Abstract
Oxidative compounds have been demonstrated to decrease the fertilization capability and viability of offspring of treated spermatozoa. As estrogen and its hydroxylated metabolites readily undergo redox cycling, this study was undertaken to determine if estrogens and other oxidants could damage DNA and impair sperm function. Sperm was preexposed to either 17β-estradiol (E2), 4-hydroxyestradiol (4OHE2) or the oxidant t-butyl hydroperoxide (t-BOOH), and allowed to fertilize untreated eggs. The fertilization rates and development of the larvae were assessed, as well as the amount of 8-oxodeoxyguanosine (8-oxodG) as an indication of oxidative DNA damage. All compounds caused significant decreases in fertilization and increases in pathological abnormalities in offspring, with 4OHE2 being the most toxic. Treatment with 4OHE2 caused a significant increase of 8-oxodG, but E2 failed to show any effect. Pathological abnormalities were significantly correlated (r2 = 0.44, p ≤ 0.05) with 8-oxodG levels in sperm treated with t-BOOH and 4OHE2, but not E2. 8-OxodG levels also were somewhat weakly correlated with impaired fertilization in 4OHE2-treated sperm (r2 = 0.33, p ≤ 0.05). The results indicate that biotransformation of E2 to 4OHE2 enhances oxidative damage of DNA in sperm, which can reduce fertilization and impair embryonic development, but other mechanisms of action may also contribute to these effects.
Keywords: Spermatozoa, DNA damage, Estradiol, Catechol estrogen, Reproduction, Echinoderm
1. Introduction
Estrogens have been linked with oxidative effects, including DNA oxidation, in a number of studies in mammals (Cavalieri et al., 2002; Wellejus et al., 2004; Wellejus and Loft, 2002) and fish (Rempel et al., 2008). Production of 2- and 4-hydroxy metabolites catalyzed primarily by cytochrome P450 1B1 (Lee et al., 2003) with subsequent transformation of 4-hydroxyestradiol (4OHE2) lead to the formation of semiquinones and quinones which through redox cycling can produce H2O2 and hydroxyl radicals that interact with DNA bases and form 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxodG). In addition, the quinone can also covalently bind to DNA through Michael addition (Zahid et al., 2006).
As the male germ cell develops it progressively loses its ability to protect itself from oxidative insult. Cellular differentiation is coupled with an increasing down-regulation of DNA repair mechanisms and a gradual loss of ability to undergo complete apoptosis (Lewis and Aitken, 2005). As spermatozoa are high in unsaturated fatty acids, they are particularly susceptible to lipid peroxidation. The effect is a decrease in the probability of successful fertilization and an increase in the probability of transmissible DNA damage upon exposure of spermatozoa to oxidizing agents.
Susceptibility to oxidative DNA damage can be influenced by the presence of antioxidants in seminal fluid as well as sperm, the structure of the DNA in spermatozoa, and the ability of the zygote to repair damaged DNA incorporated from the sperm (Ashwood-Smith and Edwards, 1996; Vernet et al., 2004; Sharma et al., 2004; Dietrich et al., 2005; Lewis and Aitken, 2005). If the DNA damage is too severe for repair, then the most likely result is embryo lethality, prior to the blastula stage (Ahmadi and Ng, 1999).
Oxidative agents have been shown to impact sperm function and viability in a number of studies. Duroquinone induced the production of reactive oxygen species (ROS) in the sperm of carp (Cyprinus carpio) causing DNA damage and lipid peroxidation, which were associated with decreases in sperm motility and hatching success (Zhou et al., 2006). UVA and UVB had significant impacts on sperm motility as well as fertilization by sea urchin (Anthocidaris crassipina) (Lu and Wu, 2005a) with an increase in ROS in the sperm related to the decrease in fertilization (Lu and Wu, 2005b).
