Abstract
The adrenergic and histaminergic systems have been reported to have analogous effects on the heart. A case of transient ventricular dysfunction with echocardiographic findings characteristic of stress-induced cardiomyopathy (also known as takotsubo cardiomyopathy) in a patient who had an urticarial transfusion reaction is described. The effect of histamine on ventricular function and its interaction with the adrenergic system are discussed.
Keywords: Catecholamine, Histamine, Stress-induced cardiomyopathy, Takotsubo cardiomyopathy, Urticarial transfusion reaction
The adrenergic and histaminergic systems have been reported to have analogous effects on the heart. We present a case of transient ventricular dysfunction with echocardiographic findings characteristic of stress-induced cardiomyopathy (SCM; also known as takotsubo cardiomyopathy) in a patient who experienced an urticarial transfusion reaction. Although the exact pathophysiology has not yet been fully elucidated, it has been proposed that this transient cardiomyopathy is mediated by catecholamine cardiotoxicity. In the present case report, we discuss the effect of histamine on ventricular function and its interaction with the adrenergic system.
CASE PRESENTATION
A 48-year-old postmenopausal woman with a history of stage IV non-Hodgkin’s lymphoma, asthma, fludarabine-induced anemia and thrombocytopenia was admitted to the Stanford University Hospital (California, USA) with persistent thrombocytopenia (serum platelet count of 9×109/L) and anemia (hemoglobin 77 g/L). She had no cardiac history and no family history of cardiomyopathy. Her medications on admission included an albuterol inhaler as needed, lorazepam as needed and diphenhydramine.
As part of her inpatient therapy, the patient received platelet transfusions for worsening thrombocytopenia. During one of the transfusions, she acutely developed shortness of breath, chills and diaphoresis, which rapidly progressed to hypoxemic and hypercarbic respiratory failure. She was afebrile, normotensive (118/73 mmHg), tachycardic (138 beats/min), tachypneic (28 breaths/min) and acutely hypoxemic, with an oxygen saturation of 80% on 5 L/min oxygen via a nasal cannula. Her lung examination was notable for expiratory wheezing and crackles, and a cardiac examination revealed a regular heart rate and rhythm with an S3 gallop and distended jugular veins. She was also noted to have developed urticaria and pruritis on her abdomen.
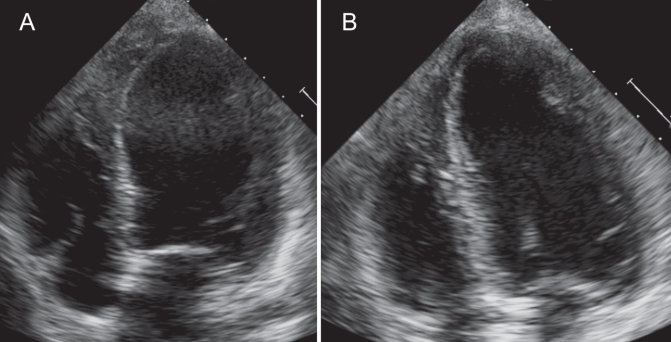
The platelet transfusion was stopped and the patient responded well to bilevel positive airway pressure, furosemide, intravenous methylprednisolone and diphenhydramine. An electrocardiogram showed normal sinus rhythm without significant ST segment changes. Troponin I peaked at 0.6 ng/mL. A chest x-ray showed bibasilar opacities with small bilateral pleural effusions and no evidence of pulmonary edema. A transthoracic echocardiogram revealed severe left ventricular dysfunction, with an ejection fraction of 30%, a left ventricular end diastolic dimension of 5.4 cm, and severe apical hypokinesis with preserved contraction of the basal segments, suggestive of SCM (Figure 1A). Cardiac catheterization was deferred due to the patient’s anemia and severe thrombocytopenia.
Figure 1).
A Transthoracic echocardiogram immediately following transfusion reaction revealing apical hypokinesis with preservation of basal segments. B Transthoracic echocardiogram seven days after transfusion reaction with resolution of systolic dysfunction and wall motion abnormalities
After stabilization, the patient was started on oral lisinopril 2.5 mg twice a day and oral carvedilol 3.125 mg twice a day. Her troponin level normalized within two days, and the patient’s symptoms progressively resolved. A transthoracic echocardiogram was repeated seven days following the event and revealed a left ventricular ejection fraction of 63%, with resolution of the wall motion abnormalities (Figure 1B). A repeat chest x-ray revealed minimally improved aeration of the bases without significant change. Her platelet count normalized. The patient was discharged home after one week, and regained good exercise capacity.
