Abstract
The hyperglycemia of maternal diabetes suppresses the glucose transporter-1 (GLUT1) facilitative glucose transporter 49–66% in preimplantation embryos. Glucose uptake is reduced and apoptosis is activated. We hypothesized that the reduction of embryonic GLUT1 may play a key role in the malformations of diabetic embryopathy. Therefore, we produced GLUT1-deficient transgenic mice [i.e., antisense-GLUT1 (GT1AS)] to determine whether GLUT1 deficiency alone could reproduce the growth defects. Early cell division of fertilized mouse eggs injected with GT1AS was markedly impaired, P < 0.001 vs. controls. Two populations of preimplantation embryos obtained from GT1AS × GT1AS heterozygote matings exhibited reduction of the 2-deoxyglucose uptake rate: one by 50% (presumed heterozygotes, P < 0.001 vs. control) and the other by 95% (presumed homozygotes, P < 0.001 vs. heterozygotes). Embryonic GLUT1 deficiency in the range reported with maternal diabetes was associated with growth retardation and developmental malformations similar to those described in diabetes-exposed embryos: intrauterine growth retardation (31.1%), caudal regression (9.8%), anencephaly with absence of the head (6.6%), microphthalmia (4.9%), and micrognathia (1.6%). Reduced body weight (small embryos, <70% of the nontransgenic body weight) was accompanied by other malformations and a 56% reduction of GLUT1 protein, P < 0.001 vs. nonsmall embryos (body weight ≥70% normal). The heart, brain, and kidneys of embryonic day 18.5 GT1AS embryos exhibited 24–51% reductions of GLUT1 protein. The homozygous GT1AS genotype was lethal during gestation. Reduced embryonic GLUT1 was associated with the appearance of apoptosis. Therefore, GLUT1 deficiency may play a role in producing embryonic malformations resulting from the hyperglycemia of maternal diabetes. Late gestational macrosomia was absent, apparently requiring a different mechanism.
Keywords: embryo, antisense, transgenic, development, apoptosis
The uncontrolled hyperglycemia of maternal diabetes is associated with early intrauterine growth retardation and deformities in embryos. Recent work indicates that facilitative glucose transporter (GLUT) expression in preimplantation embryos from hyperglycemic diabetic mothers is decreased in the range of 50%, which potentially could impair embryonic development. These GLUTs include GLUTs 1, 2 and 3, the main GLUTs of preimplantation embryos (1). GLUT8, regulated by insulin, was recently identified in mouse blastocysts although its response to maternal diabetes is unknown (2).
A group of deformities has been described in embryos from diabetic mothers, among which caudal regression syndrome is the most characteristic (3, 4). This syndrome involves dysgenesis or agenesis of organs below the diaphragm, with general impairment of caudal development (3, 4). Other embryonic deformities described with maternal diabetes include microphthalmia, micrognathia, and impaired brain development. GLUT1 is a low Km, high-affinity GLUT believed to play a key role in maintaining basal glucose uptake for metabolism in many cell types (5). GLUT1 has been identified in the developing embryo from as early as the oocyte stage on (6). In addition, its expression increases 11-fold during development from the two-cell stage to the blastocyst stage (7), and GLUT1 has been identified in both the inner cell mass and trophectoderm of late preimplantation embryos (6).
Recent work indicated that suppression of GLUT1 in preimplantation embryos by antisense-GLUT1 (GT1AS) treatment to mimic the diabetic condition led to apoptosis (8). Therefore, we hypothesized that isolated transgenic suppression of GLUT1 in embryos, to mimic exposure to the hyperglycemia of maternal diabetes, could delay or impair their development. We recently reported (9) that GLUT1 partially compensates for its own deficiency in heterozygous knockout embryonic stem cells, and that homozygous knockouts are nonviable; therefore, we suppressed GLUT1 by creating GT1AS transgenic mice. This method suppresses GLUT1 protein by 50% in cultured cells (10) and suppresses GLUT1 in preimplantation embryos (8).
Materials and Methods
Assembly of the GT1AS DNA Expression Construct. The mammalian expression vector pHβAPr1 uses the human β-actin promoter for widespread, high-level expression of transcripts (11, 12). It contains 3 kb of the human β-actin gene 5′ flanking sequence plus 5′ UTR and intervening sequence-1. An SV40 polyadenylation signal is also included (11). The full-length human GLUT1 cDNA (gift of M. Mueckler, Washington University, St. Louis) was ligated into the SalI cloning site of the pHβAPr1 vector. The antisense orientation of the GLUT1 cDNA insert was confirmed by Southern analysis.
Injection of DNA Constructs into Pronuclei of Fertilized Eggs. All animal procedures were approved by the Institutional Review Board. Fertilized mouse eggs (8 h) were obtained from superovulated (C57BL/6 × SJL)F1 egg donors mated to (C57BL/6 × SJL)F1 stud males and injected as described (13) with 2–3 pl of the GT1AS construct (7.7 kb, 3 ng/μl), a promoterless 997-bp fragment of the chloramphenicol acetyltransferase (CAT) gene (3 ng/μl), a 17-kb LacZ construct (3 ng/μl), or injection buffer alone (no DNA). The eggs were then cultured in M16 medium (Specialty Media, Lavellette, NJ) in a humidified, 5% CO2, 37°C incubator, or reimplanted into foster female mice. Development was monitored in vitro over 3.7 days. The destiny of reimplanted eggs injected with either the GT1AS or LacZ (control) DNA constructs was also determined. LacZ transgenic mice were identified by PCR (see below). GT1AS transgenic mice were identified with a specific 32P-labeled SV40 DNA probe on Southern analysis. The smallest GT1AS transgenic founder mouse was crossed back repeatedly (>20 generations) to the C57BL/6 background to produce an inbred line of mice. Additional egg injections of the GT1AS construct produced nine transgenic mouse lines for study.