The current study was undertaken to determine whether E2 and its metabolite 4OHE2 cause oxidative damage to spermatozoa in vitro, and impair their reproductive capability. Results from the exposure to E2 and 4OHE2 were compared to effects caused by the model oxidative compound, t-butyl hydroperoxide (t-BOOH) utilizing the echinoderm Dendraster excentricus, the sand dollar. Echinoderm fertilization and embryo development have been well studied, and widely used protocols exist for conducting exposures and scoring abnormalities, therefore echinoderms are useful model organisms. Sand dollars in particular were chosen because the study was conducted during their spawning season on the west coast of the United States, therefore they were the best source of quality echinoderm gametes. Endpoints included oxidative DNA damage, through measurement of 8-oxodG, fertilization and subsequent development of embryos.
2. Materials and methods
2.1. Source of chemicals
T-BOOH (70% solution) and E2 were purchased from Sigma-Aldrich, St. Louis, MO. 4OHE2 was purchased from Steraloids, Newport, RI.
2.2. Spawning of organisms
Sand dollars were collected by Marine Research and Educational Products (MREP, Carlsbad, CA) or NewFields Northwest, LLC (Port Gamble, WA) and were spawned within 24 h of receipt at the laboratory. Spawning, exposure of sperm, and development of embryos followed U.S. Environmental Protection Agency guidelines, with some modifications (Chapman et al., 1995a,b). Sand dollars were induced to spawn by injection through the oral cavity with chilled 0.5 M potassium chloride dissolved in artificial seawater. Eggs were harvested by inverting the sand dollars on beakers full of filtered (0.2 µm) artificial seawater at 15 ± 1 °C, salinity of 34 ± 1 ppt, and allowing the eggs to pool at the bottom of the beaker. Eggs were pooled from three to five females for use in the experiments. Concentrated sperm was collected directly from the surface of sand dollars submerged in a shallow volume of seawater at test temperature. The sperm was pooled from five to seven sand dollars, and held on ice. A separate batch of gametes was used for each exposure. Prior to the exposure a trial fertilization was performed to determine the minimum amount of spermper egg that would be required to achieve at least 90% fertilization. The trial was performed at egg:sperm ratios of 1:500, 1:1000, 1:2000, 1:3000 and 1:4000. Two hundred and fifty eggs were added to 10 mL of seawater. The appropriate amount of sperm was added and fertilization was allowed to take place for 10 min. After the exposure period, 0.5 mL of 5% buffered formalin in seawater was added to stop fertilization, and the first 100 eggs counted to determine the percentage fertilized. The trial fertilization resulted in approximately a 1:1500 egg:sperm ratio for the exposures.
2.3. Experimental design
Sperm, at a concentration of approximately 3750 cells/µl, was exposed to the toxicants in four replicates of 10 mL of filtered, artificial seawater in 20-mL glass scintillation vials. The t-BOOH nominal exposure concentrations were 0.1, 1, 10, 100, and 1000 mg/L plus a seawater control. The E2 and 4OHE2 nominal exposure concentrations were 0.001, 0.01, 0.1, 1, and 10 mg/L, plus a solvent control. The solvent used in the E2 and 4OHE2 exposures was ethanol at a maximum concentration of 0.05%. The exposure duration was 40 min and empirically determined through range finding studies.
After exposure 100 µl of each replicate of exposed sperm (approximately 375,000 cells) was added to a vial of 250 eggs in 10 mL of clean, filtered artificial seawater, resulting in a sperm:egg ratio of 1500:1. The sperm and egg mixtures were set up in duplicate. One set was allowed to fertilize for 20 min, then the fertilization was stopped with the addition of 0.5 mL of 10% buffered formalin in seawater. This set of eggs was used to determine the percent of eggs fertilized. The second set of eggs was allowed to develop for two days until >80% of controls reached the pluteus stage, then development was stopped by addition of 1 mL of 37% buffered formalin in seawater. This set of eggs was used to determine the effect of the exposure on the development of the larvae.