DISCUSSION
SCM is increasingly recognized as a cause of transient ventricular dysfunction occurring predominantly in postmenopausal women. It is suggested to occur in approximately 0.7% to 2.5% of suspected acute coronary syndromes. Urticarial transfusion reactions are also relatively uncommon, occurring in 1% to 3% of all blood transfusions (1). The presence of urticaria indicates histamine release. The relationship between histamine and SCM has not been well described. On the other hand, there are multiple reports of catecholamines playing an important role in the etiology of SCM. In the present case report, in which SCM appears to occur in the context of histamine release, we discuss the direct effect of histamine on the heart, as well as the interaction between the cardiac adrenergic and histaminergic systems.
Currently, catecholamines are believed to play an important role in the underlying mechanism of SCM. Patients diagnosed with SCM have been reported to present with supraphysiological levels of plasma catcholamines up to three times greater than those of patients who present with myocardial infarction (2). Excess catecholamines are believed to mediate ventricular dysfunction through coronary vasospasm, microvascular dysfunction or catecholamine cardiotoxicity. Mental stress has been reported to induce coronary vasoconstriction in patients without coronary disease (3). An early description of SCM (4) attributed this reversible cardiomyopathy to coronary vasospasm, but this hypothesis remains controversial because intracoronary acetylcholine failed to provoke coronary spasm in the majority of patients with SCM (5). Alternatively, in one study (6), microvascular dysfunction was found via abnormal thrombolysis in myocardial infarction perfusion grade in approximately two-thirds of patients presenting with SCM. Similarly, abnormal thrombolysis in myocardial infarction frame counts in three major coronary arteries in most patients presenting with SCM suggested diffuse coronary microvascular dysfunction (7). Finally, catecholamines induced direct cardiocyte toxicity via cyclic AMP-mediated calcium overload (8). More recently, Lyon et al (9) proposed that supraphysiological adrenaline stimulates a negative inotropic effect on myocyte contraction via beta-2 adrenoceptor signalling to the inhibitory G protein. According to this hypothesis, the increased density of sympathetic nerve endings in the basal myocardium relative to the apical myocardium may contribute to the characteristic ballooning of SCM.
Three histamine receptors have been described in the heart. H1 receptors have been described in epicardial vessels. H2 receptors constitute the majority of receptors in the heart, and have been found in the atria and ventricles. H3 receptors are localized presynaptically on postganglionic sympathetic fibres innervating blood vessels of the heart.
Histamine appears to affect downstream signals in the heart similar to the downstream signals in the adrenergic system.
Similar to catecholamines, histamine has direct vasoactive properties. Some groups (10) have observed H1 receptor stimulation resulting in coronary artery vasodilation, while others (11) have noted coronary vasoconstriction and vasospasm. Similarly, the H2 receptor has also been reported to mediate both coronary vasoconstriction (12) and coronary vasodilation (11). It has been proposed that the inconsistent findings may be secondary to a differential responsiveness of proximal and distal vessels (13). The vasoactive effect of histamine also appears to be related to a patient’s underlying coronary artery disease state and the stimulation of H1 receptor-mediated coronary vasodilation in patients without coronary artery disease and vasoconstriction in patients with vasospastic angina (14). Next, H2 receptors mediate a positive inotropic response. Similar to beta-1 adrenergic receptors, H2 receptors are coupled to adenylate cyclase, which increases cyclic AMP formation in myocytes, increasing myocyte contraction (15). Finally, histamine appears to affect the release of catecholamines. Histamine infusion in humans was associated with a significant rise in plasma adrenaline and noradrenaline (16). In another study (17) exemplifying the crosstalk between the histaminergic and adrenergic systems, H3 receptors on adrenergic nerve endings in the heart are believed to downregulate noradrenaline exocytosis from sympathetic nerve endings, resulting in a negative inotropic and chronotropic effect.
These common and interrelated pathways may have contributed to this patient’s transient ventricular dysfunction. Basal hyperkinesis may have resulted from the positive inotropic effects of histamine and catecholamines via the H2 and beta-adrenergic receptors, while apical hypokinesis may have been due to coronary vasospasm from H1/H2 and/or alpha-1 receptor stimulation.
CONCLUSION
We presented a case involving a postmenopausal woman who developed SCM during an urticarial transfusion reaction associated with histamine release. Although the urticarial transfusion reaction and her reversible cardiomyopathy may represent two independent events, the temporal relationship between her urticaria and new onset cardiomyopathy suggested that her reversible ventricular dysfunction may have occurred in the context of histamine release.