PCR to Detect the LacZ Transgene in Control LacZ Transgenic Mice. PCR was carried out by using a previously published protocol and LacZ primers (14). A 30-bp sense oligonucleotide (5-′ TTCACTGGCCGTCGTTTTACAACGTCGTGA-3′) and a 30-bp antisense oligonucleotide (5-′ ATGTGAGCGAGTAACAACCCGTCGGATTCT-3′) were used to amplify a 364-bp fragment from the LacZ gene corresponding to nucleotides 15–379.
Measurement of 2-Deoxyglucose (DG) Uptake Rates in Preimplantation Embryos. DG uptake rates were measured in preimplantation blastocyst embryos by using a nonradioactive microanalytic procedure described in detail (8). Experiments were performed in triplicate on 10–15 individual embryos per group for each experiment.
Measurements of Blood Glucose Concentrations. Tail blood was collected from nonfasting adult mice for measurements of blood glucose concentrations by use of an Accucheck Simplicity glucometer, following the manufacturer's instructions (Roche Molecular Biochemicals). Measurements were made in nontransgenic (NT), transgenic, and pregnant mice for comparisons.
Northern Analysis of Native and Antisense GLUT1 Transcripts from Adult Mouse Organs. Twenty micrograms of total RNA from each of eight tissues was electrophoresed on 6% formaldehyde, 1.0% agarose gels, then blotted and probed for GLUT1 mRNA as described in detail (15). Equal lane loading was confirmed by assessment of 28s and 18s rRNA band intensities on ethidium bromide-stained gels.
Gestational Interruption. Eight- to 16-week-old heterozygous GT1AS transgenic mice were mated for experiments in which gestation would be interrupted at embryonic days (E)11.5, E14.5, E16.5, and E18.5 after mating. Pregnant mice were anesthetized with Avertin (450 mg/kg i.p.) for laparotomy to expose the embryos. Whole embryos or individual organs were flash-frozen in liquid nitrogen for immunoblotting of GLUTs and β-tubulin, or fixed in 10% buffered formalin for hematoxylin/eosin staining.
Immunoblotting of GLUTs in Whole Embryos and Embryonic Organs. SDS PAGE gels (10%) were used to run 100 μg of total protein per lane as described (10). Equal lane loading was confirmed with β-tubulin antibody or by Ponceau-S staining. Specific, polyclonal antibodies against GLUT1 (rabbit anti-rat, reacts with mouse, 1:10,0000; C. Carter-Su, University of Michigan, Ann Arbor), GLUT3 (goat anti-mouse, 1:200; Santa Cruz. Biotechnology), and β-tubulin (rabbit anti-human, reacts with mouse, 1:200; Santa Cruz Biotechnology) were used to detect the respective proteins by using the enhanced chemiluminescence assay (Amersham Pharmacia). The bands were semiquantitated by optical scanning densitometry (Microtek Scanmaker-5) by using nih image v. 1.62 software.
Embryo Recovery and Culture in Preparation for Staining. Mice were fed standard chow and water ad libitum. Female mice (GT1AS heterozygotes) of 4–6 weeks of age were superovulated with 10 units of pregnant mare serum gonadotropin (Sigma) followed by 10 units of human chorionic gonadotropin (hCG). Mating with GT1AS heterozygous males was confirmed by identification of a vaginal plug. Animals were killed on E3.5, 96 h after hCG administration and mating. Blastocyst embryos were recovered as described from dissected uterine horns and ostia (8, 16, 17), immediately placed in KSOM media (Specialty Media) and cultured at 37°C in 5% CO2, 5% O2, and 90% N2.
Immunofluorescent Labeling of GLUT1 and Terminal Deoxynucleotidyltransferase-Mediated dUTP Nick End Labeling (TUNEL)-Positive Cells in Preimplantation Embryos. Heterozygous GT1AS mice were mated and blastocyst stage embryos were harvested from the uterine horns at E3.5. Blastocysts were fixed on glass slides with 3% paraformaldehyde, and were permeabilized with 0.1% Tween 20. TUNEL labeling for detection of apoptosis (FITC, green channel) was performed as described (16, 18). GLUT1 was labeled with 15 μg/ml specific rabbit anti-mouse GLUT1 Ab (16) for 1 h at room temperature, then secondary goat anti-rabbit Alexa 594-labeled antibody (1:80, red channel; ref. 16). Nuclear DNA of all cells was counterstained with To-Pro-3 (4 μM, blue channel). Washed embryos were visualized by using confocal immunofluorescent microscopy (Bio-Rad; MRC-600) at ×63 magnification. TUNEL and GLUT1 fluorescence staining were initially assessed as 0–3+, then a Z series was performed on some of the blastocysts to determine the percent TUNEL-positive nuclei per embryo.
