Abstract
Several years after the seven-valent pneumococcal conjugate vaccine (PCV7) was introduced in Canada and elsewhere, routine infant vaccination has led to near eradication of invasive pneumococcal disease caused by vaccine serotype strains in both children and adults. There have also been significant declines in pneumococcal-related disease including lobar pneumonia and otitis media. These declines have been offset, to some extent, by increases in nonvaccine serotype disease. Serotype 19A, which is often highly resistant to antibiotics, has become predominant. In most populations, however, the magnitude of replacement disease is much lower than the magnitude of decline in invasive pneumococcal disease with the use of PCV7. There is increasing evidence that three PCV7 doses provide protection that is nearly identical to that of four doses. New 10-valent and 13-valent pneumococcal conjugate vaccines were recently approved in Canada. These vaccines increase pneumococcal serotype coverage including serotype 19A (present in the 13-valent vaccine). Many provinces and territories have incorporated the 13-valent vaccine in their vaccination programs.
Keywords: Conjugate vaccine, Infant, Meningitis, Pneumococcal disease, Streptococcus pneumoniae, Vaccination
Abstract
Quelques années après l’adoption du vaccin conjugué heptavalent contre le pneumocoque (PCV7) au Canada et ailleurs, la vaccination systématique des nourrissons a suscité la quasi-éradication des pneumococcies invasives causées par les souches des sérotypes vaccinaux, tant chez les enfants que chez les adultes. On a également observé une diminution importante des maladies liées au pneumocoque, y compris la pneumonie lobaire et l’otite moyenne. Ces diminutions ont été contrebalancées, dans une certaine mesure, par l’augmentation des maladies à sérotypes non vaccinaux. Le sérotype 19A, qui est souvent hautement résistant aux antibiotiques, prédomine désormais. Dans la plupart des populations, cependant, la magnitude d’une maladie de remplacement est beaucoup plus faible que celle de la diminution des pneumococcies invasives attribuables au vaccin PCV7. De plus en plus de données probantes indiquent que trois doses du vaccin PCV7 assurent une protection presque identique à celle conférée par quatre doses. Les nouveaux vaccins conjugués 10-valent et 13-valent contre le pneumocoque ont récemment été approuvés au Canada. Ces vaccins accroissent la couverture des sérotypes pneumococciques, y compris le sérotype 19A (présent dans le vaccin 13-valent). De nombreuses provinces et de nombreux territoires ont intégré le vaccin 13-valent à leur programme de vaccination.
Français en page 237
The seven-valent pneumococcal conjugate vaccine (PCV7 [Prevnar, Pfizer Canada Inc, Canada]) was licensed in Canada in 2001. Routine infant vaccination programs began in 2002, but it was not until 2006 that all provinces and territories implemented routine programs. Currently, several years after PCV7 was introduced in Canada and the United States (US) (as well as many other countries), the routine use of this vaccine in infants and high-risk children has led to near eradication of invasive pneumococcal disease (IPD) caused by vaccine serotype strains in both children and adults. The recent approval in Canada of the new 10-valent and 13-valent pneumococcal conjugate vaccines (PCV10 and PCV13), which contain three and six additional serotypes to PCV7, respectively, is expected to prevent even more infections caused by the pneumococcus. The purpose of the present practice point is to update paediatricians and primary care physicians on the newer pneumococcal vaccines, and the potential impact they may have on decreasing morbidity and mortality caused by IPD.
REDUCTION OF VACCINE SEROTYPE INVASIVE PNEUMOCOCCAL INFECTIONS
The Calgary Area Streptococcus pneumoniae Epidemiology Research (CASPER) surveillance study (1) demonstrated a 94% decline in the incidence of vaccine serotype IPD and a 79% decline in overall IPD in children younger than two years of age in 2007, compared with 1998 to 2001 before PCV7 was introduced to Alberta. During the same time period, there was a 92% decline in vaccine serotype IPD in adults 65 to 84 years of age and a near-significant 29% decline in overall IPD. The decline seen in young children was related to the rapid and high uptake of routine four-dose PCV7, while the decline seen in adults appears to have been due to the herd effect of immunizing infants that reduces nasopharyngeal colonization and, therefore, reduces transmission to older children and adults and subsequent disease (2). There are also reports (3–7) on the decline in vaccine serotype IPD in children and adults from British Columbia, Quebec and northern Canada.
