Abstract
The cytokine cascade in pain and inflammatory processes is a tremendously complex system, involving glial, immune, and neuronal cell interactions. IL-1β is a pro-inflammatory cytokine that has been implicated in pain, inflammation and autoimmune conditions. This review will focus on studies that shed light on the critical role of IL-1β in various pain states, including the role of the intracellular complex, the inflammasome, which regulates IL-1β production. Evidence will be presented demonstrating the importance of IL-1β in both the induction of pain and in the maintenance of pain in chronic states, such as after nerve injury. Additionally, the involvement of IL-1β as a key mediator in the interaction between glia and neurons in pain states will be discussed. Taken together, the evidence presented in the current review showing the importance of IL-1β in animal and human pain states, suggests that blockade of IL-1β be considered as a therapeutic opportunity.
1. Interleukin-1
Interleukin-1 α and β are prototypic proinflammatory cytokines that exert pleiotrophic effects on a variety of cells and play key roles in acute and chronic inflammatory and autoimmune disorders. There are two IL-1 receptors, IL-1 type 1 receptor (IL-1RI) and IL-1 type 2 receptor (IL-1 RII). IL-1α and IL-1β signal through IL-1RI. Binding to IL-1RII does not lead to cell signaling and it is therefore considered a decoy receptor. Upon binding of IL-1 to IL-1RI, a second receptor termed IL1 receptor accessory protein (IL-1RAcP) gets recruited at the cell membrane to form a high affinity binding receptor complex leading to intracellular signaling. A third IL-1 family member, IL-1 receptor antagonist (IL-1ra), binds to IL-1 receptors and prevents the interaction of IL-1 with its receptors, acting as a natural IL-1 inhibitor (reviewed in Dinarello, 1996 and Braddock and Quinn, 2004) This review will focus on the role of IL-1β in painful and inflammatory conditions.
IL-1β has important homeostatic functions in the normal organism, such as in the regulation of feeding, sleep, and temperature (reviewed in Dinarello, 1996). However, overproduction of IL-1β is implicated in the pathophysiological changes that occur during different disease states, such as rheumatoid arthritis, neuropathic pain, inflammatory bowel disease, osteoarthritis, vascular disease, multiple sclerosis, and Alzheimer's disease (reviewed in Dinarello, 1996; Braddock and Quinn, 2004, and Dinarello, 2004). IL-1β can be released from keratinocytes, fibroblasts, synoviocytes, endothelial, neuronal, immune cells such as macrophages and mast cells, and glial cells such as Schwann cells, microglia and astrocytes (Watkins et al., 1995; Copray et al., 2001; Shamash et al., 2002; Sommer and Kress, 2004; Perrin et al., 2005; Clark et al., 2006; Guo et al., 2007; Thacker et al., 2007). One area of research that has shed new light into IL-1β's role in inflammation and pain during disease state is the processing of IL-1β by Caspase-1 via the inflammasome.
2. Inflammasome
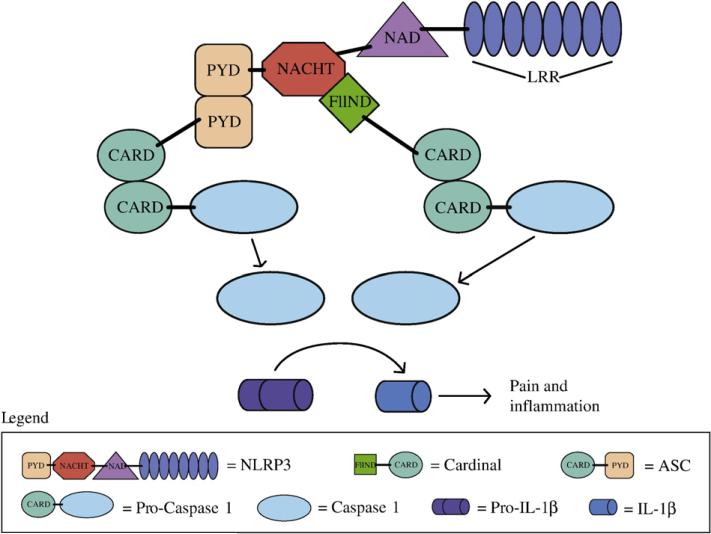
The inflammasome is an intracellular multi-protein complex that is emerging as an important regulator of inflammation (Fig. 1). The inflammasome acts as an activating scaffold for proinflammatory Caspases. One such Caspase, Caspase 1, cleaves and activates pro-IL-1β and pro-IL-18 (reviewed in Martinon and Tschopp, 2007). IL-33 has also been shown to be a possible Caspase 1 substrate (Schmitz et al., 2005). Inflammasomes play important roles in the innate immunity pathway and are active players in inflammatory disorders. As shown below, there is also evidence that they are involved in painful conditions.
Fig. 1.
NLRP3 inflammasome: The NLRP3 protein is thought to be activated by both intracellular and extracellular signals and acts as a central component of a protein complex containing ASC, Cardinal, and Pro-Caspase1. The activation of the inflammasome leads to cleavage of the Pro-caspase 1 to its active form, which results in the production of the active mature form of IL-1β.
