Abstract
Murine sperm initiate fertilization by binding to the outer covering of the egg known as the murine zona pellucida (mZP). This binding is thought to require the interaction of O-glycans linked to a specific mZP glycoprotein (mZP3) with egg-binding proteins coating the sperm plasma membrane. The precise molecular basis of this interaction remains to be resolved. In this study, we analyzed the O-glycosylation of the individual mZP glycoproteins by using ultrasensitive MS methods. We found that the majority of the O-glycans that are linked to mZP3 are core type 2 sequences terminated with sialic acid, lacNAc (Galβ1-4GlcNAc), lacdiNAc (Gal-NAcβ1-4GlcNAc), Galα1-3Gal, and NeuAcα2-3[GalNAcβ1-4]Galβ1-4 (Sda antigen). Many of these terminal sequences have been implicated previously in murine sperm–egg binding. Core type 1 O-glycans are also present and are generally unmodified, although some are terminated with sialic acid, β-linked N-acetylhexosamine, or NeuAcα2-3[GalNAcβ1-4]Galβ1-4. Eggs expressing human ZP (huZP) glycoprotein huZP3, derived from transgenic mice, bind murine but not human sperm, implying that huZP3 acquires the same O-glycans as native mZP3. Sequencing of huZP3-associated O-glycans confirms that this implication is correct. The data obtained in this investigation may prove to be very useful for studies to determine the precise molecular basis of initial murine sperm–egg binding.
The initial binding of sperm to the egg is the first committed step in metazoan fertilization (1). Currently, there is an excellent understanding of the molecular events that are necessary for gamete binding in many lower species (1, 2). However, the molecular basis for this interaction in mammals is poorly understood despite a rather substantial research effort. The predominant model for mammalian sperm–egg binding is the mouse (3, 4). The murine zona pellucida (mZP) is a specialized extracellular matrix composed of three major glycoproteins (mZP1, mZP2, and mZP3). mZP1 plays a role in organizing this matrix (5). mZP3 is responsible for initial sperm–egg binding and the induction of the acrosome reaction (3, 4). mZP2 mediates the secondary binding to the inner acrosomal membrane exposed after the induction of the acrosome reaction (6). Gene-inactivation studies demonstrate clearly that the deletion of mZP2 (7) or mZP3 (8) abrogates fertility and leads either to the complete loss of the mZP or to a functionally inactive matrix. mZP1 contributes to fecundity but not to the formation of a functional mZP or to fertility (5).
The predominant model for murine gamete interaction implicates mZP3-associated O-glycans in the initial binding (3, 4). The precise mZP O-glycans and their complementary binding proteins on sperm, however, have not been determined unambiguously (9). An alternative hypothesis is that the protein sequence of mZP glycoproteins mediates sperm–egg binding (10). Thus, in this model, the taxon-specific binding of human sperm to their homologous eggs is due to differences in the protein structures of mZP3 and its human ZP (huZP) analogue, huZP3 (10).
To test this hypothesis, transgenic mice were created in which mZP3 was replaced with huZP3. If initial gamete binding were protein-mediated, such mice would bind human sperm (10). However, murine but not human sperm were found to bind to mouse eggs expressing huZP3 (10). In another study, a mouse line was established in which both mZP2 and mZP3 were replaced with their human homologues, huZP2 and huZP3 (11). Again, eggs from these mice were found to bind murine but not human sperm.
These results are consistent with the predominant model for initial murine gamete binding but only if huZP3 acquires the same O-glycans as mZP3, as outlined in a recent review (12). This acquisition would then enable huZP3 to bind murine but not human sperm to manifest taxon-specific binding. The primary goals of the current investigation were to analyze the O-glycosylation of native mZP glycoproteins and to determine whether mZP3 and huZP3 produced in mice express similar O-glycans.
Experimental Procedures
Materials. Flash-frozen mouse ovaries from 12- to 14-week-old wild-type mice were purchased from Harlan Bioproducts for Science (Indianapolis) and stored at –80°C. A colony of huZP3 rescue mice (kindly provided by Jurrien Dean, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda) was maintained at Harlan Bioproducts for Science. Ovaries were harvested from 12- to 14-week-old female mice, flash frozen, and stored at –80°C. All other chemicals and reagents were purchased from Sigma, unless stated otherwise.