In this experiment we were interested in the effects of sperm exposure on the capabilities of sperm to fertilize unexposed eggs and produce normal larvae. Yet because of the test design, a small amount of toxicant was added to the eggs with the sperm resulting in a low exposure of eggs as well. To control against impacts on eggs as a confounding variable in the experimental design, a subset of eggs in 10 mL of seawater was fertilized with control sperm, but also had 100 µl of the highest toxicant concentration(s) added to mimic the incidental addition of compound that would have occurred if the eggs were fertilized with exposed sperm. Therefore, 100 µl of 1000 mg/L t-BOOH was added to eggs, resulting in a final exposure of 10 mg/L. One hundred µl of 10 mg/L of E2 was added to the eggs, resulting in a final exposure of 0.1 mg/L. And 100 µl of 1 or 10 mg/L of 4OHE2 was added to the eggs, resulting in a final exposure of 0.01 and 0.1 mg/L, respectively. This was done to determine if the low amount of toxicant added to the eggs during sperm addition was enough to affect fertilization and development.
All spawning, sperm exposures and egg fertilization and development tests were run in the same water bath. Temperature was held at 15 ± 1 °C, salinity was 35 ± 1 mg/L, dissolved oxygen was >90% saturation, and pH was 7.8 ± 0.3.
2.4. Egg fertilization and larval development endpoints
One hundred eggs from each replicate of the fertilization test were counted by placing the eggs on a Sedgwick-Rafter cell and examining them under 40× power with a compound microscope. The percentage of fertilized vs. unfertilized eggs was determined by the presence or absence of a fertilization membrane around the egg. All larvae from the development test were counted (usually about 250/vial), and the number of normal pluteus larvae, pathological larvae, and larvae with inhibited development were determined. Normal pluteus larvae have a roughly pyramid shape, with skeletal rods extending at least half the length of the larvae, post oral arms beginning to develop, and a lobed gut present. Pathological larvae are at the single or multi-cell stage either with (prehatch) or without (hatch) the fertilization membrane still present. Larvae with inhibited development may appear to be developing normally at the blastula, gastrula or prepluteus (prism) stage, but not at the pluteus stage (Chapman et al., 1995b). Examples of a normal pluteus larva and each type of abnormality are presented in Fig. 1. Abnormalities were presented as proportion of total larvae.
Fig. 1.
Normal pluteus larva (A), pathological larvae at the prehatched (B), and hatched (C) stages, and inhibited larvae at the blastula (D) gastrula (E) and prepluteus (F) stages.
2.5. 8-Oxo-7,8-dihydro-2'-deoxyguanosine analysis
From each exposure three concentrations were selected which represented the range of responses seen in the fertilization and development endpoints. Four replicates of sperm from each of the three concentrations selected plus a control were analyzed for 8-oxodG content. Techniques for isolation and analysis of 8-oxodG followed those outlined in Hong et al. (2006) and Rempel et al. (2008). Roughly 107 sperm cells from each replicate were centrifuged at 5.2×1000 g at 4 °C for two minutes. The resultant pellet was lysed and the DNA extracted with a Wako Chemicals (Richmond, VA) DNA Extractor WB Kit (sodium iodide method) following the kit instructions. The extracted DNA was vacuum-dried, then reconstituted in 100 µl of ddH2O prior to DNA digestion. All enzymes used in digestion of the DNA were purchased from U.S. Biological (Swampscott, MA). Ten µl of buffer (300 mM sodium acetate buffer, 10 mM zinc acetate, pH 5.0), 10 units of nuclease P1, and 0.01 units phosphodiesterase II were added to the DNA and the solution was incubated at 37 °C for 2 h. Twenty µl of a second buffer (500 mM Tris-HCl, pH 8.9) together with 0.05 units of phosphodiesterase I and 95 units of alkaline phosphatase were then added and the resulting solution was incubated for an additional 2 h at 37 °C. After enzymatic digestion the proteins were removed using a Millipore (Billerica, MA) Microcon® YM-10 centrifugal filter, 10 kDa molecular weight cut off. The filtrate was then vacuum dried then stored at −80 °C until enrichment.
8-oxodG in the nucleoside mixture was isolated chromatographically using a Shimadzu (Columbia, MD) HPLC equipped with a Waters (Milford, MA) Atlantis® dC18 column (5 µm, 4.6×250 mm). The nucleoside mixture was reconstituted in ddH2O. Then, 5 pmol of 15N labeled standard was added, and the solution injected into the HPLC. The flow rate was 0.5 ml/min. A gradient of 0–2% acetonitrile in 10 mM ammonium formate in 5 min, followed by 2–10% acetonitrile in 55 min, was employed. The fraction eluted between 44 and 48 min was collected and vacuum dried.