Inflammasomes contain NOD-like receptor (NLR) proteins, and are named based on which NLR protein is present. The NLRP3, also known as NALP3 or CIAS1, inflammasome is probably the best studied (reviewed in Tschopp et al., 2003 and Mariathasan and Monack, 2007). The NLRP3 protein contains four distinct domains, a Pyrin domain (PYD) at the N-terminus, followed by a NACHT domain (named after NAIP, CIITA, HET-E, and TP1), a NACHT-associated domain (NAD) and a Leucine-rich repeats (LRR) domain at the C-terminus (Reviewed in Church et al., 2008). It is thought that NLRP3 acts as a sensor for cell injury and microbial components and once activated it binds through the PYD region to the ASC (apoptosis-associated speck-like protein containing a CARD domain) adaptor protein, which contains a PYD domain at the N-terminus and a CARD domain at the C-terminus. Besides binding to ASC, NLRP3 is also bound through its NACHT domain to the FIIND domain at the N-terminus of the Cardinal protein (Agostino et al., 2004). The ASC and Cardinal proteins, through their CARD domains, in turn bind to the CARD domain of pro-Caspase 1, causing proteolytic cleavage yielding activated Caspase 1. Cleaved Caspase 1 can then process pro-IL-1β to its bioactive IL-1β form (Agostino et al., 2004). Besides Caspase 1, there is evidence that metalloproteases (MMPs) cleave IL-1β, therefore Caspase 1-independent pathways may also play roles in pain transmission (Kawasaki et al., 2008a).
3. IL-1β in gout and other autoinflammatory diseases
Recently, gouty arthritis has taken center stage in the inflammasome field. Gout is one of the most painful acute conditions known to man and has been described since Egyptian times (reviewed in Nuki and Simkin, 2006). Gout is an autoinflammatory disorder that is caused by hyperuricemia. Articular deposits of monosodium urate (MSU) crystals lead to gouty arthritis, which causes acute gout attacks. These attacks present clinically as a highly inflammatory arthritis with intense redness, warmth and pain surrounding an affected joint that lasts for a few days and are associated with systemic symptoms such as fever, leukocytosis and elevated markers of inflammation. Most patients will have recurring attacks and chronic tophaceus gout can occur in untreated gout (reviewed in Dalbeth and Haskard, 2005, Masseoud et al., 2005, and Terkeltaub, 2006).
For the past 20 years, it had been known that IL-1β was produced by human white blood cells stimulated by MSU crystals (Malawista et al., 1985; Di Giovine et al., 1987), but the mechanism of how the crystals increased IL-1β production was not known until the seminal work of Martinon et al. (2006). In this paper, it was demonstrated that the inflammatory effects of the MSU crystals worked through the NLRP3 inflammasome. The authors showed that there was a deficiency in the activation of IL-1β by MSU crystals in macrophages isolated from mice deficient in various components of the inflammasome. Additionally, in an in vivo model of MSU crystal-induced peritonitis, there was a reduction in the neutrophil influx in NLRP3, ASC, or Caspase 1-deficient mice as well as in IL-1R1-deficient mice (Martinon et al. 2006).
The importance of the IL-1 pathway, and not IL-18, was demonstrated by Chen et al. (2006), who showed that IL-1R1 deficient mice, and not IL-18R deficient mice, had an impairment of neutrophil influx in the MSU crystal peritonitis model. Additionally, pharmacological blockade of IL-1 pathway was shown to reduce neutrophil influx in the MSU crystal peritonitis model with blocking antibodies to IL-1 and IL-R1 or with the recombinant protein version of IL-1ra, Anakinra. This neutrophil influx decrease was not observed with an anti-TNF blocking antibody (Chen et al., 2006; So et al., 2007). Furthermore, in a small human pilot study involving 10 gouty arthritis patients, Anakinra rapidly relieved the inflammatory symptoms of gout (So et al., 2007). Anakinra is an FDA approved drug for the treatment of rheumatoid arthritis (reviewed in Braddock and Quinn, 2004 and Dinarello, 2004).
Besides the role of the inflammasome in acute gouty arthritis, there is a growing body of literature that shows that multiple auto-inflammatory diseases may result from mutations within genes that encode for different components of the inflammasome, leading to over production of IL-1β (reviewed in Stojanov and Kastner, 2005 and Church et al., 2008). For example, there are mutations within the NLRP3 gene that are associated with several hereditary periodic-fever syndromes, such as familial cold urticaria and Muckle–Wells, that lead to periodic fevers, joint inflammation and pain among other symptoms (Hoffman et al., 2001; reviewed in Hull et al., 2003). These NLRP3 gene mutations are thought to lead to excessive production of IL-1β (Agostino et al., 2004; Gattorno et al., 2007). The importance of the overproduction of IL-1β was recently demonstrated in small human pilot clinical trials showing that the recombinant protein version of IL-1ra, Anakinra, ameliorated clinical symptoms in periodic-fever syndromes (Hawkins et al., 2004, Hoffman et al., 2004). Additionally, Rilanocept (IL-1 Trap) was recently approved by the FDA for the treatment of Cryopyrin-Associated Periodic Syndromes (CAPS), including Familial Cold Autoinflammatory Syndrome (FCAS) and Muckle–Wells Syndrome (MWS) after successfully completing human clinical trials (Hoffman et al., 2008; Gold-bach-Mansky et al., 2008). The IL-1 Trap is a recombinant dimeric fusion protein that contains in a single chain the extracellular domains of IL-1RI and IL-1RAcP fused to the human Fc portion of an IgG protein (Economides et al., 2003; reviewed in Braddock and Quinn, 2004).
4. IL-1β in the periphery and pain
IL-1β is a potent mechanical and thermal hyperalgesic agent when injected into any number of peripheral tissues (Ferreira et al., 1988; Fukuoka et al., 1994; Watkins et al., 1994; Safieh-Garabedian et al., 1995; Cunha et al., 2000; Zelenka et al. 2005). Intraplantar injection of inflammatory agents, such as carrageenan, lipopolysaccharide (LPS) bacterial endotoxin, or complete Freund's adjuvant (CFA), produce mechanical or thermal hyperalgesia associated with an upregulation of IL-1β and other inflammatory cytokines in the inflamed tissue and in the dorsal root ganglia (DRG) (Safieh-Garabedian et al., 1995, 2002; Woolf et al., 1997; Cunha et al., 2000; Samad et al., 2001; Chessell et al. 2005; Menetski et al. 2007).
