified heterocyclic-peptide analogues could be performed using solid phase synthesis. To the best of our knowledge, this is the first study incorporating bioactive small molecule peptidomimetics into a potent melanocortin receptor peptide template to probe melanocortin ligand structure-activity relationships.
Figure 2.
Illustration of the chimeric hAGRP-melanocortin peptide template 1 and the bioactive heterocyclic molecule (2) utilized in this study. The side chain of His is depicted in green, DPhe in orange, Arg in blue, the heterocycle moiety of 2 in magenta, and the disulfide bridge circled in yellow.
Design of the Reverse Turn Heterocycle-Peptide Hybrid Template
This study utilized the chimeric peptide template 1 (Figure 2) we have previously identified as a potent and novel agonist peptide template for the melanocortin receptors.39,40 This template is derived from the antagonist hAGRP(109–118) decapeptide sequence with amino acid residues His-Phe-Arg-Trp from the melanocortin agonists incorporated in the place of the antagonist Arg-Phe-Phe amino acids39,40 and contains a single disulfide bridge between two Cys side chains (Figure 2). The hAGRP pharmacophore Arg-Phe-Phe42 amino acids have been postulated to mimic putative ligand-receptor interactions43 of the melanocortin agonist core domain and upon substitution with the His-DPhe-Arg-Trp residues, convert the functionality of the peptide from an antagonist to an agonist.39,44 This chimeric 1 template was reported by our laboratory to exhibit sub nM agonist potency at the hMC4R and results in the ability to functionally rescue nM potency at human MC4R polymorphisms (identified in morbidly obese humans) that do not pharmacologically respond normally to the endogenous agonists.45–47 A similar template incorporating a lactam bridge instead of a disulfide bridge Tyr-c[β-Asp-His-DPhe-Arg-Trp-Asn-Ala-Phe-Dpr]-Tyr-NH2 has been studied by our laboratory and resulted in a full agonist at the melanocortin receptors that is as potent as the endogenous α-MSH agonist.40 Biophysical studies (NMR and CAMM) of those chimeric melanocortin-AGRP peptides identified the presence of a β-turn spanning different amino acid residues, as well as unique structures postulated to differentiate MC3R antagonist versus MC4R agonist pharmacology.40
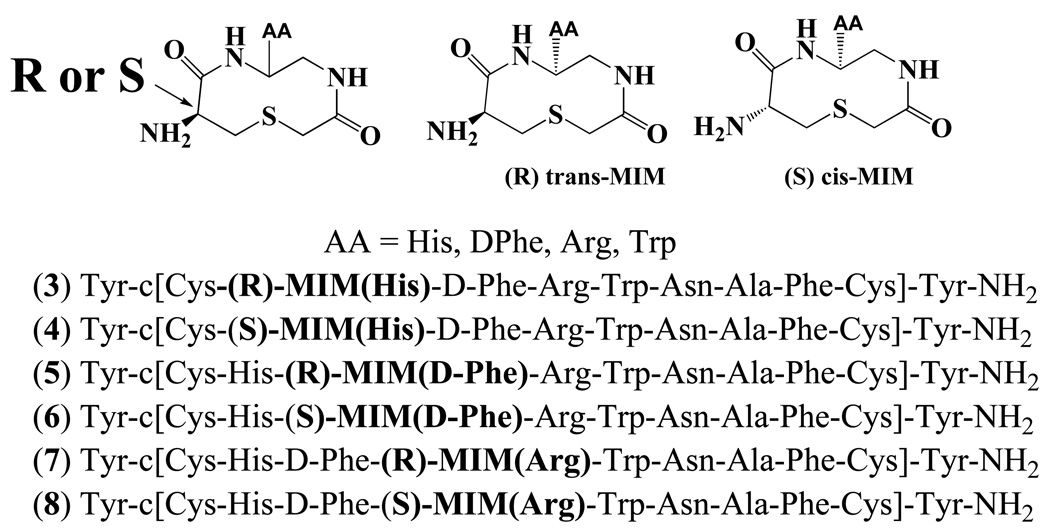
The endogenous melanocortin agonists are linear flexible molecules that are potent at the melanocortin receptors but are generally not selective at any receptor subtype, albeit with species-specific variances, and with the exception of the MC2R which only responds to ACTH. All of the endogenous melanocortin agonists have aromatic and basic side chains in the core His-Phe-Arg-Trp pharmacophore region and upon incorporation of the DPhe7 residue (α-MSH numbering), are postulated to adopt a β-turn structure.48 One possible approach to stabilize a turn structure in globally constrained peptides is to introduce a ring structure in the ligand pharmacophore domain. A cyclic thioether based mimetic (2) has been reported41 that can be synthesized using solid-phase chemistry and with side chain functionality that can be chemically modified. The advantageous feature of this solid-phase synthetic protocol is that a mild on-resin cyclization condition leaves intermediates attached to the solid support for further synthetic chemistry. The side chain functionality was introduced by commercially available Fmoc-amino acids and can be readily diversified. Herein, we have designed, synthesized, and analytically characterized a series of compounds, incorporating a bioactive peptidomimetic reverse turn heterocycle into a mono-cyclic peptide backbone. These molecules were synthesized using solid-phase chemical strategies and were pharmacologically characterized at the mouse melanocortin receptor isoforms for potency and selectivity. To probe structure-function relationships, we designed this study to examine the effect of insertion of the heterocyclic small molecule into different regions of the peptide template backbone. This approach modifies both side chain orientation as well as backbone flexibility towards improving receptor selectivity and structural determination of the role of the Arg residue for receptor potency. The position of the heterocyclic structure (Figure 3) in the chimeric AGRP-melanocortin peptide template 1 was positionally “walked” through the pharmacophore region of 1 to determine the importance of the His-DPhe-Arg elements for receptor potency and selectivity. Both structural elements (heterocycle and peptide template) used in this study have shown biological activity at the melanocortin receptors individually but without selectivity.
Figure 3.
Illustration of the peptide heterocyclic template (2) incorporating either and Lor D-Cys residue during synthesis to induce the R or S stereocenter incorporated into the peptide template 1. A positional “walking” approach was used to place the bolded amino acid side chain into the AA position indicated within the peptide template.
Results
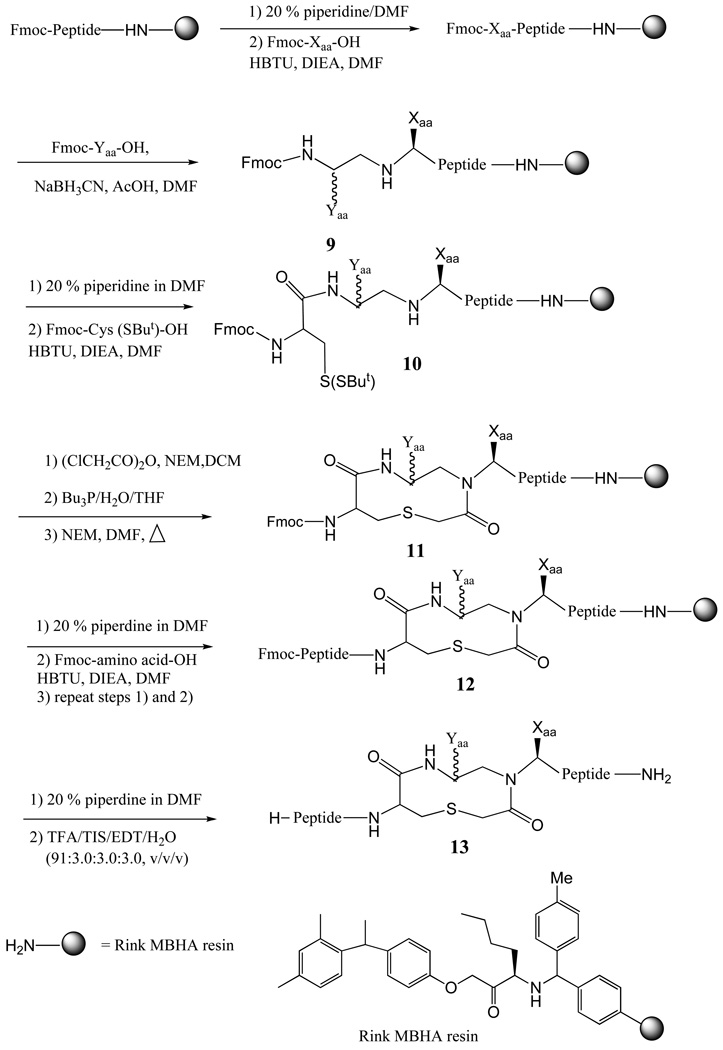
Table 2 summarizes the six peptide-heterocyclic compounds synthesized in this study (structures of the compounds are shown in Figure 3) and pharmacologically characterized at the mouse MC1 and MC3-5 receptors. Chemistry was optimized on solid-support using the Rink amide MBHA linker resin. The C-terminal ligand domain was synthesized by coupling Fmoc-protected amino acids, using standard solid phase peptide synthesis procedures49,50 (Scheme 1). The ring structure was introduced through a known procedure41 including reductive alkylation (using NaBH3CN) together with the Fmoc-protected amino aldehyde51 (prepared by LiAlH4 reduction of the corresponding Weinreb Amide).52–54 The N-fluorenylmethyloxycarbonyl (Fmoc)-α-amino aldehydes for amino acids Fmoc-His(Trt)-OH, Fmoc-Phe-OH, and Fmoc-Arg(Boc)2-OH were synthesized by a known two step procedure involving the transformation of N-Fmoc-α-amino acid to the corresponding Weinreb amide, followed by reduction with LiAlH4. The N-Fmoc-α-amino acid was first coupled with N,O-dimethylhydroxylamine to give the resulting amide, followed by LiAlH4 reduction at 0°C to yield the aldehyde. The synthesized N-Fmoc-amino aldehydes were used for the reductive alkylation to give monoalkylated product 9 at room temperature in 3 h, as indicated by negative Kaiser test.55 Following reductive alkylation, Fmoc-Cys (SBut)-OH was coupled with free amine using HBTU activation to give 10. Acylation on the secondary amine using chloroacetic anhydride was achieved in 30 min, as indicated by negative chloranil test for secondary amines.56 To avoid acylation of the unprotected nitrogen of the guanidino group, Arg(Boc)2 was used instead of Arg(Pmc) or Arg(Pbf). Deprotection of SBut from the cysteine group with Bu3P resulted in the free thiol and served as the cyclization precursor. Formation of the thioether bond was achieved by addition of N-ethylmorpholine in DMF and heating the resulting solution at 60°C for 10 h to yield 11. After deprotection of the Fmoc group, the remaining amino acids were coupled using standard Fmoc synthetic strategies to give 12. The analogues were cleaved from the resin by a 3h treatment with a cleavage cocktail (TFA/triisopropylsilane/EDT/water 91:3:3:3) to give the crude compounds (13; Scheme 1) The disulfide bond was formed by dissolving crude product in 20% DMSO/water and stirred at room temperature. Progress of the disulfide cyclization was monitored by UV-HPLC (λ=214nm) and was generally completed in 24–36 h. After disulfide bond formation, the molecules were purified to homogeneity using semi-preparative reversed-phase high-pressure liquid chromatography (RP-HPLC). The peptides possessed the correct molecular weights, as determined by mass spectrometry, and the purity of the molecules (>95%) was assessed by analytical RP-HPLC (at a wavelength of 214 nm) in two diverse solvent systems (Table 3).
