Abstract
BACKGROUND
Intermittent androgen deprivation therapy (IADT) was developed to improve the quality of life and retard prostate cancer progression to castration resistance. IADT involves regrowth of the tumor during the off cycle upon testosterone recovery. Our previous studies showed that testosterone is more potent than dihydrotestosterone (DHT) in the induction of a subset of androgen-responsive genes during rat prostate regrowth. However, it is not clear if the same phenomenon would occur during androgen-induced regrowth of prostate tumors. Understanding the differences between testosterone and DHT in inducing androgen-responsive genes during prostate tumor regrowth may provide new insight for improving IADT.
METHODS
Nude mice bearing androgen-sensitive LNCaP xenograft were castrated and followed up for 7–10 days before being randomized into various androgen manipulations, consisting of continuous castration (C) or testosterone replacement (T) in the absence or presence of dutasteride (D), a 5α-reductase inhibitor that blocks the conversion of testosterone to DHT. Testes-intact animals in the absence or presence of D were used as controls. The expression of five androgen-responsive genes, including the tumor suppressor U19/Eaf2, was determined using real-time RT-PCR, 3 days after randomization.
RESULTS
In LNCaP tumors, the expression of U19/Eaf2 was higher in the T+D group as compared with T alone (2.87-fold, P < 0.05). In contrast, dutasteride treatment in testes-intact animals inhibited the expression of U19/Eaf2.
CONCLUSIONS
Inhibition of 5α-reductase during LNCaP tumor regrowth enhanced the expression of U19/Eaf2, an androgen-regulated tumor suppressor. This finding suggests that off cycle 5α-reductase inhibition may enhance the efficacy of IADT.
Keywords: prostate cancer, intermittent androgen deprivation therapy, LNCaP, 5α-reductase inhibitors
INTRODUCTION
Huggins and Hodges first reported the importance of androgen ablation in prostate cancer treatment in 1941, since then androgen deprivation therapy (ADT) has become the main form of treatment for advanced prostate cancer, which encompasses metastatic disease and biochemical recurrence [1,2]. While ADT has proven efficacious at inducing tumor regression and improving survival, this therapy also produces deleterious side effects and leads to the eventual progression to castration-resistant disease. Intermittent androgen deprivation therapy (IADT) was first proposed by Klotz et al. in 1986 and later by Bruchovsky et al. [3–9] in an effort to circumvent the problems associated with ADT. IADT consists of multiple cycles of androgen deprivation, referred to as “on-cycle” followed by an “off-cycle” during which androgen levels are restored. IADT was based on the premise that transient androgen replacement could improve the quality of life and retard progression to castration resistance by promoting differentiation and clonal expansion of androgen-dependent cells within the tumor [3,10]. The impact of ADT on quality of life is an important issue for patients with prostate cancer and IADT appears to be a feasible alternative. Also, the cycling of androgen manipulation in IADT provides potential opportunities to enhance its therapeutic efficacy.
We have previously reported that administration of the 5α-reductase inhibitor finasteride during IADT prolonged the survival of nude mice bearing LNCaP xenograft tumors when the off-cycle intervals were fixed [11]. In subsequent experiments, we set the duration of the off-cycles based on the tumor doubling time. Finasteride administration during the off-cycle prolonged its duration; however, finasteride no longer provided a survival advantage for these animals [12]. Similarly, in patients with prostate cancer, the addition of finasteride doubled the duration of the off-cycle, but had no effect on progression to castration resistance [13]. The important question still remains as to whether 5α-reductase inhibition could improve survival in patients treated with IADT when off-cycle prolongation is not permitted. Understanding the impact of 5α-reductase inhibition on androgen action, particularly on the expression of androgen-responsive genes in prostate cancer, will help optimize the use of 5α-reductase inhibitors in IADT.
Two isoforms of 5α-reductase enzyme exist, type I and type II. In the normal prostate type II is the main form expressed, while type I is the main isoform expressed in prostate cancer [14,15]. Overexpression of both isoenzymes has been observed in prostate cancer [16]. Thus to effectively inhibit DHT synthesis both isoforms should be inhibited. Finasteride at therapeutic doses inhibits mainly isoform II, yet at higher doses it can inhibit both types [14,15]. Dutasteride on the other hand is considered a dual inhibitor at therapeutic doses.
Androgen action is mediated through the androgen receptor (AR), a ligand-dependent transcription factor that regulates the expression of androgen-responsive genes [17]. Studies from our laboratory and others have shown that many of these genes suppress growth [18–21]. One of these androgen-responsive genes encodes for U19/Eaf2, a potential tumor suppressor. Prostate cancer cells express less U19/Eaf2 as compared with benign adjacent glandular epithelial cells in clinical specimens [22]. Functional studies revealed that U19/Eaf2 overexpression induces apoptosis and inhibits proliferation in prostate cancer cells both in vitro and in tumor xenografts. Furthermore, U19/Eaf2 gene knockout mice develop B-cell lymphoma, hepatocellular carcinoma, lung adenocarcinoma, and mouse prostatic intraepithelial neoplasia (mPIN), the putative precursor of prostate cancer in mice [23]. These observations support the continuing study of the role of U19/Eaf2 as a tumor suppressor in prostate cancer, and further develop approaches to increase its expression in cancer cells.
The expression of androgen-responsive genes during prostate regrowth is enhanced in the rat model when 5α-reductase inhibition blocks the conversion of testosterone to dihydrotestosterone (DHT) [24]. Testosterone and DHT are the two major biologically active androgens. DHT and testosterone are known to differ in AR stabilization, androgen response element activation potency and also exert different actions during embryogenesis [25–27]. The finding that testosterone is more potent than DHT in inducing androgen-responsive gene expression in a regressed rat ventral prostate provides additional evidence for functional differences between testosterone and DHT [24]. Growth inhibition by finasteride during prostate regrowth is associated with elevated expression of growth suppressive androgen-responsive genes such as U19/Eaf2 in the rat model. Based on Huggins’ conclusion that malignant prostatic cells and normal prostatic epithelium respond similarly to androgen manipulation [1], we hypothesize that blocking the conversion of testosterone to DHT would enhance the induction of a subset of androgen-responsive genes, particularly those with growth suppressive properties such as U19/Eaf2, during androgen-stimulated regrowth of a regressed tumor in castrated animals. The studies herein tested the above hypothesis, using the androgen-dependent LNCaP xenograft tumor model.
MATERIALS AND METHODS
Xenograft Tumor Implantation
Early passage LNCaP cells from American Type Culture Collection (ATCC) were maintained in RPMI 1640 media supplemented with 10% fetal bovine serum (FBS), glutamine, penicillin and streptomycin. Cells underwent 4–8 passages prior to mouse inoculation. Approximately 106 LNCaP cells suspended in 250 µl media were mixed with 250 µl Matrigel (Becton Dickinson labware, Bedford, MA) and then inoculated subcutaneously in the flank region of 6–8 weeks old male athymic mice (BALB/c strain, Charles River Laboratory, Montreal, PQ, Canada) using a 25-gauge needle. Animal experiments were approved by the Institutional Animal Care Use Committee (IACUC) at the University of Pittsburgh.