One mechanism of action for IL-1β is through upregulation of other pro-nociceptive mediators. For example, administration of IL-1ra significantly reduced mechanical hyperalgesia produced by a CFA intraplantar injection as well as CFA-induced upregulation of Nerve Growth Factor (NGF), a neurotrophic factor known to play a crucial role in a variety of acute and chronic pain states (Safieh-Garabedian et al., 1995). Interestingly, anti-NGF pre-treatment was able to reduce the CFA-induced hyperalgesia but not the elevation in IL-1β (Safieh-Garabedian et al., 1995) suggesting indirect mechanisms may be responsible for the changes in behavior. This upregulation of NGF by IL-1β occurred at both the transcriptional and post-transcriptional levels (Lindholm et al., 1987, 1988; Vige et al., 1991). IL-1β can additionally signal through complex signaling cascades that lead to the release and/or activation of other nociceptive molecules such as Prostaglandin, Interleukin-6, Substance-P, and MMP9 (Inoue et al. 1999; Samad et al., 2001; Economides et al., 2003; Kawasaki et al., 2008a).
Despite the anti-NGF results, there is also evidence that IL-1β's actions can occur directly on nociceptors. RT-PCR and in situ hybridization studies have demonstrated that IL-1R1 is expressed in sensory neurons (Copray et al. 2001, Obreja et al., 2002). IL-1β is known to modulate neuronal excitability by affecting neuronal receptors such as TRPV1, sodium channels, GABA receptors, and NMDA receptors (reviewed in Schäfers and Sorkin, 2008). As evidence of IL-1β's direct actions on nociceptors, it has been shown that IL-1β in a nerve-skin in vitro preparation can excite nociceptive fibers in as little as 1 min (Fukuoka et al., 1994). Additionally, IL-1β has been shown to cause an increase in the heat-evoked release of Calcitonin Gene Related Peptide (CGRP) from rat cutaneous nociceptors in vitro (Opree and Kress, 2000). In a separate study, brief application of IL-1β to isolated neurons yielded a potentiation of heat-activated excitatory inward currents (Iheat)(Obreja et al., 2002).
5. IL-1β's role during neuropathic pain
Neuropathic pain arises from dysfunction of the nervous system. The interplay between the immune and nervous systems is thought to be critical for the development and maintenance of neuropathic pain, and the proinflammatory cytokines, including IL-1β, appear to be contributory to the pain state (reviewed in Scholz and Woolf, 2007, and Uceyler and Sommer, 2008). Low back pain is a common and debilitating painful disorder which can arise from nerve injury. In degenerate and herniated human intervertebral discs, IL-1β expression is higher than in non-degenerate intervertebral disc controls (LeMaitre and Hoyland, 2007). In various animal models of neuropathic pain, IL-1β expression is increased in the injured sciatic nerve, DRG, and spinal cord (Rotshenker et al., 1992; Hashizume et al 2000; Lee et al., 2004; Perrin et al., 2005; Ruohonen et al., 2005; Uceyler et al., 2007; Kawasaki et al., 2008a). Immediately after peripheral nerve injury, Schwann cells are activated and macrophages are recruited to the injury site and both secrete IL-1β (reviewed in Scholz and Woolf, 2007) . The ipsilateral upregulation of IL-1β at the site of injury in the sciatic nerve has been detected as early as 1 h post-surgery in the chronic constriction injury (CCI) model in mice (Uceyler et al., 2007). In a rat transected sciatic nerve model, upregulation of IL-1β has been detected as long as 35 days post-surgery (Ruohonen et al., 2005).
In the CCI model in mice, sciatic nerve epineural injections of IL-1R1 neutralizing antibodies were shown to reduce both thermal hyperalgesia and mechanical allodynia, suggesting a role for the upregulated IL-1β in the induction of neuropathic pain (Sommer et al. 1999; Schafers et al., 2001). Additionally, in the same CCI model, mechanical allodynia was reduced by intrathecally administered IL-1β neutralizing antibody (Kawasaki et al., 2008a) suggesting that neuropathic pain is mediated by IL-1β activity at several sites. That IL-1β may act in concert with other mediators is suggested by the observation that in another neuropathic pain model, the L5 spinal nerve transection in rats, the combination of intrathecal (i.t.) injections of IL-1ra and soluble TNF Receptor (sTNFR) dose-dependently attenuated mechanical allodynia (Sweitzer et al., 2001). Using genetically-engineered models, it was shown that both IL-1R1 knockout mice and mice genetically overexpressing IL-1ra in astrocytes had reduced thermal hyperalgesia and mechanical allodynia in the L5 spinal nerve transection model, and reduced autotomy in a complete sciatic denervation model. Additionally, both lines of engineered mice had a reduction in the amount of spontaneous ectopic activity in isolated DRG, a phenomenon previously associated with the development of neuropathic pain (Wolf et al., 2006).
Recently, new mechanisms of neuropathic pain have been revealed involving a complex pathway with MMP9, MMP2 and IL1-β. Kawasaki et al., 2008a showed that in the CCI model cleavage of IL-1β by MMP subtypes contributed to different phases of neuropathic pain behavior. After nerve injury, MMP-9 induced neuropathic pain through IL-1β cleavage and microglial activation at early times, whereas MMP-2 maintained neuropathic pain through IL-1β cleavage and astrocyte activation at later times. This well-orchestrated sequential activation of microglia followed by activation of astrocytes in the spinal cord during neuropathic pain has been previously documented (reviewed in Scholz and Woolf, 2007). Additionally, IL-1β was shown to activate MMPs, suggesting a circular regulation between MMPs and IL-1β (Kawasaki et al., 2008a). Therefore, IL-1β is likely part of a complex signaling cascade involving MMPs in the CCI model.