Table 2.
Mouse melanocortin receptor agonist EC50 (nM) values of the peptide-heterocyclic molecules and reference compounds.
| Compound | mMC1R | mMC3R | mMC4R | mMC5R | |
|---|---|---|---|---|---|
| EC50 (nM) | EC50 (nM) | EC50 (nM) | EC50 (nM) | ||
| NDP-MSH | 0.018 ± 0.003 | 0.14 ± 0.03 | 0.20 ± 0.03 | 0.03 ± 0.05 | |
| 1 | AMW3–130 | 0.35 ± 0.17 | 2.00 ± 0.45 | 0.27 ± 0.09 | 2.3 ± 0.57 |
| 2* | Heterocycle | 164 ± 22 | 7600 ± 1890 | 650 ± 126 | 335 ± 106 |
| 3 | 190 ± 76 | 3900 ± 1380 | 350 ± 76 | 305 ± 106 | |
| 4 | 1180 ± 155 | >100,000 | 3060 ± 2330 | 390 ± 24 | |
| 5 | 540 ± 290 | >100,000 | 2730 ± 310 | 1595 ± 1960 | |
| 6 | 240 ± 48 | 3200 ± 610 | 3210 ± 1470 | 990 ± 440 | |
| 7 | 33 ± 9 | 495 ± 215 | 85 ± 13 | 72 ± 16 | |
| 8 | 495 ± 180 | 4620 ± 1060 | 780 ± 340 | 925 ± 360 | |
The indicated errors represent the standard error of the mean determined from at least three independent experiments. >100,000 denotes that some stimulatory activity was observed at 100 µM concentrations, but not enough to determine an EC50 value since the curve did not plateau.
The heterocycle starting template has been previously reported in reference 41 as the 1n compound.
Scheme 1.
Table 3.
Analytical data of synthesized compounds.
| Peptide | K’ MeCN |
K’ MeOH |
Purity % |
HRMS m/z |
|---|---|---|---|---|
| (1) Tyr-c[Cys-His-D-Phe-Arg-Trp-Asn-Ala-Phe-Cys]-Tyr-NH2 | 4.5 | 8.0 | >99 | 1506.6 |
| (3) Tyr-c[Cys-(R)-MIM(His)-D-Phe-Arg-Trp-Asn-Ala-Phe-Cys]-Tyr-NH2 | 5.7 | 9.3 | >96 | 1635.0 |
| (4) Tyr-c[Cys-(S)-MIM(His)-D-Phe-Arg-Trp-Asn-Ala-Phe-Cys]-Tyr-NH2 | 6.2 | 9.2 | >98 | 1636.8 |
| (5) Tyr-c[Cys-His-(R)-MIM(D-Phe)-Arg-Trp-Asn-Ala-Phe-Cys]-Tyr-NH2 | 5.4 | 10.2 | >99 | 1636.4 |
| (6) Tyr-c[Cys-His-(S)-MIM(D-Phe)-Arg-Trp-Asn-Ala-Phe-Cys]-Tyr-NH2 | 5.9 | 9.3 | >95 | 1635.3 |
| (7) Tyr-c[Cys-His-D-Phe-(R)-MIM(Arg)-Trp-Asn-Ala-Phe-Cys]-Tyr-NH2 | 6.1 | 11.0 | >96 | 1637.2 |
| (8) Tyr-c[Cys-His-D-Phe-(S)-MIM(Arg)-Trp-Asn-Ala-Phe-Cys]-Tyr-NH2 | 5.5 | 10.2 | >95 | 1636.2 |
The HPLC k’ value equals [(peptide retention time – solvent retention time)/ solvent retention time]. Two different solvent systems were used. Solvent system 1 is 10% acetonitrile in 0.1% trifluoroacetic acid/H2O with a gradient to 90% acetonitrile over 35 min. Solvent system 2 is 10% methanol in 0.1% trifluoroacetic acid/ H2O with a gradient to 90% methanol over 35 min. An analytical Vydac C18 column (Vydac 218TP104) with a flow rate of 1.5 ml/min was used for analytical characterization. The peptide purity was determined by HPLC in both solvent systems at a wavelength of 214 nm.
Melanocortin Receptor Pharmacology
All the compounds were tested for agonist activity at the mouse melanocortin receptors using a cAMP based β-galactosidase reporter gene bioassay.57 The MC2R is only stimulated by the ACTH agonist and was excluded from the present study. The heterocyclic template 2 pharmacology previously reported by our laboratory41 is included as a control in addition to the standard NDP-MSH (Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2)34 reference peptide.
Incorporation of the His6 (α-MSH numbering) side chain into the heterocyclic-peptide template at the AA position (Figure 3) in the R-configuration 3, resulted in essentially an equipotent melanocortin receptor profile (Table 2) as the heterocyclic template (incorporates the Arg side chain at the AA position) upon incorporation into template 1. Comparison of 3 with the peptide 1 results in 130- to 1950-fold decreased melanocortin receptor agonist potency at the different isoforms. Compound 4, with incorporation of the His6 (α-MSH numbering) side chain into the heterocyclic template at the AA position (Figure 3) in the S-configuration, resulted in the inability to stimulate the mMC3R at up to 100 µM concentrations. However, at the mMC5R, 4 possessed a full agonist potency of 390 nM, ca 170-fold less then 1, but the same potency as the R-configured molecule 3. Compound 4 possessed 3370- and 11330-fold decreased full agonist potency compared to the flexible peptide 1 at the mMC1R and mMC4R, respectively. Incorporation of the Phe7 (α-MSH numbering) side chain into the heterocyclic-peptide template at the AA position (Figure 3) in the R-configuration 5, resulted in a loss of agonist activity at the mMC3R at up to 100 µM concentrations, decreased potency at the mMC1R, mMC4R, and mMC5R as compared to the heterocyclic template 2, and 690- to 10111-fold decreased potency at the same receptors relative to the flexible template 1. Inversion of stereochemistry of the S-configured Phe7 containing heterocyclic-peptide 6, similar to 5, possessed mMC1R, mMC4R, and mMC5R decreased full agonist potency as compared to 2. However, 6 functioned as a full µM agonist at the mMC3R while the R-configured 5 was devoid of stimulatory activity.
The peptide-heterocyclic hybrid compounds 7 and 8 that incorporate the Arg side chain moiety at the AA position (Figure 3) result in full agonists at all the melanocortin receptor isoforms examined in this study. Molecule 7 containing the R-configuration, possessed nM full agonist pharmacology at the mMC1R, mMC4R, and mMC5R that was more potent than the corresponding heterocyclic template 2 alone, but is 30- to 310-fold less potent than peptide 1. Compound 8 containing the S-configuration, while possessing full agonist activity at all the melanocortin receptors examined, was less potent compared to the R-configured 7. These data led us to study the structural differences between the R- and S- configured heterocycle-peptide moieties that incorporate the Arg side chain at the AA position (Figure 3).
Biophysical studies: 2D 1H NMR and computer assisted molecular modeling (CAMM) structures
In an attempt to correlate the melanocortin receptor functional studies with peptide structure, we performed 2D 1H NMR and CAMM studies of the most potent derivative 7 at the melanocortin receptors. This compound contains the Arg side chain at the i+2 position in the ring AA position (Figure 3) and was compared with the less potent analogue 8. Analogues 7 and 8 are sequentially identical and differ configurationally at only one stereo center. We therefore wanted to probe the conformational differences between these two compounds and compare them with the reference peptide 1 to aid in understanding the effect of insertion of the heterocyclic small molecule into the peptide backbone template and correlate the structure with function.
