Abstract
The contribution of the stromal microenvironment to the progression of endometrial cancer (EC) has not been well explored. We have conditionally expressed a mutant allele of adenomatous polyposis coli (APCcKO) in murine uterine stroma cells to study its effect on uterine development and function. In addition to metrorrhagia, the mice develop complex atypical endometrial gland hyperplasia that progresses to endometrial carcinoma in situ and endometrial adenocarcinoma as evidenced by myometrial invasion. Stromal cells subjacent to the carcinoma cells express αSMA with fewer cells expressing PDGFR-α compared to normal stromal cells suggesting that the mutant stromal cells have acquired a more myofibroblastic phenotype, which have been described as cancer-associated fibroblasts and have been shown to induce carcinogenesis in other organ systems. Analyses of human EC specimens showed substantial αSMA expression in the stroma compared with normal endometrial stroma cells. We also show that APCcKO mutant uteri and human EC have decreased stromal levels of TGFβ and BMP activities and that the mutant uteri failed to respond to exogenous estradiol stimulation. The mutant stroma cells also had higher levels of VEGF and SDF signaling components and diminished expression of ERα and PR which is common in advanced stages of human EC and is an indicator of poor prognosis. Our results indicate that de novo mutation or loss of heterozygosity in stromal APC is sufficient to induce endometrial hyperplasia and endometrial carcinogenesis by mechanisms that are consistent with unopposed estrogen signaling in the endometrial epithelium.
Introduction
The tissue microenvironment plays an important role in normal organogenesis, tissue homeostasis, and can lead to carcinogenesis when pathologically disrupted (1). For example, tissue recombination studies have shown that combining urogenital sinus mesenchyme with embryonic or adult urinary bladder epithelium changes its fate to prostatic epithelium (2). Similarly, combining uterine mesenchyme with vaginal epithelium causes development of uterine epithelium (3). Remarkably, transplantation of neural stem cells or spermatogenic cells into the mammary fat pad is sufficient to reprogram both into mammary epithelial cells (4, 5). These studies highlight the importance of stromal cells during morphogenesis of adjacent epithelial cells and the ability of stromal signals to direct cell fate determination.
The importance of the microenvironment in cancer has been reported in human studies showing tumor development and progression in the presence of tumor-associated fibroblast cells with genetic mutations in tumor suppressor genes (6). For example, mutations in TP53 and PTEN are frequently present in the stroma of breast carcinomas (7). In accordance with these findings, stromal mutations in CTNNB1 and APC accompanied by nuclear accumulation of β-catenin in stromal cells have been observed in patients with non-metastasizing breast tumors (8). Lastly, fibroblast cells collected from Down's syndrome patients, who have a lower incidence of breast cancer inhibit the proliferation of breast cancer cells in vivo and in vitro (9).
Endometrial cancer (EC) is a one of the most common gynecological malignancies of the female reproductive tract with over 40,000 new cases diagnosed and nearly 8,000 deaths every year (10). Mutations and/or alterations in PTEN, TP53, KRAS, mismatch repair genes, β-catenin, and adenomatous polyposis coli (APC) have all been observed in human EC patients and have been associated with the etiology or progression of this disease (11). Mutations in APC, which are frequently observed in patients with familial colon cancer (12), have been detected in 43% of human EC patients (13). APC is a large multi-domain protein that interacts with several other proteins to regulate various cellular processes including cell proliferation, death, differentiation, migration, and adhesion (12). The best-studied role of APC is its regulation of Wnt signaling by controlling the availability of β-catenin (12). It is still unclear how APC mutations contribute to the development of EC.
In vitro normal human endometrial stromal cells have been shown to decrease proliferation and invasion, and to promote differentiation of endometrial adenocarcinoma cell lines (14, 15). Additionally, some human endometrial polyps, which are benign but have been associated with the development of EC in age dependent manner (16), have genetic mutations in the stromal component (17, 18). However, the tumor-promoting potential of endometrial stroma on EC has not been reported. We have examined the effects of expressing a truncated from of APC that lacks β-catenin binding domains in murine endometrial stromal cells and show endometrial hyperplasia and EC development, indicating that stromal APC plays an important role in controlling the proliferative potential and plasticity of adjacent endometrial epithelium.
Materials and Methods
Mouse genetics and husbandry
The mice used in this study were maintained under standard animal housing conditions. All protocols involving animal experimentation were approved by the Institutional Animal Care and Use Committee at Massachusetts General Hospital. Mice used in this study were maintained on C57BL/6;129/SvEv mixed genetic background. The following parental mice strains: Amhr2tm3(cre)Bhr (Amhr2-Cre) (19), APCflox/flox (20), Gt(ROSA)26Sortm1(EYFP)Cos (Yfpflox/flox) Gt(ROSA)26Sortm1Sor (LacZ) (both from Jackson Labs, Bar Harbor ME) were mated to generate Amhr2-Cre/+;APCflox/flox (APCcko), Amhr2-Cre/+;APCflox/+, APCflox/flox, Amhr2-Cre/+;Gt(ROSA)26Sortm1(EYFP)Cos, Amhr2-Cre/+;LacZ mice. The genotyping of mice was performed with standard PCR protocols using DNA collected from tail biopsies. The gross images were taken with a Nikon SMZ1500 microscope equipped with an attached Spot camera (Diagnostic Instruments, Sterling Heights, MI) or with a Nikon D60 camera with a macro lens.
Estradiol treatments
To investigate E2 response, control (APCflox/flox, n=4) and APCcko (n=5) mice were injected intramuscularly with 10 ug of a depo formulation of E2 (Estradiol valerate, JHP Pharmaceuticals, Rochester, MI) one week post-oophorectomy. Animals were euthanized 30 days later and their uteri were collected for further analyses.
Laser capture microdissection and genomic PCR
For laser-cut microdissection (MMI Cellcut from Molecular Devices, Sunnyvale, CA), 10 um sections of frozen uterine EC tissue from APCcko mice was mounted on PEP-membrane slides. Genomic DNA from microdissected cells from uterine epithelium and stroma and from ovary, oviduct, uterus and tails was collected using DNeasy (Qiagen, Valencia, CA). The recombined and flox APC alleles (500 and 430 bp, respectively) were detected by PCR with the following primers Apc-Int13F2 (GAGAAACCCTGTCTCGAAAAAA), Apc-Int14R4 (TTGGCAGACTGTGTATATAAGC), Apc-Int13R2 (AGTGCTGTTTCTATGAGTCAAC) as described (20).