The enriched 8-oxodG was then reconstituted in ddH2O and analyzed via LC-MS/MS. A Zorbax SB-C18 column (Agilent, Santa Clara, CA), 5 µm in particle size, 0.5×150 mm, was used for separation of the enriched 8-oxodG solution utilizing an Agilent 1100 capillary HPLC pump, coupled to a Thermo Finnigan (San Jose, CA) LCQ Deca XP ion-trap mass spectrometer set up for monitoring the fragmentation of the [M+H]+ ions of the labeled and unlabeled 8-oxodG. A 60-minute gradient of 0–25% acetonitrile in 20 mM ammonium acetate was employed, at a flow rate of 8.0 µl/min. The estimated limit of detection was 0.125 pmol. The quantity of 2'-deoxyguanosine calculated from the peak area on the HPLC chromatogram during enrichment was used for normalization of 8-oxodG values.
2.6. Statistics
Proportional data (fertilization and abnormality) were Arcsin Square Root transformed before analysis. Data was analyzed for normality of distribution by Shapiro-Wilk's test, and for equality of variance by Bartlett's Test. If the data were normally distributed and had equal variance, a one-way ANOVA was initially performed with a post-hoc Dunnett's Test to determine significant difference from control (1 — tailed for proportion fertilized and pathological, 2 — tailed for 8-oxodG, α = 0.05). If the data had unequal variance and were not normally distributed, Steel's Many-One Rank Test was performed. A linear regression was used to calculate r2 to examine the relationship between percentages of fertilization, abnormal development, and 8-oxodG levels.
3. Results
3.1. Fertilization
Average fertilization was greater than 80% for all controls. All three compounds caused a significant decrease in the fertilization capability of the sand dollar sperm (Fig. 2, p<0.05). The decrease in the proportion of eggs fertilized was more gradual for the sperm treated with t-BOOH than those treated with E2 or 4OHE2. 4OHE2, with a no-observed-effect concentration (NOEC) of 0.01 mg/L, was the most toxic compound for this endpoint. E2 was the second most toxic with a NOEC of 1 mg/L and t-BOOH was the least toxic with a NOEC of 10 mg/L.
Fig. 2.
Fertilization success for sand dollar sperm exposed to A) t-butyl hydroperoxide, B) 17β-estradiol, and C) 4-hydroxyestradiol. Bars are S.E. Asterisk indicates significant difference from control (p<0.05).
Incidental egg exposure to t-BOOH and E2 had no effect on the fertilization endpoint (data not shown). There was a significant decrease in fertilization when eggs were exposed to treatments corresponding with 1 and 10 mg/L 4OHE2, with fertilization at 92% and 9%, respectively(p<0.05). As fertilization was 0% in both of these concentrations for exposed sperm, it was believed that most if not all of the toxicity demonstrated was due to effects on the sperm prior to addition to the eggs rather than effects on eggs.
3.2. Larval development
Average normal development was greater than 90% for all controls. All of the compounds caused a significant increase in the proportion of larvae with abnormal development, but had no effect on the proportion of larvae with inhibited growth (Fig. 3, p<0.05). No gut or skeletal abnormalities were observed. There was again a more gradual increase in the proportion of larvae with abnormal development from sperm exposed to t-BOOH than for sperm exposed to E2 or 4OHE2. 4OHE2 was again the most toxic compound for this endpoint, with a NOEC of 0.01 mg/L, the same NOEC as for the fertilization endpoint. E2 also had the same NOEC as the fertilization endpoint, 1 mg/L. The development endpoint was more sensitive to t-BOOH exposure than the fertilization endpoint, as the NOEC dropped from 10 mg/L to 0.1 mg/L.
Fig. 3.
Developmental abnormalities in eggs fertilized with sand dollar sperm exposed to A) t-butyl hydroperoxide, B) 17β-estradiol, and C) 4-hydroxyestradiol. Bars are S.E. Asterisk indicates significant difference from control (p<0.05).