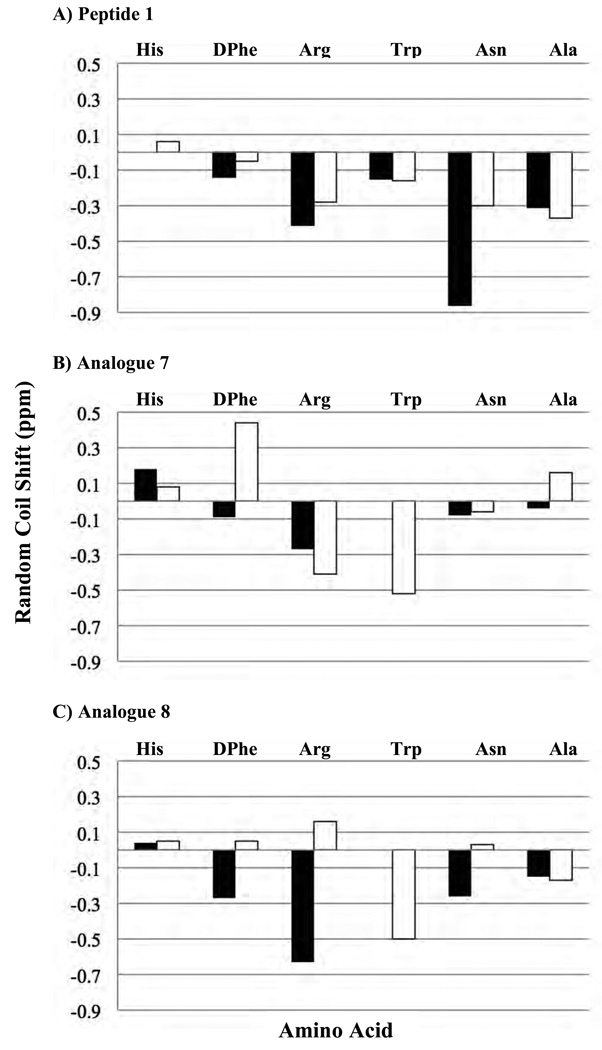
NMR chemical shifts are extremely sensitive to the electronic and chemical environment, therefore providing useful information about molecular structure. However, in the case of flexible peptides, they may not be as singularly informative because they represent a population-weighted average of rapidly inter-converting structures with different averaged chemical shifts. Comparative analyses of peptide chemical shift values are generally derived from the use of aqueous solvents and not a mixed solvent system (acetonitrile and water), as used herein. Nonetheless, by subtracting a common reference value from each chemical shift, a qualitative comparison of the analogues in this study can be made. Figure 4 shows the differences between our mixed experimental solvent (acetonitrile and water) and literature reported aqueous random coil amide and alpha proton chemical shift values58 for the conserved His-DPhe-Arg-Trp-Asn-Ala region of the three compounds examined. A noticeable feature of Figure 4 is that each of the compounds looks different by NMR. Both 7 and 8 are different from the reference compound 1. Comparisons of the chemical shift values are presented in Tables 4 and 5.
Figure 4.
Histogram of the difference between the random coil (water solvent) and experimental chemical shifts of A) peptide 1, B) analogue 7, and C) analogue 8 (acetonitrile/water solvent). The black bar indicates the amide protons and the and α protons are represented white bars.
Table 4.
1H NMR and Chemical Shifts Assignment for Reference Peptide 1.
| amino acid | HN | Hα | Hβ |
|---|---|---|---|
| Peptide 1 | |||
| Tyr1 | - | - | - |
| Cys2 | - | - | - |
| His3 | 8.28 | 4.66 | 2.98, 3.11 |
| DPhe4 | 8.16 | 4.58 | 2.92, 3.00 |
| Arg5 | 7.86 | 4.07 | 1.36, 1.54 |
| Trp6 | 8.03 | 4.50 | 3.19, 3.27 |
| Asn7 | 7.52 | 4.44 | 2.7 |
| Ala8 | 7.84 | 3.96 | 1.13 |
| Phe9 | 7.92 | 4.44 | 2.97, 3.18 |
| Cys10 | 7.70 | 4.50 | - |
| Tyr11 | 7.68 | 4.45 | 2.88, 3.03 |
A – indicates that the chemical shift values could not be determined due to overlapping peaks.
Table 5.
1H NMR and Chemical Shifts Assignment for Analogues 7 and 8.
| amino acid | HN | Hα | Hβ | Others |
|---|---|---|---|---|
| Analogue 7 | ||||
| Tyr1 | 4.63 | 3.07, 2.91 | 6.89, 7.28 | |
| Cys2 | 8.04 | 4.9 | 2.78, 2.90 | |
| His3 | 8.46 | 4.68 | 2.88, 3.01 | 6.93, 8.27 |
| DPhe4 | 8.21 | 5.07 | 2.88, 3.01 | 7.23 |
| Cys5* | 8.06 | 4.74 | 2.71, 3.15 | |
| Arg6 | 7.91 | 3.94 | 1.26, 1.26 | 1.05, 1.05; 2.91, 2.91; 6.89 |
| Trp7 | 4.14 | 3.33, 3.48 | 7.09, 7.43; 9.93 | |
| Asn8 | 8.3 | 4.68 | 2.88, 2.88 | |
| Ala9 | 8.11 | 4.49 | 1.18 | |
| Phe10 | 8.21 | 4.63 | 2.88, 3.01 | 7.29 |
| Cys11 | 8.02 | 4.88 | 2.78, 2.90 | |
| Tyr12 | 7.91 | 4.51 | 2.82, 3.01 | 6.86, 7.36 |
| Ring CH2 Arg | 2.33, 3.01 | |||
| Ring CH2 Acetyl | 3.36, 3.43 | |||
| Analogue 8 | ||||
| Tyr1 | ||||
| Cys2 | 7.96 | 4.54 | 2.78, 2.95 | |
| His3 | 8.32 | 4.65 | 2.87, 2.99 | 6.19, 8.30 |
| DPhe4 | 8.03 | 4.68 | 2.80, 3.04 | 7.27 |
| Cys5* | 7.83 | 4.37 | 2.67, 2.90 | |
| Arg6 | 7.64 | 4.51 | 1.40, 1.40 | 1.24, 1.24; 3.01, 3.01; 6.96 |
| Trp7 | 4.16 | 3.41, 3.51 | 7.15, 7.53; NH 10.04 | |
| Asn8 | 8.12 | 4.77 | 2.83, 2.90 | |
| Ala9 | 8 | 4.16 | 1.4 | |
| Phe10 | 7.94 | 4.56 | 2.97, 3.06 | 7.34 |
| Cys11 | 7.94 | 4.51 | 2.78, 2.95 | |
| Tyr12 | 7.7 | 4.45 | 2.83, 3.01 | 6.80, 7.26 |
| Ring CH2 Arg | 2.36, 2.71 | |||
| Ring CH2 Acetyl | 3.41, 3.53 |
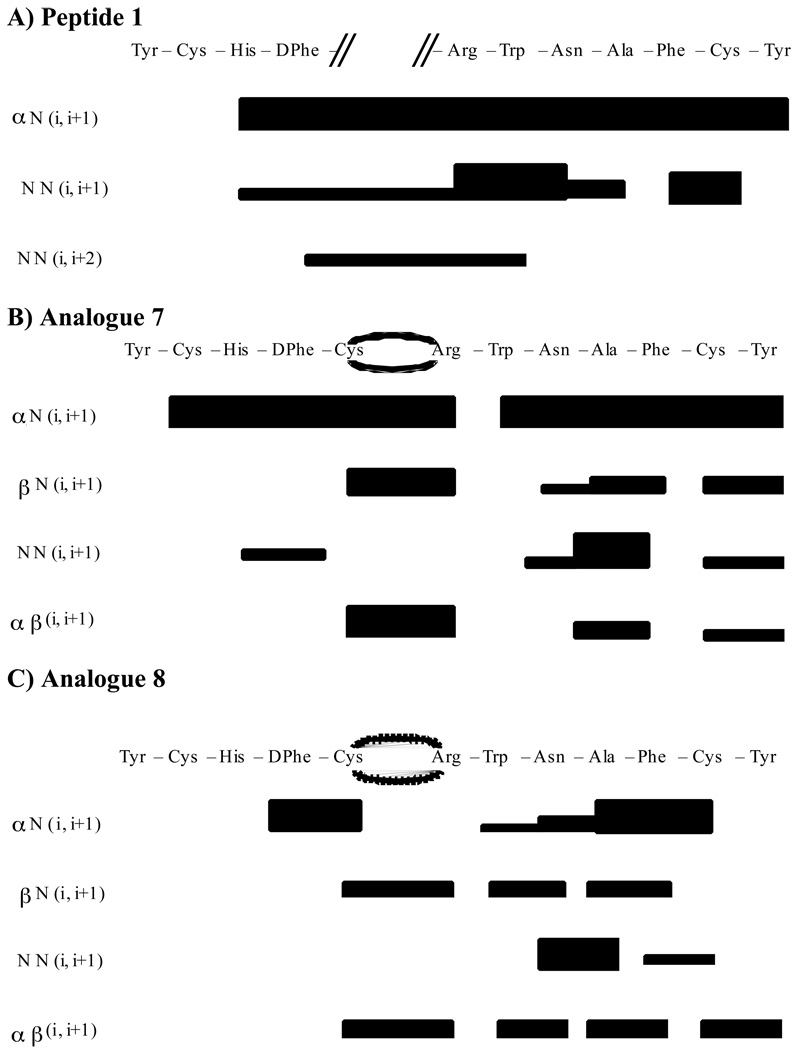
The Nuclear Overhauser Effect (NOE) measures the distance between atoms in space and is useful to characterize peptide secondary structure features like reverse turns. Two-dimensional NOESY experiments can be utilized to identify cross peaks and interactions between protons closer than 5Å in space. Different patterns of NOEs indicate different molecular secondary structures and can be used as tool to identify regular structure in molecules and/or to distinguish structural differences between analogues. Figure 5 summarizes the NOE intensities observed for these peptide analogues. Consistent with chemical shifts above, compounds 7 and 8 have different NOE patterns. As illustrated in Figure 5, compound 7 has more NOE interactions in the His-Phe-Arg-Trp domain than 8. Moreover, compound 7 has regular and strong Hα(i) to N(i+1) NOEs across the entire sequence.