Statistics. The Student t test was used for comparisons between two experimental groups. One-way analysis of variance was performed to determine significance for data involving more than two groups. The Bonferroni t test correction was used to confirm statistical differences in situations where more than one group comparison was made, and χ2 analyses were performed for studies of birth rates employing the Yates continuity correction. The significance levels for statistical comparisons were P < 0.05. Data are presented as mean ± SEM in the text and graphs.
Results
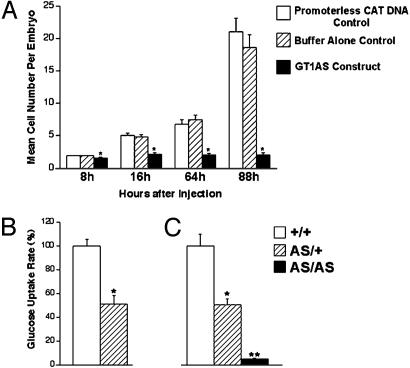
First, preimplantation embryonic development was evaluated in vitro (Fig. 1A). Fertilized mouse oocytes from matings of (C57BL/6 × SJL male) × (C57BL/6 × SJL female) mice were injected with the linearized GT1AS DNA, a promoterless CAT control DNA, or with injection buffer alone. GT1AS-injected embryos exhibited fewer cells than controls at each time point observed (P < 0.001), indicating severe growth delay, and 9.3% of GT1AS-injected embryos died (lysed) vs. 0% of controls.
Fig. 1.
(A) Mean cell number per embryo after injection of the GT1AS expression construct, promoterless CAT DNA, or buffer alone, into 8-h fertilized mouse eggs. (B) Suppression of glucose uptake rate in GT1AS transgenic preimplantation embryos. DG uptake rates into preimplantation embryos are shown. Mating of GT1AS heterozygous males (AS/+) × nontransgenic (NT) females (+/+) led to a 49% suppression of the glucose uptake rate in approximately half (45%) of the preimplantation embryos recovered. These embryos were concluded to be the GT1AS heterozygotes (AS/+, n = 10). The remaining 55% of the preimplantation embryos with a higher glucose uptake rate were concluded to be the nontransgenics (+/+, n = 12). (C) Mating of GT1AS heterozygotes produced three distinct groups of embryos as shown (no overlap), in a 1:3.7:1.3 ratio, close to the ideal 1:2:1 ratio expected from heterozygous parents.
Subsequently, DG uptake rates were measured in NT and transgenic (GT1AS) preimplantation embryos. Nearly half (45%) of embryos obtained from mating GT1AS transgenic heterozygotes with NT C57BL/6 mice (background strain) exhibited a mean 49% suppression of the DG uptake rate, P < 0.001 vs. control, which was consistent with heterozygotes (Fig. 1B). In comparison, matings of GT1AS heterozygotes together (Fig. 1C) produced preimplantation embryos with 50% suppression (presumed heterozygotes, 61% of embryos obtained, P < 0.001 vs. control) and 95% suppression (presumed homozygotes, 22% of embryos obtained, P < 0.001 vs. heterozygotes) of the DG uptake rate. The substantial reduction of glucose uptake rates suggested this was the cause of the severely delayed embryonic development.
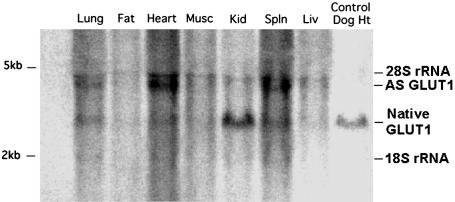
Next, the capability for GT1AS-injected fertilized eggs to produce live transgenic pups was evaluated (Table 1). The birth rate from such eggs was 46.5% lower than that from LacZ DNA (control)-injected eggs (P < 0.01). Similarly, the fraction of liveborn pups identified as transgenic was 47.3% less from GT1AS-injected eggs than from LacZ control-injected eggs (P < 0.05). Four live transgenic mice were born from the initial series of GT1AS-injected and reimplanted eggs. They were observed until 10 weeks of age, at which time the smallest transgenic mouse (a female with body weight that was 69% of female NT sibling weight) was selected to develop the line of GT1AS mice reported here. This founder mouse was chosen for breeding, because its small size suggested the best antisense suppression of GLUT1. The mouse was bred into the C57BL/6 background for >20 generations to obtain an inbred line of mice carrying the GT1AS transgene. Adult, nonfasting blood glucose concentrations for NT, GT1AS transgenic, and pregnant GT1AS transgenic mice are shown in Table 2. GT1AS transgenic mice reaching adulthood were all heterozygotes, as described below. Blood glucose concentrations in transgenic and pregnant transgenic mice did not differ significantly from the concentrations in NT control mice, and were consistent with those reported in nondiabetic C57BL/6 mice (19). All adult tissues examined from GT1AS transgenic mice expressed the GT1AS transcript (Fig. 2), with heart expression > spleen > lung > skeletal muscle, liver, kidney > adipose tissue.
Table 1. Destiny of DNA-injected fertilized oocytes reimplanted into foster mothers.
| Fertilized oocytes injected with | LacZ DNA (control) | GT1AS DNA |
|---|---|---|
| No. of injected oocytes reimplanted | 266 | 296 |
| No. of liveborn pups obtained (%)* | 70 (26.3) | 41 (13.9)† |
| No. of transgenic liveborn pups (%)* | 13 (4.8) | 4 (1.4)‡ |
Numbers shown in parentheses represent percent of injected oocytes reimplanted into foster mothers.