Separation of ZP Glycoproteins. ZP from wild-type and huZP3 rescue mice was isolated as described (13). Native mZP glycoproteins were separated on a YMC-Pack Diol 300 column (Waters) as outlined (6, 14). Proteomic analysis confirmed the identity of the separated native mZP glycoproteins and the presence of huZP3 in the humanized mZP preparation (15).
Reductive Elimination. O-glycans were released by reductive elimination in 400 μl of sodium borohydride (38 mg/ml in 0.05 M sodium hydroxide) at 45°C for 16 h. Reactions were terminated by dropwise addition of glacial acetic acid, followed by Dowex 50W-X8 (H) 50–100 mesh (BDH, Poole, U.K.) chromatography and borate removal (16).
Matrix-Assisted Laser Desorption Ionization–Time-of-Flight (MALDI-TOF) Analysis. Glycans were permethylated by using the sodium hydroxide procedure and purified on a Sep-Pak C18 cartridge by using an acetonitrile gradient, as described (13). MALDI-TOF data were acquired by using a Voyager-DE STR mass spectrometer (PerSeptive Biosystems, Framingham, MA) in the reflectron mode with delayed extraction. Permethylated samples were dissolved in 10 μl of methanol, and 1 μl of dissolved sample was premixed with 1 μl of matrix/2,5-dihydroxybenzoic acid before loading onto a metal plate.
Collisionally Activated Nanoelectrospray Tandem MS (CAD ES-MS/MS) Analysis. Spectra were acquired by using quadrupole orthogonal acceleration TOF MS (Micromass, Manchester, U.K.) and Q-STAR (Applied Biosystems) mass spectrometers. The permethylated glycans were dissolved in methanol before loading into a spray capillary (Proxeon Biosystems, Odense, Denmark), coated with a thin layer of gold/palladium, in a final volume of 2 μl. A potential of 1.5 kV was applied to the nanoflow tip to produce a flow rate of 10–30 nl/min. The drying gas was N2, and the collision gas was Ar maintained at 10–4 millibars (1 bar = 100 kPa). Collision energies [30–90 eV (1 eV = 1.602 × 10–19 J)] varied depending on the size of the carbohydrate.
Results
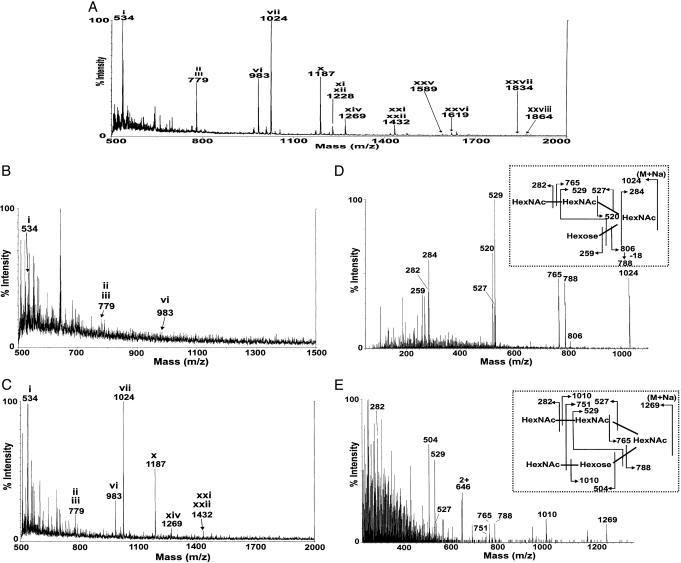
Definition of the O-Glycans Linked to mZP Glycoproteins. Initially, we performed MALDI-TOF MS analysis of the O-glycans associated with mZP (Fig. 1A). mZP was also separated to obtain mZP1, mZP2, and mZP3, as described (refs. 6 and 14 and data not shown). O-glycans associated with each glycoprotein were subjected to MALDI-TOF analysis. mZP1 gave no observable O-glycan signals, which was likely because of its low abundance (data not shown). mZP2 showed only traces of O-glycans (Fig. 1B), consistent with a previous study (15). The MALDI-TOF spectrum of mZP3 (Fig. 1C) confirmed that it is enriched in the same O-glycans as the total mZP matrix (Fig. 1 A). With the exception of m/z 1,024 and 1,269, which were shown by CAD ES-MS/MS analysis (Fig. 1 D and E) to have the sequences shown in Fig. 2 (vii and xiv), all molecular ions in Fig. 1 A and C are consistent with the mZP glycans reported previously when variations in sialylation levels are taken into account. Because the majority of the O-glycosylation is limited to mZP3, O-glycans observed in the global screens of humanized mZP could then be assigned confidently to huZP3.