Figure 5.
Summary of the NOE intensities from 400 ms NOESY data observed for molecules A) 1, B) 7, and C) 8. The height of the bar indicates the strength of the NOE. The NOE volumes were categorized as strong (1.8–3.0 Å), medium (1.8–3.5 Å), or weak (1.8–5.0 Å).
Computer-Assisted Molecular Modeling (CAMM)
Relative proton distances derived from the 2D NOESY NMR spectra were used as constraints for conformational studies using CAMM for the reference peptide 1 and analogues 7 and 8. In attempts to avoid the risk of becoming “trapped” in local energy minima induced by the formation of the disulfide bond, we performed restrained molecular-dynamics (RMD) simulations with unambiguous NMR distance restraints before we covalently formed this cyclic constraint. At the end of the initial NOE-based RMD simulations, the Cys amino acid side chains were identified to be located in a favorable low energy orientation to allow for the formation of the disulfide bond. Following the formation of disulfide bond, the analogues were allowed to fully relax by energy minimization before performing a more robust RMD simulation using the cyclized compounds and incorporating all the experimentally identified NMR distance restraints (Figure 5). Conformational families of structures were identified by energy minimizing 200 evenly spaced points along a 10 ns RMD trajectory. The identical procedure was followed for all three compounds studied herein.
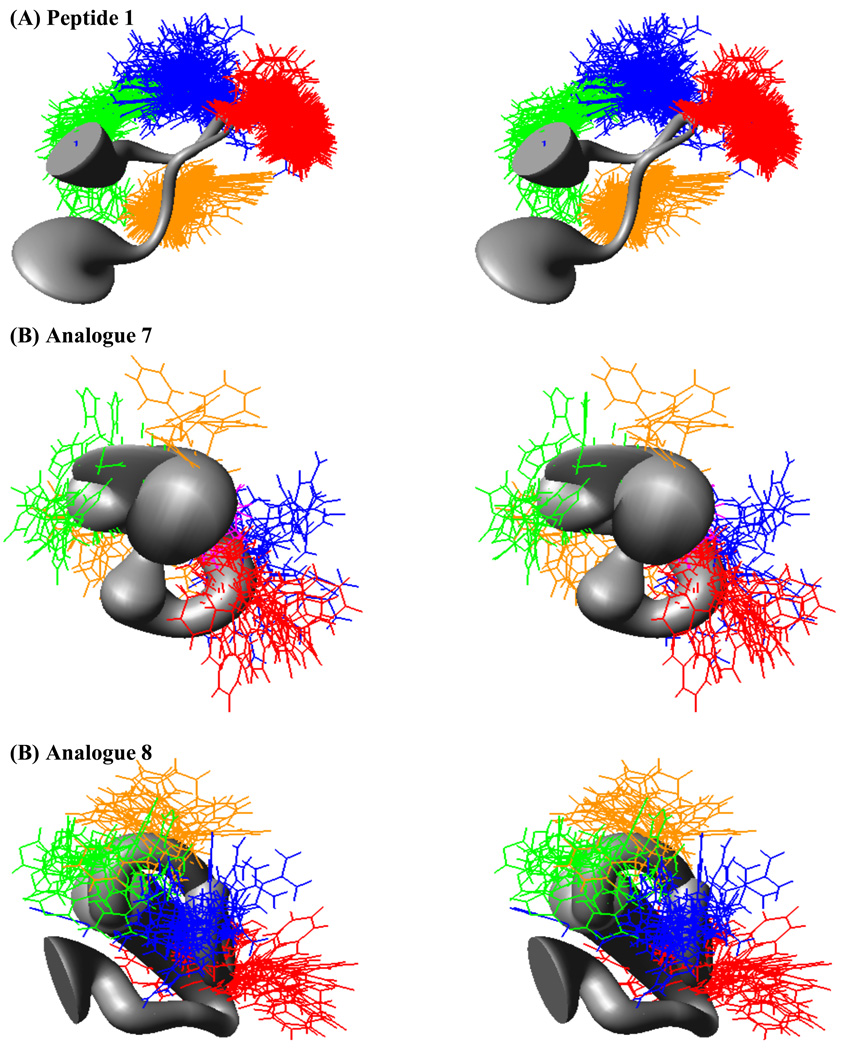
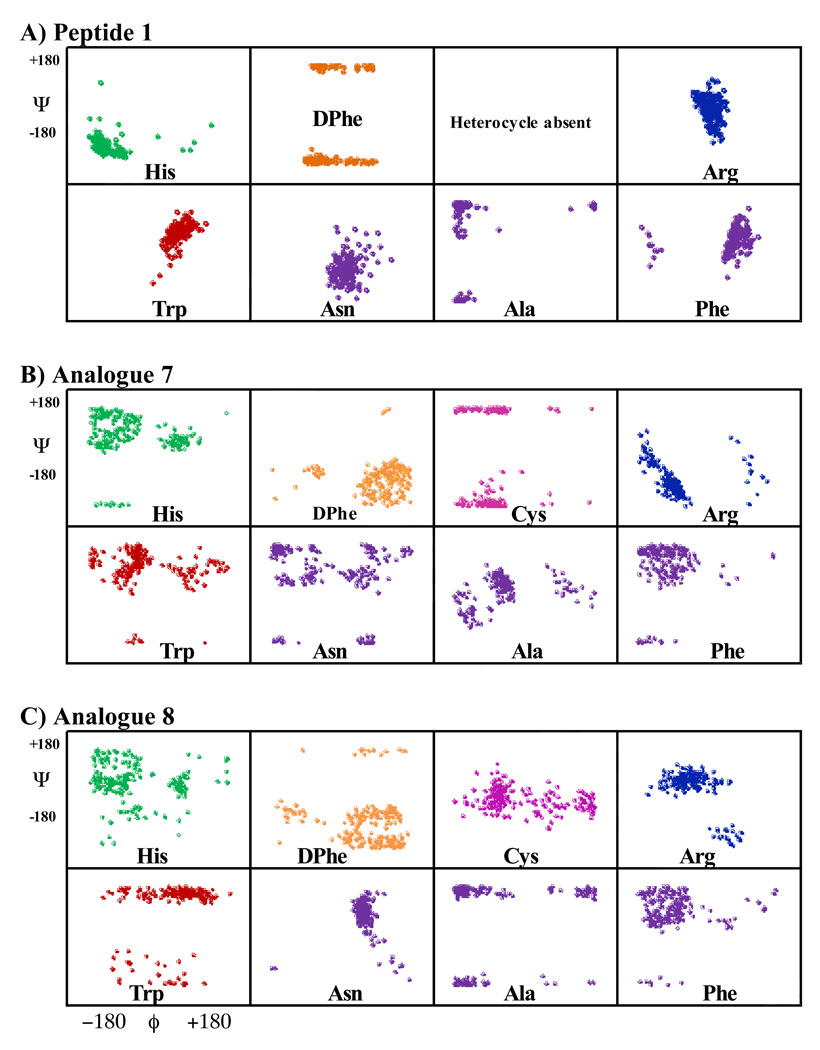
Molecular dynamics simulation experiments of the reference peptide 1 resulted in three major conformational families, with the largest family possessing ca 60% of the sampled conformations. A sausage diagram of the superimposed members of this family is shown in Figure 6. This representative conformational family of 1 has a β-turn structure containing the Arg and Trp residues at i+1 and i+2 positions, respectively, and possesses a β-hairpin-like structure. The phi (ϕ) and psi (ψ) values of the most highly populated 1 representative structure are: ϕ(i+1) ~52, ψ(i+1) ~31, ϕ(i+2) ~95, ψ(i+2) ~106, which may be classified as a type I’ reverse turn [idealized values for this type of turn have been reported as: ϕ(i+1)=60, ψ(i+1)=30, ϕ(i+2)=90, ψ(i+2)=0].59 Figure 7 represents ϕ-ψ distribution of all the residues in the sequence with the exception of the terminal Tyr residues. Comparison of ϕ-ψ distributions of analogues 7 and 8 reveal that while consistent patterns are observed for the His, DPhe, and Phe residues, notable differences are observed for the other residues within the two analogues.
Figure 6.
Stereoview illustration of the “sausage” representations of the backbone superposition of the major family members for (A) reference peptide 1, (B) analogue 7, and (C) analogue 8. The His side chain is indicated in green, the DPhe side chain in orange, the Arg side chain in blue, and the Trp side chain in red.
Figure 7.
Illustrates the CAMM based Phi-Psi angle distribution for the A) reference peptide 1, B) analogue 7, and C) analogue 8 conformational families. The His residue is indicated in green, the DPhe residue in orange, the Arg residue in blue, and the Trp residue in red. All other residues are indicated in purple.
The CAMM structures of analogues 7 and 8 resulted in multiple conformational families, all with very low populations. Therefore a cluster analysis was performed which grouped the structures by backbone (φ, ψ) dihedral angles in the region corresponding to His-DPhe-Arg-Trp residues. Analogue 7 possessed six major families with the largest family having only 9% of the sampled conformations. The total number of conformers of the subfamilies is very small and only represents 36% of the total number of total identified conformers. Analogue 8 possessed four major families, with the most highly populated having only ca 11% of the total sampled population. In summary, both 7 and 8 are highly flexible in comparison to 1.