Fig. 4 depicts the type of pathological abnormality that was prevalent in the high concentrations of each treatment. Hatched larvae with abnormal development were the predominant effect observed in the offspring derived from t-BOOH-treated sperm. Prehatched larvae with abnormal development were more common in the offspring of E2-treated sperm. 4OHE2 treatment resulted in effects at both stages, with the effects in hatched larvae predominating. Incidental exposure of eggs to t-BOOH and E2 had no effect on development (data not shown). The treatment corresponding to 10 mg/L for 4OHE2 caused an increase in larval abnormalities, but as there were no eggs fertilized at this concentration development was not affected. There was no significant difference in abnormalities in the treatment corresponding with 1 mg/L.
Fig. 4.
Pattern of pathological abnormalities in offspring of sperm treated with t-butyl hydroperoxide (t-BOOH), 17β-estradiol (E2), and 4-hydroxyestradiol (4OHE2). Bars are S.E.
3.3. 8-Oxo-7,8-dihydro-2'-deoxyguanosine
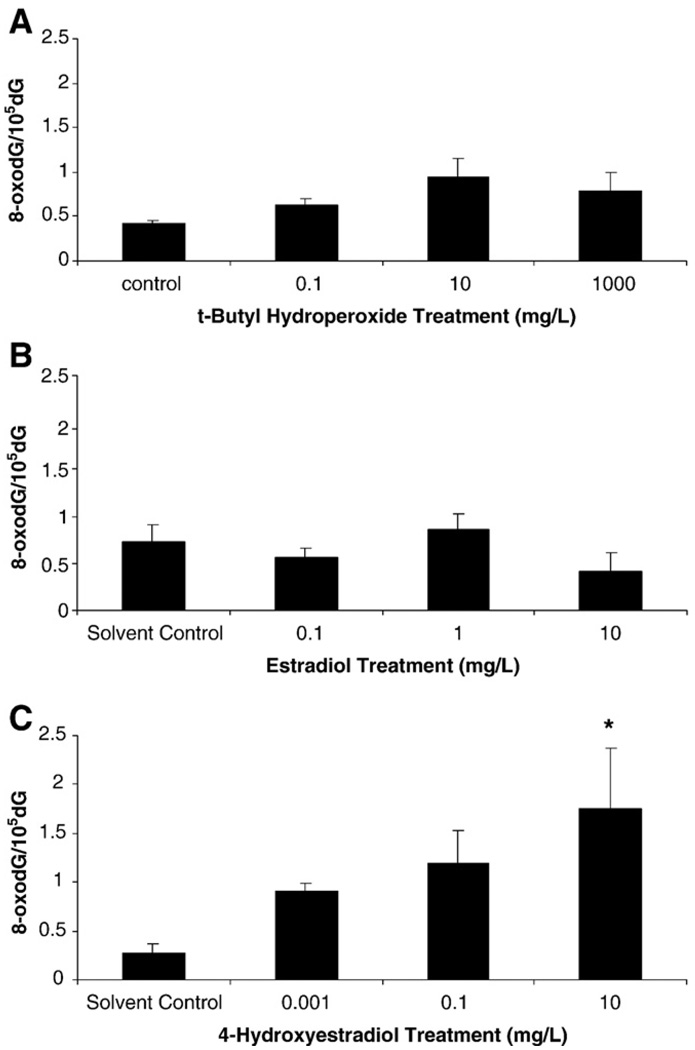
There was a slight but non-significant increase of 8-oxodG levels in sperm exposed to 0.1 and 10 mg/L t-BOOH (Fig. 5). There was no dose response in 8-oxodG levels in sperm exposed to E2. The strongest dose response was in sperm exposed to 4OHE2, with the 10 mg/L exposure being significantly different from the control (p<0.05).
Fig. 5.
8-oxo-7,8-dihydro-2'-deoxyguanosine in sand dollar sperm exposed to A) t-butyl hydroperoxide, B) 17β-estradiol, and C) 4-hydroxyestradiol. Bars are S.E. Asterisk indicates significant difference from control (p<0.05).