Fig. 1.
MS analysis of O-glycans linked to native mZP, mZP2, and mZP3. Permethylated O-glycans released from mZP derived from 240 ovaries (A), mZP2 (B), and mZP3 (C) were subjected to MALDI-TOF analysis. Signals attributable to O-glycans are annotated with roman numerals, corresponding to structures shown in Fig. 2. D and E show the CAD ES-MS/MS spectra of m/z 1,024 and 1,269 (see Fig. 2, vii and xiv), which indicate the presence of the two HexNAc residues linked in tandem to the 6 position of core type 2 structures (for assignments, see Fig. 2 Insets).
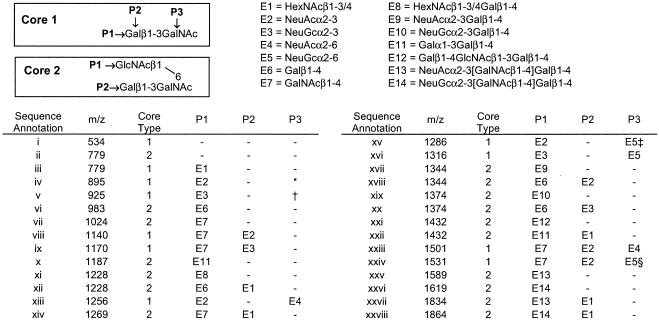
Fig. 2.
Sequence of O-glycans linked to mZP3 and huZP3. The O-glycans (roman numerals) are either core type 1 or 2 structures (boxes) that can be modified at the indicated positions (P1–P3), with different carbohydrate extensions (E1–E14). Structures are assigned based on data (13) complemented by the CAD ES-MS/MS experiments reported here and by applying the rules reported recently (35). A high level of sialylation was observed in a previous study (13), with structures iv and v (rather than structure i, as shown in this study) representing major components. Similarly, structure vi is the desialylated counterpart of structures xvii/xix and xviii/xx, as reported (13). The exact terminal N-acetylhexosamine in E1 and E8 has not yet been defined but is likely to be terminal GalNAc in β1-4 linkage arising from desialylation of the structures terminated with NeuAcα2-3[GalNAcβ1-4]Galβ1-4 (viii and ix). Possible structural isomers: *, E4 may be added at P3 instead of E2 at P1; †, E5 can be added at P3 instead of E3 at P1; ‡, E3 and E4 may be added at P1 and P3, respectively; §, E3 and E4 may be added at P2 and P3, respectively.
Analysis of the O-Glycans Linked to the Humanized mZP. The O-glycans identified by the MALDI-TOF analysis of humanized mZP (Fig. 3A) are identical to the O-glycans that are most abundant in mZP3 (Fig. 1C). CAD ES-MS/MS analysis of the molecular ions observed in the MALDI-TOF spectra was performed to confirm the sequences as shown (Fig. 3 B–E Insets). These sequences correspond to O-glycans identified in native mZP (Fig. 1 A), and each MALDI-TOF signal in Fig. 3A is annotated accordingly (Fig. 2).