Discussion
The use of peptidomimetics has emerged as a powerful tool to overcome the inherent limitations of the peptides and can improve a ligands therapeutic potential.60 Linear peptides in their native form can exist in multiple solution phase conformations in equilibrium and, consequently, posses the potential to interact with different shapes at the site of the target receptor. It has been widely recognized that by incorporating structural restrictions (i.e. cyclizations, N-methylation, modified amino acid side chains, and modified backbone amide bonds) into peptides decreased peptide conformational flexibility can result in potent and selective ligands. Using biophysical studies such as 2D NMR and CAMM, “bioactive” conformations of these structurally restricted molecules can be deduced. The approach presented herein, involves the integration of a bioactive heterocyclic peptidomimetic moiety (2)41 into a highly potent and non-selective peptide agonist 146 with the intention to improve the compound potency, receptor selectivity, and perform structure-activity relationship studies. It was also a goal to develop a synthetic strategy so that all the building block assembly chemistry associated with the synthesis of these modified heterocyclic-peptide analogues could be performed using solid phase synthesis. Both structural elements in this novel template possess biological activity at the melanocortin receptors individually but without selectivity (Table 2).
It was hypothesized that by inserting a ten-membered heterocyclic moiety into the peptide amide bond region, the resulting compound could be used as a tool to probe the “bioactive” peptide backbone conformation as well as the amino acid orientation resulting from positioning of the different side chain moieties along the heterocycle (Figure 3). The use of solid phase chemistry for the synthesis in addition to the nM agonist potency at the melanocortin receptors made this peptidomimetic scaffold (2)41 an attractive choice to use as a template. The ten-membered thioether cyclized scaffold was introduced in the peptide template using mild on-resin cyclization method as previously reported.41 Unexpectantly, the heterocycle moiety made the entire compound more flexible than the parent peptide scaffold alone.
The peptide-small molecule hybrid analogue 7 resulted in the most potent full agonist at the melanocortin receptors and possessed a modest 5-fold MC4R versus MC3R selectivity (Table 2). Compounds 7 and 8 differ in the configuration at one stereocenter of the 10-membered ring mimetic (Figure 3) and variances in receptor potency as anticipated. Reversing the orientation of the Cys residue involved in the ring formation allowed us to make subtle changes in the orientation of the Arg moiety. The Arg side chain has been established to play an important role in ligand binding and stimulation of the melanocortin receptors.65 The replacement of Arg with Ala in the MTII cyclic template (Ac-Nle-c[Asp-His-DPhe-Arg-Trp-Lys]-NH2)61 has been reported to cause at least 100-fold drop in affinity at the MC3, MC4 and MC5 receptors due to a lack of interactions between the guanidinyl functionality and melanocortin receptor residues.62 In another example, replacement of Arg with Ala in the linear Ac-His-DPhe-Arg-Trp-NH2 tetrapeptide resulted in decreased potency ranging from 170- to 1,740-fold at the mouse MC1R and MC3R–MC5R.63 Receptor mutagenesis studies have postulated interactions between positively charged basic residues of melanocortin peptides and negatively charged acidic residues in the TM2 and TM3 region of melanocortin receptors.43,64
Orientation of the heterocycle stereocenter in compounds 7 and 8 changes the pharmacology, the NMR spectral parameters, and the resulting conformational families of these two compounds. Contrary to our expectations, incorporation of the heterocycle into the peptide template made both 7 and 8 very flexible. Given the extremely low populations of structures in either compound, it is difficult to perform a direct structural comparison. However, the NMR data that are most distinct between 7 and 8 are for the chemical shift values of arginine. We can speculate that the orientation of the arginine side chain is more important for the potency than the selectivity at the melanocortin receptors since there is at least 10-fold decreased potency for 8 than 7 but the selectivity profile is same in both the compounds.
The reference peptide 1 possessed a relatively constrained structure in the molecular dynamics studies, as compared to 7 and 8. Superposition of conformers of the major family of 1 was relatively good (backbone RMSD = 1.80 ± 0.54 Å in the H,F,RW region RMSD=0.66 +/−0.30 A). A β –hairpin-like structure was identified in 1, which was consistent with structure obtained for similar peptides having lactam bridge instead of disulfide bridge.42 A putative type I’ β-turn was identified based on phi-psi dihedral angles involving Arg and Trp residues.
In conclusion, to our knowledge, this is the first report of integrating a bioactive heterocyclic turn moiety into a highly potent but non-selective melanocortin receptor peptide agonist. The synthesis of six analogues and the assessment of their efficacy in stimulating melanocortin receptors resulted in analogues with nM potency to inactive molecules. This study has resulted in the identification of a novel lead compound (7) for further structure-activity studies, both from the in vitro ligand-receptor standpoint as well as providing novel tools to study the in vivo melanocortin pathways.
Experimental Section
Reagents and General Methods
p-Methylbenzhydrylamine Resin (p-MBHA Resin, 0.47 mequiv/g substitution), Nα 9-fluorenylmethoxycarbonyl (Fmoc) protected amino acids Cys(Trt), Tyr(tBu), Arg(Pbf), His(Trt), Phe, Asn(Trt), Ala and the coupling reagents: benzotriazol-1-yl-N-oxy-tris- (dimethylamino)phosphonium hexafluorophosphate (BOP), and 1-hydroxybenzotriazole (HOBt) were purchased from Peptides International (Louisville, KY). Fmoc-Arg(Boc)2-OH was obtained form the Bachem (Torrance, CA). Glacial acetic acid (HOAc), dichloromethane (DCM), methanol (MeOH), acetonitrile (ACN), and anhydrous ethyl ether were purchased from Fisher (Fair Lawn, NJ). N,N Dimethylformamide (DMF) was purchased from Burdick and Jackson (McGaw Park, IL). Trifluoroacetic acid (TFA), 1,3-diisopropylcarbodiimide (DIC), and piperidine were purchased from Sigma (St. Louis, MO). N,N-Diisopropylethylamine (DIEA) was purchased from Aldrich (Milwaukee, WI). All reagents and chemicals were ACS grade or better and were used without further purification.
Solid-phase reactions were performed using a manual synthesis reaction vessel (flat-bottom polyethylene syringes equipped with sintered Teflon filters, Teflon valves for flow control, and suction to drain the syringes from below, at room temperature. Amino acid couplings consisted of the following steps: (i) removal of the Nα- Fmoc group by 20% piperidine in DMF (1 × 2 min, 1 × 18 min) (ii) single 2 h coupling of Fmoc-amino acid (3 equiv) using BOP (3 equiv), HOBt (3 equiv), and DIEA (6 equiv) in DMF. The presence or absence of the Nα free amino group was monitored using the Kaiser test. After the completed synthesis, the peptides were cleaved from the resin and deprotected using a cleavage cocktail consisting of 82.5% TFA, 5% H2O, 5% EDT, 5% phenol, and 2.5% TIS for 3 h at room temperature. After cleavage and side chain deprotection, the solution was concentrated and the peptide was precipitated and washed using cold (4 °C), anhydrous diethyl ether. The crude, linear peptides were purified by reversed-phase HPLC using a Shimadzu chromatography system with a photodiode array detector and a semi preparative RP-HPLC C18 bonded silica column (Vydac 218TP1010, 1.0 ×25 cm).
Reactions at elevated temperatures were conducted in automated synthesizer (Advanced ChemTech 440MOS, Louisville, KY). MALDI-TOF spectra were recorded on a Voyager MALDI-TOF MS, using α -cyano-4-hydroxycinnamic acid as matrix. TLC plates used were Merck silica gel 60F254 on aluminum. Visualization was achieved with UV light.
General Procedure for Preparation of Weinreb Amides
To a dried flask flushed with N2 gas, the Fmoc-protected amino acid (3 mmol), BOP (3.3 mmol), N,N’-diisoproplethylamine (DIEA, 4.4 mmol) and 6 mL of DMF was added. After stirring for 30 min at room temperature, N,O-dimethylhydroxylamine (3.3 mmol) and DIEA (3.3 mmol) and an additional 4 mL DMF were added and mixed for 3h. Subsequently, 40 mL ethyl acetate was added to the reaction mixture and washed with saturated sodium bicarbonate solution, a 1 M potassium hydrogen sulfate (KHSO4) solution, and brine. The organic layer was dried over MgSO4, concentrated and used in the next step without further purification.
General Procedure for Reduction of Weinreb Amides
The Weinreb amide was dissolved in dry THF (20 mL) and added drop wise to a suspension of LiAlH4 (2 equiv) in THF (30 mL) at −78 °C. The suspension was stirred at −78 °C for 1–2 h, or when judged complete by TLC, the reaction was quenched at −78 °C with water (3 mL). Subsequently, MgSO4 was added and the solution was filtered. The solvent was removed in vacuo to give an oil or a sticky solid. Titration with diethyl ether usually resulted in a white solid, which was essentially pure and could be used directly without further purification in the next step.