6. IL-1β in the CNS and pain
As suggested by the spinal cord data mentioned previously, the involvement of cytokines in persistent pain is not limited to peripheral sensitization. Proinflammatory cytokines and their receptors have been found in the CNS. For example, IL-1β's receptor IL-1R1 has been localized to the spinal dorsal horn and brain (Samad et al., 2001, Guo et al., 2007; Zhang et al., 2008).
Direct injection of IL-1β into the CNS has been shown to produce hyperalgesia and enhanced neuronal responses in animals (Oka et al., 1993; Oka et al., 1994; Watkins et al., 1994; Reeve et al., 2000). For example, intracerebroventricular (i.c.v.) injection of IL-1β has been shown to decrease response latency in the hot plate test in rats (Oka et al., 1993). A separate study showed that an i.t. injection of IL-1β led to a decrease in hind paw withdrawal thresholds in the von Frey test (Reeve et al., 2000). To assess the effects of IL-1β in neuronal responses, Oka et al. (1994) microinjected IL-1β in the lateral cerebral ventricle of rats. This resulted in potentiated responses of wide dynamic range neurons in the trigeminal subnucleus caudalis to noxious pinching of the facial skin. However, the same dose of IL-1β did not affect the responses of low threshold mechanoreceptive neurons to skin brushing, suggesting some specificity of action. The IL-1β-induced enhancement of nociceptive neuron responses was completely abolished by pretreatment with IL-1ra (Oka et al., 1994). In a separate study, Reeve et al. (2000) showed that an i.t. administration of rat IL-1β produced enhanced dorsal horn neuronal activity, including enhancement of responses to C-fiber stimulation, wind-up and after-discharges.
In addition to the evidence cited above suggesting that CNS administration of IL-1β can induce pain states, data have been generated which suggest that IL-1β acting in the CNS can contribute to nociceptive responses in animal models of pain. For example, i.t. delivery of IL-1ra has been demonstrated to relieve HIV-1 gp120-induced mechanical allodynia and thermal hyperalgesia in the hind paw (Milligan et al., 2001). In addition, Zhang et al. (2008) showed that IL-1ra given by an i.t. injection decreased inflammatory hyperalgesia in the hind paw induced by a CFA injection. While the source of increased IL-1β in the CNS is not clear, IL-1β has been shown to be present in the spinal cord and brain following a CFA hind paw injection (Samad et al., 2001; Raghavendra et al., 2004; Zhang et al., 2008). Work with IL-1β and other cytokines has led to the notion that the central cytokine cascade could be an important contributor to the development of persistent pain states (Samad et al., 2001; Guo et al., 2007; Kawasaki et al., 2008b; Zhang et al., 2008).
7. IL-1β in central glia-neuronal interaction
Injury-induced central neuronal hyperexcitability, or central sensitization, has been identified as an important mechanism underlying persistent pain. Evidence suggests that glia, particularly astroglia, are intimately involved in the control of neuronal activity (Jourdain et al., 2007; Parri and Crunelli, 2007). Convergent evidence suggests that inflammatory cytokines act as mediators between glia and neurons and assume roles as neuromodulators (reviewed in Watkins and Maier, 2003).
Inflammatory cytokines are known to be released by activated glia and have been implicated in persistent hyper-algesia (DeLeo and Yezierski, 2001; Watkins et al., 2003). Injection of CFA into the masseter muscle of the rat produces muscle inflammation and hyperalgesia. After CFA injection, rats exhibited an increased responsiveness and reduced response threshold to mechanical stimuli, characteristic of mechanical hyperalgesia and allodynia (Sugiyo et al., 2005; Watanabe et al., 2005). Masseter inflammation induced glial activation in the spinal trigeminal nucleus, as indicated by increased immunoreactivity of glial fibrillary acidic protein (GFAP, astroglial marker), astroglial gap junction protein connexin 43 and CD11b, a marker of activated microglia (Guo et al., 2007). Activation of glia by masseter inflammation was accompanied by an increase in IL-1β levels. Interestingly, IL-1β was selectively induced in astroglia as shown by double immunofluorescence staining: IL-1β colocalized with GFAP, but not CD11b and Neu-N, a neuronal marker (Guo et al., 2007). Similar selective induction of IL-1β in astrocytes was also observed in a bone cancer pain model and after intracerebral hemorrhage (Zhang et al., 2005; Wasserman and Zhu, 2007). These results suggest that astrocytes are a source of IL-1β release under these conditions. Further evidence indicated that both inflammation-induced astroglial activation and IL-1β were dependent on nerve input and were inhibited by the glial modulator propentofylline, suggesting that glial activation is upstream and critical to cytokine induction in the CNS after inflammation (Guo et al., 2007).
Studies have also indicated that IL-1β is produced in microglia in the CNS (Clark et al. 2006; Van Dam et al., 1995). Application of LPS to an ex vivo dorsal horn slice preparation induced rapid secretion of IL-1β from activated spinal micro-glial cells (Clark et al., 2006). Additionally, an i.t. injection of LPS in the lumbar spinal cord produced mechanical hyper-algesia in the rat hindpaw that was attenuated by the concomitant i.t. injection of IL-1ra (Clark et al., 2006). These data suggest a critical role of IL-1β and activated microglia in enhancing nociceptive transmission in spinal cord inflammation.