3.4. Correlations between 8-oxo-7,8-dihydro-2'-deoxyguanosine and the fertilization and development endpoints
Correlations between 8-oxodG levels in sperm and decreases in fertilization and development are presented in Table 1. There were no strong correlations between 8-oxodG levels and fertilization capability for sperm exposed to t-BOOH or E2, but there was a weak but significant correlation for 4OHE2. Even though there was a weak dose response for 8-oxodG in t-BOOH-exposed sperm, the strongest correlation between 8-oxodG levels and the proportion of pathological larvae was observed with the t-BOOH treatment. A weaker correlation between 8-oxodG levels and proportion of pathological larvae was observed in the 4OHE2 treatment, but there was no correlation in E2-exposed sperm.
Table 1.
Correlations of 8-oxo-7,8-dihydro-2'-deoxyguanosine with fertilization and abnormal larval development
| Treatment | Proportion fertilized | Proportion abnormal | ||
|---|---|---|---|---|
| r2 | Significance (p) | r2 | Significance (p) | |
| t-Butyl hydroperoxide | 0.10 | >0.05 | 0.76 | <0.001 |
| 17β-estradiol | 0.17 | >0.05 | 0.22 | >0.05 |
| 4-Hydroxyestradiol | 0.33 | <0.05 | 0.44 | <0.05 |
4. Discussion
Estrogens can have a negative impact on embryo development. Exposure of sea urchin (Strongylocentrotus purpuratus) zygotes to 17β-estradiol (E2) led to decreased cleavage and exogastrulation at a concentration of 0.5 mg/L (Mwatibo and Green, 1998). At a concentration of 1.0 mg/L E2 caused a total inhibition of cleavage in embryos. In a separate study with both S. purpuratus and Lytechinus anamesus concentrations up to 0.25 mg/L led to delayed and abnormal development in sea urchin embryos (Roepke et al., 2005). Although studies demonstrated effects of E2 exposure on embryos, the effects of estrogen exposure prior to fertilization and the relationship with oxidative damage on the reproductive capacity of sperm has not been well studied.
Reactive oxygen species (ROS) are necessary for the proper functioning of sperm in the fertilization process. Stimulation of H2O2 generation in human sperm is associated with the enhanced expression of phosphotyrosyl proteins, which are involved in the cascade of cellular events that eventually leads to fertilization of the egg (Aitken et al., 1995). There is a delicate balance achieved between the prooxidants produced in the sperm and the antioxidant defenses present in the cell and the seminal fluid that is necessary to maintain the proper function of the sperm. Therefore, it is not simply the presence of ROS in sperm that leads to deleterious effects, but the imbalance between pro- and antioxidants.
Exposure of DNA to t-BOOH causes single-strand breaks, and moderate increases in 8-oxodG (Latour et al., 1995). The effect of t-BOOH on 8-oxodG levels varies with concentration (more effective increase at lower concentrations) and cell type (Lazzé et al., 2003). T-BOOH undergoes decomposition in the presence of transition metals to produce tert-butyloxyl or tert-butylperoxyl radicals. Organic peroxyl radical can induce lesions in DNA that are substrates for the formadiopyrimidine glycosylase; however, the oxidation of dG by peroxyl radical does not give rise to 8-oxodG (Valentine et al., 1998). In addition, oxazolone and imidazolone were found to be the major products of guanine when DNA or dG was exposed to a chemically generated alkoxyl radical, and alkoxyl radical does not appear to generate 8-oxodG (Adam et al., 1998). Thus, this may explain the failure of t-BOOH to cause a significant increase in 8-oxodG. However, even though there was not a strong dose response, there was a fairly strong correlation between 8-oxodG levels in t-BOOH treated sperm and pathological abnormalities in offspring, indicating that the abnormalities were associated with oxidative damage to the sperm DNA. However, the effect on fertilization did not correlate with oxidative DNA damage suggesting other types of oxidative damage may have caused the decrease in fertility. Exposure of sea urchin (A. crassispina) sperm to ultraviolet light caused increases in ROS and lipid peroxidation, impaired mitochondrial function and caused concomitant decreases in sperm motility and membrane stability (Lu and Wu, 2005a,b). There were significant positive correlations between sperm mitochondrial function and motility and fertilization success. Therefore, it appears that ROS effects on mitochondria can directly affect fertilization (Lu and Wu, 2005a). Lipid peroxidation can also lead to a decrease in sperm membrane fluidity hampering motility, as well as ion leakage leading to a loss of membrane potential. As the events leading up to fertilization involve an influx of calcium followed by a loss of membrane potential (see below) the premature loss of membrane potential could lead to an impairment of fertilization. T-BOOH caused a loss of membrane potential in mitochondria in a mammalian cell line (Sestili et al., 1999). It also caused an increase in lipid peroxidation and decrease in cell membrane fluidity and membrane potential in hamster cells (Lapshina et al., 2005). Disruption of calcium homeostasis in rat lung epithelial cells has also been previously observed (Choi et al., 1997). Therefore t-BOOH could be adversely affecting fertilization through several mechanisms including effects on mitochondrial function, membrane stability and/or calcium homeostasis.