Construction of Testosterone, Finasteride, and Dutasteride Pellets
Testosterone, finasteride, and dutasteride pellets were made as previously described [11,12]. Briefly, approximately 7.5mg of testosterone (Sigma Chemical, St. Louis, MO) was tightly packed into a silicone tube with an inner and outer diameter of 1.58 and 3.18mm, respectively (Helix Medical, Carpenteria, CA). The ends were plugged with wood sticks and sealed with a silicone adhesive (Dow Corning, Midland, MI). Following overnight air-drying, they were sterilized with 70% ethanol for 10 min and stored in a light-free environment. The 15mg finasteride and 8mg dutasteride (gift from GlaxoSmithKline) pellets were made similarly, except the silicone tubing had an inner and outer diameter of 1.47 and 1.96mm, respectively.
Treatment Protocol and Measurement of Tumor Growth
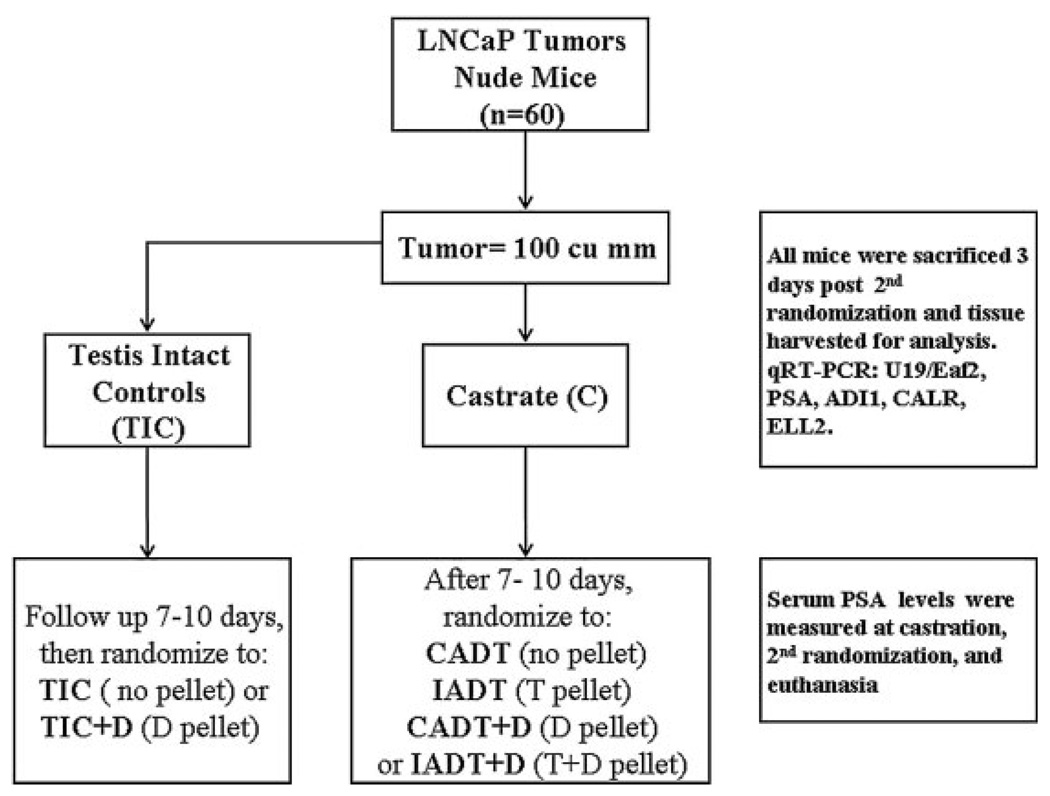
Once established at 8–12 weeks after injection, tumors were measured biweekly. Tumor volume was calculated by the modified ellipsoid formula: length × width2 × 0.52. For the initial set of experiments (Fig. 1), tumors were allowed to grow to 0.5 cm in diameter, or 0.0625 cm3 in the case of amorphous tumors, before randomization. Mice were randomized into three experimental groups: (1) castrated (C) for 10 days, (2) castrated for 10 days followed by testosterone replacement (T) for 3 days, and (3) castrated for 10 days followed by testosterone replacement in the presence of finasteride (T+F) for 3 days. For the subsequent experiments (the experimental design is illustrated in Fig. 2), mice were randomized into two groups when tumor volume equaled 100mm3: Testis Intact and Castration. Seven to 10 days after the first randomization the testis-intact mice were randomized a second time to receive either no treatment (TIC) or 3-day dutasteride treatment (TIC+D). For the castration group, trans-scrotal castration was performed under isoflurane anesthesia with proper aseptic and antiseptic technique. Castrated mice were randomized into four groups seven to 10 days post-castration: (1) Castration only (C), (2) Castration plus dutasteride (C+D), (3) Castration followed by testosterone replacement alone (T), and (4) Castration followed by testosterone replacement plus dutasteride (T+D). Response to castration was evidenced by tumor growth arrest or a decrease in tumor volume. Tumors that continued to grow within the 7–10-day period after castration were considered castration resistant and thus excluded from the study. All pellets were implanted subcutaneously in the flank contralateral to the tumor bearing side. According to our observation in the rat ventral prostate [24], maximum gene expression changes were observed 2–3 days after 5α-reductase inhibition, thus we decided to use a 3-day treatment protocol. Mice were sacrificed and tumor tissues were harvested for further studies 3 days after the second randomization. Blood sampling at the 1st and 2nd randomization was collected by saphenous phlebotomy. At sacrifice, blood collection was carried out by terminal cardiac puncture.
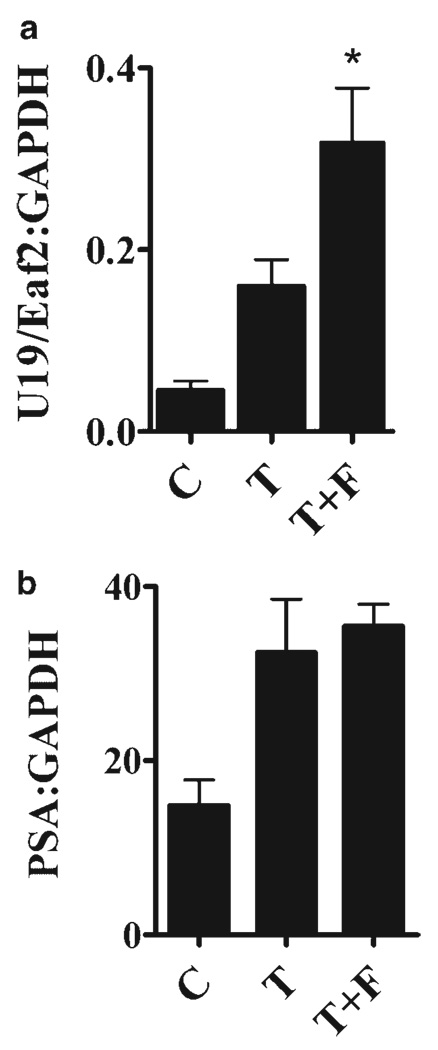
Fig. 1.