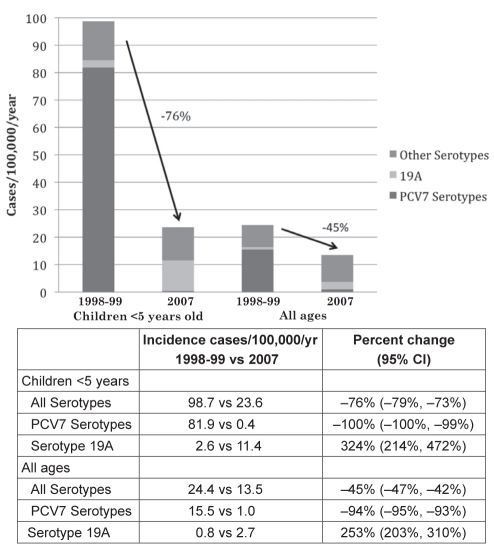
PCV7 was introduced to the US in 2000. The Active Bacterial Core surveillance (ABCs) study (8) of more than 40 million Americans demonstrated a 100% decline in PCV7 serotype IPD cases and a 76% decline in all IPD cases in children younger than five years of age in 2007, compared with in 1998/1999 before PCV7 was introduced in the US (Figure 1). In addition, as shown in Figure 1, by 2007 there was a 45% decline in all IPD cases and a 94% decline in PCV7 serotype IPD cases at all ages combined. Overall, since the introduction of PCV7 in the US in 2000, it was estimated that 211,000 IPD cases and 13,000 deaths were prevented by 2007 (8).
Figure 1).
Changes in the incidence of invasive pneumococcal disease for children younger than five years of age and all ages combined in the United States (1998/1999 versus 2007). PCV7 Seven-valent pneumococcal conjugate vaccine; vs Versus; yr Year. Data from reference 8
REDUCTION OF MENINGITIS
Although meningitis accounts for only 7% of IPD cases overall (1), it is associated with serious morbidity and mortality. In the US (8), by 2005 to 2007 compared with 1998/1999 in children younger than five years of age, PCV7 serotype meningitis and all pneumococcal meningitis cases decreased by 97% (from 3.8 to 0.1 cases/100,000/year) and by 64% (from 4.7 to 1.7 cases/100,000/year), respectively (P<0.001 for both). However, in children younger than five years of age, there was a 78% increase in non-PCV7 serotype meningitis cases at all ages, from 0.9 to 1.6 cases/100,000/year.
REDUCTION OF PNEUMONIA HOSPITALIZATIONS
The association of PCV7 implementation on hospitalization for pneumonia (in which pneumococcus is a prominent cause) has been evaluated in Canada and the US. In Quebec, in children younger than five years of age, during the first 15 months after the start of universal infant PCV7 vaccination, there was a 72% decline in hospital admissions for lobar pneumonia and a 13% decline in admissions for all-cause pneumonia (3). In the US, an evaluation of a nationwide survey (9) of hospital admissions found that during the first four years after PCV7 implementation, in children younger than two years of age, there was a 65% decline in hospital admissions for pneumococcal pneumonia and a 39% decline in admissions for all-cause pneumonia. In addition, for adults 65 years of age and older, there was a 20% decline in admissions for pneumococcal pneumonia and a 15% decline in admissions for all-cause pneumonia. While findings such as these, from health services databases, do not prove a cause-and-effect relationship resulting from PCV7 implementation, it is biologically likely that an effect exists, the effect is strong, there is no alternative explanation and the change has been noted in two different countries.
REDUCTION OF OTITIS MEDIA CASES AND MYRINGOTOMY TUBE PLACEMENT
Similar to pneumonia, the association of PCV7 implementation with the incidence of otitis media (for which pneumococcus is the predominant cause) has been evaluated using administrative data. In the US, evaluation of cohorts of children born after the introduction of PCV7 found 17% and 28% reductions in frequent otitis media, and 16% and 23% declines in myringotomy tube placement in children up to two years of age from Tennessee and New York, respectively (10).