P < .001 vs. LacZ
P < 0.05 vs. LacZ
Table 2. Adult nonfasting blood glucose concentrations.
| Adult genotype | NT (male and female) | GT1AS heterozygotes (male and female) | Pregnant GT1AS heterozygotes |
|---|---|---|---|
| Blood glucose, mg/dL | 169 ± 10 (n = 14) | 144 ± 8 (n = 9) | 145 ± 11 (n = 11) |
| P | — | >0.05 vs. NT | >0.1 vs. NT |
Fig. 2.
Northern blot identifying expression of the native (2.8 kb) and GT1AS transgenic (3.6 kb) transcripts in organs from an adult GT1AS heterozygous transgenic mouse. Every tissue examined expressed the GT1AS transcript. The relative levels of expression in the organs shown were similar to the expected pattern of expression for this promoter (12). Even lane loading was confirmed as described in Materials and Methods.
To evaluate GT1AS transgene effects on litter size (Table 3), newborn pup body mass (Table 4), and parental fertility (Table 3), GT1AS heterozygotes were mated together, or with NT mice. The former matings produced litters reduced in size by 47% vs. litter sizes from GT1AS × NT and NT × NT matings, and were associated with a 7-fold higher stillborn rate than in the GT1AS × NT matings (Table 3). The mean body mass of stillborn transgenic pups (Table 4) was 35.0% less than the mass of liveborn NT pups, and 32.3% less than the mass of liveborn transgenic pups. However, liveborn transgenic and NT pups had similar body masses (Table 4), indicating the demise of growth-impaired transgenic embryos. This result is consistent with our finding in GT1AS transgenic mice surviving to adulthood where only 2 of 149 adult transgenics (1.3%) demonstrated overt growth impairment, with a maximum 40% reduction in body mass. The percentages of liveborn pups identified as transgenic is shown in Table 3 for matings involving GT1AS mice.
Table 3. Liveborn and stillborn pups obtained from mouse matings.
| Matings | GT1AS × GT1AS | GT1AS × NT | GT1AS male × NT female | GT1AS female × NT male | NT × NT |
|---|---|---|---|---|---|
| No. of pups born per litter | 4.4 ± 0.6 (n = 14) | 7.1 ± 0.6 (n = 15) | 7.3 ± 0.6 (n = 10) | 7.0 ± 1.6 (n = 5) | 7.3 ± 0.7 (n = 4) |
| No. of liveborn per litter | 3.7 ± 0.7* | 7.0 ± 0.6 | 7.1 ± 0.7 | 7.0 ± 1.6 | 7.3 ± 0.7 |
| No. of stillborn per litter | 0.7 ± 0.2* | 0.1 ± 0.1 | 0.2 ± 0.1 | 0 | 0 |
| Percent liveborn GT1AS | 77 ± 7* (n = 6) | 53 ± 7 (n = 4) | — | — | — |
| Percent liveborn NT | 23 ± 7 | 47 ± 7 | — | — | — |
GT1AS parents were heterozygous (homozygotes nonviable). n, no. of litters examined. *, P < 0.05 vs. GT1AS × NT.
Table 4. Newborn and adult body weights.
| Newborn body weights from GT1AS × GT1AS matings
|
Adult body weights† (percent of NT same-sex sibling weights)
|
|||||
|---|---|---|---|---|---|---|
| Liveborn NT pups, g | Liveborn GT1AS pups, g | Stillborn GT1AS pups, g | NT Males | GT1AS Males (heterozygotes) | NT Females | GT1AS Females (heterozygotes) |
| 1.60 ± 0.05 (n = 21) | 1.54 ± 0.05 (n = 24) | 1.04 ± 0.07* (n = 4) | 100 ± 1% (n = 7) | 103 ± 2% (n = 3) | 100 ± 2% (n = 11) | 99 ± 3% (n = 10) |
All GT1AS adults were heterozygous (homozygotes nonviable). n = No. of mice examined. *, P < 0.001 vs. both liveborn NT pups and liveborn GT1AS pups.
Mice surviving to adulthood were taken from randomly selected litters and weighed at 2-5 months of age. Therefore, the adult weights are expressed as percent of NT same-sex sibling controls for comparison.
When the stillborns were counted as part of the newborn litters from matings of GT1AS heterozygotes together, the decrease in litter size was 37.6%, P < 0.005, still greater than the expected 25% decrease if GT1AS homozygotes alone succumbed during gestation. Although some heterozygotes may have had sufficient suppression of GLUT1 to cause their demise during gestation, heterozygous embryos typically survived from matings of male and female heterozygotes with NT mice (Table 3). The latter matings gave the expected fraction of transgenics in normal-sized litters. This finding suggested that, above and beyond the effects of GLUT1 suppression in individual embryos, matings of GT1AS heterozygous transgenic mice together produced an additional adverse effect on the gestations, resulting in reduced survival of both transgenic and NT embryos. This concept is supported by preservation of the 3:1 ideal ratio of live transgenic and NT pups in litters from GT1AS × GT1AS matings (Table 3), despite the documented reduction in litter size. We hypothesize that the demise of homozygous GT1AS embryos in utero may in some way interfere with the survival of other embryos in the same gestation. This hypothesis will be tested in future investigations. Eleven GT1AS transgenic mice born from GT1AS × GT1AS matings, and 46 randomly selected transgenics, were all proven to be heterozygous by genotyping pups born from their matings with NT mice.