Effect of finasteride on the expression of U19/Eaf2 and PSA during LNCaP tumor regrowth. Tumor bearing mice were castrated when the tumor sizes were 0.5 cm in diameter. After 2 weeks, they were randomized into three groups: castrated with no intervention (C), with testosterone-replacement (T), and with testosterone-replacement plus finasteride (T+F). Drug pellets were implanted for 3 days and tumors harvested for gene expression analysis. The expression of U19/Eaf2 was enhanced 1.8-fold in the T+F group (n = 9) versus the T group (n = 12). *P < 0.05, independent samples t-test. Error bars depict SEM.
Fig. 2.
Flowchart of experimental design. Tumor bearing mice were castrated and followed up for 7–10 days before been randomized to receive testosterone (T), testosterone + dutasteride (T+D), dutasteride (D) or no intervention (C).T implantation mimicked intermittent androgen deprivation therapy (IADT),while T+D implantation mimicked IADT + OFF cycle 5α-reductase inhibition. Testes-intactmice, with or without dutasteride implantation, were kept as controls (TIC, TIC+D).
Determination of Serum PSA and Tissue DHT
Blood samples were centrifuged at 2,500 rpm for 5 min at room temperature to collect serum, which was stored at −80°C until measurement. Serum PSA levels were measured using a sandwich enzyme immunoassay kit (CAN-tPSA-4300, Diagnostics Biochem Canada Inc., Ontario, Canada) with a lower limit of detection of 0.1 ng/ml. DHT levels in tumors were assayed as previously described [17]. Briefly, tissue was homogenized in 1 × PBS plus 10mM EDTA, 100 µM PMSF, 100 µM leupeptin, 1 µM pepstatin, and antifoam B emulsion. Following homogenization, samples were centrifuged and the supernatant was collected for analysis. Hormone levels were determined using an enzyme immunoassay kit (CAN-DHT-280, Diagnostics Biochem Canada Inc.) with a lower limit of detection of 6.0 pg/ml for DHT.
Quantitative Reverse Transcriptase Real-Time PCR (qPCR)
Tumor tissue was collected at sacrifice, flash frozen in liquid Nitrogen, and stored at −80°C until further use. Total RNA was extracted using Trizol (Invitrogen, Carlsbad, CA). Approximately 4 µg of RNA was reverse transcribed with random primers using the high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Exon-exon junction spanning primers and Taqman probes were designed using the Primer 3 software (Totowa, NJ) and synthesized by Integrated DNA Technologies (Coralville, IA). Ex Taq™ 2 × premix (Takara Bio Inc.) was used to set up the real-time PCR reactions with 0.25 µM of forward and reverse primers each, and 0.5 µM of probe. Reactions were run in triplicates on a Bio-Rad IQ5 machine (Bio-Rad Laboratories, Hercules, CA), and repeated on an ABI Step-One Plus machine (Applied Biosystems). Rox was used as passive reference dye when using the ABI machine. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the endogenous control. The specificity of the primer-probe combinations for their cDNA targets was confirmed by the lack of amplification of human genomic DNA, mouse genomic DNA or mouse cDNA. The genes analyzed were ELL2, U19/Eaf2, calreticulin, PSA and aci-reductone dioxygenase-like protein (ADI1). The primers and probes for each gene are listed in Table I.
TABLE I.
Primers and Probes for Real-Time qRT-PCR Analysis
| Target | Primers and probe | Efficiency (%) |
|---|---|---|
| ADI1 | For: 5′-GAGGGACAAGGAGGACCAGT-3′ | 95 |
| Rev: 5′-TGGCCTTCGTGTAGTTCTTCTC-3′ | ||
| Probe: 5′-6FATCTTCATGGAGAAGGGAGACATGGTGACTAMRA-3′ | ||
| CALR | For: 5′-GGATCGAATCCAAACACAAGTC-3′ | 98 |
| Rev: 5′-TGGCTTGTCTGCAAACCTTTAT-3′ | ||
| Probe: 5′-6FAM TGGCAAATTCGTTCTCAGTTCCGGCAA TAMRA-3′ | ||
| EAF2/U19 | For: 5′-CCAGGACTCCCAATCTTGTAAA-3′ | 93 |
| Rev: 5′-TAGCTTCTGCCTTCAGTTCTCTT-3′ | ||
| Probe: 5′-6FAM CTCCATCTGAAGATAAGATGTCCCCAGCA TAMRA-3′ | ||
| ELL2 | For: 5′-TGACTGCATCCAGCAAACAT-3′ | 98 |
| Rev: 5′-TCGTTTGTTGCACACACTGTAA-3′ | ||
| Probe: 5′-6FAM TCTCCAGCTCTGGAGCCTCCCA TAMRA-3′ | ||
| GAPDH | For: 5′-CATGTTCGTCATGGGTGTGA-3′ | 95 |
| Rev: 5′-GGTGCTAAGCAGTTGGTGGT-3′ | ||
| Probe: 5′-6FAM ACAGCCTCAAGATCATCAGCAATGCCTC TAMRA-3′ | ||
| PSA | For: 5′-GTCCCGGTTGTCTTCCTCA-3′ | 94 |
| Rev: 5′-CACAATCCGAGACAGGATGAG-3′ | ||
| Probe: 5′-6FAM TGTCCGTGACGTGGATTGGTGCTG TAMRA-3′ |
For the set of experiments in Figure 1 using Finasteride as the 5α-reductase inhibitor, quantitative PCR (qPCR) was performed with SYBR Green dye on a MJ Chromo4™ System (Bio-Rad) to determine U19/Eaf2 and PSA expression. Briefly, total RNA was isolated from LNCaP xenograft tumor using the acid guanidinium/CsCl gradient method or RNeasy RNA purification system (Qiagen, Valencia, CA). cDNA was synthesized from 1–4 µg of total RNA using Super-Script III first-strand Synthesis System (Invitrogen) in the presence of random primers. All qPCR reactions were performed with iQ™ SYBR Green Supermix (Bio-Rad). The amount of each target gene relative to the housekeeping gene (GAPDH) for each sample was determined using the ΔCP method. All reactions were subjected to melting curve analysis and products from selected experiments were resolved by electrophoresis on 2% agarose gels. Randomly selected amplification products were sent for sequencing to confirm that the correct target was amplified. Primer pairs for the amplification of human specific U19/Eaf2, PSA and GAPDH are as below:
U19/Eaf2 Forward: 5′-ggaagcagtaaaattcagtatcgtaa-3′;
U19/Eaf2 Reverse: 5′-gacacaattccctgtatcag-3′;
PSA Forward: 5′-cgctctacgatatgagcctcc-3′;
PSA Reverse: 5′-ttgatccacttccggtaatgc-3′;
GAPDH Forward: 5′-tggggagtccctgccacactc-3′;
GAPDH Reverse: 5′-gatggtacatgacaaggtgc-3′.