REPLACEMENT IPD WITH NONVACCINE SEROTYPES
The PCV7 vaccine reduces pneumococcal disease by inducing a protective immune response. In addition, PCV7 inhibits asymptomatic colonization with vaccine serotype strains of pneumococcus (2). Because there are 91 known serotypes of pneumococcus, it was anticipated that PCV7 use would lead to some replacement with nonvaccine serotype strains in the nasopharynx of children (who are the main reservoir of pneumococcus), thus causing disease (11). Indeed, the large declines in PCV7 serotype infections have been offset, to some extent, by increased infections caused by a limited number of nonvaccine serotypes, particularly serotype 19A, which is often resistant to multiple antibiotics (8,12,13).
The Immunization Monitoring Program, ACTive (IMPACT) study (14) of IPD in 12 paediatric tertiary care centres across Canada has found that the proportion of IPD caused by nonvaccine serotypes has increased since 2000, both relatively (as vaccine serotype IPD has decreased) and absolutely (as the actual number of cases has increased). From 2000 to 2007, in children younger than five years of age, the proportion of IPD caused by nonvaccine serotypes increased from 17% to 82%, and the number of cases nearly doubled. The main replacement serotypes included 19A (21% of all cases in 2007), as well as 3, 5, 6A, 7F and 22F. In 2007, 17% and 63% of all isolates and serotype 19A isolates, respectively, had reduced susceptibility to penicillin. Of note, all of these serotypes, except 22F, are included in one or both of two new expanded-valency conjugate vaccines, as discussed later in the present article. More recent IMPACT data indicated that serotype 19A caused 44% of all IPD cases in children younger than five years of age in 2008/2009 (15).
Province-wide surveillance in Quebec found that by 2009, 96% of all IPD cases in children younger than five years of age were caused by nonvaccine serotypes. Of these cases, 42% were caused by serotype 19A and 51% of the 19A strains had reduced susceptibility to penicillin (6).
In the US, the ABCs study found that by 2007 compared with 1998/1999, the proportion of all IPD cases caused by nonvaccine serotypes in children younger than five years of age increased from 17% to 98%, and for serotype 19A the proportion increased from 3% to 47% (8). During the same time period, for all ages combined, the proportion of all IPD cases caused by nonvaccine serotypes, and serotype 19A specifically, increased from 36% to 93% and from 3% to 20%, respectively.
It is important to reiterate that, in most populations, the absolute increase in the incidence of nonvaccine serotype IPD has been much smaller than the decline in incidence of vaccine serotype IPD disease, and that the changes have leveled off over several years (1,8). However, it is notable that in Quebec, the number of cases of nonvaccine IPD in children and at all ages has continued to increase each year since 2006 (6).
There is uncertainty regarding whether the emergence of serotype 19A is only related to PCV7 or whether it represents a periodic secular change in predominant serotypes. Common pneumococcal serotypes have been observed to change cyclically in frequency over a period of years and even decades in different age groups and different geographical regions (16). With 19A specifically, an increased number of infections were described in South Korea and Israel before the introduction of PCV7 in those countries (17,18).
TRENDS IN COMPLICATED EMPYEMA
Studies from Canada, Scotland and Israel (19–21) have described increases in empyema in children (with or without microbiological information) before or soon after the introduction of PCV7. Other studies from the US (22,23) have described increases in culture-negative or pneumococcal (particularly caused by nonvaccine serotypes) empyema since the introduction of PCV7. The impact of PCV7 on these trends is not clear. Obviously, increases before the introduction of PCV7 are unrelated. Whether increases that continued or started after the introduction of PCV7 are due to replacement and/or longer term cyclical trends (as noted above) is not yet known.
REDUCED DOSE SCHEDULES OF PCV7
The main clinical trials of PCV7 were all conducted using a four-dose schedule, which includes a three-dose series in infancy followed by a fourth booster dose after one year of age. The high cost of PCV7 relative to other vaccines, vaccine shortages in the US and immunogenicity studies comparing two or three doses in infancy have led to evaluations suggesting that three doses (two in infancy followed by a third booster dose in the second year of life) may be as effective as a four-dose schedule (24–26).