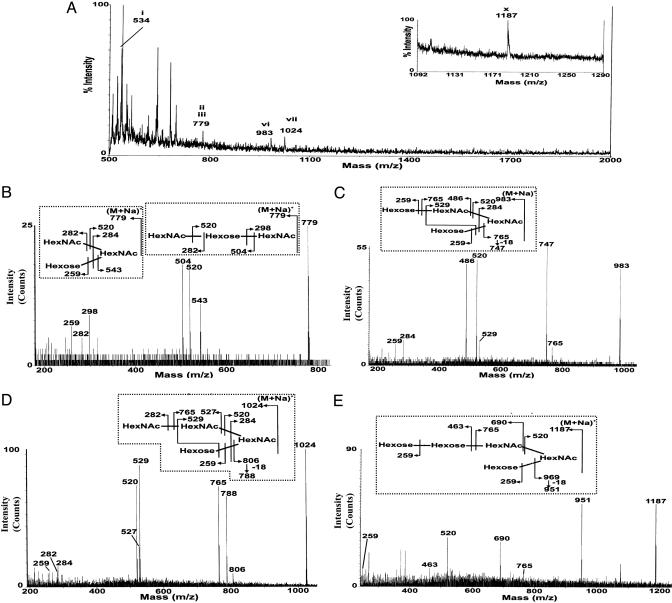
Fig. 3.
MS analysis of O-glycans derived from humanized mZP. O-glycans were isolated from humanized mZP derived from 100 ovaries, permethylated, and subjected to reverse-phase chromatography on Sep-Pak C18 cartridges, as described (13). The MALDI-TOF mass spectrum of the 35% aqueous acetonitrile fraction from the cartridge is shown in A. An additional signal (m/z 1187) corresponding to Hex3HexNAc2 was observed in the 50% aqueous acetonitrile fraction and is shown in the Inset to A. Signals attributable to O-glycans are annotated with roman numbers, as outlined in Fig. 2. The sequences of each of these components were confirmed by CAD ES-MS/MS. Data from these experiments are presented in B–E for m/z 779, 983, 1,024, and 1,187, respectively, and fragment ions are assigned on the structures shown in Insets.
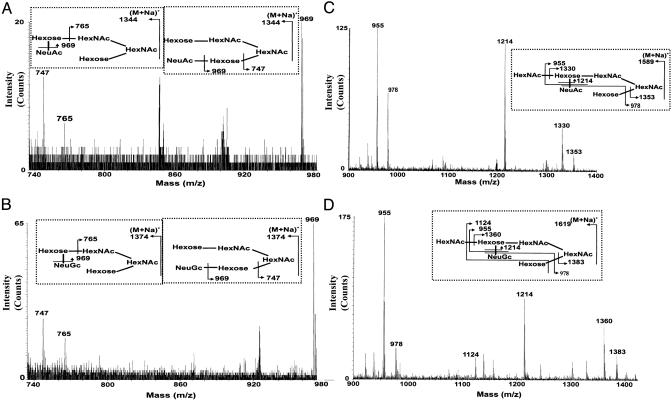
Minor O-Glycans in Humanized mZP Identified by CAD-ES-MS/MS. Our earlier studies (13) indicate that oligosaccharides larger than pentasaccharides are not readily detectable in MALDI-TOF screens of mZP-associated O-glycans derived from ≤200 ovaries. To determine whether the humanized mZP contained these larger glycans, we exploited the enhanced sensitivity of CAD ES-MS/MS on quadrupole orthogonal acceleration TOF MS-type instruments (17, 18). We had already identified the higher molecular mass components that are present in mZP (Fig. 1 A; ref. 13). Each of the m/z values obtained in our analysis of the humanized mZP O-glycans corresponding to molecular ions observed in the m/z 1,200–1,900 region of the mZP spectrum (Fig. 1 A) was selected for collisional activation studies. The m/z values for sialylated glycans were chosen because the loss of sialic acid is a favored cleavage in MS/MS experiments. Despite the very small quantity of material available for analysis and the absence of detectable molecular ions, our MS/MS studies were remarkably successful. Thus, spectra that were rich in fragment ions were obtained when m/z values corresponding to the sialylated components that were the most abundant in the native mZP (13) (m/z 1,344, 1,374, 1,589, and 1,619) were selected for collisional activation (Fig. 4). These MS/MS data confirmed the presence of glycans in humanized mZP (Fig. 2, xvii, xviii, xix, xx, xxv, and xxvi). We selected additional m/z values corresponding to minor sialylated components in mZP (m/z 1,140, 1,256, 1,316, 1,823, 1,834, and 1,864) (Fig. 1 A). In each case, we observed characteristic fragment ions resulting from the loss of sialic acid (data not shown), confirming that these very minor glycans are present also in humanized mZP. The nonsialylated components (m/z 1,228, 1,269, and 1,432) also afforded characteristic fragment ions (data not shown), confirming the presence of the glycans (Fig. 2, xi, xii, xiv, xxi, and xxii). Thus, both low- and high-mass glycans identified in native mZP are present also in the humanized mZP preparation.