Statistical Analysis
GraphPad Prism 4.0 (GraphPad Software, Inc.) and SPSS 15.0 (SPSS Inc., Chicago, IL) were used for statistical analysis and MS Excel 2003 was used for graphical composition. All data were expressed as the Means ± SEM of the samples examined, and values of P < 0.05 were considered statistically significant. The qPCR data were exported into MS Excel and the expression of transcripts relative to GAPDH calculated by the ΔCP method: Relative Expression = 2−ΔCP, where ΔCP is the difference between the crossing point thresholds of target gene versus GAPDH [28,29]. The results were depicted on scatter plots to convey the expression patterns.
RESULTS
Finasteride Enhanced the Expression of the Androgen-Responsive Gene U19/Eaf2 During Testosterone-Stimulated Regrowth of LNCaP Xenograft Tumors in Castrated Nude Mice
Our previous studies showed that blocking the conversion of testosterone to DHT by finasteride, a selective type II 5α-reductase inhibitor at therapeutic doses and a dual inhibitor at higher doses, impedes regrowth of both LNCaP xenograft tumors and regressed rat prostate [11,12,24]. We also observed an association between the finasteride-mediated inhibition of regressed rat prostate regrowth and the elevated expression of a subset of androgen-responsive genes that are growth suppressive in the prostate [24]. These observations suggest that finasteride inhibition of LNCaP xenograft tumor regrowth may also be associated with elevated expression of growth suppressive genes. To test this hypothesis, we first evaluated whether finasteride could enhance the testosterone induction of the androgen-responsive genes U19/Eaf2 and PSA in an LNCaP model. We chose U19/Eaf2 and PSA in this initial experiment because these two genes have different properties: U19/Eaf2 is a very potent growth inhibitory and tumor suppressive gene while the PSA gene expresses a secreted protein associated with differentiation [22,23,30]. Testosterone replacement enhanced the expression of both U19/Eaf2 and PSA relative to their expression in the castrated control group (Fig. 1), indicating that LNCaP tumors in this experiment were responsive to androgens. Interestingly, finasteride further enhanced testosterone replacement-induced expression of U19/Eaf2, but not PSA, in LNCaP tumor regrowth in castrated mice (Fig. 1). This finding suggests that, as in the rat ventral prostate, inhibition of 5α-reductase can also enhance the testosterone-induction of some androgen-responsive genes, particularly those involved in growth suppression, in prostate tumor regrowth.
Androgen Sensitivity of LNCaP Xenograft Tumors
We next wanted to validate and further explore the effect of 5α-reductase inhibition on the expression of androgen-responsive genes during tumor regrowth. We thus repeated the experiment using additional control groups. Also, instead of using finasteride, we used dutasteride, which is considered a dual inhibitor of both type I and II 5α-reductase at therapeutic doses, to determine if our finding was reproducible with a different 5α-reductase inhibitor.
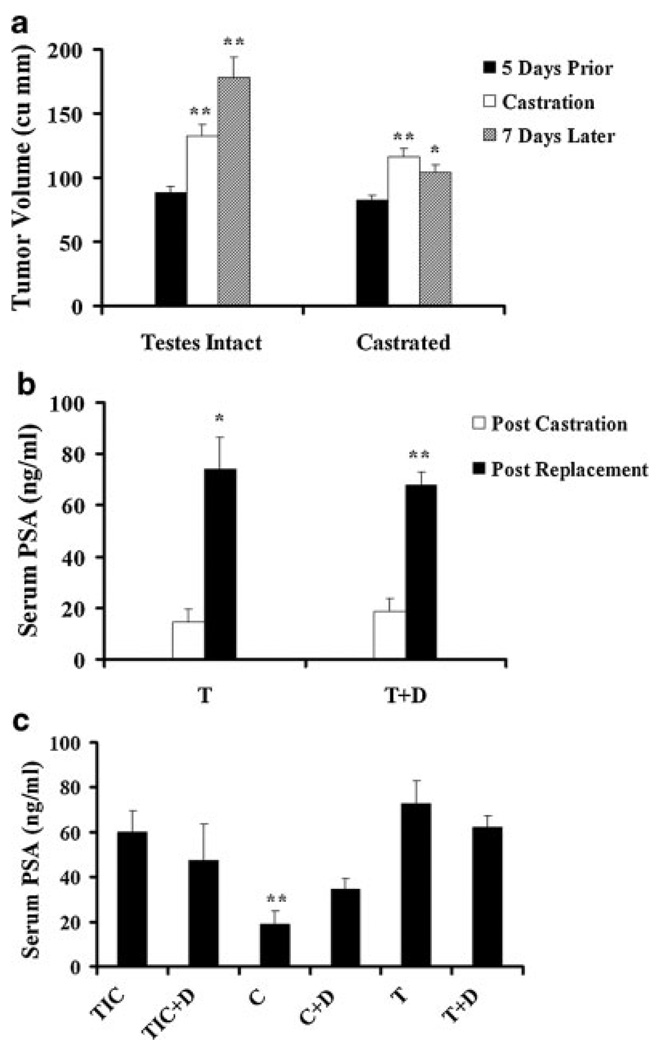
A key requirement of the experiment is that the established LNCaP tumors in nude mice be androgen-dependent. Tumor response to castration allows for the selection of androgen-sensitive/dependent tumors. To assess the effects of androgen manipulation, we monitored tumor volume and serum PSA at various time points. Castration-resistant tumors, defined by continuous growth during the 7–10-day period following castration were excluded. Of the castrated mice, only one was not included in the analysis for this reason. Testis intact controls showed progressive increase in volume, while castrated mice had modest decrease or arrest of tumor growth. One week following castration, the mean tumor volume decreased from 116.34 to 104.6mm3 (P < 0.05), while the mean tumor volume in the testes intact control group increased from 131.87 to 177.58mm3 (P < 0.01) during the same time frame (Fig. 3a).
Fig. 3.
LNCaP tumor response to androgen manipulation. a: Effect of castration on tumor volume. Tumor bearing mice were castrated, or were followed up without intervention. Tumors in both groups showed a significant increase in volume from 5 days before castration until castration day (116.34mm3 vs. 82.26mm3 in the castrated group; 131.87mm3 vs. 88.21mm3 in testes intact group, **P < 0.01 for both).Castration led to an arrest of tumor growth, and after 1 week, the mean tumor volume (104.6mm3)was modestly lower than that at castration day (116.34mm3, *P < 0.05).Over the same period, testes intact controls showed a significant increase in tumor volume, from 131.87 to 177.58mm3 (**P < 0.01). Error bars depict SEM, and the paired t test was used for p value calculation. b: Effect of androgen replacement on serum PSA. Tumor bearing mice castrated for 7–10 days were implanted with testosterone (T)or testosterone + dutasteride(T+D)pellets. Three days after pellet implantation, both groups showed increases in serum PSA levels, from 14.54 to 73.99 ng/ml in the T group (*P < 0.05) and from 18.68 to 68.01 ng/ml in the T+D group (**P < 0.01).Error bars depict SEM, and the paired t test was used for p value calculation. c: Serum PSA levels at sacrifice. Serum PSA was significantly different across the groups (P = 0.002). Serum PSA was lower in the castrated (C) group versus the testes intact control (TIC) group (18.89ng/ml vs. 59.82ng/ml, *P < 0.05), the T group (18.89 ng/ml vs. 72.63 ng/ml, **P < 0.01) and the T+D group (18.89 ng/ml vs.62.31 ng/ml, **P < 0.01). Error bars indicate SEM. One-way ANOVA with Tukey’s post-hoc test was used for statistical analyses.