There was a shortage of PCV7 in the US from 2001 to 2004. During this time, modified PCV7 schedules were often given, and anywhere from one to four doses were given to many children. A case-control study (24) comparing the effectiveness of different schedules found that all schedules containing at least two doses of PCV7 had similar levels of effectiveness of 95% or greater, for at least six months after vaccination. There were too few cases to accurately compare specific schedules.
Several studies (24,26) have shown that two infant doses, compared with three infant doses, of PCV7 in the first six months of life, produce similar levels of antibodies after the infant series. Whether two or three infant doses are given, a booster dose at 12 months of age or later is needed to produce the best immune response in toddlers.
In Canada, Quebec implemented a three-dose PCV7 schedule (at two, four and 12 months) when the universal PCV7 program was started. High-risk children received four doses. In British Columbia, the initial four-dose universal program was changed to the same three-dose program as Quebec, also with a four-dose provision for high-risk children. Data from Quebec, as well as other countries that have implemented three-dose programs, show that the effectiveness of three-dose programs is similar to jurisdictions with four-dose programs (6,27,28). Similar to the US results, a case-control study (25) from Quebec found that the effectiveness of PCV7 against vaccine serotype IPD was 98% in children who received at least two doses of the vaccine.
It is important to note that no data support the use of three-dose PCV7 in children with underlying conditions that increase the risk of IPD, and that all jurisdictions that evaluated three-dose PCV7 for healthy infants used four-dose PCV7 for children at increased risk for IPD.
THE POTENTIAL IMPACT OF THE NEW PCV10 AND PCV13, AND NEW CANADIAN RECOMMENDATIONS
The new PCV10 (Synflorix, GlaxoSmithKline Inc, Canada) has been approved for use in Canada (29). This vaccine contains the same serotypes as PCV7 (4, 6B, 9V, 14, 18C, 19F and 23F), as well as serotypes 1, 5 and 7F. PCV13 (Prevnar 13, Pfizer Canada Inc) has also been approved in Canada. It contains the same serotypes as PCV10, as well as serotypes 3, 6A and 19A (30).
In 2010, the National Advisory Committee on Immunizations (NACI) published two statements (26,31) on pneumococcal conjugate vaccines, referring to both of the new vaccines. Based on the changing epidemiology of IPD in Canada and the current predominance of serotype 19A in particular (which is in PCV13 but not PCV10), the most recent recommendations include the following:
There is good evidence to recommend PCV13 for a routine infant immunization schedule.
There is good evidence for a three-dose PCV7 schedule in healthy infants; however, there is insufficient evidence for a three-dose PCV10 or PCV13 schedule. Therefore, currently, a four-dose routine schedule is recommended.
There is good evidence that all healthy children 12 to 35 months of age should receive one dose of PCV13 and fair evidence that children at high risk for IPD should additionally receive a dose of 23-valent polysaccharide pneumococcal vaccine after two years of age.
There is good evidence that, for children 36 to 59 months of age, healthy Aboriginal children, children attending group daycare and children at high risk for IPD should receive a dose of PCV13, and that a dose should be considered for all other children in this age group.
There is fair evidence to suggest that children at high risk for IPD who are five years of age and older should receive a dose of PCV13.
Although the NACI recommended a four-dose PCV13 schedule, there was recognition that some provinces and territories have implemented a three-dose schedule for healthy infants, likely based on the PCV7 data. Evidence does not yet exist to definitively conclude that a three-dose PCV10 or PCV13 schedule is as effective as a four-dose schedule. For infants who are given three doses, it is important that the third dose is given early in the second year of life, but not any earlier. The complete NACI recommendations should be consulted for full details (26).