Fig. 4.
CAD ES-MS/MS data from high-molecular-mass O-glycans derived from humanized mZP. Shown are fragment ion data obtained from analysis of several of the O-glycans in the remainder of the humanized sample of which the MALDI-TOF profile is shown in Fig. 3A. Data correspond to the ES-MS/MS analysis of m/z 1,344 (A), 1,374 (B), 1,589 (C), and 1,619 (D). Despite the absence of observable molecular ions, each analysis gave excellent MS/MS spectra, enabling the unambiguous assignment of the sequences shown in Insets. The roman-numeral annotations correspond to the O-glycan structures shown in Fig. 2.
Discussion
Sexual reproduction requires the binding of sperm to their homologous eggs. Biochemical definition of this interaction is more difficult in mammals because of the limited number of eggs available for study. To determine the potential carbohydrate-binding specificity of murine sperm–egg binding, many investigators used known glycan sequences as inhibitors in sperm–egg binding assays (4, 9, 19, 20). Although supportive evidence for carbohydrate mediation was acquired, the egg glycans that are necessary for binding were not identified. Gene inactivation and transgenesis studies in mice were used also to define the functional roles of mZP glycoproteins and to substitute huZP glycoproteins for their murine counterparts (21).
While these developments were being made, the MS methods for performing glycomic analysis were increasing steadily in their sensitivity and precision. We previously characterized both the N- and O-glycans linked to mZP from a relatively large number of mice by using fast atom bombardment MS strategies (13). However, these methods did not allow the characterization of the glycans from the individual mZP glycoproteins. We have now used ultrasensitive MS methods to study the O-glycans linked to individual mZP glycoproteins with limited amounts of starting material. The O-glycans were characterized first because of their proposed functional role in initial gamete binding (22, 23). We addressed another relevant issue by comparing the O-glycosylation of native mZP3 with huZP3 produced in transgenic mice (12).
Based on the current study, identical O-glycans are found on the whole native mZP and on mZP3. mZP2 carries few, if any, O-glycans, as established in ref. 15. Thus, mZP3 is the major carrier of O-glycans in mZP. However, some differences were observed between the current data and our earlier study (13). These differences are at the level of sialylation that may arise from the occurrence of desialylation during the handling and storage of samples (Fig. 2) and the identification of two previously unreported O-glycans (13). These previously uncharacterized O-glycans, present in both native and humanized mZP, have sequences consistent with core type 2 structures carrying a GalNAcβ1-4GlcNAc (lacdiNAc) sequence on the 6-arm (Fig. 2, vii and xiv). The lacdiNAc sequence is rare in mammalian glycoproteins and, when observed, is often sulfated (24). The sulfated analogue of structure vii (Fig. 3) is expressed also on bovine proopiomelanocortin (25). Therefore, the two major lacdiNAc-containing O-glycans are likely to be sulfated in native ZP, thus explaining their lack of detection in our previous study (13). Complete characterization of these lacdiNAc-containing O-glycans will be described elsewhere.
Terminal NeuGc and Galα1-3Gal sequences are not expressed on human glycoproteins because of the loss of functional modification enzymes (26, 27). However, huZP3 from rescue mice expresses these sequences, indicating acquisition of the mZP3-type glycosylation profile. This result suggests that mZP3 and huZP3 are modified by the same glycosyltransferases in the Golgi apparatus of mouse eggs.