Human EC tissue analyses
Paraffin-embedded human EC (n=9) and normal/benign endometrium (n=4) were obtained from the Department of Pathology, MGH using Institutional Review Board-approved protocols. Sections were cut and stained for αSMA, PR, ERα pSmad 1/5/8 and pSmad2.
Histological analyses, immunofluorescence (IF) and immunohistochemistry (IHC)
Tissues were collected at different developmental stages and fixed by immersion in 4% paraformaldehyde for 10–12 h at 4C, then transferred to 70% ethanol until processing. H&E staining was performed using standard protocols. The methods used to perform IF/IHC are described in our previous studies (21, 22). The primary and secondary antibodies used in this study are β-catenin (BD Transduction Laboratories, San Jose, CA), pSmad 1/5/8, pSmad2, LEF1, pMet (Cell Signaling Technology, Danvers, MA), αSMA-CY3-conjugated (Sigma, St. Louis, MO), Cytokeratin 8 (Developmental Studies Hybridoma Bank, Iowa City, IA), CD133, CD31, PDGFRα CXCL12, CXCR4 (Santa Cruz Biotechnology, Santa Cruz, CA), PR, ERα (Dako, Carpinteria, CA), VEGFR2/ FLK1/ KDR (Neomarkers, Fremont, CA), AlexaFluor secondary antibodies (Invitrogen, Carlsbad, CA), biotinylated donkey secondary F(ab)2 fragments (Jackson ImmunoResearch, West Grove, PA).
Western analyses
Protein extracts were prepared in RIPA buffer. Protein concentrations were measured by the Bradford assay and an equivalent amount of protein from each sample was analyzed. The following antibodies were used: ®-catenin (Sigma, St. Louis, MO), LEF1, TCF1, pSmad 1/5/8, pSmad2, pSmad 3, Foxo1 (Cell Signaling Technology), HGF (R&D Systems, Minneapolis, MN), PR, ERα VEGF (Santa Cruz Biotechnology), Cyclin d1, β-actin (Neomarkers, Fremont, CA).
Statistical analyses
The unpaired t-test was performed using Prism (Graphpad Software, LaJolla, CA) to estimate the differences between groups and a p value < 0.05 was considered to be statistically significant.
Results
Uterine stroma-specific expression of truncated APC
In order to generate mice with a floxed allele of APC in uterine stroma, we used mice with the Cre recombinase gene inserted in the Amhr2 locus. Amhr2 is the anti-Müllerian hormone (AMH) type II receptor (also known as Müllerian inhibiting substance type II receptor), which is expressed in the mesenchyme of fetal Müllerian ducts, the anlagen of the uterus, oviducts, cervix and upper portion of the vagina. Expression of the receptor is required in fetal males for Müllerian duct regression in response to testicular AMH (23). To confirm that the expression of Amhr2-Cre in mice uteri was limited to cells derived from Müllerian duct mesenchyme and not observed in uterine epithelial cells, we crossed Amhr2-Cre mice with LacZ reporter mice and collected the reproductive tracts of the female progeny of analysis of β-galactosidase activity. Consistent with previous studies (23, 24), strong Cre-induced β-galactosidase activity was detected in the stromal cells but not in endometrial and glandular epithelial cells (Fig.1A, a). These results were confirmed by crossing Amhr2-Cre and Yellow Fluorescence Protein (YFP) reporter mice. Similar to LacZ activity, YFP expression was observed in stromal cells but not in epithelial cells of mice uteri (Fig.1A, b). No expression of β-galactosidase or YFP was observed in control mice (Fig.1A inset in a & b). Similar to our previous observations (23), YFP and β-galactosidase staining was also observed in the myometrium (data not shown).
Figure 1.
Analyses of Amhr2-Cre-induced recombination in murine uteri. (Panel A) Amhr2-Cre mice were crossed with LacZ and Yfp reporter mice. Amhr2-Cre induced β-galactosidase activity and direct YFP fluorescence was detected in stroma (ES) but not in epithelial cells of uteri (arrowheads and asterisks) or in their respective controls (A, insets). (Panel B, a) PCR of genomic DNA collected from the epithelium and stroma of APCcko tumors using laser capture microdissection and from whole uterus, ovary, oviduct, and tail was used to detect recombined 500 bp floxed allele. The unrecombined flox APC allele (430 bp) was present in all tissue examined. (Panel B, b) Western blot analyses of uterine lysates showing increased expression of β-catenin, TCF1, LEF1, and Cyclin d1 in APCcko compared to control mice. β-actin was used a loading control. (Panel C) Immunolocalization of β-catenin in 4-wk-old (a, b) and 5-month old (c, d) uteri of control and APCcko mice. Inset in b is a higher magnification image of area outlined by dotted lines in same panel. Arrowheads in panel d show nuclear accumulation of β-catenin in stroma. Epi: epithelium; ES: endometrial stroma. Bar equals 50 μm.
To generate conditional homozygous mutation of APC, Amhr2-Cre mice were crossed with APCflox/flox mice leading to the deletion of exon 14 of the APC gene, which causes expression of a truncated APC protein in mutant APCcko mice (20). To confirm deletion of the APC conditional allele specifically in the stromal compartment but not in the endometrial epithelium, we collected genomic DNA from epithelial and stromal cells of adult mice uteri using laser capture microdissection (Fig. S1) for comparison by PCR (20) with genomic DNA from the tissues where Amhr2-Cre is known to induce recombination (uteri, ovary and oviduct) (23). We observed bands corresponding to the floxed APC allele (500 bp) in APCcko uterine stromal cells, whole uteri, ovaries, and oviducts but not in the uterine epithelial cells and tail (Fig. 1B, a). A band corresponding to the unfloxed APC allele (430 bp) was observed in all the cells and tissues examined for genomic PCR (Fig. 1B, a). Tail DNA was used as negative control for the floxed allele because Amhr2-Cre is not expressed in this tissue (23).