Peptide Synthesis
The peptides were assembled on Rink Amide MBHA resin purchased from Peptides International (Louisville, KY). The synthesis (0.2 mmol scale) was performed using a manual synthesis reaction vessel. Each synthetic cycle consisted of the following steps: (i) removal of the Nα- Fmoc group by 20% piperidine in DMF (1 × 2 min, 1 × 20 min) (ii) single 2 h coupling of Fmoc-amino acid (3 equiv) using BOP (3 equiv), HOBt (3 equiv), and DIEA (6 equiv) in DMF and repeated until the peptide synthesis was complete. The presence or absence of the Nα-free amino group was monitored using the Kaiser test.55
Disulfide bridge formation in solution
The linear peptides were cleaved from the resin and fully deprotected using a cleavage cocktail consisting of 91% TFA, 3% TIS, 3% EDT, and 3% H2O for 3 h at room temperature. After cleavage and side chain deprotection, the solution was concentrated and the peptide was precipitated and washed using cold (4°C), anhydrous diethyl ether. The crude linear peptides were dissolved in 20 % DMSO in water (1.0 mg/mL) and stirred at room temperature. Progress of the disulfide cyclization was monitored by UV-HPLC that generally completed in 24–36 h. The resulting solution was lyophilized to give the crude cyclic peptide and purified by reversed-phase HPLC using a Shimadzu chromatography system with a photodiode array detector and a semi preparative RP-HPLC C18 bonded silica column (Vydac 218TP1010, 1.0 × 25 cm). The purified peptides were at least >95% pure as determined by RP-HPLC (at a wavelength of 214 nm) in two diverse solvent systems and had the correct molecular mass (University of Florida Protein Core Facility), Table 3.
Cell Culture and Transfection
The HEK-293 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal calf serum and seeded 1 day prior to transfection at 1–2 × 106 cell/100-mm dish. Melanocortin receptor DNA in the pCDNA3 expression vector (20 µg) were transfected using the calcium phosphate method.65 Stable receptor populations were generated using G418 selection (1 mg/mL) for subsequent bioassay analysis.
Functional Bioassay
The HEK-293 cells stably expressing the mouse melanocortin receptors were transfected with 4µg CRE/β-galactosidase reporter gene as previously described.39,43,57 Briefly, 5,000 to 15,000 post-transfection cells were plated into collagen treated 96-well plates (Nunc) and incubated overnight. Forty-eight hours post-transfection the cells were stimulated with 100 µL of peptide (10−4 to 10−12 M) or forskolin (10−4 M) control in assay medium (DMEM containing 0.1 mg/mL BSA and 0.1 mM isobutylmethylxanthine) for 6 h. The assay media was aspirated and 50 µL of lysis buffer (250 mM Tris-HCl pH=8.0 and 0.1% Triton X-100) was added. The plates were stored at −80 °C overnight. The plates containing the cell lysates were thawed the following day. Aliquots of 10 µL were taken from each well and transferred to another 96-well plate for relative protein determination. To the cell lysate plates, 40 µL of phosphate-buffered saline with 0.5% BSA was added to each well. Subsequently, 150 µL of substrate buffer (60 mM sodium phosphate, 1 mM MgCl2, 10 mM KCl, 5 mM β-mercaptoethanol, 2 mg/ mL ONPG) was added to each well and the plates were incubated at 37°C. The sample absorbance, OD405, was measured using a 96-well plate reader (Molecular Devices). The relative protein was determined by adding 200 µL of 1:5 dilution Bio Rad G250 protein dye:water to the 10 µL cell lysate sample taken previously, and the OD595 was measured on a 96-well plate reader (Molecular Devices). Data points were normalized both to the relative protein content and non-receptor dependent forskolin stimulation. Maximal efficacy was compared to that observed for the NDP-MSH control peptide tested simultaneously on each 96-well plate. All compounds that were observed to result in full agonists with the same maximal efficacy as observed for the NDP-MSH control.
Data Analysis
The EC50 values represent the mean of duplicate wells performed in quadruplet or more independent experiments. EC50 value estimates, and their associated standard errors, were determined by fitting the data to a nonlinear least-squares analysis using the PRISM program (v4.0, GraphPad Inc.). The results are not corrected for peptide content.
NMR Spectroscopy
Peptide NMR samples were prepared by dissolving 1.0–2.0 mg of peptide in a 750 µL solution containing 400 µL of CD3CN and 350 µL of H2O, and adding DSS as an internal standard (0.0 ppm). The NMR data were collected using Bruker Avance spectrometer operating at 500 MHz at the Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) facility at the University of Florida. Standard proton TOCSY and NOESY 2D NMR data were collected, processed, and analyzed as described previously.66 NOESY data were collected at both 250 and 400 ms mixing times, and proton-proton distances were obtained from the 400 ms dataset. The chemical shifts of each of the peptides in this study were assigned using standard TOCSY and NOESY 1H-based strategies.67 This approach utilizes TOCSY spectra to identify resonances within a given amino acid and NOESY spectra to correlate one amino acid with the next through interactions of the α and/or β protons of residue i with the amide proton of residue i + 1.
Computer-Assisted Molecular Modeling (CAMM)
Proton-proton distances were calibrated using the well resolved methylene protons based on the relationship r= rref × (ηref/η)1/6, where r is the distance between atoms, η is the NOESY cross-peak volume, rref is the known distance, and ηref is the corresponding volume of the NOESY calibration cross-peak. The NOE volumes were categorized as strong (1.8–3.0 Å), medium (1.8–3.5 Å), or weak (1.8–5.0 Å). All conformational modeling was performed using SYBYL v7.0 software from Tripos Inc. (St. Louis, MO) on a Silicon Graphics workstation. Restrained molecular dynamics (RMD) simulations were run in vacuo with a dielectric constant of 4.0 and a temperature of 500 K using the Tripos force field and Gastaiger-Hückel partial atomic charges. The peptides were built in fully extended linear conformations and in the first step of modeling, RMD simulations were run for 1 ns. Following the initial 1 ns RMD trajectory, the cysteine residues were oriented next to each other, and disulfide bonds were manually formed and energy minimized without restraints. Finally, all the NOE restraints were included, and 10 ns RMD trajectories were collected. Following the RMD simulations, structures from 200 equally spaced points along the dynamics trajectory were energy minimized, analyzed, and grouped into conformational families. The clustering was done by comparison of backbone φ and ψ dihedral angles in the region corresponding to His-DPhe-Arg-Trp residues.
Acknowledgements
This work has been supported by NIH Grant DK064250.
List of Abbreviations
- ACTH
Adrenocorticotropin Hormone
- AGRP
Agouti-Related Protein
- ASIP
agouti-signaling protein
- BAL
Backbone amide linker resin
- CAMM
computer-assisted molecular modeling
- cAMP
cyclic 5’-adenosine monophosphate
- DCM
Dichloromethane
- DMF
N,N-Dimethylformamide
- DMS
Dimethylsulfide
- Fmoc
Nα 9-fluorenylmethoxycarbonyl
- GPCR
G Protein Coupled Receptor
- MC1R
Melanocortin-1 Receptor
- MC2R
Melanocortin-2 Receptor
- MC3R
Melanocortin-3 Receptor
- MC4R
Melanocortin-4 Receptor
- MC5R
Melanocortin-5 Receptor
- MCR
Melanocortin Receptor
- MeOH
Methanol
- MSH
Melanocyte Stimulating Hormone
- nM
Nanomolar
- NOE
Nuclear Overhauser Effect
- POMC
Proopiomelanocortin
- ϕ
phi
- ψ
psi
- SAR
Structure Activity Relationship
- SEM
Standard Error of the Mean
- TFA
Trifluoroacetic acid
- TM
transmembrane
- α-MSH
Alpha-Melanocyte Stimulating Hormone
- β-MSH
Beta-Melanocyte Stimulating Hormone
- γ-MSH
Gamma-Melanocyte Stimulating Hormone
- µM
Micromolar
- RMD
restrained molecular dynamics
- NDP-MSH
Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2
- AM3-130 (1)
Tyr-c[Cys-His-DPhe-Arg-Trp-Asn-Ala-Phe-Cys]-Tyr-NH2
References
- 1.Lerner AB, McGuire JS. Effect of Alpha- and Beta-Melanocyte Stimulating Hormones on the Skin Colour of Man. Nature. 1961;189:176–179. doi: 10.1038/189176a0. [DOI] [PubMed] [Google Scholar]
- 2.Lindner E, Scholkens B. ACTH and alpha-MSH: cardiovascular and antiarrhythmic properties. Arch Int Pharmacodyn Ther. 1974;208:19–23. [PubMed] [Google Scholar]