8. IL-1β signaling, NMDA receptor phosphorylation, and persistent pain
Neuronal glutamate receptors, particularly ionotropic NMDA receptors, play major roles in activity-dependent synaptic plasticity and persistent pain (reviewed in Woolf and Salter, 2000, Guo et al., 2006, and Ji and Woolf, 2001). NMDA receptors are heteromers of NR1/NR3 and NR2 subunits (reviewed in Paoletti and Neyton, 2007). Several amino acid residues on the intracellular C-termini of the NR1 and NR2 proteins are phosphorylated upon activation of protein kinases. These phosphorylation sites of the NMDA receptor subunits facilitates its trafficking, modulates channel kinetics and enhances function (Chen and Huang, 1992; Tingley et al., 1997; Yu et al., 1997; Brenner et al., 2004). The NMDA receptor phosphorylation is increased after tissue or nerve injury and this correlated with increased pain sensitivity (Guo et al., 2002; Zou et al., 2002; Brenner et al., 2004; Caudle et al., 2005).
Recent studies have indicated that the signal transduction cascade involving IL-1β and NMDA receptors are linked in the ascending nociceptive circuit (Viviani et al., 2003; Yang et al., 2005; Guo et al., 2007; Zhang et al., 2008; Kawasaki et al., 2008b). The glial inhibitor fluorocitrate and IL-1ra inhibited inflammatory hyperalgesia and inflammation-induced NMDA receptor phosphorylation. Additionally, IL-1R1 and the NR1 subunit of the NMDA receptor were shown to colocalize in neurons (Guo et al. 2007, Zhang et al., 2008). In another set of experiments, direct application of IL-1β to an in vitro brain stem slice preparation induced an enhanced NMDA receptor phosphorylation in regions involved in trigeminal nociceptive processing. The effect of IL-1β on NMDA receptor was selective since another prototype inflammatory cytokine, TNFα, did not affect P-ser896-NR1 levels at the dose tested. This IL-1β-induced NR1 phosphorylation was blocked by IL-1ra, but not by fluorocitrate, the glial inhibitor, suggesting that the effect of IL-1β on NMDA receptor is downstream to glial activation (Guo et al. 2007). Taken together, these findings provide evidence that IL-1β leads to NMDA receptor phosphorylation through IL-1R1 signaling to facilitate pain transmission.
9. Conclusions
In summary, a growing number of studies show that peripheral injury activates both neuronal and non-neuronal or glial components of the peripheral and central cellular circuitry. The subsequent interactions between the injury site, neurons, and glia cells lead to increased excitability and persistent pain. Proinflammatory cytokines are also induced after injury, and may act on neurons to facilitate central sensitization and hyperalgesia. Recent findings implicate IL-1β in painful and inflammatory processes at multiple levels, both peripherally and centrally. IL-1β may explain how glial cells affect CNS neuronal activity and promote hyperalgesia. The mediation of interactions between cells at the injury site, such as glia and neurons, by IL-1β may facilitate synaptic activity and pain transmission, and contribute to the development of chronic pain. Taken together, these findings suggest that IL-1β inhibition could represent a broad-acting and efficacious method for managing pain and inflammation across a wide variety of conditions.
Acknowledgments
Dr. Ken Ren's work is supported by NIH grants DE11964, DE15374, NS060735. We thank Drs. Susan Croll and Lynn Macdonald for critical reading of this manuscript.
REFERENCES
- Agostino L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1β processing inflammasome with increased activity in Muckle–Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- Braddock M, Quinn A. Targeting IL-1 in inflammatory disease: new opportunities for therapeutic intervention. Nat. Rev. Drug Discov. 2004;3:1–10. doi: 10.1038/nrd1342. [DOI] [PubMed] [Google Scholar]
- Brenner GJ, Ji RR, Shaffer S, Woolf CJ. Peripheral noxious stimulation induces phosphorylation of the NMDA receptor NR1 subunit at the PKC-dependent site, serine-896, in spinal cord dorsal horn neurons. Eur. J. Neurosci. 2004;20:375–384. doi: 10.1111/j.1460-9568.2004.03506.x. [DOI] [PubMed] [Google Scholar]
- Caudle RM, Perez FM, Del Valle-Pinero AY, Iadarola MJ. Spinal cord NR1 serine phosphorylation and NR2B subunit suppression following peripheral inflammation. Mol. Pain. 2005;1:25. doi: 10.1186/1744-8069-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Shi Y, Hearn A, Fitzgerald K, Golenbock D, Reed G, Akira S, Rock K. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J. Clin. Invest. 2006;116(8):2262–2271. doi: 10.1172/JCI28075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Huang LY. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CBA, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN. Disruption of P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Church LD, Cook GP, McDermott MF. Primer: inflammasomes and interleukin-1β in inflammatory disorders. Nat. Clin. Pract. Rheumatol. 2008;4(1):34–42. doi: 10.1038/ncprheum0681. [DOI] [PubMed] [Google Scholar]
- Clark AK, D, Aquisto F, Gentry C, Marchand F, McMahon SB, Malcangio M. Rapid co-release of interleukin 1beta and caspase 1 in spinal cord inflammation. J. Neurochem. 2006;99:868–880. doi: 10.1111/j.1471-4159.2006.04126.x. [DOI] [PubMed] [Google Scholar]
- Copray JCVM, Mantingh I, Brouwer N, Biber K, Kust BM, Liem RSB, Huitinga I, Tilders FJH, Van Dam A-M, Boddeke HWGM. Expression of interleukin-1 beta in rat dorsal root ganglia. J. Neuroimmunol. 2001;118:203–211. doi: 10.1016/s0165-5728(01)00324-1. [DOI] [PubMed] [Google Scholar]