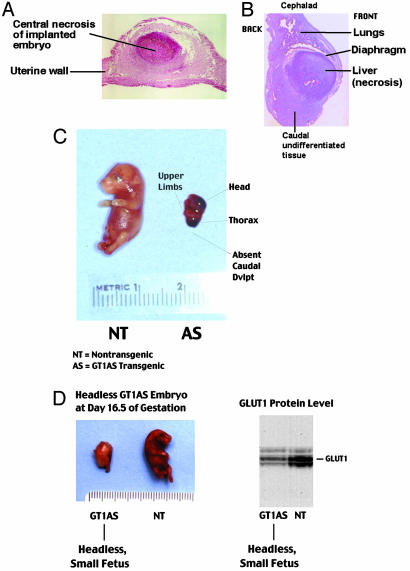
Next, the fate of developing embryos was investigated by interruption of gestation at E11.5, E14.5, E16.5, and E18.5 after mating GT1AS heterozygotes together (Table 5). In an analysis of 72 embryos (10 gestations) from these time points, 18 (35.3%) of the transgenic embryos were malformed. Furthermore, 100% of the malformations occurred in transgenic embryos. Subsequently, specific malformations were documented in a population of 61 embryos (nine gestations) from matings of GT1AS heterozygotes together (Table 5). A wide range of developmental delay was observed. Embryos dying shortly after implantation into the uterine wall were typically very small, round, or oblong in shape, and demonstrated large areas of necrosis (Fig. 3 A and B). In contrast, embryos which continued to grow and develop limbs typically did not demonstrate necrosis. GLUT1 protein levels in the embryos dying early postimplantation could not be accurately assessed due to the presence of necrosis. However, sufficient viable tissue remained to identify the GT1AS transgene in these small embryos, which were presumed to be homozygotes.
Table 5. Embryos and malformations resulting from matings of GT1AS heterozygotes together.
| GT1AS × GT1AS (10 gestations, 72 embryos)
|
GT1AS × GT1AS (9 gestations, 61 embryos)
|
|||
|---|---|---|---|---|
| NT Embryos | GT1AS embryos | Specific malformations | No. (percent of all embryos)* | |
| No. of embryos | n = 21 | n = 51 | Body mass < 70% control | 19 (31.1) |
| Percent total embryos | 29 | 71† | Microphthalmia | 3 (4.9) |
| No. malformed, % | 0 (0) | 18 (35.3)‡ | Caudal regression | 6 (9.8) |
| No. not malformed, % | 21 (100) | 33 (64.7) | Absent head | 4 (6.6) |
| — | — | — | Micrognathia | 1 (1.6) |
Embryos with malformations typically had more than one type of malformation.
Approximates the calculated ideal value of 75% GT1AS transgenic.
P < 0.01 vs. NT.
Fig. 3.
(A and B) Examples of impaired development in a subpopulation of GT1AS transgenic embryos harvested on E18.5, when the head and abdominal organs would otherwise normally have been present. (A) Necrosis early postimplantation. (B) Severe caudal dysgenesis, including impaired organogenesis below the diaphragm with liver necrosis. The head was absent. (C) Severe example of the caudal regression syndrome, with complete absence of caudal development at E16.5. (D) An example of absent head and caudal regression phenotypes at E16.5. GLUT1 protein (immunoblot) was typically reduced in the small (i.e., <70% of NT sibling body weight) transgenic embryos.
A transgenic embryo with severely impaired organogenesis is shown in Fig. 3B exhibiting the caudal regression syndrome, where organs below the diaphragm develop abnormally or not at all. This embryo exhibited liver necrosis, and impaired caudal development with lack of kidneys, intestines, and hindlimbs. Fig. 3 C and D also demonstrate caudal regression syndrome. Multiple developmental defects unique to GT1AS transgenic embryos were identified (Table 5), including: stunted growth (Fig. 3 C and D), absence of the head (Fig. 3D), caudal regression (Fig. 3 C and D) with impaired organ development (Fig. 3 B and C), particularly organs below the diaphragm such as the kidneys and intestines, microphthalmia (Fig. 6. which is published as supporting information on the PNAS web site), and micrognathia (Fig. 7, which is published as supporting information on the PNAS web site). Four additional lines of transgenic mice (called GT1AS2 lines) developed from separate injections of the GT1AS transgene into fertilized eggs were examined, and the embryos exhibited severe developmental impairment, malformations, and reduced GLUT1 similar to the original GT1AS line.