APC plays an important role in canonical Wnt signaling and its loss leads to the nuclear accumulation of β-catenin (12). We examined the level of β-catenin expression in control and mutant uteri (n=3 each) by western blot (Fig. 1B, b). As expected, increased expression of β-catenin was observed in the APCcko compared with control APCflox/flox uteri (Fig. 1B, b). We also observed increased expression of β-catenin transcriptional targets (TCF1, LEF1 and Cyclin d1) in uterine lysates of mutant mice (Fig. 1B, b), indicating that the nuclear β-catenin was functionally active. To confirm that Amhr2-Cre induced recombination of the flox APC allele and therefore stabilization of β-catenin only occurred in stromal cells, we analyzed β-catenin expression in 4-wk old and adult uteri by immunofluorescence and observed nuclear accumulation of β-catenin in stromal cells of APCcko uteri at both ages (Fig. 1C b and d). In contrast, mainly membranous expression of β-catenin was observed in the endometrial epithelium of both control and APCcko uteri (Fig. 1C).
Gross abnormalities and metrorrhagia were observed in the reproductive tracts of APCcko female mice
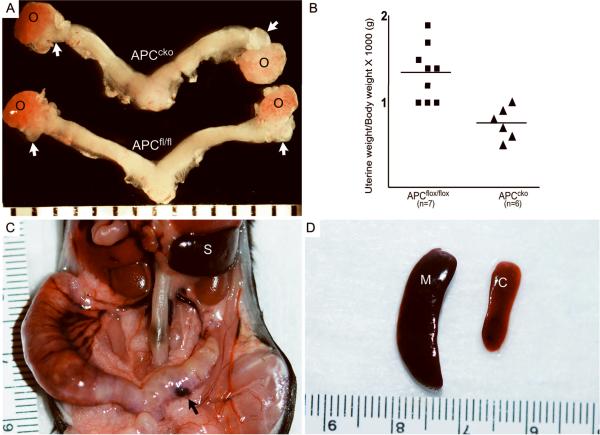
Examination of the gross morphology of reproductive tracts from 4-week-old control and APCcko mice showed that the oviducts were either absent or smaller with less coiling (100%, 5/5), but otherwise looked normal (Fig. 2A). The weight of mutant uteri was also significantly smaller than the controls at 4wks of age (Fig. 2B). Abnormal uterine enlargement and blood filled cysts were also observed in older (>8 months) APCcko mice (Fig. 2C). Additionally, these mice displayed abnormal uterine hemorrhagia (metrorrhagia), which could explain the splenomegaly (Fig. 2D), suggesting splenic extramedullary erythropoiesis in response to hemolytic anemia.
Figure 2.
Gross phenotypic changes in the reproductive tracts of APCflox/flox, APCcko female mice. (Panel A) 4wk old uteri from representative APCflox/flox and APCcko mice are shown. White arrows point to the oviducts of control and mutant mice, which are shorter and exhibit less coiling in mutants. (Panel B) The average uterine wet weight of 4-week-old APCcko/ mice was also significantly smaller (p < 0.05) when compared with controls (0.75 g ± 0.76 vs. 1.34 g ± 0.11, respectively). (Panel C) Grossly enlarged APCcko uteri with blood filled cyst (black arrow) (D) Splenomegaly in APCcko mice compared to controls. o: ovary; M: mutant; C: control; S: spleen.
APCcko mice progressively develop endometrial hyperplasia and cancer
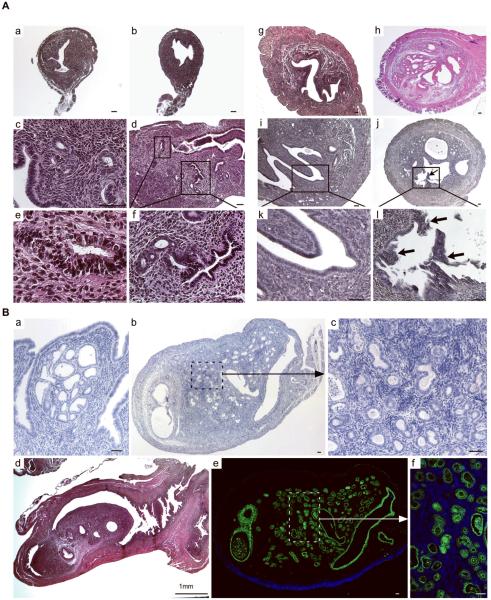
Histological examination of APCcko and control uteri at different stages of development was performed to assess the effect of stromal APC loss on the uterine parenchyma. At 4 weeks, control and APCcko uteri look very similar at low magnification (Fig. 3A a & b); however, closer examination revealed that endometrial glands adjacent to the mutant stroma were hyperplastic compared to controls (Fig. 3A c–f). By 5 months, we observed complex hyperplasia of both the glandular and luminal endometrial epithelial lining in 100% of mutant mice (Fig. 3A g & h). At 7 months of age, endometrial epithelium showed complex atypical hyperplasia with polyp-like projections into the lumen of uteri (Fig. 3A i–l). After 7 months, hyperplasia progressed to endometrial carcinoma in situ in some (4/7) of the mutant animals (Fig. 3B a). At one year of age, the uteri of some mutant mice (3/8) were greatly enlarged and developed endometrial adenocarcinomas (Fig. 3B b–f). Examination of their uteri revealed that the uterine lumen was occluded by tumors consisting of admixed epithelial and desmoplastic stromal cells (Fig. 3B b–d). Expression of cytokeratin 8, an epithelial specific marker, confirmed a diagnosis of endometrial adenocarcinoma (Fig. 3B e & f). No abnormalities were observed in the age-matched control mice (data not shown).
Figure 3.
Histological analyses of uteri collected at different developmental stages from APCflox/flox and APCcko mice. Cross sections (Panel A, a & b) and longitudinal sections (Panel A, c & d) of 4wk old control (Panel A, a & c) and mutant (Panel A, b & d) uteri. Rectangular areas in Panel A, d are shown in higher magnification in e and f. Hyperplasia of epithelial lining of 5 month old APCcko mice uteri (Panel A, h) is shown compared with control (Panel A, g). (Panel A, i & k), polyp like outgrowths (Arrow) were observed from endometrium of some 7-month old mutant uteri (Panel A, j & l). Panel B, a shows development of carcinoma in situ in APCcko uteri. Panel B, b–d show mutant uteri with occlusion of uterine lumen by carcinogenic growth. Higher magnification image from Panel B, b showing admixed endometrial epithelial and stroma cells is shown in Panel B, c. Staining of mutant uterus with cytokeratin 8, an epithelial cell-specific marker. (Panel B, e). The boxed area is shown at higher magnification in Panel B, f. Bar equals 50 μm unless otherwise indicated.