- Cunha JM, Cunha FQ, Poole S, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-1 receptor antagonist. Br. J. Pharmacol. 2000;130:1418–1424. doi: 10.1038/sj.bjp.0703434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalbeth N, Haskard DO. Mechanism of inflammation in gout. Rheumatology. 2005;44:1090–1096. doi: 10.1093/rheumatology/keh640. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Biological basis for Interleukin-1 in disease. Blood. 1996;87(6):2095–2147. [PubMed] [Google Scholar]
- Dinarello CA. Therapeutic strategies to reduce IL-1 activity in treating local and systemic inflammation. Curr. Opin. Pharmacol. 2004;4:378–385. doi: 10.1016/j.coph.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Di Giovine FS, Malawista SE, Nuki G, Duff GW. Interleukin 1 (IL-1) as a mediator of crystal arthritis. J. Immunol. 1987;138(10):3213–3218. [PubMed] [Google Scholar]
- Economides AN, Carpenter LR, Rudge JS, Wong V, Koehler-Stec E, Hartnett C, Pyles EA, Xu X, Daly TJ, Young MR, Fandl JP, Lee F, Carver S, McNay J, Bailey K, Ramakanth S, Hutabarat R, Huang TT, Radziejewski C, Yancopoulos GD, Stahl N. Cytokine traps: multi-component, high-affinity blockers of cytokine action. Nat. Med. 2003;9(1):47–52. doi: 10.1038/nm811. [DOI] [PubMed] [Google Scholar]
- Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- Fukuoka H, Kawatani M, Hisamitsu T, Takeshige C. Cutaneous hyperalgesia induced by peripheral injection of interleukin-1 beta in the rat. Brain Res. 1994;657:133–140. doi: 10.1016/0006-8993(94)90960-1. [DOI] [PubMed] [Google Scholar]
- Gattorno M, Tassi S, Carta S, Delfino L, Ferlito F, Pelagatti MA, D, Osualdo A, Buoncompagni A, Alpigiani MG, Alessio M, Martini A, Rubartelli A. Pattern of interleukin-1beta secretion in response to lipopolysaccharide and ATP before and after interleukin-1 blockade in patients with CIAS1 mutations. Arthritis Rheum. 2007;56(9):3138–3148. doi: 10.1002/art.22842. [DOI] [PubMed] [Google Scholar]
- Goldbach-Mansky R, Shroff SD, Wilson M, Snyder C, Plehn S, Barham B, Pham T-H, Pucino F, Wesley RA, Papadopoulos JH, Weinstein SP, Mellis SJ, Kastner DL. A pilot study to evaluate the safety and efficacy of the long-acting Interleukin-1 inhibitor Rilonacept (Interleukin-1 Trap) in patients with familial cold autoinflammatory syndrome. Arthritis Rheum. 2008;58(8):2432–2442. doi: 10.1002/art.23620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Zou S, Guan Y, Ikeda T, Tal M, Dubner R, Ren K. Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord during the development and maintenance of inflammatory hyperalgesia. J. Neurosci. 2002;22:6208–6217. doi: 10.1523/JNEUROSCI.22-14-06208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Dubner R, Ren K. Spinal N-methyl-D-aspartate receptor mechanisms of central sensitization and persistent pain following tissue injury. In: Mao JR, editor. Translational Pain Research. Nova Science Publishers; New York: 2006. pp. 45–78. [Google Scholar]
- Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J. Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume H, DeLeo JA, Colburn RW, Weinstein JN. Spinal glial activation and cytokine expression after lumbar root injury in the rat. Spine. 2000;25:1206–1217. doi: 10.1097/00007632-200005150-00003. [DOI] [PubMed] [Google Scholar]
- Hawkins PN, Lachmann HJ, Aganna E, McDermott MF. Spectrum of clinical features in Muckle–Wells syndrome and responses to anakinra. Arthritis Rheum. 2004;50(2):607–612. doi: 10.1002/art.20033. [DOI] [PubMed] [Google Scholar]
- Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial autoinflammatory syndrome and Muckle–Wells syndrome. Nat. Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HM, Rosengren S, Boyle DL, Cho JY, Nayar J, Mueller JL, Anderson JP, Wanderer AA, Firestein GS. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1-receptor antagonist. Lancet. 2004;364:1779–1785. doi: 10.1016/S0140-6736(04)17401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HM, Throne ML, Amar NJ, Sebai M, Kivitz AJ, Kavanaugh A, Weinstein SP, Belomestnov P, Yancopoulos GD, Stahl N, Mellis SJ. Efficacy and safety of Rilonacept (Interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes. Arthritis Rheum. 2008;58(8):2443–2452. doi: 10.1002/art.23687. [DOI] [PubMed] [Google Scholar]
- Hull KM, Shoham N, Chae JJ, Aksentijevich I, Kastner DL. The expanding spectrum of systemic autoinflammatory disorders and their rheumatic manifestations. Curr. Opin. Rheumatol. 2003;15:61–69. doi: 10.1097/00002281-200301000-00011. [DOI] [PubMed] [Google Scholar]
- Inoue A, Ikoma K, Morioka N, Kumagai K, Hashimoto T, Hide I, Nakata Y. Interleukin-1beta induces substance P release from primary afferent neurons through the cycloxygenase-2 system. J. Neurochem. 1999;73(5):2206–2213. [PubMed] [Google Scholar]
- Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol. Dis. 2001;8:1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat. Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, Ji RR. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat. Med. 2008a;14:331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 2008b;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-L, Lee K-M, Son S-J, Hwang S-H, Cho HJ. Temporal expression of cytokines and their receptors mRNAs in a neuropathic pain model. NeuroReport. 2004;15(18):2807–2811. [PubMed] [Google Scholar]