The present case emphasizes the potential interaction of the histaminic and adrenergic systems in stress cardiomyopathy. It also demonstrates the importance of considering transient ventricular dysfunction in patients presenting with respiratory distress following histamine-mediated transfusion reactions.
Footnotes
DISCLOSURE: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Domen RE, Hoeltge GA. Allergic transfusion reactions: An evaluation of 273 consecutive reactions. Arch Pathol Lab Med. 2003;127:316–20. doi: 10.5858/2003-127-0316-ATR. [DOI] [PubMed] [Google Scholar]
- 2.Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–48. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 3.Lacy CR, Contrada RJ, Robbins ML, et al. Coronary vasoconstriction induced by mental stress (simulated public speaking) Am J Cardiol. 1995;75:503–5. doi: 10.1016/s0002-9149(99)80590-6. [DOI] [PubMed] [Google Scholar]
- 4.Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. Myocardial stunning due to simultaneous multivessel coronary spasms: A review of 5 cases. J Cardiol. 1991;21:203–14. [PubMed] [Google Scholar]
- 5.Tsuchihashi K, Ueshima K, Uchida T, et al. Transient left ventricular apical ballooning without coronary artery stenosis: A novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol. 2001;38:11–8. doi: 10.1016/s0735-1097(01)01316-x. [DOI] [PubMed] [Google Scholar]
- 6.Elesber A, Lerman A, Bybee KA, et al. Myocardial perfusion in apical ballooning syndrome correlate of myocardial injury. Am Heart J. 2006;152:469.e9–e13. doi: 10.1016/j.ahj.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Bybee KA, Prasad A, Barsness GW, et al. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004;94:343–6. doi: 10.1016/j.amjcard.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 8.Mann DL, Kent RL, Parsons B, Cooper G., IV Adrenergic effects on the biology of the adult mammalian cardiocyte. Circulation. 1992;85:790–804. doi: 10.1161/01.cir.85.2.790. [DOI] [PubMed] [Google Scholar]
- 9.Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE. Stress (Takotsubo) cardiomyopathy – a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008;5:22–9. doi: 10.1038/ncpcardio1066. [DOI] [PubMed] [Google Scholar]
- 10.Matsuyama K, Yasue H, Okumura K, et al. Effects of H1-receptor stimulation on coronary arterial diameter and coronary hemodynamics in humans. Circulation. 1990;81:65–71. doi: 10.1161/01.cir.81.1.65. [DOI] [PubMed] [Google Scholar]
- 11.Ginsburg R, Bristow MR, Stinson EB, Harrison DC. Histamine receptors in the human heart. Life Sci. 1980;26:2245–9. doi: 10.1016/0024-3205(80)90209-x. [DOI] [PubMed] [Google Scholar]
- 12.Rosen R, Hegner-Mai M, Klaus W. Response to histamine of the intact coronary system in an isolated rabbit heart preparation. Eur Heart J. 1989;10(Suppl F):164–7. doi: 10.1093/eurheartj/10.suppl_f.164. [DOI] [PubMed] [Google Scholar]
- 13.Miller WL, Bove AA. Differential H1- and H2-receptor-mediated histamine responses of canine epicardial conductance and distal resistance coronary vessels. Circ Res. 1988;62:226–32. doi: 10.1161/01.res.62.2.226. [DOI] [PubMed] [Google Scholar]
- 14.Vigorito C, Poto S, Picotti GB, Triggiani M, Marone G. Effect of activation of the H1 receptor on coronary hemodynamics in man. Circulation. 1986;73:1175–82. doi: 10.1161/01.cir.73.6.1175. [DOI] [PubMed] [Google Scholar]
- 15.Klein I, Levey GS. Activation of myocardial adenyl cyclase by histamine in guinea pig, cat, and human heart. J Clin Invest. 1971;50:1012–5. doi: 10.1172/JCI106571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vigorito C, Russo P, Picotti GB, Chiariello M, Poto S, Marone G. Cardiovascular effects of histamine infusion in man. J Cardiovasc Pharmacol. 1983;5:531–7. doi: 10.1097/00005344-198307000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Endou M, Poli E, Levi R. Histamine H3-receptor signaling in the heart: Possible involvement of Gi/Go proteins and N-type Ca++ channels. J Pharmacol Exp Ther. 1994;269:221–9. [PubMed] [Google Scholar]