The present study is enlightening as to the diversity of O-glycans in mZP3. Core type 1 sequences were primarily unmodified, although some were terminated with sialic acid or β1-3-linked HexNAc. Core type 2 sequences were terminated with sialic acid, Galβ1-4GlcNAc (lacNAc), lacdiNAc, Galα1-3Gal, and NeuAcα2-3[GalNAcβ1-4]Galβ1-4 (Sda antigen) sequences (13). All of these sequences except terminal lacdiNAc have been implicated either in the primary (9) or secondary (28) binding interaction.
It is very unlikely that sialylated mZP glycans participate in gamete binding, because desialylation of the mZP increases sperm binding by 30% in vitro (29). Terminal lacNAc sequences are likely to act as ligands because the digestion of murine eggs with a β-galactosidase that is highly specific for terminal β1-4-linked Gal residues inhibits murine sperm–egg binding by 70–75% (29). Artificial oligosaccharide constructs capped with lacNAc also inhibit murine sperm–egg binding by 75–80% (30). There is also other circumstantial evidence indicating that N- or O-linked oligosaccharides terminated with β1-6-linked lacNAc sequences mediate initial murine sperm–egg binding (13). A sperm surface β1-4-galactosyltransferase was also postulated to mediate initial sperm–egg binding by recognizing mZP3-associated O-glycans terminated with β-linked GlcNAc (23, 31). However, murine eggs bind three to four times as many sperm from transgenic mice lacking this specific β1-4-galactosyltransferase as they do sperm from wild-type mice (32). Oligosaccharide constructs capped with terminal β1-3 and -4 linked GlcNAc are also very poor inhibitors of murine sperm–egg binding (20, 30). Gene-inactivation experiments indicate that terminal Galα1-3Gal sequences are not required for murine sperm–egg binding (33), but constructs capped with this disaccharide inhibit murine sperm–egg binding in vitro, suggesting potential ligand redundancy in this binding interaction (20, 30). Antibodies directed against GalNAcβ1-4Gal sequences inhibit the secondary but not primary binding interaction (28), indicating the possible participation of NeuAcα2-3[GalNAcβ1-4]Galβ1-4. Therefore, substantial evidence supports a role for carbohydrate recognition in murine sperm–egg binding. We have now shown that huZP3 acquires mZP3-associated O-glycans when expressed in murine eggs, a finding consistent with the concept that the O-glycans represent the essential “functional groups” that confer taxon-specific binding (12).
Not all investigators share this point of view, however. Dean and colleagues (11) suggested recently that murine sperm–egg binding is mediated by the formation of a supramolecular complex of all three mZP glycoproteins. The accuracy of this model now rests on a major functional difference between native mZP and humanized mZP containing huZP2/huZP3. In native eggs, the cortical reaction releases a protease that cleaves mZP2, generating a 23-kDa peptide that remains in disulfide linkage to this glycoprotein (34). This cleavage is associated with the loss of native murine sperm binding activity to two cell stage embryos. huZP2 does not undergo this cleavage in eggs from the rescue mice, coinciding with continued sperm binding to the embryos (11).
Dean and colleagues (11) propose that this postfertilization binding should have been disrupted by the release of a cortical granule-associated N-acetylglucosaminidase if it were carbohydrate-dependent. However, this model is predicated on the unsupported concept that a sperm-specific β-galactosyltransferase mediates sperm–egg binding (23, 31). The presence of decreased ovulatory capacity in the huZP2/huZP3 rescue mice suggests that they could have other defects in their cortical granule enzymes or proteins (11). In addition, mZP2 is proposed to interact with the inner acrosomal membrane to mediate the tight sperm–mZP binding (3, 4). This secondary binding may (28) or may not (6) be carbohydrate-dependent. Therefore, the observation that sperm binding continues even after the cortical reaction in the huZP2/huZP3 rescue mice could be simply due to the fact that huZP2 does not undergo postfertilization cleavage, which is necessary to inhibit the secondary binding (12).