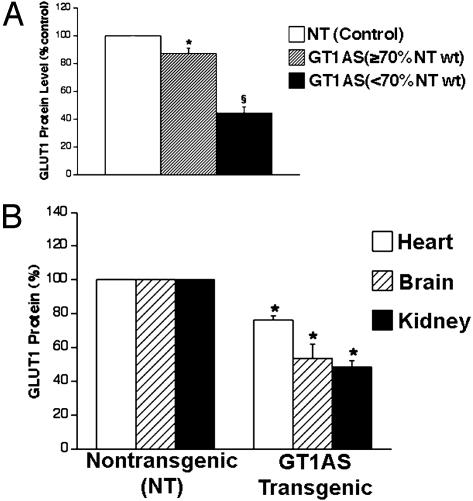
GLUT1 protein levels were determined in 68 NT and transgenic day E18.5 whole embryos obtained from GT1AS × GT1AS matings. Transgenic embryos which were overtly small, i.e., < 70% of NT embryonic mass, typically exhibited additional malformations (Fig. 3 C and D and Table 5). Whole transgenic embryo GLUT1 was determined in embryos which had not necrosed, i.e., those that survived beyond the early postimplantation stage. GLUT1 protein levels determined in whole embryos by immunoblotting, were reduced 56% in the small GT1AS transgenic embryos when compared to NT sibling embryos (Fig. 4A, P < 0.001, E18.5 embryos). In contrast, the nonsmall GT1AS transgenic embryos (i.e., ≥70% of NT body mass) demonstrated less severe GLUT1 suppression (mean suppression 13%, Fig. 4A, P < 0.001 vs. small embryos). The range of GLUT1 suppression observed in GT1AS transgenic whole embryos was 0–70%, with the smaller embryos having the lower GLUT1 protein levels (Fig. 4A). GLUT3 protein levels in whole transgenic embryos were unchanged (data not shown, P > 0.1, NS).
Fig. 4.
(A) GLUT1 protein suppression in E18.5 GT1AS transgenic whole embryos (n = 5–32 each group). The small embryos (i.e., <70% of NT sibling body weight), exhibited 56% reduction of GLUT1 protein (§, P < 0.001). (B) GLUT1 protein levels from three vital organs of E18.5 embryos, obtained by immunoblotting (n = 5–10 each group). The kidneys exhibited the greatest reduction of GLUT1 (51%, *, P < 0.001 vs. NT) and failed to develop in some transgenic embryos.
GLUT1 protein levels were next examined in the heart, brain, and kidneys (Fig. 4B) of E18.5 embryos obtained from GT1AS × GT1AS matings. In the GT1AS transgenic embryos, GLUT1 was reduced by 24% in the heart (P < 0.001), 46% in the brain (P < 0.001), and 51% in the kidneys (P < 0.001), when compared to NT sibling embryos, n = 5–10 for each group. This finding may account for the lack of kidney or brain development in some of the transgenic embryos. In contrast, another embryonic kidney GLUT (GLUT3) was unchanged in transgenics (P > 0.1, NS). In GT1AS transgenic mice surviving to adulthood (only heterozygotes survived), mean body mass was not significantly different from same-sex, NT siblings (Table 4), although rare (<2%), transgenic adults survived with severe growth impairment.
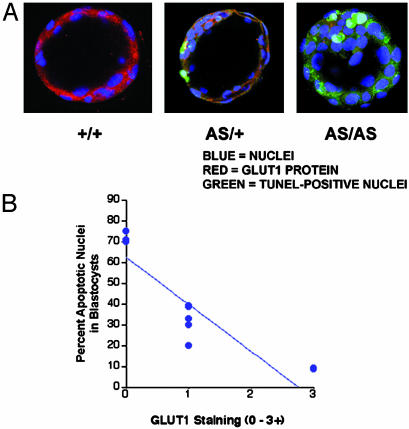
To determine how suppressed glucose uptake into the embryos may cause developmental impairment, blastocyst embryos were assessed for apoptosis and GLUT1 protein expression (Fig. 5A). Three distinct populations of embryos were identified when GT1AS heterozygotes were mated together: those with 3+ GLUT1 (red) and 0 TUNEL (green) labeling, which was consistent with the NT genotype; those with 1+ GLUT1 and 1+ TUNEL labeling, which was consistent with heterozygotes; and those with 0 (minimal to none) GLUT1 protein and 3+ TUNEL labeling, which was consistent with homozygotes. These three populations of embryos were observed in a 1.2:2.0:0.9 ratio, respectively (n = 27 embryos from three separate experiments), which was similar to the expected 1:2:1 Mendelian pattern. Analysis of all embryos from a gestation emphasized the inverse relationship between percent TUNEL-positive (apoptotic) nuclei per embryo and the degree of GLUT1 staining (Fig. 5B, r = 0.88, P = 0.002, n = 9 embryos), which may have accounted for developmental impairment in the GLUT1-deficient embryos.
Fig. 5.
(A and B) Detection of GLUT1 protein and apoptosis by confocal microscopy in blastocyst embryos from matings of GT1AS heterozygotes together (A; ×63 magnification). Cell nuclei are stained blue, GLUT1 is stained red, and apoptotic cells are stained green (TUNEL assay). Three populations of embryos were detected in these matings: those with high GLUT1 expression and minimal apoptosis (+/+), those with intermediate GLUT1 expression and intermediate apoptosis (AS/+), and those with trace to no detectable GLUT1 expression and a high frequency of apoptosis (AS/AS). The results indicated an inverse relationship between GLUT1 protein and apoptosis (B).