We also analyzed the expression of CD133, which has been associated with “cancer stem cells” in a variety of cancers (25), in control and mutant mice (Fig 4A & B). Similar to human endometrial tumors (25), we found increased expression of CD133 in the epithelial cells of uterine tumors in APCcko mice compared with controls, suggesting expansion of these cells with deletion of stromal APC.
Figure 4.
Myometrial invasion by endometrial adenocarcinoma in adult APCcko mice. CD133 expression, which is highly expressed in human endometrial carcinoma, was detected at higher levels in endometrial tumors of APCcko mice compared with controls (Panels A & B). Inset in Panel B is a higher magnification of area boxed in white dashed line. H&E sections of 8 month-old APCcko uteri with endometrial adenocarcinoma showing fully formed endometrial glands (arrows) present in the myometrium (M) (Panels C–D). (Panels E & F) Colocalization of αSMA (red, a smooth muscle specific marker) and Cytokeratin 8 (ck, green, an epithelial specific marker) confirmed the presence of endometrial glands (white arrows) in the myometrium. Control uteri are shown in Panels G & H. White dotted line in Panel C shows demarcation of smooth muscle layer from the endometrial stromal compartment. Bar equals 50 μm.
The murine uterus has three main layers, endometrium, stroma, and myometrium, and is covered by a peritoneal serosa layer. Normally all endometrial glands are limited to the stromal compartment and myometrial invasion by endometrial epithelial cells is considered one of the hallmarks of endometrial adenocarcinomas (11). In our study, we found that in some (3/8) of mutant APCcko mice with endometrial adenocarcinomas, endometrial glands invaded the myometrium layer (Fig. 4C & D). We performed colocalization of αSMA (smooth muscle marker) and cytokeratin 8 (marker of epithelial cells) on mutant uterine tissue sections to confirm the presence of endometrial glands inside the myometrium (Fig. 4E & F). Control uteri did not show any evidence of endometrial glands in the myometrium (Fig. 4G & H).
Estrogen receptor alpha (ERα) expression and the uterine estrogenic response are suppressed in APCcko mice
Uterine tissue recombination experiments using ER knockout and control mice have shown that estrogen activates stromal ERα to induce epithelial cell proliferation indirectly (26). We investigated the effects of E2 on uteri of oophorectomized APCcko and control mice maintained on depo estradiol for 30 days. We observed that APCcko mice showed minimal response to E2 treatment and gained significantly less uterine weight compared to controls (Fig. 5A & B). The normal uterine response to E2 also requires ERα-mediated induction of the progesterone receptor (PR), which then induces Foxo1 expression (27). We performed western blot analyses on uterine lysates collected from APCcko and control mice to determine whether the lack of an E2 response in APCcko mice was due to uninduced ERα expression or of its downstream targets, PR and Foxo1 and observed lower expression of ERα, PR, and Foxo1 in APCcko uteri compared to controls (Fig. 5C), suggesting that loss of APC inhibited the response of stromal cells to E2. Of note, loss of Foxo1 expression is a common event in human EC (28).
Figure 5.
APCcko uterine stroma is insensitive to estradiol treatment. APCflox/flox uterine size (Panel A, a) and weight (Panel B) is greatly increased after treatment compared to APCcko (Panel A, b & B) mice uteri. Histological sections from untreated control (Panel A, c) and mutant (Panel A, d) uteri were compared with age-matched, estradiol-treated control (Panel A, e) and mutant (Panel A, f) uteri. (Panel C) Western blot analyses of ERα, PR, and Foxo1 in mutant and control uterine lysates (n=3 each). β-actin was used as a loading control. Bar equals 50 μm.
Loss of APC increases the myofibroblast population in endometrial stroma
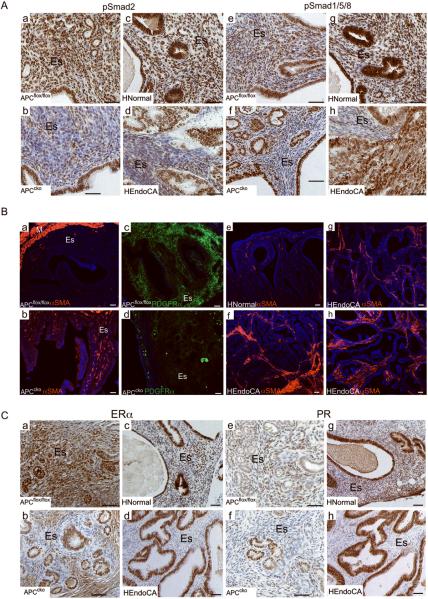
Dysregulated BMP, TGFβ, and Wnt signaling has been shown to induce myofibroblast phenotypic changes in stromal fibroblast cells, which is also a characteristic of cancer-associated fibroblast cells (6, 29). Additionally, crosstalk between TGFβ family members and Wnt signaling plays an important role in cancer progression of various organs. For example, compound deletion of Smad4 and APC has a synergistic effect that leads to the formation of highly malignant intestinal tumors (30). To determine whether stromal-specific deletion of APC affects TGFβ and BMP signaling between the stromal and epithelial compartments of murine uteri and human EC, we examined whether the expression of the downstream mediators of TGFβ (pSmad2/3) and BMP (pSmad1/5/8) signaling. IHC of pSmad2 and pSmad1/5/8 in mice and human tissues revealed that TGFβ and BMP signaling was suppressed in the stromal cells of APCcko mice tumor and human EC compared to control and normal tissues (Fig. 6A). In contrast, no differences were observed in epithelial-specific expression of pSmad2 and pSmad1/5/8 in both mice and human EC compared to controls (Fig. 6A).
Figure 6.
APC deletion suppresses paracrine signaling and increases the myofibroblast population in mutant uterine stroma. (Panel A) Reduced expression of pSmad2 (a–d) and pSmad 1/5/8 (e–h) in the stroma, but not the epithelium, of mutant uteri and in human EC when compared with control uteri and in normal human endometrium was obaserved by IHC. (Panel B) Immunofluorescence was performed for αSMA (a & b) and PDGFRα (c & d) on 7 month-old APCflox/flox and APCcko uterus. In normal/benign human endometrium (n=4), αSMA is normally detected in vascular smooth muscle cells only (e) but in patients with EC (7/9), αSMA was detected in stromal cells (f–h). (Panel C) Suppression of ERα (a–d) and PR (e–h) expression in stroma of APCcko uteri and human EC patients compared to control or normal tissues was observed by IHC. HNormal: normal human endometrium HEndoCA: human EC. ES: endometrial stroma; M: myometrium. Bar equals 50 μm.