E2 had no effect on 8-oxodG levels, and 8-oxodG levels were not correlated with either fertilization or embryotoxicity. In order for oxidative damage to occur, E2 would need to be metabolized to 4OHE2 to form reactive metabolites. Although there is some cytochrome P450 (CYP) activity in sperm (Baker et al., 2004; Carreau et al., 2001) it may be that the specific CYP enzymes necessary for biotransformation of E2 to 4OHE2, such as CYP1B (Lee et al., 2003), are not active in sperm, or at least not in this species.
Despite the lack of effect on 8-oxodG levels, E2 still caused a decrease in fertilization and increase in embryotoxicity at high doses. As male germ cells mature, P450 aromatase (CYP19) activity increases, reaching the highest level in spermatozoa (Carreau et al., 2001). CYP19 is the enzyme responsible for the conversion of testosterone to E2, therefore the increase in activity implies a function for E2 in sperm. Both alpha and beta estrogen receptors have been found in sperm, as well as a smaller, membrane-bound receptor (Luconi et al., 2004; Luconi et al., 1999; Wu et al., 2001). The partial estrogen-receptor agonist, tamoxifen slightly reduced embryotoxicity in sea urchins, but the full estrogen receptor antagonist (ICI 182780) actually enhanced toxicity (Roepke et al., 2005). Tamoxifen has varied biological effects ranging from being a complete estrogen antagonist to an agonist, depending on dose, organ and sex in mammals. It exhibits estrogen agonist properties in testis, and does not inhibit E2 actions on spermatozoa in mammals (Balasinor et al., 2001; Luconi et al., 1999). Exposure of sea urchin (Paracentrotus lividus) sperm to tamoxifen decreased fertilization and increased deformities and developmental arrest in offspring at high doses (Pagano et al., 2001). Thus, the contribution of estrogen receptor activation to the adverse effects observed in sand dollar is unclear. Alternatively, E2 may modulate calcium levels in sperm affecting sperm mobility and subsequent fertilization processes. E2 causes a rapid sustained rise in intracellular calcium levels which interferes with the acrosome reaction (Baldi et al., 2000; Luconi et al., 1999).
While E2 was inactive with regard to oxidative DNA damage, 4OHE2 was significantly effective in generating 8-oxodG, which was significantly correlated with reduced fertilization and developmental abnormalities in offspring. Following the initial oxidation of E2 to 4OHE2 subsequent conversion of 4OHE2 to semiquinones and quinones can occur through additional CYP or peroxidase pathways. Although echinoderm sperm have peroxidase activity (Boldt et al., 1984), it is unclear whether CYP activity or expression is present.