We also measured serum PSA, a marker reflecting the activity of the androgen receptor, in castrated animals before and after testosterone replacement (Fig. 3b). In the castrated groups, with and without dutasteride, 3-day testosterone replacement exerted no effect on tumor volume (data not shown), yet serum PSA was significantly increased. Without dutasteride, serum PSA increased from 14.54 to 73.99 ng/ml (P = 0.022) and with dutasteride, PSA levels increased from 18.68 to 68.01 ng/ml (P < 0.0001; Fig. 3b). This result provides further evidence of the androgen sensitivity of the LNCaP tumors in our study.
We also measured serum PSA level at sacrifice (Fig. 3c). Serum PSA was lowest in the castrated groups and significantly different from all other groups (P = 0.002). Overall, we observed a trend towards a decrease in serum PSA in the testes-intact and the testosterone replacement groups treated with dutasteride. Again, these findings are consistent with LNCaP tumors being responsive to androgen manipulation in our experiment.
Dutasteride Enhanced the Expression of Androgen-Responsive Gene U19/Eaf2 and Calreticulin During Regrowth of LNCaP Xenograft Tumors
To study gene expression patterns in response to androgen manipulation and 5α-reductase inhibition, we used real-time qRT-PCR to investigate the expression of five known androgen-responsive genes: U19/Eaf2, PSA, ELL2, calreticulin, and ADI1. The results are graphed in scatter plots as gene expression relative to GAPDH, determined using the delta Ct method (Fig. 4).
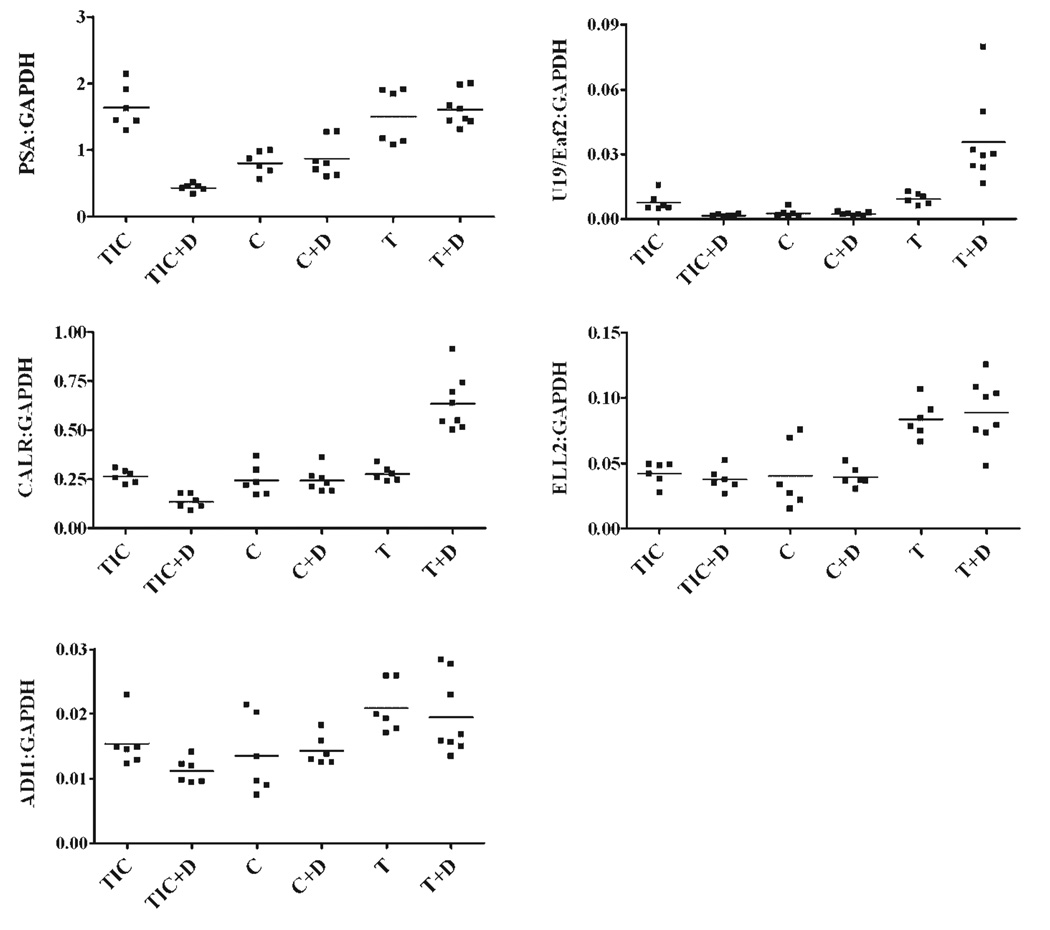
Fig. 4.
Effect of dutasteride on the expression of indicated androgen-responsive genes. Tumor bearing mice were castrated when the tumors reached a volume of 100 mm3.After castration, they were randomized to C,C+D,T, or T+D.TIC and TIC+D mice were followed up concurrently. Transcript levels for the assayed genes were normalized to GAPDH using the ΔCP method. Each dot represents a single sample, and the horizontal line depicts the median. PSA (**P < 0.01) and U19/Eaf2 (*P < 0.05) expression was significantly decreased in castrated mice when compared to TIC. Expression of PSA, U19/Eaf2, Calreticulin and ADI1 was significantly decreased, **P < 0.01, in TIC mice treated with D. Expression of U19/Eaf2 and calreticulin was enhanced in the T+D group, **P < 0.01. Unpaired t-test was used for P-value calculation.
This study showed that dutasteride can enhance the testosterone-induced expression of a subset of the androgen-responsive genes during the regrowth of LNCaP xenograft tumors in castrated nude mice (Fig. 4). For example, dutasteride treatment resulted in an increase in U19/Eaf2 expression (2.8-fold, P < 0.01), but not PSA, during tumor regrowth. This selective response may reflect the difference in promoter and/or enhancer(s) present in different androgen-responsive genes. This finding is similar to that observed in Figure 1, indicating that the effects of dutasteride and finasteride were comparable in androgen-responsive gene expression induction. Among the other three additional androgen-responsive genes tested, calreticulin also displayed elevated expression in the presence of dutasteride during testosterone replacement (2.15-fold, **P<0.01).However, dutasteride had no significant effect on the testosterone-induced expression of ELL2 or ADI1during LNCaP tumor regrowth. The above findings indicate that testosterone is more potent than DHT in the induction of a subset of androgen-responsive genes during LNCaP regrowth.