Discussion
GLUT1 is a major GLUT of mouse embryos (1). Maternal diabetes reduces its expression and glucose uptake in preimplantation embryos, leading to apoptosis (16), which is potentially involved in diabetic embryopathy. Therefore, we produced transgenic mice with suppressed GLUT1 in the range (50% normal) observed in embryos of diabetic mothers (8). The GT1AS transgene significantly suppressed early embryonic glucose uptake rates and markedly delayed development, indicating an important role for GLUT1 early in gestation. Although older studies (21) suggested glucose might not be needed at the earliest developmental stages of the embryo (e.g., two-cell stage), recent data indicate that glucose in fact may play an important role in the survival of these early embryos (1). The reduced birth rates observed from GT1AS-injected eggs were consistent with the delayed embryonic development.
This work is consistent with previous reports (10, 22) suggesting an important role for GLUT1 in cell growth. It is stimulated by multiple growth factors involved in mitogenesis (5), and is increased in many tumors (23), whereas antisense suppression of GLUT1 can slow nontumor and tumor cell growth (10, 23). It is therefore understandable that suppression of GLUT1 is harmful to the developing embryo. High extracellular glucose with maternal diabetes suppresses GLUT1 in preimplantation embryos (1), resulting in diminished glucose uptake and intraembryonic free glucose levels. These embryos exhibit excess apoptosis mediated by increased Bax expression. Similarly, in the present report, antisense suppression of embryonic GLUT1 was associated with the appearance of apoptosis, a potential cause for the impaired development observed. Gestational interruption revealed impaired development in many transgenic embryos and the homozygous genotype was lethal. GLUT1 protein was reduced to varying degrees in whole GT1AS transgenic embryos, with the lowest levels in the small embryos, which typically exhibited additional malformations.
Suppression of GLUT1 protein in individual vital organs of GT1AS embryos provided clues to some of the malformations and contributed to their overall growth impairment. The substantially reduced levels of GLUT1 in embryonic brain and kidneys may have been responsible for absence of the brain, microphthalmia, or absence of the kidneys in some GT1AS embryos. Dysgenesis or agenesis of the kidneys is a previously reported feature of more severe cases of caudal regression syndrome (3, 4, 24).
The individual developmental abnormalities detected in GT1AS embryos are not specific to diabetes; however, the combination of defects observed is typical for diabetic embryopathy (3, 4). In particular, caudal regression syndrome is highly characteristic of diabetic embryopathy (3, 4), and was observed in nearly 10% of GT1AS embryos from GT1AS heterozygote matings in the current study. This syndrome includes varying degrees of impaired development of the hind portion of the embryo, some mild with only impaired hindlimb development, and others severe including dysgenesis and agenesis of organs below the diaphragm, such as the kidneys. This syndrome occurs 200 times more frequently in infants of diabetic mothers than in infants of nondiabetic mothers (4), suggesting the maternal hyperglycemia may play a role in its development. A similar variation in severity of caudal regression was observed in the GT1AS transgenic embryos without maternal hyperglycemia, where the most severe malformations were associated with the greatest suppression of GLUT1 protein.
In contrast to the diabetic state, pregnant GT1AS transgenic females had normal nonfasting blood glucose levels. Their lack of hyperglycemia may partly explain the absence of late macrosomia. Macrosomia has been observed in 10–27% of human fetuses exposed to maternal diabetes (25) and has been associated with the following: maternal hyperglycemia (26), fetal hyperinsulinemia (25), and increased insulin-like growth factors in neonates (20). Therefore, GLUT1 deficiency does not appear to explain late gestational macrosomia in the offspring of diabetic mothers, although it does impair early embryonic growth. The mechanisms for the two growth abnormalities appear to be different.
In summary, isolated GLUT1 suppression in mice impaired embryonic development from the earliest stages on, leading to developmental malformations and embryonic demise in those with the lowest levels of the GLUT. Multiple organs were affected by the reduced GLUT1 expression, and the embryonic malformations were similar in many respects to those previously described in embryos exposed to the hyperglycemia of maternal diabetes where embryonic GLUT1 is known to be reduced. The appearance of apoptosis in the embryos with reduced GLUT1 protein suggested this was a mechanism contributing to their impaired development. Therefore, hyperglycemic suppression of embryonic GLUT1 may play an important role in the malformations that develop in response to maternal diabetes.
Supplementary Material
Acknowledgments
We thank Drs. Ying Jin and Minghui Xiang for their assistance with mouse breeding and surgery. This work was supported by National Institutes of Health Grant RO1 DK54507 (to C.W.H.), the American Diabetes Association, the American Heart Association, the National Kidney Foundation, a University of Rochester Buswell Award, and a Fund of Henry Ford Hospital Award. F.C.B. was supported by National Kidney Foundation and Juvenile Diabetes Foundation awards.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: NT, nontransgenic; GLUT, glucose transporter; GT1AS, antisense-GLUT1; DG, 2-deoxyglucose; En, embryonic day n; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling.