Therefore, the recent transgenic manipulations support the concept that initial murine sperm–egg binding is carbohydrate-dependent. The current analysis also highlights the diversity of O-glycosylation within the mZP, suggesting that redundant systems may be used in this crucial process required for species propagation. Attention should now shift to the egg-binding proteins. Is there one promiscuous egg-binding protein that can accommodate many different carbohydrate sequences, or do several egg-binding proteins exist, each with a restricted binding specificity that contributes to the overall affinity? Such questions may now be addressed because the mysteries of mZP glycosylation are finally being revealed. Future genetic manipulations, in combination with the ultrasensitive MS methods of carbohydrate sequencing used in this study, will be essential to confirm the role of carbohydrate recognition in mediating initial murine sperm–egg binding.
Acknowledgments
This work was supported by grants from the Biotechnology and Biological Sciences Research Council and the Wellcome Trust (to A.D. and H.R.M.), National Institutes of Health Grant HD 35652 (to G.F.C.), and grants from the Elsa U. Pardee Foundation (to M.S.P.). A.D. is a Biological Sciences Research Council Professorial Fellow, and S.C. was supported by a Biological Sciences Research Council Studentship.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CAD ES-MS/MS, collisionally activated nanoelectrospray tandem MS; huZP, human zona pellucida; lacdiNAc, GalNAcβ1-4GlcNAc; lacNAc, Galβ1-4GlcNAc; MALDI-TOF, matrix-assisted laser desorption ionization–time-of-flight; mZP, murine ZP.
References
- 1.Ohlendieck, K. & Lennarz, W. J. (1996) Curr. Top. Dev. Biol. 32, 39–58. [DOI] [PubMed] [Google Scholar]
- 2.Mengerink, K. J. & Vacquier, V. D. (2001) Glycobiology 11, 37R–43R. [DOI] [PubMed] [Google Scholar]
- 3.Wassarman, P. M. (1999) Cell 96, 175–183. [DOI] [PubMed] [Google Scholar]
- 4.Talbot, P., Shur, B. D. & Myles, D. G. (2003) Biol. Reprod. 68, 1–9. [DOI] [PubMed] [Google Scholar]
- 5.Rankin, T., Talbot, P., Lee, E. & Dean, J. (1999) Development (Cambridge, U.K.) 126, 3847–3855. [DOI] [PubMed] [Google Scholar]
- 6.Bleil, J. D., Greve, J. M. & Wassarman, P. M. (1988) Dev. Biol. 128, 376–385. [DOI] [PubMed] [Google Scholar]
- 7.Rankin, T. L., O'Brien, M., Lee, E., Wigglesworth, K., Eppig, J. & Dean, J. (2001) Development (Cambridge, U.K.) 128, 1119–1126. [DOI] [PubMed] [Google Scholar]
- 8.Rankin, T., Familari, M., Lee, E., Ginsberg, A., Dwyer, N., Blanchette-Mackie, J., Drago, J., Westphal, H. & Dean, J. (1996) Development (Cambridge, U.K.) 122, 2903–2910. [DOI] [PubMed] [Google Scholar]
- 9.Tulsiani, D. R. P., Yoshida-Komiya, H. & Araki, Y. (1997) Biol. Reprod. 57, 487–494. [DOI] [PubMed] [Google Scholar]
- 10.Rankin, T. L., Tong, Z. B., Castle, P. E., Lee, E., Gore-Langton, R., Nelson, L. M. & Dean, J. (1998) Development (Cambridge, U.K.) 125, 2415–2424. [DOI] [PubMed] [Google Scholar]
- 11.Rankin, T. L., Coleman, J. S., Epifano, O., Hoodbhoy, T., Turner, S. G., Castle, P. E., Gore-Langton, R. & Dean, J. (2003) Dev. Cell 5, 33–43. [DOI] [PubMed] [Google Scholar]