Dutasteride significantly down-regulated the gene expression of PSA, U19/Eaf2, calreticulin, and ADI1, in LNCaP tumors naїve to androgen manipulation in testes-intact mice (Fig. 4). This finding indicates that blocking testosterone conversion to DHT in testes-intact animals is inhibitory to the expression of some androgen-responsive genes in LNCaP tumors, which is consistent with finasteride inhibition of androgen-responsive gene expression in the intact prostate of the rat model [24]. Our findings suggest that 5α-reductase inhibitors could suppress the expression of a selected group of androgen-responsive genes in LNCaP xenograft tumors naїve to androgen manipulation.
As expected, dutasteride had virtually no effect on the expression of all the tested androgen-responsive genes in LNCaP tumors in castrated mice. This observation verified that dutasteride does not influence androgen-responsive expression in LNCaP tumors under castrated conditions.
Castration, with or without dutasteride treatment, down-regulated the expression of PSA and U19/Eaf2, but not of ELL2, ADI1, or calreticulin (Fig. 4). It is important to consider that serum testosterone levels in nude mice are approximately 0.2 ng/ml, according to our previous study [12], which is comparable to the castrated serum levels observed in men [31]. This fact could account for the lack of dramatic down-regulation in androgen-responsive gene expression in response to castration in the LNCaP tumors. However, the expression of all tested genes in castrated conditions, with or without dutasteride, was lower than that in the testosterone replacement groups, with or without dutasteride. Testosterone supplementation after castration is expected to achieve physiological levels of serum testosterone ranging from 2 to 3 ng/ml [12]. Taken together, these data argue that the expression of the tested androgen-responsive genes was sensitive to androgen manipulation.
Tissue Dihydrotestosterone Levels
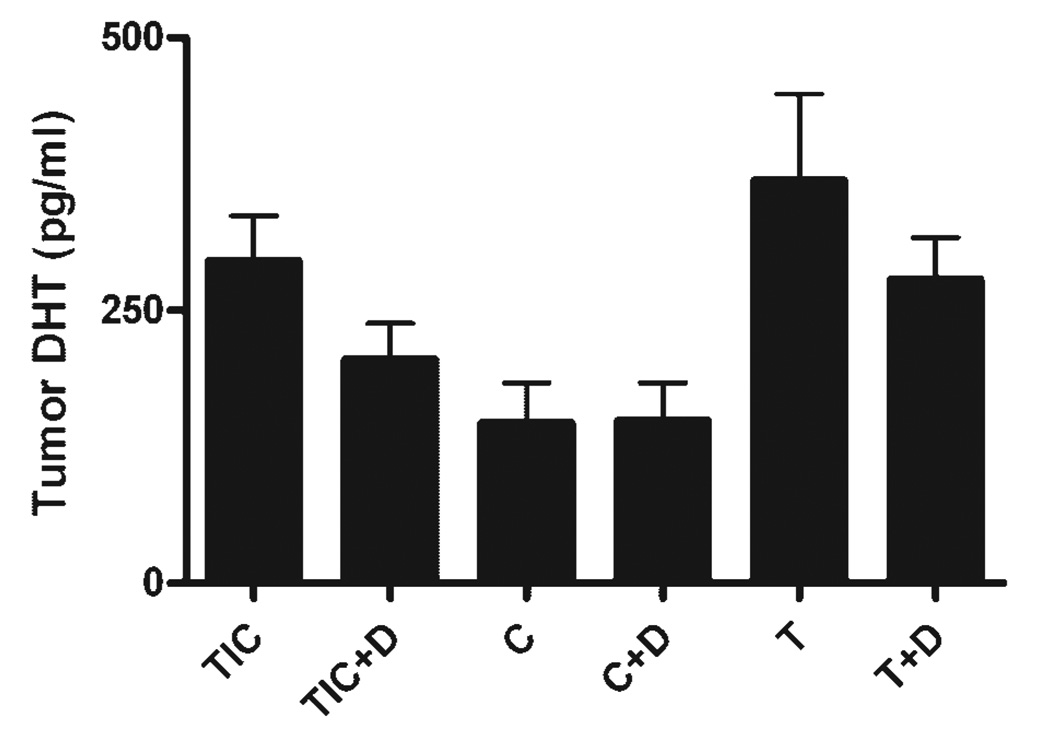
In order to examine the inhibitory effect of dutasteride on conversion of testosterone to DHT, tumor tissues were assayed for DHT levels. The drug pellet design was previously used in murine models with satisfactory results, as evidenced by the drug’s effect on the target organ, for example, seminal vesicles and prostate lobes in the presence of testosterone [11]. Figure 5 shows that dutasteride reduced the tumor tissue DHT levels in testis-intact animals from 296 to 206 pg/ml (P = 0.107). Similarly, dutasteride reduced the tumor tissue DHT levels in testosterone-replacement groups from 556 and 279 pg/ml (Fig. 5, P = 0.142). Due to the limited sample size, dutasteride inhibition of DHT levels in the testes-intact and testosterone-replacement groups were not statistically significant. However, the trend of tumor tissue DHT inhibition by dutasteride was clear. As expected, castrated mice exhibited a significant decrease in tumor DHT levels as compared with the testis-intact group, with a mean DHT concentrations of 296.18 and 124.27 pg/ml in testes-intact and castrated groups, respectively (Fig. 5, P = 0.017).
Fig. 5.
DHT levels in tumor tissues the DHT levels were significantly different across the groups (P = 0.0085, one-way ANOVA). The testes intact mice had a mean tumor DHT concentration of 296.2 pg/ml, and in castrated mice it was 124.27 pg/ml. Testosterone pellet implantation (T) restored the intra tumor DHT levels to a mean of 370 pg/ml and the addition of dutasteride to the off-cycle (T+D) caused a modest decline to 278.78 pg/ml. Error bars depict SEM.
DISCUSSION
The present study shows that 5α-reductase inhibition can enhance the expression of some androgen-responsive genes, particularly those with growth suppressive properties such as U19/Eaf2, during the regrowth of LNCaP xenograft tumors. This suggests that testosterone is more potent than DHT in the induction of U19/EAF2 in regressed prostate tumors in the castrated host. This phenomenon is similar to the observation that 5α-reductase inhibition enhanced the expression of a subset of androgen-responsive genes in the regressed rat ventral prostate upon testosterone replacement [24]. Thus, the mechanism responsible for the differential regulation of androgen-responsive genes by testosterone and DHT exists in regressed androgen-sensitive LNCaP tumors, and is a phenomenon conserved from rodent to human.
Interestingly, the expression level of most of the androgen responsive genes we studied was not significantly affected by castration when compared to testes-intact controls. This could be attributed to the low concentration of serum testosterone that is present in nude mice. According to our previous publication [12], serum testosterone in testis-intact nude mice is approximately 0.2 ng/ml, while in the Sprague–Dawley rat and in men are 3 and 3–10 ng/ml, respectively [24,31]. The serum testosterone level in nude mice is slightly higher than the castrated testosterone levels in men [12,31], which could be responsible for the weak down-regulation in androgen-responsive gene expression observed following castration. The testosterone delivery system used in the present experiments has been previously validated [11,12] and is expected to achieve serum testosterone concentration of ~3 ng/ml, which corresponds to physiological levels in men and ~10 times higher than in testis-intact nude mice.