GLOBAL IMPLEMENTATION OF THE PNEUMOCOCCAL CONJUGATE VACCINE
Worldwide, it is estimated that pneumococcal infections cause more than 800,000 deaths each year in children younger than five years of age, mostly in developing countries (32). Historically, the introduction of new vaccines to low-income countries occurs 15 to 20 years after the introduction to high-income countries. The Global Alliance for Vaccines and Immunization (33) has supported an advance market commitment to accelerate the implementation of pneumococcal conjugate vaccines in developing countries. With funding and support from governments (including in Canada), the Bill & Melinda Gates Foundation and other nongovernmental organizations, funding for 600 million doses of PCV10 and PCV13 for low-income countries has been approved, as well as funding to support two India-based vaccine manufacturers to develop and manufacture pneumococcal vaccines. Vaccine introduction to Gambia and Rwanda began in 2009. By 2015, vaccine introduction to more than 40 countries is planned (33).
Footnotes
INFECTIOUS DISEASES AND IMMUNIZATION COMMITTEE (2009/2010)
Members: Drs Robert Bortolussi, IWK Health Centre, Halifax, Nova Scotia (Chair); Jane Finlay, Richmond, British Columbia; Jane C McDonald, Montreal, Quebec; Heather Onyett, Queen’s University, Kingston, Ontario; Joan L Robinson, Edmonton, Alberta; Élisabeth Rousseau-Harsany, Sainte-Justine UHC, Montreal, Quebec (Board Representative)
Consultants: Drs James Kellner, Calgary, Alberta; Noni E MacDonald, IWK Health Centre, Halifax, Nova Scotia; Dorothy L Moore, The Montreal Children’s Hospital, Montreal, Quebec
Liaisons: Drs Upton D Allen, The Hospital for Sick Children, Toronto, Ontario (Canadian Pediatric AIDS Research Group); Charles PS Hui, Children’s Hospital of Eastern Ontario, Ottawa, Ontario (CPS Representative to the Committee to Advise on Tropical Medicine and Travel); Nicole Le Saux, Children’s Hospital of Eastern Ontario, Ottawa, Ontario (Canadian Immunization Monitoring Program, ACTive); Larry Pickering, Atlanta, Georgia, USA (American Academy of Pediatrics); Marina I Salvadori, Children’s Hospital of Western Ontario, London, Ontario (CPS Representative to the National Advisory Committee on Immunization)
Principal author: Dr James D Kellner, Calgary, Alberta
The recommendations in this statement do not indicate an exclusive course of treatment or procedure to be followed. Variations, taking into account individual circumstances, may be appropriate. All Canadian Paediatric Society position statements and practice points are reviewed, revised or retired as needed on a regular basis. Please consult the “Position Statements” section of the CPS website (www.cps.ca/english/publications/statementsindex.htm) for the most current version.
REFERENCES
- 1.Kellner JD, Vanderkooi OG, MacDonald J, Church DL, Tyrrell GJ, Scheifele DW. Changing epidemiology of invasive pneumococcal disease in Canada, 1998–2007: Update from the Calgary-Area Streptococcus pneumoniae Research (CASPER) study. Clin Infect Dis. 2009;49:205–12. doi: 10.1086/599827. [DOI] [PubMed] [Google Scholar]
- 2.Kellner JD, Scheifele D, Vanderkooi OG, Macdonald J, Church DL, Tyrrell GJ. Effects of routine infant vaccination with the 7-valent pneumococcal conjugate vaccine on nasopharyngeal colonization with Streptococcus pneumoniae in children in Calgary, Canada. Pediatr Infect Dis J. 2008;27:526–32. doi: 10.1097/INF.0b013e3181658c5c. [DOI] [PubMed] [Google Scholar]
- 3.De Wals P, Robin E, Fortin E, Thibeault R, Ouakki M, Douville-Fradet M. Pneumonia after implementation of the pneumococcal conjugate vaccine program in the province of Quebec, Canada. Pediatr Infect Dis J. 2008;27:963–8. doi: 10.1097/INF.0b013e31817cf76f. [DOI] [PubMed] [Google Scholar]
- 4.Bjornson G, Scheifele DW, Bettinger J, et al. Effectiveness of pneumococcal conjugate vaccine in Greater Vancouver, Canada: 2004–2005. Pediatr Infect Dis J. 2007;26:540–2. doi: 10.1097/INF.0b013e31803c56df. [DOI] [PubMed] [Google Scholar]
- 5.Paulus S, David ST, Tang W, et al. Incidence of invasive pneumococcal disease after introduction of the Universal Infant Immunization Program, British Columbia (2002–2005) Can Commun Dis Rep. 2006;32:157–61. [PubMed] [Google Scholar]
- 6.Lefebvre B, Bourgault AM. Programme de Surveillance du Pneumocoque, Rapport 2009. Institut national de santé publique du Québec. < www.inspq.qc.ca/pdf/publications/1180_SurveillancePneumocoque_2009> (Accessed on February 6, 2011).