References
- 1.Moley, K. H., Chi, M. M. & Mueckler, M. M. (1998) Am. J. Physiol. 275, E38–E47. [DOI] [PubMed] [Google Scholar]
- 2.Carayannopoulos, M. O., Chi, M. M., Cui, Y., Pingsterhaus, J. M., McKnight, R. A., Mueckler, M., Devaskar, S. U. & Moley, K. H. (2001) Proc. Natl. Acad. Sci. USA 97, 7313–7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindstrom, J. A. (1971) Birth. Defects. Orig. Artic. Ser. 7, 278–279. [PubMed] [Google Scholar]
- 4.Mills, J. L. (1982) Teratology 25, 385–394. [DOI] [PubMed] [Google Scholar]
- 5.Devaskar, S. & Mueckler, M. (1992) Pediatr. Res. 31, 1–13. [DOI] [PubMed] [Google Scholar]
- 6.Aghayan, M., Rao, L. V., Smith, R. M., Jarett, L., Charron, M. J., Thorens, B. & Heyner, S. (1992) Development (Cambridge, U.K.) 115, 305–312. [DOI] [PubMed] [Google Scholar]
- 7.Morita, Y., Tsutsumi, O., Oka, Y. & Taketani, Y. (1994) Biochem. Biophys. Res. Commun. 199, 1525–1531. [DOI] [PubMed] [Google Scholar]
- 8.Chi, M. M., Pingsterhaus, J., Carayannopoulos, M. & Moley, K. H. (2001) J. Biol. Chem. 275, 40252–40257. [DOI] [PubMed] [Google Scholar]
- 9.Heilig, C., Brosius, F., III, Siu, B., Concepcion, L., Mortensen, R., Heilig, K., Zhu, M., Weldon, R., Wu, G. & Conner, D. (2003) Am. J. Pathol. 163, 1873–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heilig, C. W., Kreisberg, J. I., Freytag, S., Murakami, T., Ebina, Y., Guo, L., Heilig, K., Loberg, R., Qu, X., Jin, Y., Henry, D. & Brosius, F. C. (2001) Am. J. Physiol. Renal. Physiol. 280, F657–F666. [DOI] [PubMed] [Google Scholar]
- 11.Gunning, P., Leavitt, J., Muscat, G., Ng, S. Y. & Kedes, L. (1987) Proc. Natl. Acad. Sci. USA 84, 4831–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oberley, T. D., Coursin, D. B., Cihla, H. P., Oberley, L. W., el-Sayyad, N. & Ho, Y. S. (1993) Histochem. J. 25, 267–279. [DOI] [PubMed] [Google Scholar]
- 13.Kendall, S. K., Saunders, T. L., Jin, L., Lloyd, R. V., Glode, L. M., Nett, T. M., Keri, R. A., Nilson, J. H. & Camper, S. A. (1991) Mol. Endocrinol. 5, 2025–2036. [DOI] [PubMed] [Google Scholar]
- 14.Kendall, S. K., Gordon, D. F., Birkmeier, T. S., Petrey, D., Sarapura, V. D., O'Shea, K. S., Wood, W. M., Lloyd, R. V., Ridgway, E. C. & Camper, S. A. (1994) Mol. Endocrinol. 8, 1420–1433. [DOI] [PubMed] [Google Scholar]
- 15.Brosius, F. C., Liu, Y., Nguyen, N., Sun, D., Bartlett, J. & Schwaiger, M. (1997) J. Mol. Cell. Cardiol. 29, 1675–1185. [DOI] [PubMed] [Google Scholar]
- 16.Chi, M. M., Schlein, A. L. & Moley, K. H. (2003) Endocrinology 141, 4784–4792. [DOI] [PubMed] [Google Scholar]
- 17.Wyman, A. H., Chi, M., Riley, J., Carayannopoulos, M. O., Yang, C., Coker, K. J., Pessin, J. E. & Moley, K. H. (2003) Mol. Endocrinol. 17, 2096–2102. [DOI] [PubMed] [Google Scholar]
- 18.Moley, K. H., Chi, M. M., Knudson, C. M., Korsmeyer, S. J. & Mueckler, M. M. (1998) Nat. Med. 4, 1421–1424. [DOI] [PubMed] [Google Scholar]
- 19.Devedjian, J. C., George, M., Casellas, A., Pujol, A., Visa, J., Pelegrin, M., Gros, L. & Bosch, F. (2001) J. Clin. Invest. 105, 731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan-Jun, L., Tsushima, T., Minei, S., Sanaka, M., Nagashima, T., Yanagisawa, K. & Omori, Y. (1996) Endocr. J. 43, 221–231. [DOI] [PubMed] [Google Scholar]
- 21.Martin, K. L. & Leese, H. J. (1995) Mol. Reprod. Dev. 40, 436–443. [DOI] [PubMed] [Google Scholar]
- 22.Burstein, D. E., Reder, I., Weiser, K., Tong, T., Pritsker, A. & Haber, R. S. (1998) Mod. Pathol. 11, 392–396. [PubMed] [Google Scholar]
- 23.Choi, J. W., Yoon, D. J., Lee, H. W., Han, D. P. & Ahn, Y. H. (1995) Yonsei. Med. J. 36, 480–486. [DOI] [PubMed] [Google Scholar]
- 24.Perrot, L. J., Williamson, S. & Jimenez, J. F. (1987) Ann. Clin. Lab. Sci. 17, 211–220. [PubMed] [Google Scholar]
- 25.Schwartz, R., Gruppuso, P. A., Petzold, K., Brambilla, D., Hiilesmaa, V. & Teramo, K. A. (1994) Diabetes Care 17, 640–668. [DOI] [PubMed] [Google Scholar]
- 26.Rudge, M. V., Calderon, I. M., Ramos, M. D., Abbade, J. F. & Rugolo, L. M. (2001) Gynecol. Obstet. Invest. 50, 108–112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.