- 3.Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O'Rahilly S, Farooqi IS. Modulation of Blood Pressure by Central Melanocortinergic Pathways. N. Engl. J Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 4.Bertolini A, Vergoni W, Gessa GL, Ferrari W. Induction of Sexual Excitement by the Action of Adrenocorticotrophic Hormone in Brain. Nature. 1969;221:667–669. doi: 10.1038/221667a0. [DOI] [PubMed] [Google Scholar]
- 5.Wessells H, Fuciarelli K, Hansen J, Hadley ME, Hruby VJ, Dorr R, Levine N. Synthetic Melanotropic Peptide Initiates Erections in Men with Psychogenic Erectile Dysfunction: Double-blind, Placebo Controlled Crossover Study. J. Urol. 1998;160:389–393. [PubMed] [Google Scholar]
- 6.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of Melanocortinergic Neurons in Feeding and the Agouti Obesity Syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- 7.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Smith FJ, Kesterson RA, Boston BA, Fang Q, Berkemeir LR, Gu W, Cone RD, Campfield LA, Lee F. Targeted Disruption of the Melanocortin-4 Receptor Results in Obesity in Mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 8.Chhajlani V, Wikberg JES. Molecular Cloning and Expression of the Human Melanocyte Stimulating Hormone Receptor cDNA. FEBS Lett. 1992;309:417–420. doi: 10.1016/0014-5793(92)80820-7. [DOI] [PubMed] [Google Scholar]
- 9.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The Cloning of a Family of Genes that Encode the Melanocortin Receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 10.Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD. Identification of a Receptor for γ Melanotropin and Other Proopiomelanocortin Peptides in the Hypothalamus and Limbic System. Proc. Natl. Acad. Sci. USA. 1993;90:8856–8860. doi: 10.1073/pnas.90.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the Melanocortin-4 Receptor (MC4-R) in Neuroendocrine and Autonomic Control Circuits in the Brain. Mol. Endo. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 12.Gantz I, Konda Y, Tashiro T, Shimoto Y, Miwa H, Munzert G, Watson SJ, DelValle J, Yamada T. Molecular Cloning of a Novel Melanocortin Receptor. J. Biol. Chem. 1993;268:8246–8250. [PubMed] [Google Scholar]
- 13.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. Molecular Cloning, Expression, and Gene Localization of a Fourth Melanocortin Receptor. J. Biol. Chem. 1993;268:15174–15179. [PubMed] [Google Scholar]
- 14.Gantz I, Shimoto Y, Konda Y, Miwa H, Dickinson CJ, Yamada T. Molecular Cloning, Expression, and Characterization of a Fifth Melanocortin Receptor. Biochem. Biophys. Res. Commun. 1994;200:1214–1220. doi: 10.1006/bbrc.1994.1580. [DOI] [PubMed] [Google Scholar]
- 15.Eipper BA, Mains RE. Structure and Biosynthesis of Pro-ACTH/Endorphin and Related Peptides. Endocrin. Rev. 1980;1:1–26. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- 16.Smith AI, Funder JW. Proopiomelanocortin Processing in the Pituitary, Central Nervous System and Peripheral Tissues. Endocrin. Rev. 1988;9:159–179. doi: 10.1210/edrv-9-1-159. [DOI] [PubMed] [Google Scholar]
- 17.Bultman SJ, Michaud EJ, Woychick RP. Molecular Characterization of the Mouse Agouti Locus. Cell. 1992;71:1195–1204. doi: 10.1016/s0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- 18.Ollmann MM, Wilson BD, Yang Y-K, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of Central Melanocortin Receptors in Vitro and in Vivo by Agouti-Related Protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 19.Castrucci AML, Hadley ME, Sawyer TK, Wilkes BC, Al-Obeidi F, Staples DJ, DeVaux AE, Dym O, Hintz MF, Riehm J, Rao KR, Hruby VJ. α-Melanotropin: The Minimal Active Sequence in the Lizard Skin Bioassay. Gen. Comp. Endocrinol. 1989;73:157–163. doi: 10.1016/0016-6480(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 20.Hruby VJ, Wilkes BC, Hadley ME, Al-Obeidi F, Sawyer TK, Staples DJ, DeVaux A, Dym O, Castrucci AM, Hintz MF, Riehm JP, Rao KR. α-Melanotropin: The Minimal Active Sequence in the Frog Skin Bioassay. J. Med. Chem. 1987;30:2126–2130. doi: 10.1021/jm00394a033. [DOI] [PubMed] [Google Scholar]
- 21.Haskell-Luevano C, Sawyer TK, Hendrata S, North C, Panahinia L, Stum M, Staples DJ, Castrucci AM, Hadley ME, Hruby VJ. Truncation Studies of α-Melanotropin Peptides Identifies Tripeptide Analogues Exhibiting Prolonged Agonist Bioactivity. Peptides. 1996;17:995–1002. doi: 10.1016/0196-9781(96)00141-6. [DOI] [PubMed] [Google Scholar]
- 22.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A Unique Metabolic Syndrome Causes Obesity in the Melanocortin-3 Receptor-deficient Mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 23.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, Metzger JM, Strack AM, Camacho RE, Mellin TN, Nunes CN, Min W, Fisher J, Gopal-Truter S, MacIntyre DE, Chen HY, Van Der Ploeg LH. Inactivation of the Mouse Melanocortin-3 Receptor Results in Increased Fat Mass and Reduced Lean Body Mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD. Exocrine Gland Dysfunction In MC5-R Deficient Mice: Evidence For Coordinated Regulation Of Exocrine Gland Functions By Melanocortin Peptides. Cell. 1997;91:789–798. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 25.Haskell-Luevano C, Monck EK. Agouti-related Protein (AGRP) Functions as an Inverse Agonist at a Constitutively Active Brain Melanocortin-4 Receptor. Regulatory Peptides. 2001;99:1–7. doi: 10.1016/s0167-0115(01)00234-8. [DOI] [PubMed] [Google Scholar]
- 26.Nijenhuis WA, Oosterom J, Adan RA. AGRP(83–132) Acts as an Inverse Agonist on the Human-Melanocortin-4 Receptor. Mol. Endocrinol. 2001;15:164–171. doi: 10.1210/mend.15.1.0578. [DOI] [PubMed] [Google Scholar]
- 27.Al-Obeidi F, Hadley ME, Pettitt BM, Hruby VJ. Design of a New Class of Superpotent Cyclic α-Melanotropins Based on Quenched Dynamic Stimulations. J. Am. Chem. Soc. 1989;111:3413–3416. [Google Scholar]
- 28.Sawyer TK, Hruby VJ, Darman PS, Hadley ME. [half-Cys4,half-Cys10]-α-Melanocyte-Stimulating Hormone: A Cyclic a-Melanotropin Exhibiting Superagonist Biological Activity. Proc. Natl. Acad. Sci. USA. 1982;79:1751–1755. doi: 10.1073/pnas.79.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hess S, Linde Y, Ovadia O, Safrai E, Shalev DE, Swed A, Halbfinger E, Lapidot T, Winkler I, Gabinet Y, Faier A, Yarden D, Xiang Z, Portillo FP, Haskell-Luevano C, Gilon C, Hoffman A. Backbone Cyclic Peptidomimetic Melanocortin-4 Receptor Agonist as a Novel Orally Administrated Drug Lead for Treating Obesity. J. Med. Chem. 2008;51:1026–1034. doi: 10.1021/jm701093y. [DOI] [PubMed] [Google Scholar]
- 30.Hruby VJ, Lu D, Sharma SD, Castrucci AML, Kesterson RA, Al-Obeidi FA, Hadley ME, Cone RD. Cyclic Lactam α-Melanotropin Analogues of Ac-Nle4-c[Asp5, DPhe7, Lys10]-α-MSH(4–10)-NH2 With Bulky Aromatic Amino Acids at Position 7 Show High Antagonist Potency and Selectivity at Specific Melanocortin Receptors. J. Med. Chem. 1995;38:3454–3461. doi: 10.1021/jm00018a005. [DOI] [PubMed] [Google Scholar]
- 31.Haskell-Luevano C, Toth K, Boteju L, Job C, Castrucci AML, Hadley ME, Hruby VJ. β-Methylation of Phe7 and Trp9 Melanotropin Side Chain Pharmacophores Affect Ligand-Receptor Interactions and Prolonged Biological Activity. J. Med. Chem. 1997;40:2740–2749. doi: 10.1021/jm970018t. [DOI] [PubMed] [Google Scholar]
- 32.Todorovic A, Holder JR, Scott JW, Haskell-Luevano C. Synthesis and Activity of the Melanocortin Xaa-DPhe-Arg-Trp-NH2 Tetrapeptides with Amide Bond Modifications. J. Pept. Res. 2004;63:270–278. doi: 10.1111/j.1399-3011.2004.00137.x. [DOI] [PubMed] [Google Scholar]
- 33.Doedens L, Opperer F, Cai M, Beck JG, Dedek M, Palmer E, Hruby VJ, Kessler H. Multiple N-Methylation of MT-II Backbone Amide Bonds Leads to Melanocortin Receptor Subtype hMC1R Selectivity: Pharmacological and Conformational Studies. J. Am. Chem. Soc. 2010;132:8115–8128. doi: 10.1021/ja101428m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawyer TK, Sanfillippo PJ, Hruby VJ, Engel MH, Heward CB, Burnett JB, Hadley ME. 4-Norleucine, 7-D-Phenylalanine-α-Melanocyte-Stimulating Hormone: A Highly Potent α-Melanotropin with Ultra Long Biological Activity. Proc. Natl. Acad. Sci. USA. 1980;77:5754–5758. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haskell-Luevano C, Boteju LW, Miwa H, Dickinson C, Gantz I, Yamada T, Hadley ME, Hruby VJ. Topographical Modifications of Melanotropin Peptide Analogues with β-Methyltryptophan Isomers at Position 9 Leads to Differential Potencies and Prolonged Biological Activities. J. Med. Chem. 1995;38:4720–4729. doi: 10.1021/jm00023a012. [DOI] [PubMed] [Google Scholar]