- LeMaitre CL, Hoyland JA. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res. Ther. 2007;9(4):R7. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D, Heumann R, Meyer M, Thoenen H. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature. 1987;330:658–659. doi: 10.1038/330658a0. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Heurmann R, Hengerer B, Thoenen H. Interleukin-1 increases stability and transcription of mRNA encoding nerve growth factor in culture rat fibroblasts. J. Biol. Chem. 1988;263(31):16348–16351. [PubMed] [Google Scholar]
- Malawasti SE, Duff GW, Atkins E, Cheung HS, McCarthy DJ. Crystal-induced endogenous pyrogen production. Arthritis Rheum. 1985;28(9):1039–1046. doi: 10.1002/art.1780280911. [DOI] [PubMed] [Google Scholar]
- Mariathasan S, Monack D. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat. Rev., Immunol. 2007;7:31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- Martinon F, Tschopp J. Inflammator caspases and inflammasomes: master switches of inflammation. Cell Death and Differentiation. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Masseoud D, Rott K, Liu-Bryan R, Agudelo C. Overview of hyperuricemia and gout. Curr. Pharm. Des. 2005;11:4117–4124. doi: 10.2174/138161205774913318. [DOI] [PubMed] [Google Scholar]
- Menetski J, Mistry S, Lu M, Mudgett JS, Ransohoff RM, Demartino JA, Macintyre DE, Abbadie C. Mice overexpressing chemokine ligand 2 (CCL2) in astrocytes display enhanced nociceptive responses. Neuroscience. 2007;149:706–714. doi: 10.1016/j.neuroscience.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Milligan ED, O, Connor KA, Nguyen KT, Armstrong CB, Twining C, Gaykema RP, Holguin A, Martin D, Maier SF, Watkins LR. Intrathecal HIV-1 envelope glycoprotein gp120 induces enhanced pain states mediated by spinal cord proinflammatory cytokines. J. Neurosci. 2001;21:2808–2819. doi: 10.1523/JNEUROSCI.21-08-02808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuki G, Simkin PA. A concise history of gout and hyperuricemia and their treatment. Arthritis Res. Ther. 2006;8(Suppl.1):S1. doi: 10.1186/ar1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obreja O, Rathee PK, Lips KS, Distler C, Kress M. IL-1β potentiates heat-activated currents in rat sensory neurons: involvement of IL-1R1, tyrosine kinase, and protein kinase C. FASEB. 2002;16:1497–1502. doi: 10.1096/fj.02-0101com. [DOI] [PubMed] [Google Scholar]
- Oka T, Aou S, Hori T. Intracerebroventricular injection of interleukin-1 beta induces hyperalgesia in rats. Brain Res. 1993;624:61–68. doi: 10.1016/0006-8993(93)90060-z. [DOI] [PubMed] [Google Scholar]
- Oka T, Aou S, Hori T. Intracerebroventricular injection of interleukin-1 beta enhances nociceptive neuronal responses of the trigeminal nucleus caudalis in rats. Brain Res. 1994;656:236–244. doi: 10.1016/0006-8993(94)91466-4. [DOI] [PubMed] [Google Scholar]
- Opree A, Kress M. Involvement of the protoinflammatory cytokines Tumor Necrosis Factor-α, IL-1β, and IL-6 but not IL-8 in the development of heat hyperalgesia: effects on heat-evoked Calcitonin Gene-Related Peptide release from rat skin. J. Neurosci. 2000;20(16):6289–6293. doi: 10.1523/JNEUROSCI.20-16-06289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr. Opin. Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Parri R, Crunelli V. Astrocytes target presynaptic NMDA receptors to give synapses a boost. Nat. Neurosci. 2007;10:271–273. doi: 10.1038/nn0307-271. [DOI] [PubMed] [Google Scholar]
- Perrin FE, Lacroix S, Aviles-Trigueros M, Davis S. Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1α and interleukin-1β in Wallerian degeneration. Brain. 2005;128:854–866. doi: 10.1093/brain/awh407. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur. J. Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Reeve AJ, Patel S, Fox A, Walker K, Urban L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur. J. Pain. 2000;4:247–257. doi: 10.1053/eujp.2000.0177. [DOI] [PubMed] [Google Scholar]
- Rotshenker S, Aamar S, Barak V. Interleukin-1 activity in lesioned nerve. J. Neuroimmunol. 1992;39:75–80. doi: 10.1016/0165-5728(92)90176-l. [DOI] [PubMed] [Google Scholar]
- Ruohonen S, Khademi M, Jagodic M, Taskinen H-S, Olsson T, Roytta M. Cytokine responses during chronic denervation. J. Neuroinflamm. 2005;18(2):26–37. doi: 10.1186/1742-2094-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safieh-Garabedian B, Poole S, Allchorne A, Winter J, Woolf CJ. Contribution of interleukin-1β to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br. J. Pharmacol. 1995;115:1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safieh-Garabedian B, Poole S, Haddad JJ, Massaad CA, Jabbur SJ, Saade NE. The role of the sympathetic efferents in endotoxin-induced localized inflammatory hyperalgesia and cytokine upregulation. Neuropharmacology. 2002;42:864–872. doi: 10.1016/s0028-3908(02)00028-x. [DOI] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Schäfers M, Sorkin L. Effect of cytokines on neuronal excitability. Neurosci. Lett. 2008;437:188–193. doi: 10.1016/j.neulet.2008.03.052. [DOI] [PubMed] [Google Scholar]