- 7.Bruce MG, Deeks SL, Zulz T, et al. International Circumpolar Surveillance System for Invasive Pneumococcal Disease, 1999–2005. Emerg Infect Dis. 2008;14:25–33. doi: 10.3201/eid1401.071315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pilishvili T, Lexau C, Farley MM, et al. for the Active Bacterial Core Surveillance/Emerging Infections Program Network Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 9.Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: A time-series analysis. Lancet. 2007;369:1179–86. doi: 10.1016/S0140-6736(07)60564-9. [DOI] [PubMed] [Google Scholar]
- 10.Poehling KA, Szilagyi PG, Grijalva CG, et al. Reduction of frequent otitis media and pressure-equalizing tube insertions in children after introduction of pneumococcal conjugate vaccine. Pediatrics. 2007;119:707–15. doi: 10.1542/peds.2006-2138. [DOI] [PubMed] [Google Scholar]
- 11.Lipsitch M. Bacterial vaccines and serotype replacement: Lessons from Haemophilus influenzae and prospects for Streptococcus pneumoniae. Emerg Infect Dis. 1999;5:336–45. doi: 10.3201/eid0503.990304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beall B, McEllistrem MC, Gertz RE, Jr, et al. Active Bacterial Core Surveillance Team Pre- and postvaccination clonal compositions of invasive pneumococcal serotypes for isolates collected in the United States in 1999, 2001, and 2002. J Clin Microbiol. 2006;44:999–1017. doi: 10.1128/JCM.44.3.999-1017.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pelton SI, Huot H, Finkelstein JA, et al. Emergence of 19A as virulent and multidrug resistant Pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007;26:468–72. doi: 10.1097/INF.0b013e31803df9ca. [DOI] [PubMed] [Google Scholar]
- 14.Bettinger J, Scheifele DW, Kellner JD, et al. for Members of the Canadian Immunization Monitoring Program, ACTive (IMPACT) The effect of routine vaccination on invasive pneumococcal infections in Canadian children, Immunization Monitoring Program, Active 2000–2007. Vaccine. 2010;28:2130–6. doi: 10.1016/j.vaccine.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 15.Bettinger JA, Scheifele DW, Kellner JD, et al. Emergence of Serotype 19A in Canadian Children: IMPACT 2000–2009. Oral Presentation at 9th Canadian Immunization Conference, Quebec City, December 2010. Can J Infect Dis Med Microbiol. 2010;21:178. [Google Scholar]
- 16.Pelton SI. Replacement pneumococcal disease in perspective. Clin Infect Dis. 2008;46:1353–5. doi: 10.1086/586748. [DOI] [PubMed] [Google Scholar]
- 17.Choi EH, Kim SH, Eun BW, et al. Streptococcus pneumoniae serotype 19A in children, South Korea. Emerg Infect Dis. 2008;14:275–81. doi: 10.3201/eid1402.070807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dagan R, Givon-Lavi N, Leibovitz E, Greenberg D, Porat N. Introduction and proliferation of multidrug-resistant Streptococcus pneumoniae serotype 19A clones that cause acute otitis media in an unvaccinated population. J Infect Dis. 2009;199:776–85. doi: 10.1086/597044. [DOI] [PubMed] [Google Scholar]
- 19.Finley C, Clifton J, Fitzgerald JM, Yee J. Empyema: An increasing concern in Canada. Can Respir J. 2008;15:85–9. doi: 10.1155/2008/975312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldbart AD, Leibovitz E, Porat N, et al. Complicated community acquired pneumonia in children prior to the introduction of the pneumococcal conjugated vaccine. Scand J Infect Dis. 2009;41:182–7. doi: 10.1080/00365540802688378. [DOI] [PubMed] [Google Scholar]