Next, we examined whether the mutant endometrial stromal cells were becoming more myofibroblast-like, which is characteristic of cancer-associated fibroblasts (1), by analyzing the expression of αSMA, a myofibroblast marker (31), and PDGFRα, a fibroblast marker (32). We observed more αSMA-positive and fewer PDGFRα-positive in the stromal compartment of mutant mice compared with controls (Fig. 6B, a–d). In control uteri, most of the αSMA positive staining was observed in the myometrium and the vascular smooth muscle cells (Fig. 6B, a). Because normal stromal cells maintain the nonmalignant phenotype of adjacent epithelial cells, we predicted that the conversion to a more myofibroblastic stroma we observed in APCcko mice might be contributing to carcinogenesis in human EC. To confirm that similar changes also occur in human EC patients, we collected and examined human endometrial carcinoma and normal/benign tissues for upregulated αSMA expression in uterine stroma cells, a transformation that has been linked to carcinogenesis in other systems (6). Similar to APCcko mice, αSMA-positive myofibroblast cells were greatly increased in the stroma of EC patients (7/9) (Fig. 6B, f–h) but only observed in vascular smooth muscle cells in normal/benign human endometrium (n=4) (Fig. 6B, e).
Stromal ERα plays an important role in induction of PR expression, which is thought to subsequently counteract the mitogenic effect of estrogen on epithelium through paracrine mechanisms (26, 33). We examined whether the increase in stromal myofibroblasts in murine and human EC affects ERα and PR expression by IHC (Fig. 6C). We observed decreased expression of ERα and PR in stromal cells adjacent to the epithelium of both mutant mice and human EC compared to controls or normal endometrium, but expression was unchanged in the epithelial cells (Fig. 6C). Loss of stromal ERα and PR expression suggests that unopposed estrogen stimulation of epithelium is a contributing factor in the development of endometrial hyperplasia and cancer in APCcko mice.
Increased expression of intercellular growth factors (VEGF and CXCL12) in APCcko mice
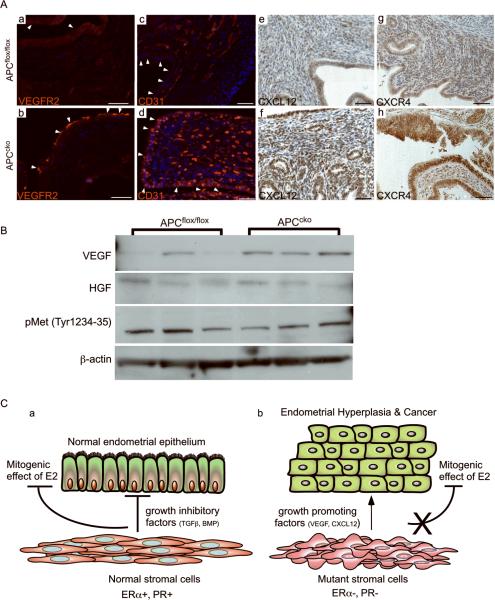
Other intercellular signaling mechanisms are known to be involved in tumorigenesis. For example, expression of VEGF and its receptor VEGFR2 is upregulated in the stroma of human EC patients and is correlated with poor outcome (34, 35). In this study, we observed increased expression of VEGFR2 on the leading edge of the APCcko mice tumors (Fig. 7A, a & b). VEGF expression itself was assessed by western blot of uterine lysates and showed increased VEGF expression in mutant uteri compared to controls (Fig. 7B). Additionally, more CD31-positive endothelial cells were observed in murine tumors compared to controls, indicating increased vasculogenesis in mutant tumors in response to increased VEGF signaling (Fig. 7A, c & d). Similarly, stromal derived factor-1 (SDF-1)/CXCL12 is a paracrine factor secreted by cancer-associated fibroblast in various cancers including breast and EC (36, 37), whose receptor, CXCR4, has been shown to be overexpressed in the epithelial cells of human EC (38). In vitro, CXCL12 increases proliferation and invasion of human endometrial adenocarcinomas cells lines (39). In this study, we observed increased expression of CXCL12 in the stroma and CXCR4 in the epithelium of APCcko tumors, suggesting that, similar to human EC, disrupted CXCL12/CXCR4 signaling might also play an important role in endometrial carcinogenesis in mutant mice.
Figure 7.
Increased carcinogenic signaling in APCcko uteri. (Panel A, a &b) Expression of VEGFR2 on the leading edge of tumor (arrowheads) by immunofluorescence in APCcko mice compared to controls (arrowheads). Increased numbers of CD31 positive-endothelial cells were present in mutant uteri (arrowheads) compared to controls (arrowheads) by immunofluorescence (c & d). Increased expression of CXCL12 in stroma (e & f) and CXCR4 in epithelium (g & h) in APCcko mice by IHC compared with controls. (Panel B) Western blot analyses of VEGF, HGF, pMet (tyr 1234–1235) and β-actin in control and mutant APCcko uteri. (Panel C) Schematic summarizing the effect of stromal-specific APC deletion on epithelial cells. Normal fibroblastic stromal cells (PR+ and ERα+) maintain uterine epithelial homeostasis by secreting growth inhibitory factors (TGFβ and BMPs) to regulate the mitogenic effect of estrogen on epithelial proliferation (a). Mutant myofibroblastic stromal cells (PR-neg and ERneg) express reduced levels of growth inhibitory factors (TGFβ and BMPs) and express more growth-promoting factors (VEGF and CXCL12), and are unable to oppose estrogen-induced ERα+ epithelial proliferation, which leads to hyperplasia and carcinogenesis. Bar equals 50 μm.
Suppression of TGFβ signaling in stromal cells increases hepatocyte growth factor (HGF) secretion and has been associated with prostate and forestomach neoplasia (6). Since we observed suppression of TGFβsignalinginstroma of APCcko mice uteri, we examined expression of HGF and its activated receptor pMET in mutant and control uteri. No difference in the expression of these two proteins between control and mutant mice was observed (Fig. 7B). Additionally, pMet was not detected in 8/9 human EC patients tissues examined (Fig. S3). These findings suggest that HGF/MET may not play an important role in stromal-epithelial crosstalk in this model system or in human EC. Collectively, these data suggest that unopposed estrogen stimulation of epithelium due to suppression of ERα and PR in stroma increases secretion of growth promoting factors, VEGF and CXCL12, and suppression of growth inhibitory signals, TGFβ and BMPs, are key mechanisms for the development of EC in APCcko mice and in humans (Fig. 7C).