- 36.Hruby VJ, Li G, Haskell-Luevano C, Shenderovich M. Design of Peptides, Proteins, and Peptidomimetics in Chi Space. Biopolymers. Peptide Science. 1997;43:219–266. doi: 10.1002/(SICI)1097-0282(1997)43:3<219::AID-BIP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 37.Verhelst SH, Witte MD, Arastu-Kapur S, Fonovic M, Bogyo M. Novel Aza Peptide Inhibitors and Active-Site Probes of Papain-Family Cysteine Proteases. Chembiochem. 2006;7:943–950. doi: 10.1002/cbic.200600001. [DOI] [PubMed] [Google Scholar]
- 38.Holder JR, Bauzo RM, Xiang Z, Scott JW, Haskell-Luevano C. Design and Pharmacology of Peptoids and Peptide-Peptoid Hybrids Based on the Melancortin Agonist Core Tetrapeptide Sequence. Bioorg. Med. Chem. Lett. 2003;13:4505–4509. doi: 10.1016/j.bmcl.2003.08.078. [DOI] [PubMed] [Google Scholar]
- 39.Wilczynski A, Wang XS, Joseph CG, Xiang Z, Bauzo RM, Scott JW, Sorensen NB, Shaw AM, Millard WJ, Richards NG, Haskell-Luevano C. Identification of Putative Agouti-Related Protein(87–132)-Melanocortin-4 Receptor Interactions by Homology Molecular Modeling and Validation Using Chimeric Peptide Ligands. J. Med. Chem. 2004;47:2194–2207. doi: 10.1021/jm0303608. [DOI] [PubMed] [Google Scholar]
- 40.Wilczynski A, Wilson KR, Scott JW, Edison AS, Haskell-Luevano C. Structure-Activity Relationships of the Unique and Potent Agouti-Related Protein (AGRP)-Melanocortin Chimeric Tyr-c[β-Asp-His-DPhe-Arg-Trp-Asn-Ala-Phe-Dpr]-Tyr-NH2 Peptide Template. J. Med. Chem. 2005;48:3060–3075. doi: 10.1021/jm049010r. [DOI] [PubMed] [Google Scholar]
- 41.Bondebjerg J, Xiang Z, Bauzo RM, Haskell-Luevano C, Meldal M. A Solid Phase Approach to Mouse Melanocortin Receptor Agonists Derived From a Novel Thioether Cyclized Peptidomimetic Scaffold. J. Am. Chem. Soc. 2002;124:11046–11055. doi: 10.1021/ja0123913. [DOI] [PubMed] [Google Scholar]
- 42.Tota MR, Smith TS, Mao C, MacNeil T, Mosley RT, Van der Ploeg LHT, Fong TM. Molecular Interaction of Agouti Protein and Agouti-Related Protein with Human Melanocortin Receptors. Biochemistry. 1999;38:897–904. doi: 10.1021/bi9815602. [DOI] [PubMed] [Google Scholar]
- 43.Haskell-Luevano C, Cone RD, Monck EK, Wan Y-P. Structure Activity Studies of the Melanocortin-4 Receptor by In Vitro Mutagenesis: Identification of Agouti-Related Protein (AGRP), Melanocortin Agonist and Synthetic Peptide Antagonist Interaction Determinants. Biochemistry. 2001;40:6164–6179. doi: 10.1021/bi010025q. [DOI] [PubMed] [Google Scholar]
- 44.Jackson PJ, Yu B, Hunrichs B, Thompson DA, Chai B, Gantz I, Millhauser GL. Chimeras of the Agouti-Related Protein: Insights into Agonist and Antagonist Selectivity of Melanocortin Receptors. Peptides. 2005;26:1978–1987. doi: 10.1016/j.peptides.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 45.Xiang Z, Litherland SA, Sorensen NB, Proneth B, Wood MS, Shaw AM, Millard WJ, Haskell-Luevano C. Pharmacological Characterization of 40 Human Melanocortin-4 Receptor Polymorphisms with the Endogenous Proopiomelanocortin-Derived Agonists and the Agouti-Related Protein (AGRP) Antagonist. Biochemistry. 2006;45:7277–7288. doi: 10.1021/bi0600300. [DOI] [PubMed] [Google Scholar]
- 46.Xiang Z, Pogozheva ID, Sorenson NB, Wilczynski AM, Holder JR, Litherland SA, Millard WJ, Mosberg HI, Haskell-Luevano C. Peptide and Small Molecules Rescue the Functional Activity and Agonist Potency of Dysfunctional Human Melanocortin-4 Receptor polymorphisms. Biochemistry. 2007;46:8273–8287. doi: 10.1021/bi7007382. [DOI] [PubMed] [Google Scholar]
- 47.Xiang Z, Proneth B, Dirain ML, Litherland SA, Haskell-Leuvano C. Pharmacological Characterization of 30 Human Melanocortin-4 Receptor Polymorphisms with Endogenous Proopiomelanocortin Derived Agonists, Synthetic Agonists, and the Endogenous Agouti-Related Protein (AGRP) Antagonist. Biochemistry. 2010;49:4583–4600. doi: 10.1021/bi100068u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hruby VJ, Wilkes BC, Cody WL, Sawyer TK, Hadley ME. Melanotropins: Structural, Conformational and Biological Considerations in the Development of Superpotent and Superprolonged Analogs. Peptide Protein Rev. 1984;3:1–64. [Google Scholar]
- 49.Carpino LA, Han GY. The 9-Fluorenylmethoxycarbonyl Function, a New Base-Sensitive Amino-Protecting Group. J. Am. Chem. Soc. 1970;92:5748–5749. [Google Scholar]
- 50.Carpino LA, Han GY. The 9-Fluorenylmethyloxycarbonyl Amino-Protecting Group. J. Org. Chem. 1972;37:3404–3409. [Google Scholar]
- 51.Wang G, Mahesh U, Chen GYJ, Yao SQ. Solid-Phase Synthesis of Peptide Vinyl Sulfones as Potential Inhibitors and Activity-Based Probes of Cysteine Proteases. Org. Lett. 2003;5:737–740. doi: 10.1021/ol0275567. [DOI] [PubMed] [Google Scholar]
- 52.Nahm SS, Weinreb MS. N-Methyoxy-n-Methylamides as Effective Acylating Agents. Tetrahedron Lett. 1981;22:3815–3818. [Google Scholar]
- 53.Wen JJ, Crews CM. Synthesis of 9-Fluorenylmetoxycarbonyl-Procted Amino Aldehydes. Tetrahedron Asymmetry. 1998;9:1855–1858. [Google Scholar]
- 54.Ede NJ, Eagle SN, Wickham G, Bray AM, Warne B, Shoemaker K, Rosenberg S. Solid phase synthesis of peptide aldehyde protease inhibitors. Probing the proteolytic sites of hepatitis C virus polyprotein. J. Pept. Sci. 2000;6:11–18. doi: 10.1002/(SICI)1099-1387(200001)6:1<11::AID-PSC229>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 55.Kaiser E, Colescott RL, Bossinger CD, Cook PI. Color Test for Detection of Free Terminal Amino Groups in the Solid-Phase Synthesis of Peptides. Anal. Biochem. 1970;34:595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- 56.Stewart JM, Young JD. Solid Phase Peptide Synthesis. Second ed. Rockford, Illinois: Pierce Chemical Co.; 1984. [Google Scholar]
- 57.Chen W, Shields TS, Stork PJS, Cone RD. A Colorimetric Assay for Measuring Activation of Gs- and Gq-Coupled Signaling Pathways. Anal. Biochem. 1995;226:349–354. doi: 10.1006/abio.1995.1235. [DOI] [PubMed] [Google Scholar]
- 58.Wishart DS, Sykes BD. Chemical Shifts as a Tool for Structure Determination. Methods Enzymol. 1994;239:363–392. doi: 10.1016/s0076-6879(94)39014-2. [DOI] [PubMed] [Google Scholar]
- 59.Hutchinson EG, Thornton JM. A Revised Set of Potentials for Beta-Turn Formation in Proteins. Protein Sci. 1994;3:2207–2216. doi: 10.1002/pro.5560031206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamman JH, Enslin GM, Kotze AF. Oral Delivery of Peptide Drugs: Barriers and Developments. BioDrugs. 2005;19:165–177. doi: 10.2165/00063030-200519030-00003. [DOI] [PubMed] [Google Scholar]
- 61.Al-Obeidi F, Castrucci AM, Hadley ME, Hruby VJ. Potent and Prolonged Acting Cyclic Lactam Analogues of α-Melanotropin: Design Based on Molecular Dynamics. J. Med. Chem. 1989;32:2555–2561. doi: 10.1021/jm00132a010. [DOI] [PubMed] [Google Scholar]
- 62.Bednarek MA, Silva MV, Arison B, MacNeil T, Kalyani RN, Huang RR, Weinberg DH. Structure-Function Studies on the Cyclic Peptide MT-II, Lactam Derivative of α-melanotropin. Peptides. 1999;20:401–409. doi: 10.1016/s0196-9781(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 63.Holder JR, Xiang Z, Bauzo RM, Haskell-Luevano C. Structure-Activity Relationships of the Melanocortin Tetrapeptide Ac-His-DPhe-Arg-Trp-NH2 at the Mouse Melanocortin Receptors: Part 3 Modifications at the Arg Position. Peptides. 2003;24:73–82. doi: 10.1016/s0196-9781(02)00278-4. [DOI] [PubMed] [Google Scholar]
- 64.Yang Y, Fong TM, Dickinson CJ, Mao C, Li JY, Tota MR, Mosley R, Van Der Ploeg LH, Gantz I. Molecular Determinants of Ligand Binding to the Human Melanocortin-4 Receptor. Biochemistry. 2000;39:14900–14911. doi: 10.1021/bi001684q. [DOI] [PubMed] [Google Scholar]
- 65.Chen CA, Okayama H. Calcium Phosphate-Medicated Gener Transfer: A Highly Efficient Transfections System for Stably Transforming Cells with Plasmid DNA. Biotechniques. 1988;6:632–638. [PubMed] [Google Scholar]
- 66.Thirumoorthy R, Holder JR, Bauzo RM, Richards NGJ, Edison AS, Haskell-Luevano C. Novel Agouti-related Protein (AGRP) Based Melanocortin-1 Receptor Antagonist. J. Med. Chem. 2001;44:4114–4124. doi: 10.1021/jm010215z. [DOI] [PubMed] [Google Scholar]
- 67.Wüthrich K. NMR of Proteins and Nucleic Acids. New York: John Wiley & Sons; 1986. [Google Scholar]