To the Editor:
Disseminated nocardiosis due to Nocardia farcinica has mostly been reported in immunocompromised patients with underlying malignancies, HIV infection, solid-organ transplants or in those receiving long-term corticosteroid therapy (1). We present a second case of disseminated nosocomial nocardiosis following iliac artery stent infection occurring in an immunocompetent patient.
A previously healthy 57-year-old man presented with a two-week history of generalized weakness, fever, petechial rash from the right knee down, and right knee and ankle joint pain. Empirical antibiotic therapy was initiated with intravenous (IV) vancomycin (2 g daily) and rifampin (1200 mg daily), without effect. One month before symptom appearance, he had undergone an angioplasty with stenting for a symptomatic right iliac stenosis. Multiple blood cultures, knee synovial fluid cultures and cultures of petechial lesion skin biopsies were all negative for pyogenic organisms, mycobacteria and fungi. There was no history, clinical examination or standard laboratory findings suggestive of an immunocompromised state; HIV testing was negative. At admission, the laboratory findings included a white blood cell count of 10.5×109/L and an elevated C-reactive protein level of 95 mg/L. Antibiotic therapy was discontinued to perform new investigations. At the same time, the patient underwent a second angioplasty for a new stenosis of the right iliac artery.
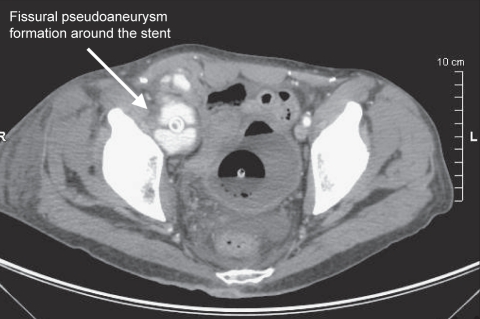
One week later, the patient’s clinical state deteriorated. Physical examination revealed a painful, tender, palpable, right ilioinguinal mass. A computed tomography (CT) scan revealed a fissured pseudoaneurysm formation (6.2 cm × 4 cm in diameter) around the stent (Figure 1). The dilated iliac stented segment was immediately excised and the patient underwent femoral bypass grafting. Abdominal and lower body CT scans showed multiple abscesses of the right lower limb, right knee arthritis, quadricipital myofasciitis and right femoral osteolytic lesions.
Figure 1).
Computed tomography scan showing a fissural pseudoaneurysm formation around the stent (arrow)
Right knee synovial fluid culture yielded Gram-positive branching rods, and the patient was treated simultaneously with IV imipenem (1 g every 8 h) and IV amikacin (1250 mg daily), but he remained septic with fever (39°C), and had a white blood cell count of 28×109/L and a further elevated C-reactive protein level. 16S ribosomal sequencing (reverse transcription, real-time polymerase chain reaction using SYBR green fluorescent dye) of the patient’s synovial fluid was performed, and N farcinica was identified four days later. This bacteria was also isolated in large amounts in excised pseudoaneurysm and stent cultures. One week later, the isolate was found to be resistant in vitro to ticarcillin, cephalosporins, imipenem, trimethoprim-sulfamethoxazole (TMP-SMZ), vancomycin, rifampin, erythromycin and gentamicin; moderately susceptible to amoxicillin and ciprofloxacin; and susceptible to amikacin, minocycline and linezolid by the National Reference Laboratory (Institut Pasteur, France) standards. We initiated IV amoxicillin (12 g daily), ciprofloxacin (1200 mg daily) and tigecycline (100 mg daily) in combination with TMP-SMZ (TMP 240 mg and SMZ 1200 mg daily). The patient improved clinically while on this treatment. Following six months of this regimen, a repeat CT scan showed an improvement, which allowed a switch to oral TMP-SMX (TMP 240 mg and SMZ 1200 mg daily), ciprofloxacin (1500 mg daily) and linezolid for a total of 12 months. No evidence of recurrent infection was noted in the two years following the discontinuation of antibiotic therapy. Femoral bypass grafting is scheduled in the near future.
Nocardia species are ubiquitous, soilborne, aerobic Actinomyces. The main route of acquisition is usually through direct inhalation of contaminated particles from soil or water, or by direct inoculation through the skin (1). Nosocomial outbreaks of nocardiosis have been reported most frequently for immunocompromised patients in heart or liver transplantation units (2), with possible transmission via health care workers. Our observation suggests nosocomial intra- or perioperative contamination of the initial stent.
Saubolle and Sussland (1) estimated that less than 10% of patients with disseminated nocardiosis have no identified underlying predisposing factors (1). Ours is the second reported case of N farcinica infection of a vascular prosthesis in an immunocompetent patient (3). Compared with other Nocardia asteroides complex organisms, N farcinica infections manifest themselves not only as pulmonary or systemic disease, but also as severe postoperative wound infections due to their virulence, affinity to medical material and resistance to antibiotics (4).
The described case was also rather atypical in implemented therapy. Combination therapy with a sulpha-containing agent or imipenem in combination with amikacin has been recommended for disseminated nocardiosis (3). However, our strain was subsequently found to be resistant to both antibiotics, by which time the patient was severely ill with severe sepsis and osteoarticular abscesses, leading us to consider linezolid and tigecycline therapy as alternatives. We decided to continue TMP-SMZ because of reports (4,5) of the clinical success of TMP-SMZ therapy despite its in vitro resistance. Treatment options were further complicated because the infection occurred on a vascular prosthesis. The necessary duration of nocardiosis therapy is uncertain, but it has often been reported to have continued for many months after clinical cure because of high relapse rates (1).
The number of patients suffering from nocardiosis is constantly rising worldwide. Our case highlights the fact that Nocardia infection can occur in an immunocompetent patient, particularly in case of persisting surgical site infection that fail to respond to conventional antimicrobial therapy. Molecular methods could improve diagnosis and the chances of survival.
REFERENCES
- 1.Saubolle MA, Sussland D. Nocardiosis: Review of clinical and laboratory experience. J Clin Microbiol. 2003;41:4497–501. doi: 10.1128/JCM.41.10.4497-4501.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wenger PN, Brown JM, McNeil MM, Jarvis WR. Nocardia farcinica sternotomy site infections in patients following open heart surgery. J Infect Dis. 1998;178:1539–43. doi: 10.1086/314450. [DOI] [PubMed] [Google Scholar]
- 3.Torres OH, Domingo P, Pericas R, Boiron P, Montiel JA, Vazquez G. Infection caused by Nocardia farcinica: Case report and review. Eur J Clin Microbiol Infect Dis. 2000;19:205–12. doi: 10.1007/s100960050460. [DOI] [PubMed] [Google Scholar]
- 4.Benes J, Viechova J, Picha D, Horova B, Zatloukal P. Disseminated Nocardia asteroides infection in an immunocompetent woman following an arm injury. Infection. 2003;31:112–4. doi: 10.1007/s15010-003-3073-x. [DOI] [PubMed] [Google Scholar]
- 5.Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:89–905. doi: 10.1093/clinids/22.6.891. [DOI] [PubMed] [Google Scholar]