Discussion
Stromal and epithelial crosstalk plays an important role in organogenesis as well as carcinogenesis of uterus, prostate, mammary gland, and other vital organs (6). Disruption of the signaling pathways responding to cellular communication is believed to be the initial events in cancer development (31, 40). For example, stromal cell only deletion of TGFβ receptor II leads to the formation of aggressive tumors in forestomach and prostate epithelium (40). Similarly, inactivation of BMP receptor II in intestinal stromal cells increases myofibroblast cell number and causes development of hamartomatous polyps (31). Human patients suffering from hamartomatous polyposis syndrome also harbor mutations in Smad4 in mesenchymal cells and show expansion of the stromal component (41). Additionally, Smad4 loss in the stroma but not in the epithelium of colon, rectum, and stomach cancers suggests that paracrine signaling from the mutated cells in the microenvironment can drive carcinogenesis in the adjacent epithelial cells (42). In the present study, we have demonstrated that APC-deletion in uterine endometrial stromal cells alone is sufficient for endometrial carcinogenesis.
One of the functions of APC is the control of Wnt/β-catenin signaling, which when dysregulated plays an important role in development of various cancers including colon, breast, ovarian, prostate, and uterine cancers. Wnt family members, such as Wnt2 and Wnt5a, are overexpressed in the stroma of human breast and colon cancers (43). Overexpression of Wnt1 in mice causes development of mammary adenocarcinomas, and in coculture experiments, Wnt1 expressing-fibroblasts induce transformation of C57MG epithelial cells without being affected themselves (6, 43). These data suggest that Wnt signaling in stromal cells might be an important therapeutic target for cancer treatment.
Wnt family members also play an important role in normal uterine organogenesis (44, 45). Deletion of Wnt7a, which is only expressed in uterine epithelium, causes abnormal development of both endometrial stroma and myometrium (44). Another Wnt ligand, Wnt5a, is expressed in uterine stroma where it is required for the development of endometrial epithelial glands (45). Similarly, conditional β-catenin knockout studies have revealed that mesenchymal β-catenin plays an important role in formation of uterine glandular epithelium (23). These studies indicate that Wnt signaling plays an important role in uterine epithelial stromal crosstalk but to date, there are no reports suggesting that dysregulated Wnt signaling causes EC. Recently, we have shown that targeted deletion of exon 3 of β-catenin (Ctnnb1(ex3)/+) to form a constitutively activated (CA) allele of β-catenin in the Müllerian duct mesenchyme by Amhr2-Cre leads to the development of uterine leiomyomas, endometrial stromal sarcomas and adenomyosis, but not EC (22). We also observed uterine leiomyomas in APCcko mice, but endometrial stromal sarcomas were not observed (data not shown). In another study, expression of CA β-catenin in endometrial epithelium using progesterone receptor (PR)-driven Cre causes endometrial hyperplasia, but not EC (46). In contrast to these mice with CA β-catenin, we have shown here that stromal-specific APCcko mice develop EC. These phenotypic differences between APCcko and Ctnnb1(ex3)/+ mice suggest that APC deletion is targeting more functions associated with APC than simply regulating the availability and/or accumulation of nuclear β-catenin and its subsequent transcriptional regulation of target genes.
The proliferation-promoting activity of estrogens and ERα expression in the uterus has been well established (47) as has the risk of EC from unopposed estrogens (48), which can occur with the absence of available ERα to mediate E2-induced expression of stromal PR. A recent report has also shown that progesterone inhibits Wnt/β-catenin signaling in endometrial carcinoma cells in vitro, suggesting a mechanism for maintaining tissue homeostasis to counteract the proliferative potential of nuclear β-catenin (49). In human EC patients, ERα and PR expression is usually limited to early, moderately or well-differentiated stages of carcinogenesis, but, paradoxically, it is the ERα- and PR-negative tumors that are usually found in relatively undifferentiated malignancies and indicate a poor prognosis (50, 51) as they do in breast cancer patients (52). Here, we showed that APCcko mice have decreased expression of stromal ERα and are nonresponsive to E2 treatment, suggesting the tumor-inhibiting effects of E2 via the induction of the PR are not available in the uteri of APCcko mice. Various epidemiological studies in postmenopausal women undergoing hormone replacement therapy and investigations with mouse models of colon or intestinal cancer with APC deletions have shown that estrogens inhibit carcinogenesis and tumor progression (53, 54). Deletion of ERα in mice with an APC hypomorphic allele increases intestinal tumor development by increasing activation of the Wnt/β-catenin signaling pathway and its targeted genes (54). Similarly, oophorectomy of APC+/− mice leads to increased tumor formation, whereas treatment with estradiol had an opposite affect (53). Additionally, western blot analyses of intestinal tumors in APCmin/+ mice showed decreased expression of ERα (53). Collectively, these observations indicate that estrogen signaling plays a role in regulating Wnt/β-catenin activities.
The current studies, along with our previous report showing that mice with uterine stromal expression of CA β-catenin do not develop EC (22), suggest that nuclear accumulation of β-catenin by the expression of a truncated form of APC that is missing its β-catenin binding domains might not be the mechanism involved in carcinogenesis in APCcko mice. Our data also suggest that deletion of APC activity leads to conversion of the stromal cells to a more myofibroblastic phenotype, which diminishes ERα expression and the ability of the cells to express PR to mediate the suppressive effects of progesterone-induced paracrine signaling on mitogenic E2 activity in the in epithelium. In vitro, estrogen treatment has been shown to increase expression of VEGF and CXCR12 / CXCR4 (55, 56). However, effect of progesterone on secretion of these growth factors is currently unclear. Because crosstalk between estrogen and progesterone plays an important role in uterine functions and endometrial carcinogenesis, deducing the mechanisms used by these ovarian steroids to control secretion of these intercellular growth factors will be critical for our understanding of endometrial tumorigenesis.
In summary, we have shown that deletion of APC activity in murine uterine stroma cells causes their transdifferentiation to a more myofibroblastic phenotype, which is accompanied by reduced ERα expression and is sufficient to induce EC. We speculate that conversion of the stromal cells with perturbed APC activity might be a common mechanism for the effects of unopposed estrogen in endometrial carcinogenesis. Our future goals will be to determine which of the non-β-catenin-related function of APC are mediating the conversion of the stromal fibroblasts to myofibroblasts and the molecular mechanisms subsequently leading to development of endometrial hyperplasia and carcinogenesis.