- Schafers M, Brinkhoff J, Neukirchen S, Maziniak M, Sommer C. Combined epineural therapy with neutralizing antibodies to tumor necrosis factor-alpha and interleukin-1 receptor has an additive effect in reducing neuropathic pain in mice. Neurosci. Lett. 2001;310:113–116. doi: 10.1016/s0304-3940(01)02077-8. [DOI] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat. Neuroscience. 2007;10(11):1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Shamash S, Reichert F, Rotshenker S. The cytokine network of Wallerian degeneration: Tumor Necrosis Factor-α, Interleukin-1α, and Interleukin-1β. J. Neurosci. 2002;22(8):3052–3060. doi: 10.1523/JNEUROSCI.22-08-03052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- So A, DeSmedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res. Ther. 2007;9:R28. doi: 10.1186/ar2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci. Lett. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Sommer C, Petrausch S, Lindenlaub T, Toyka KV. Neutralizing antibodies to interleukin-1 receptor reduce pain associated behavior in mice with experimental neuropathy. Neurosci. Lett. 1999;270:25–28. doi: 10.1016/s0304-3940(99)00450-4. [DOI] [PubMed] [Google Scholar]
- Stojanov S, Kastner DL. Familial autoinflammatory diseases: genetics, pathogenesis and treatment. Curr. Opin. Rheumatol. 2005;17:586–599. doi: 10.1097/bor.0000174210.78449.6b. [DOI] [PubMed] [Google Scholar]
- Sugiyo S, Takemura M, Dubner R, Ren K. Trigeminal transition zone-rostral ventromedial medulla connection and facilitation of orofacial hyperalgesia after masseter inflammation in rats. J. Comp. Neurol. 2005;493:510–523. doi: 10.1002/cne.20797. [DOI] [PubMed] [Google Scholar]
- Sweitzer S, Martin D, DeLeo JA. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience. 2001;103(2):529–539. doi: 10.1016/s0306-4522(00)00574-1. [DOI] [PubMed] [Google Scholar]
- Terkeltaub R. Gout in 2006. Bull. NYU Hosp. Jt. Dis. 2006;64(1–2):82–86. [PubMed] [Google Scholar]
- Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J. Biol. Chem. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- Thacker MA, Clark AK, Marchand F, MacMahon SB. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. 2007;105(3):838–847. doi: 10.1213/01.ane.0000275190.42912.37. [DOI] [PubMed] [Google Scholar]
- Tschopp J, Martinon F, Burns K. NALPs: a novel protein family involved in inflammation. Nat. Rev., Mol. Cell Biol. 2003;4:95–104. doi: 10.1038/nrm1019. [DOI] [PubMed] [Google Scholar]
- Uceyler N, Sommer C. Cytokine regulation in animal models of neuropathic pain and human diseases. Neurosci. Lett. 2008;437:194–198. doi: 10.1016/j.neulet.2008.03.050. [DOI] [PubMed] [Google Scholar]
- Uceyler N, Tscharke A, Sommer C. Early cytokine expression in mouse sciatic nerve after chronic constriction nerve injury depends on calpain. Brain Behav. Immun. 2007;21:553–560. doi: 10.1016/j.bbi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Van Dam AM, Bauer J, Tilders FJ, Berkenbosch F. Endotoxin-induced appearance of immunoreactive interleukin-1 beta in ramified microglia in rat brain: a light and electron microscopic study. Neuroscience. 1995;65:815–826. doi: 10.1016/0306-4522(94)00549-k. [DOI] [PubMed] [Google Scholar]
- Vige X, Costa E, Wise BC. Mechanism of nerve growth factor mRNA regulation by interleukin-1 and basic fibroblast growth factor in primary cultures of rat astrocytes. Mol. Pharmacol. 1991;40(2):186–192. [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J. Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman JK, Zhu X, Schlichter LC. Evolution of the inflammatory response in the brain following intracerebral hemorrhage and effects of delayed minocycline treatment. Brain Res. 2007;1180:140–154. doi: 10.1016/j.brainres.2007.08.058. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Guo W, Zou S, Sugiyo S, Dubner R, Ren K. Antibody array analysis of peripheral and blood cytokine levels in rats after masseter inflammation. Neurosci. Lett. 2005;382:128–133. doi: 10.1016/j.neulet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat. Rev. Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Wiertelak EP, Goehler LE, Smith KP, Martin D, Maier SF. Characterization of cytokine-induced hyperalgesia. Brain Res. 1994;654:15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, Goehler LE. Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain. 1995;63:289–302. doi: 10.1016/0304-3959(95)00186-7. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv. Exp. Med. Biol. 2003;521:1–21. [PubMed] [Google Scholar]
- Wolf G, Gabay E, Tal M, Yirmiya R, Shavit Y. Genetic impairment of interleukin-1 signaling attenuates neuropathic pain, autotomy, and spontaneous ectopic neuronal activity, following nerve injury in mice. Pain. 2006;120:315–324. doi: 10.1016/j.pain.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1768. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br. J. Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Liu ZW, Wen L, Qiao HF, Zhou WX, Zhang YX. Interleukin-1beta enhances NMDA receptor-mediated current but inhibits excitatory synaptic transmission. Brain Res. 2005;1034:172–179. doi: 10.1016/j.brainres.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Yu XM, Askalan R, Keil GJ, II, Salter MW. NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]
- Zelenka M, Schafers M, Sommer C. Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain. 2005;116(3):257–263. doi: 10.1016/j.pain.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Liu B, Wang L, Ren K, Qiao JT, Berman BM, Lao L. Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. Pain. 2005;118:125–136. doi: 10.1016/j.pain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Li A, Liu B, Wang L, Ren K, Zhang H, Berman BM, Lao L. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain. 2008;135:232–239. doi: 10.1016/j.pain.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Role of protein kinase A in phosphorylation of NMDA receptor 1 subunits in dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. Neuroscience. 2002;115:775–786. doi: 10.1016/s0306-4522(02)00490-6. [DOI] [PubMed] [Google Scholar]