PSA gene expression was the most sensitive transcript to androgen manipulation, being significantly down-regulated by castration and by dutasteride treatment in testes-intact animals. However, during androgen stimulated tumor regrowth dutasteride exerted no effect on PSA expression levels. Contrary to PSA, U19/EAF2 and calreticulin both demonstrated significant up-regulation in response to dutasteride during tumor regrowth. The mechanism behind the transcript specific differential response to 5α-reductase inhibition is still unclear. It is likely due to differences in promoters and/or enhancers present in each gene.
Both dutasteride and finasteride enhanced the expression of U19/Eaf2 during the regrowth of LNCaP xenograft tumors (Figs. 1 and 4), suggesting that the effect of these 5α-reductase inhibitors on U19/Eaf2 gene expression is mediated through the inhibition of the 5α-reductase enzyme rather than other unknown off-target effects. Our studies also showed that dutasteride did not influence the expression of androgen-responsive genes in LNCaP tumors in castrated animals without testosterone replacement (Fig. 4), suggesting that the influence of dutasteride on U19/Eaf2 and other androgen-responsive genes requires testosterone and is mediated by blocking the conversion of testosterone to DHT. While inhibition of 5α-reductase enzyme by either dutasteride or finasteride can enhance U19/Eaf2 expression, we are not able to state which inhibitor works more effectively. Dutasteride administration caused a slightly higher elevation in U19/Eaf2 expression in LNCaP model than finasteride. However, this difference in level of expression could be due to the fact that these experiments were carried out using different batch of animals.
Regressed LNCaP xenograft tumors in castrated mice responded to androgens differently from LNCaP tumors naїve to castration in testes-intact animals. We observed elevated expression of U19/Eaf2 and calreticulin genes by dutasteride treatment during the regrowth of regressed LNCaP tumors, but not in LNCaP tumors naїve to castration. The mechanism responsible for this difference is not clear. Variability in the expression of cofactors after castration and their recruitment by the androgen receptor in response to testosterone or DHT might explain the differential response to dutasteride of genes like U19/EAF2 and calreticulin in a regressed versus naїve tumor. However, presently this is only speculative and further studies are needed to elucidate the exact mechanisms involved. Nonetheless, this differential expression provides a potential explanation for the observation that finasteride significantly retarded the regrowth of regressed LNCaP xenograft tumors upon testosterone replacement, but not the growth of LNCaP tumors in testes-intact mice [11]. U19/Eaf2 is a potent growth inhibitor and a potential tumor suppressor [22,23]. The elevated expression of U19/Eaf2 as well as other growth suppressive androgen-responsive genes by 5α-reductase inhibition during testosterone-induced regrowth likely contributes to the retardation of LNCaP tumor regrowth observed with finasteride. In contrast, 5α-reductase inhibition did not increase the expression of U19/Eaf2 or other androgen-responsive genes in testes-intact animals, which correlates with the lack of LNCaP tumor growth inhibition observed in this group of animals upon treatment with 5α-reductase inhibitor [12]. Since testosterone-stimulated tumor regrowth occurs during the off-cycles in intermittent androgen deprivation therapy (IADT), the off-cycles provide an opportunity for using 5α-reductase inhibitor to enhance the expression of tumor suppressive androgen-responsive genes such as U19/Eaf2, and may improve the therapeutic efficacy of IADT.
Our studies show that 5α-reductase inhibition only elevated the expression of a subset of androgen-responsive genes during LNCaP xenograft tumor regrowth. Among five assayed androgen-responsive genes, U19/Eaf2 and calreticulin displayed elevated expression during LNCaP tumor regrowth in the presence of dutasteride. The expression of the other three androgen-responsive genes was not increased by dutasteride. While dutasteride may increase the expression of additional androgen-responsive genes, it is not clear whether it will increase the expression of a majority of the growth-suppressive androgen-responsive genes during LNCaP xenograft tumor regrowth. Since 5α-reductase inhibition retarded LNCaP tumor regrowth, the elevated expression of a subset of growth suppressive androgen-responsive genes is likely to be in part responsible for the growth inhibition. Given the limited number of genes studied, it is also possible that other genes could behave similarly to U19/EAF2 and further contribute to the growth inhibitory effects associated with dutasteride treatment during tumor regrowth. In addition, it will be important to determine the time frame of this selective gene up-regulation tomaximize the benefits of 5α-reductase inhibition in IADT.
In summary, our studies indicate that 5α-reductase inhibition can upregulate tumor suppressor U19/Eaf2 during testosterone-induced regrowth of LNCaP xenograft tumor in castrated mice, but not in LNCaP tumors naїve to androgen-deprivation in testes-intact animals. This finding has potential clinical implications, particularly pertaining to use of 5α-reductase inhibitors in IADT, as the off-cycles in IADT involve testosterone-stimulated prostate tumor regrowth. The possibility of enhancing the expression of growth suppressive androgen-responsive genes by inhibiting testosterone conversion to DHT during prostate tumor regrowth is likely beneficial to patients with prostate cancer. Therefore, further studies are warranted to elucidate the mechanism by which growth suppressive androgen-responsive genes are induced to a higher level by testosterone than DHT during regrowth of normal and cancerous prostate.
ACKNOWLEDGMENTS
We thank Merck and GlaxoSmithKline (GSK) for providing finasteride and dutasteride, respectively, Moira Hitchens for editing, Roger S. Rittmaster for discussion, and members of Wang lab for critical reading. This study was supported by grants from the National Institute of Health, Prostate Cancer Specialized Program of Research Excellence (SPORE), CA90386, R37 DK51193, and Department of Defense Prostate Cancer Research Program, DAMD17-02-1-0113, and also by funding from GSK.