- 21.Roxburgh CS, Youngson GG, Townend JA, Turner SW. Trends in pneumonia and empyema in Scottish children in the past 25 years. Arch Dis Child. 2008;93:316–8. doi: 10.1136/adc.2007.126540. [DOI] [PubMed] [Google Scholar]
- 22.Byington CL, Korgenski K, Daly J, Ampofo K, Pavia A, Mason EO. Impact of the pneumococcal conjugate vaccine on pneumococcal parapneumonic empyema. Pediatr Infect Dis J. 2006;25:250–4. doi: 10.1097/01.inf.0000202137.37642.ab. [DOI] [PubMed] [Google Scholar]
- 23.Hendrickson DJ, Blumberg DA, Joad JP, Jhawar S, McDonald RJ. Five-fold increase in pediatric parapneumonic empyema since introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2008;27:1030–2. doi: 10.1097/INF.0b013e31817e5188. [DOI] [PubMed] [Google Scholar]
- 24.Whitney CG, Pilishvili T, Farley MM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: A matched case-control study. Lancet. 2006;368:1495–502. doi: 10.1016/S0140-6736(06)69637-2. [DOI] [PubMed] [Google Scholar]
- 25.Deceuninck G, De Wals P, Boulianne N, De Serres G. Effectiveness of pneumococcal conjugate vaccine using a 2+1 infant schedule in Quebec, Canada. Pediatr Infect Dis J. 2010;29:546–9. doi: 10.1097/INF.0b013e3181cffa2a. [DOI] [PubMed] [Google Scholar]
- 26.Desai S, McGeer A, Quach-Thanh, Elliott D. An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI) Update on the Use of Conjugate Pneumococcal Vaccines in Childhood. Can Commun Dis Rep. 2010;36(ACS 12):1–21. doi: 10.14745/ccdr.v36i00a12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vestrheim DF, Løvoll O, Aaberge IS, et al. Effectiveness of a 2+1 dose schedule pneumococcal conjugate vaccination programme on invasive pneumococcal disease among children in Norway. Vaccine. 2008;26:3277–81. doi: 10.1016/j.vaccine.2008.03.087. [DOI] [PubMed] [Google Scholar]
- 28.Roche PW, Krause V, Cook H, et al. Enhanced Invasive Pneumococcal Disease Surveillance Working Group; Pneumococcal Working Party of the Communicable Diseases Network Australia Invasive pneumococcal disease in Australia, 2006. Commun Dis Intell. 2008;32:18–30. doi: 10.33321/cdi.2008.32.3. [DOI] [PubMed] [Google Scholar]
- 29.Health Canada Drugs and Health Products. Notice of Decision for SYNFLORIX™. < www.hc-sc.gc.ca/dhp-mps/prodpharma/sbd-smd/phase1-decision/drug-med/nd_ad_2009_synflorix_119056-eng.php> (Accessed on February 6, 2011).
- 30.Health Canada Drugs and Health Products. Notice of Decision for PREVNAR® 13. < www.hc-sc.gc.ca/dhp-mps/prodpharma/sbd-smd/phase1-decision/drug-med/nd_ad_2010_prevnar_13_122881-eng.php> (Accessed on February 6, 2011).
- 31.McGeer A, Desai S. An Advisory Committee Statement (ACS). National Advisory Committee on Immunization (NACI). Update on pediatric pneumococcal disease and recommended use of conjugate pneumococcal vaccines. Can Commun Dis Rep. 2010;36(ACS-3):1–30. doi: 10.14745/ccdr.v36i00a12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Brien KL, Wolfson LJ, Watt JP, et al. for the Hib and Pneumococcal Global Burden of Disease Study Team Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 33.Advance Market Commitments for Vaccines GAVI Alliance. < www.vaccineamc.org/index.html> (Accessed on February 6, 2011).