- 12.Jungnickel, M. K., Sutton, K. A. & Florman, H. M. (2003) Cell 114, 401–404. [DOI] [PubMed] [Google Scholar]
- 13.Easton, R. L., Patankar, M. S., Lattanzio, F. A., Leaven, T. H., Morris, H. R., Clark, G. F. & Dell, A. (2000) J. Biol. Chem. 275, 7731–7742. [DOI] [PubMed] [Google Scholar]
- 14.Nakano, M., Hatanaka, Y., Sawai, T., Kobayashi, N. & Tobita, T. (1987) Biochem. Int. 14, 417–423. [PubMed] [Google Scholar]
- 15.Boja, E. S., Hoodbhoy, T., Fales, H. M. & Dean, J. (2003) J. Biol. Chem. 278, 34189–34202. [DOI] [PubMed] [Google Scholar]
- 16.Besra, G. S., McNeil, M. R., Khoo, K. H., Dell, A., Morris, H. R. & Brennan, P. J. (1993) Biochemistry 32, 12705–12714. [DOI] [PubMed] [Google Scholar]
- 17.Morris, H. R., Paxton, T., Panico, M., McDowell, R. & Dell, A. (1997) J. Protein Chem. 16, 469–479. [DOI] [PubMed] [Google Scholar]
- 18.Morris, H. R., Paxton, T., Dell, A., Langhorne, J., Berg, M., Bordoli, R. S., Hoyes, J. & Bateman, R. H. (1996) Rapid. Commun. Mass Spectrom. 10, 889–896. [DOI] [PubMed] [Google Scholar]
- 19.Miller, D. J. & Ax, R. L. (1990) Mol. Reprod. Dev. 26, 184–198. [DOI] [PubMed] [Google Scholar]
- 20.Johnston, D. S., Wright, W. W., Shaper, J. H., Hokke, C. H., Van den Eijnden, D. H. & Joziasse, D. H. (1998) J. Biol. Chem. 273, 1888–1895. [DOI] [PubMed] [Google Scholar]
- 21.Castle, P. E. & Dean, J. (1999) Hum. Reprod. 14, 1927–1939. [DOI] [PubMed] [Google Scholar]
- 22.Florman, H. M. & Wassarman, P. M. (1985) Cell 41, 313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller, D. J., Macek, M. B. & Shur, B. D. (1992) Nature 357, 589–593. [DOI] [PubMed] [Google Scholar]
- 24.Green, E. D., van Halbeek, H., Boime, I. & Baenziger, J. U. (1985) J. Biol. Chem. 260, 15623–15630. [PubMed] [Google Scholar]
- 25.Siciliano, R. A., Morris, H. R., Bennett, H. P. & Dell, A. (1994) J. Biol. Chem. 269, 910–920. [PubMed] [Google Scholar]
- 26.Galili, U. & Swanson, K. (1991) Proc. Natl. Acad. Sci. USA 88, 7401–7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou, H. H., Hayakawa, T., Diaz, S., Krings, M., Indriati, E., Leakey, M., Paabo, S., Satta, Y., Takahata, N. & Varki, A. (2002) Proc. Natl. Acad. Sci. USA 99, 11736–11741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cahova, M. & Draber, P. (1992) J. Reprod. Immunol. 21, 241–256. [DOI] [PubMed] [Google Scholar]
- 29.Mori, E., Mori, T. & Takasaki, S. (1997) Biochem. Biophys. Res. Commun. 238, 95–99. [DOI] [PubMed] [Google Scholar]
- 30.Litscher, E. S., Juntunen, K., Seppo, A., Penttila, L., Niemela, R., Renkonen, O. & Wassarman, P. M. (1995) Biochemistry 34, 4662–4669. [DOI] [PubMed] [Google Scholar]
- 31.Shur, B. D. & Hall, N. G. (1982) J. Cell Biol. 95, 574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu, Q., Hasty, P. & Shur, B. D. (1997) Dev. Biol. 181, 257–267. [DOI] [PubMed] [Google Scholar]
- 33.Liu, D. Y., Baker, H. W., Pearse, M. J. & d'Apice, A. J. (1997) Mol. Hum. Reprod. 3, 1015–1016. [DOI] [PubMed] [Google Scholar]
- 34.Moller, C. C. & Wassarman, P. M. (1989) Dev. Biol. 132, 103–112. [DOI] [PubMed] [Google Scholar]
- 35.Wong, N. K., Easton, R. L., Panico, M., Sutton-Smith, M., Morrison, J. C., Lattanzio, F. A., Morris, H. R., Clark, G. F., Dell, A. & Patankar, M. S. (2003) J. Biol. Chem. 278, 28619–28635. [DOI] [PubMed] [Google Scholar]