REFERENCES
- 1.Huggins C, Hodges C. Studies on prostatic cancer. I. The effects of castration of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 2.Huggins C, Stevens RJ, Hodges C. Studies on prostatic cancer II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209–223. [Google Scholar]
- 3.Klotz LH, Herr HW, Morse MJ, Whitmore WF., Jr Intermittent endocrine therapy for advanced prostate cancer. Cancer. 1986;58(11):2546–2550. doi: 10.1002/1097-0142(19861201)58:11<2546::aid-cncr2820581131>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Akakura K, Bruchovsky N, Goldenberg SL, Rennie PS, Buckley AR, Sullivan LD. Effects of intermittent androgen suppression on androgen-dependent tumors. Apoptosis and serum prostate-specific antigen. Cancer. 1993;71:2782–2790. doi: 10.1002/1097-0142(19930501)71:9<2782::aid-cncr2820710916>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Bruchovsky N, Klotz LH, Sadar M, Crook JM, Hoffart D, Godwin L, Warkentin M, Gleave ME, Goldenberg SL. Intermittent androgen suppression for prostate cancer: Canadian Prospective Trial and related observations. Mol Urol. 2000;4(3):191–199. discussion 201. [PubMed] [Google Scholar]
- 6.Gleave M, Bruchovsky N, Goldenberg SL, Rennie P. Intermittent androgen suppression for prostate cancer: Rationale and clinical experience. Eur Urol. 1998;34 Suppl 3:37–41. doi: 10.1159/000052297. [DOI] [PubMed] [Google Scholar]
- 7.Higano CS, Ellis W, Russell K, Lange PH. Intermittent androgen suppression with leuprolide and flutamide for prostate cancer: A pilot study. Urology. 1996;48(5):800–804. doi: 10.1016/S0090-4295(96)00381-0. [DOI] [PubMed] [Google Scholar]
- 8.Klotz L, Sogani P, Block N. Summary of intermittent endocrine therapy for advanced prostate cancer. Semin Urol Oncol. 1997;15:117–122. [PubMed] [Google Scholar]
- 9.Pether M, Goldenberg SL, Bhagirath K, Gleave M. Intermittent androgen suppression in prostate cancer: An update of the Vancouver experience. Can J Urol. 2003;10(2):1809–1814. [PubMed] [Google Scholar]
- 10.Sato N, Gleave ME, Bruchovsky N, Rennie PS, Goldenberg SL, Lange PH, Sullivan LD. Intermittent androgen suppression delays progression to androgen-independent regulation of prostate-specific antigen gene in the LNCaP prostate tumour model. J Steroid Biochem Mol Biol. 1996;58:139–146. doi: 10.1016/0960-0760(96)00018-0. [DOI] [PubMed] [Google Scholar]
- 11.Eggener SE, Stern JA, Jain PM, Oram S, Ai J, Cai X, Roehl KA, Wang Z. Enhancement of intermittent androgen ablation by “off-cycle” maintenance with finasteride in LNCaP prostate cancer xenograft model. Prostate. 2006;66(5):495–502. doi: 10.1002/pros.20297. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Gupta S, Hua V, Ramos-Garcia R, Shevrin D, Jovanovic BD, Nelson JB, Wang Z. Prolongation of off-cycle interval by finasteride is not associated with survival improvement in intermittent androgen deprivation therapy in LNCaP tumor model. Prostate. 2009 doi: 10.1002/pros.21046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scholz MC, Jennrich RI, Strum SB, Johnson HJ, Guess BW, Lam RY. Intermittent use of testosterone inactivating pharmaceuticals using finasteride prolongs the time off period. J Urol. 2006;175(5):1673–1678. doi: 10.1016/S0022-5347(05)00975-4. [DOI] [PubMed] [Google Scholar]
- 14.Russell DW, Wilson JD. Steroid 5 alpha-reductase: Two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, Dalrymple SL, Becker RE, Denmeade SR, Isaacs JT. Pharmacologic basis for the enhanced efficacy of dutasteride against prostatic cancers. Clin Cancer Res. 2006;12(13):4072–4079. doi: 10.1158/1078-0432.CCR-06-0184. [DOI] [PubMed] [Google Scholar]
- 16.Thomas LN, Lazier CB, Gupta R, Norman RW, Troyer DA, O’Brien SP, Rittmaster RS. Differential alterations in 5alpha-reductase type 1 and type 2 levels during development and progression of prostate cancer. Prostate. 2005;63(3):231–239. doi: 10.1002/pros.20188. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Z, Wong C, Sar M, Wilson E. The androgen receptor: An overview [Review] Recent Prog Horm Res. 1994;49:249–274. doi: 10.1016/b978-0-12-571149-4.50017-9. [DOI] [PubMed] [Google Scholar]
- 18.Bolton EC, So AY, Chaivorapol C, Haqq CM, Li H, Yamamoto KR. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev. 2007;21(16):2005–2017. doi: 10.1101/gad.1564207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clegg N, Eroglu B, Ferguson C, Arnold H, Moorman A, Nelson PS. Digital expression profiles of the prostate androgen-response program. J Steroid Biochem Mol Biol. 2002;80(1):13–23. doi: 10.1016/s0960-0760(01)00167-4. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Tufts R, Haleem R, Cai X. Genes regulated by androgen in the rat ventral prostate. Proc Natl Acad Sci USA. 1997;94:12999–13004. doi: 10.1073/pnas.94.24.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang F, Wang Z. Identification and characterization of PLZF as a prostatic androgen-responsive gene. Prostate. 2004;59(4):426–435. doi: 10.1002/pros.20000. [DOI] [PubMed] [Google Scholar]
- 22.Xiao W, Zhang Q, Jiang F, Pins M, Kozlowski JM, Wang Z. Suppression of prostate tumor growth by U19, a novel testosterone-regulated apoptosis inducer. Cancer Res. 2003;63(15):4698–4704. [PubMed] [Google Scholar]
- 23.Xiao W, Zhang Q, Habermacher G, Yang X, Zhang AY, Cai X, Hahn J, Liu J, Pins M, Doglio L, Dhir R, Gingrich J, Wang Z. U19/Eaf2 knockout causes lung adenocarcinoma, B-cell lymphoma, hepatocellular carcinoma and prostatic intraepithelial neoplasia. Oncogene. 2008;27(11):1536–1544. doi: 10.1038/sj.onc.1210786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dadras SS, Cai X, Abasolo I, Wang Z. Inhibition of 5alpha-reductase in rat prostate reveals differential regulation of androgen-response gene expression by testosterone and dihydrotestosterone. Gene Expr. 2001;9(4–5):183–194. doi: 10.3727/000000001783992551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grino P, Griffin J, Wilson J. Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone. Endocrinology. 1990;126:1165–1172. doi: 10.1210/endo-126-2-1165. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Z, Lane M, Kemppainen J, French F, Wilson E. Specificity of ligand-dependent androgen receptor stabilization: Receptor domain interactions influence ligand dissociation and receptor stability. Mol Endocrinol. 1995;9(2):208–218. doi: 10.1210/mend.9.2.7776971. [DOI] [PubMed] [Google Scholar]
- 27.George F. Androgen metabolism in the prostate of the finasteride-treated, adult rat: A possible explanation for the differential action of testosterone and 5 alpha-dihydrotestosterone during development of the male urogenital tract. Endocrinology. 1997;138:871–877. doi: 10.1210/endo.138.3.5009. [DOI] [PubMed] [Google Scholar]
- 28.Livak K. ABI Prism 7700 Sequence Detection System User Bulletin #2 Relative quantification of gene expression. ABI company publication. 1997 & 2001 [Google Scholar]
- 29.O’Malley KJ, Dhir R, Nelson JB, Bost J, Lin Y, Wang Z. The expression of androgen-responsive genes is up-regulated in the epithelia of benign prostatic hyperplasia. Prostate. 2009;69(16):1716–1723. doi: 10.1002/pros.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J, Coetzee GA. Prostate specific antigen gene regulation by androgen receptor. J Cell Biochem. 2004;93(2):233–241. doi: 10.1002/jcb.20228. [DOI] [PubMed] [Google Scholar]
- 31.Sedelaar JP, Isaacs JT. Tissue culture media supplemented with 10% fetal calf serum contains a castrate level of testosterone. Prostate. 2009;69(16):1724–1729. doi: 10.1002/pros.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]