Supplementary Material
Acknowledgments
We very grateful to Dr. Richard Behringer for supplying us with the Amhr2-Cre mice and to Drs. James Pru, Patricia K. Donahoe, John O. Schorge, and George L. Mutter for reviewing an early version of this manuscript. These studies were supported in part by a grant from NICHD, HD057201, and by the Vincent Memorial Research Fund to JMT.
References
- 1.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunha GR, Hayward SW, Wang YZ. Role of stroma in carcinogenesis of the prostate. Differentiation. 2002;70:473–85. doi: 10.1046/j.1432-0436.2002.700902.x. [DOI] [PubMed] [Google Scholar]
- 3.Kurita T, Cooke PS, Cunha GR. Epithelial-stromal tissue interaction in paramesonephric (Mullerian) epithelial differentiation. Dev Biol. 2001;240:194–211. doi: 10.1006/dbio.2001.0458. [DOI] [PubMed] [Google Scholar]
- 4.Booth BW, Mack DL, Androutsellis-Theotokis A, McKay RD, Boulanger CA, Smith GH. The mammary microenvironment alters the differentiation repertoire of neural stem cells. Proc Natl Acad Sci U S A. 2008;105:14891–6. doi: 10.1073/pnas.0803214105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulanger CA, Mack DL, Booth BW, Smith GH. Interaction with the mammary microenvironment redirects spermatogenic cell fate in vivo. Proc Natl Acad Sci U S A. 2007;104:3871–6. doi: 10.1073/pnas.0611637104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–7. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurose K, Gilley K, Matsumoto S, Watson PH, Zhou XP, Eng C. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat Genet. 2002;32:355–7. doi: 10.1038/ng1013. [DOI] [PubMed] [Google Scholar]
- 8.Abraham SC, Reynolds C, Lee JH, et al. Fibromatosis of the breast and mutations involving the APC/beta-catenin pathway. Hum Pathol. 2002;33:39–46. doi: 10.1053/hupa.2002.30196. [DOI] [PubMed] [Google Scholar]
- 9.Benard J, Beron-Gaillard N, Satge D. Down's syndrome protects against breast cancer: is a constitutional cell microenvironment the key? Int J Cancer. 2005;113:168–70. doi: 10.1002/ijc.20532. [DOI] [PubMed] [Google Scholar]
- 10.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010 doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 11.Friel AM, Growdon WB, McCann CK, et al. Mouse models of uterine corpus tumors: clinical significance and utility. Front Biosci (Elite Ed) 2010;2:882–905. doi: 10.2741/e149. [DOI] [PubMed] [Google Scholar]
- 12.Aoki K, Taketo MM. Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J Cell Sci. 2007;120:3327–35. doi: 10.1242/jcs.03485. [DOI] [PubMed] [Google Scholar]
- 13.Pijnenborg JM, Kisters N, van Engeland M, et al. APC, beta-catenin, and E-cadherin and the development of recurrent endometrial carcinoma. Int J Gynecol Cancer. 2004;14:947–56. doi: 10.1111/j.1048-891X.2004.014534.x. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida S, Harada T, Iwabe T, et al. Induction of hepatocyte growth factor in stromal cells by tumor-derived basic fibroblast growth factor enhances growth and invasion of endometrial cancer. J Clin Endocrinol Metab. 2002;87:2376–83. doi: 10.1210/jcem.87.5.8483. [DOI] [PubMed] [Google Scholar]
- 15.Arnold JT, Lessey BA, Seppala M, Kaufman DG. Effect of normal endometrial stroma on growth and differentiation in Ishikawa endometrial adenocarcinoma cells. Cancer Res. 2002;62:79–88. [PubMed] [Google Scholar]
- 16.Hileeto D, Fadare O, Martel M, Zheng W. Age dependent association of endometrial polyps with increased risk of cancer involvement. World J Surg Oncol. 2005;3:8. doi: 10.1186/1477-7819-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fletcher JA, Pinkus JL, Lage JM, Morton CC, Pinkus GS. Clonal 6p21 rearrangement is restricted to the mesenchymal component of an endometrial polyp. Genes Chromosomes Cancer. 1992;5:260–3. doi: 10.1002/gcc.2870050315. [DOI] [PubMed] [Google Scholar]
- 18.Vanni R, Marras S, Andria M, Faa G. Endometrial polyps with predominant stromal component are characterized by a t(6;14)(p21;q24) translocation. Cancer Res. 1995;55:31–3. [PubMed] [Google Scholar]
- 19.Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet. 2002;32:408–10. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- 20.Kuraguchi M, Wang XP, Bronson RT, et al. Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PLoS Genet. 2006;2:e146. doi: 10.1371/journal.pgen.0020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanwar PS, Kaneko-Tarui T, Zhang L, Rani P, Taketo MM, Teixeira J. Constitutive WNT/beta-catenin signaling in murine Sertoli cells disrupts their differentiation and ability to support spermatogenesis. Biol Reprod. 2010;82:422–32. doi: 10.1095/biolreprod.109.079335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanwar PS, Lee HJ, Zhang L, et al. Constitutive activation of Beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol Reprod. 2009;81:545–52. doi: 10.1095/biolreprod.108.075648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol. 2005;288:276–83. doi: 10.1016/j.ydbio.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 24.Petit FG, Jamin SP, Kurihara I, et al. Deletion of the orphan nuclear receptor COUP-TFII in uterus leads to placental deficiency. Proc Natl Acad Sci U S A. 2007;104:6293–8. doi: 10.1073/pnas.0702039104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutella S, Bonanno G, Procoli A, et al. Cells with characteristics of cancer stem/progenitor cells express the CD133 antigen in human endometrial tumors. Clin Cancer Res. 2009;15:4299–311. doi: 10.1158/1078-0432.CCR-08-1883. [DOI] [PubMed] [Google Scholar]
- 26.Cooke PS, Buchanan DL, Young P, et al. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci U S A. 1997;94:6535–40. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Labied S, Kajihara T, Madureira PA, et al. Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol Endocrinol. 2006;20:35–44. doi: 10.1210/me.2005-0275. [DOI] [PubMed] [Google Scholar]
- 28.Goto T, Takano M, Albergaria A, et al. Mechanism and functional consequences of loss of FOXO1 expression in endometrioid endometrial cancer cells. Oncogene. 2008;27:9–19. doi: 10.1038/sj.onc.1210626. [DOI] [PubMed] [Google Scholar]
- 29.Klapholz-Brown Z, Walmsley GG, Nusse YM, Nusse R, Brown PO. Transcriptional program induced by Wnt protein in human fibroblasts suggests mechanisms for cell cooperativity in defining tissue microenvironments. PLoS One. 2007;2:e945. doi: 10.1371/journal.pone.0000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takaku K, Oshima M, Miyoshi H, Matsui M, Seldin MF, Taketo MM. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell. 1998;92:645–56. doi: 10.1016/s0092-8674(00)81132-0. [DOI] [PubMed] [Google Scholar]
- 31.Beppu H, Mwizerwa ON, Beppu Y, et al. Stromal inactivation of BMPRII leads to colorectal epithelial overgrowth and polyp formation. Oncogene. 2008;27:1063–70. doi: 10.1038/sj.onc.1210720. [DOI] [PubMed] [Google Scholar]
- 32.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17:135–47. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 33.Kurita T, Lee K, Saunders PT, et al. Regulation of progesterone receptors and decidualization in uterine stroma of the estrogen receptor-alpha knockout mouse. Biol Reprod. 2001;64:272–83. doi: 10.1095/biolreprod64.1.272. [DOI] [PubMed] [Google Scholar]
- 34.Orimo A, Tomioka Y, Shimizu Y, et al. Cancer-associated myofibroblasts possess various factors to promote endometrial tumor progression. Clin Cancer Res. 2001;7:3097–105. [PubMed] [Google Scholar]
- 35.Kamat AA, Merritt WM, Coffey D, et al. Clinical and biological significance of vascular endothelial growth factor in endometrial cancer. Clin Cancer Res. 2007;13:7487–95. doi: 10.1158/1078-0432.CCR-07-1017. [DOI] [PubMed] [Google Scholar]
- 36.Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 37.Allinen M, Beroukhim R, Cai L, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Gelmini S, Mangoni M, Castiglione F, et al. The CXCR4/CXCL12 axis in endometrial cancer. Clin Exp Metastasis. 2009;26:261–8. doi: 10.1007/s10585-009-9240-4. [DOI] [PubMed] [Google Scholar]
- 39.Mizokami Y, Kajiyama H, Shibata K, Ino K, Kikkawa F, Mizutani S. Stromal cell-derived factor-1alpha-induced cell proliferation and its possible regulation by CD26/dipeptidyl peptidase IV in endometrial adenocarcinoma. Int J Cancer. 2004;110:652–9. doi: 10.1002/ijc.20183. [DOI] [PubMed] [Google Scholar]
- 40.Bhowmick NA, Chytil A, Plieth D, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–51. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 41.Wirtzfeld DA, Petrelli NJ, Rodriguez-Bigas MA. Hamartomatous polyposis syndromes: molecular genetics, neoplastic risk, and surveillance recommendations. Ann Surg Oncol. 2001;8:319–27. doi: 10.1007/s10434-001-0319-7. [DOI] [PubMed] [Google Scholar]
- 42.Kim BG, Li C, Qiao W, et al. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature. 2006;441:1015–9. doi: 10.1038/nature04846. [DOI] [PubMed] [Google Scholar]
- 43.Macheda ML, Stacker SA. Importance of Wnt signaling in the tumor stroma microenvironment. Curr Cancer Drug Targets. 2008;8:454–65. doi: 10.2174/156800908785699324. [DOI] [PubMed] [Google Scholar]
- 44.Miller C, Sassoon DA. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development. 1998;125:3201–11. doi: 10.1242/dev.125.16.3201. [DOI] [PubMed] [Google Scholar]
- 45.Mericskay M, Kitajewski J, Sassoon D. Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development. 2004;131:2061–72. doi: 10.1242/dev.01090. [DOI] [PubMed] [Google Scholar]
- 46.Jeong JW, Lee HS, Franco HL, et al. beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene. 2008 doi: 10.1038/onc.2008.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 48.Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gynecol. 1995;85:304–13. doi: 10.1016/0029-7844(94)00383-O. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Hanifi-Moghaddam P, Hanekamp EE, et al. Progesterone inhibition of Wnt/beta-catenin signaling in normal endometrium and endometrial cancer. Clin Cancer Res. 2009;15:5784–93. doi: 10.1158/1078-0432.CCR-09-0814. [DOI] [PubMed] [Google Scholar]
- 50.Kauppila AJ, Isotalo HE, Kivinen ST, Vihko RK. Prediction of clinical outcome with estrogen and progestin receptor concentrations and their relationships to clinical and histopathological variables in endometrial cancer. Cancer Res. 1986;46:5380–4. [PubMed] [Google Scholar]
- 51.Collins F, MacPherson S, Brown P, et al. Expression of oestrogen receptors, ERalpha, ERbeta, and ERbeta variants, in endometrial cancers and evidence that prostaglandin F may play a role in regulating expression of ERalpha. BMC Cancer. 2009;9:330. doi: 10.1186/1471-2407-9-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bardin A, Boulle N, Lazennec G, Vignon F, Pujol P. Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocr Relat Cancer. 2004;11:537–51. doi: 10.1677/erc.1.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weyant MJ, Carothers AM, Mahmoud NN, et al. Reciprocal expression of ERalpha and ERbeta is associated with estrogen-mediated modulation of intestinal tumorigenesis. Cancer Res. 2001;61:2547–51. [PubMed] [Google Scholar]
- 54.Cleveland AG, Oikarinen SI, Bynote KK, et al. Disruption of estrogen receptor signaling enhances intestinal neoplasia in Apc(Min/+) mice. Carcinogenesis. 2009;30:1581–90. doi: 10.1093/carcin/bgp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsutsumi A, Okada H, Nakamoto T, Okamoto R, Yasuda K, Kanzaki H. Estrogen induces stromal cell-derived factor 1 (SDF-1/CXCL12) production in human endometrial stromal cells: a possible role of endometrial epithelial cell growth. Fertil Steril. 2010 doi: 10.1016/j.fertnstert.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 56.Fujimoto J, Toyoki H, Jahan I, et al. Sex steroid-dependent angiogenesis in uterine endometrial cancers. J Steroid Biochem Mol Biol. 2005;93:161–5. doi: 10.1016/j.jsbmb.2004.12.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.