Production of oxidative metabolites was confirmed by the dose response in 8-oxodG content in sperm. However, correlation analysis between oxidative DNA damage and impaired fertilization and development only explained 30–40% of the variance for 4OHE2, indicating alternative mechanisms may be involved. Formation of depurinating adducts of DNA by estradiol-3,4-quinone has been demonstrated and is believed to be linked to the production of tumors in hamsters (Zahid et al., 2006). Alternatively, like other catechol estrogens, 4OHE2 may bind critical proteins or deplete glutathione, leading indirectly to ROS formation and other forms of DNA damage (Yao et al., 2002). The effects of 4OHE2 on fertilization are consistent with the known oxidative effects of catechol estrogens, including lipid peroxidation and glutathione depletion (Markides et al., 1998; Yao et al., 2002). In addition, 4OHE2 has been shown to bind to estrogen receptors with relative binding affinities of 26 to 42%, relative to E2 (Schutze et al., 1994). 4-OHE2-specific binding proteins, separate from the classical nuclear estrogen receptors, have also been characterized and may provide unique estrogenic activity (Das et al., 1998; Markides and Liehr, 2005).
In conclusion, t-BOOH, E2 and 4OHE2 caused significant decreases in fertilization and significant increases in pathological abnormalities in offspring of treated sperm. Effects of t-BOOH on sperm were correlated with oxidative damage, although overall the magnitude of oxidative DNA damage was limited. Since there was no relationship between E2 and oxidative DNA damage, effects may have occurred through either receptor- or second messenger/signal transduction-based pathways. Oxidation of E2 to 4OHE2 clearly enhanced developmental toxicity through oxidative DNA damage and is an important step in the bioactivation of E2. However, additional pathways appear to contribute to impairment as well, and warrant additional study.
References
- Adam W, Grimm GN, Saha-Moeller CR, Dall'Acqua F, Miolo G, Vedaldi D. DNA damage by tert-butoxyl radicals generated in the photolysis of a water-soluble, DNA-binding peroxyester acting as a radical source. Chem Res Toxicol. 1998;11:1089–1097. doi: 10.1021/tx980089a. [DOI] [PubMed] [Google Scholar]
- Ahmadi A, Ng SC. Fertilizing ability of DNA-damaged spermatozoa. J Exp Zool. 1999;284:696–704. doi: 10.1002/(sici)1097-010x(19991101)284:6<696::aid-jez11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Paterson M, Fisher H, Buckingham DW, van Duin M. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci. 1995;108:2017–2025. doi: 10.1242/jcs.108.5.2017. [DOI] [PubMed] [Google Scholar]
- Ashwood-Smith MJ, Edwards RG. DNA repair by oocytes. Mol Hum Reprod. 1996;2:46–51. doi: 10.1093/molehr/2.1.46. [DOI] [PubMed] [Google Scholar]
- Baker MB, Krutskikh A, Curry BJ, McLaughlin EA, Aitken RJ. Identification of cytochrome P450-reductase as the enzyme responsible for NADPH-dependent lucigenin and tetrazolium salt reduction in rat epididymal sperm preparations. Biol Reprod. 2004;71:307–318. doi: 10.1095/biolreprod.104.027748. [DOI] [PubMed] [Google Scholar]
- Balasinor N, Parte P, Gill-Sharma MK, Juneja HS. Effect of tamoxifen on sperm fertilising ability and preimplantation embryo development. Mol Cell Endocrinol. 2001;178:199–206. doi: 10.1016/s0303-7207(01)00428-2. [DOI] [PubMed] [Google Scholar]
- Baldi E, Luconi M, Muratori M, Forti G. A novel function estrogen receptor on human sperm membrane interferes with progesterone effects. Mol Cell Endocrinol. 2000;161:31–35. doi: 10.1016/s0303-7207(99)00220-8. [DOI] [PubMed] [Google Scholar]
- Boldt J, Alliegro MC, Schuel H. A separate catalase and peroxidase in sea urchin sperm. Gamete Res. 1984;10:267–281. [Google Scholar]
- Carreau S, Bourguiba S, Lambard S, Galeraud-Denis I, Genissel C, Bilinska B, et al. Aromatase expression in male germ cells. Steroid Biochem Mol Biol. 2001;79:203–208. doi: 10.1016/s0960-0760(01)00137-6. [DOI] [PubMed] [Google Scholar]
- Cavalieri E, Devanesan P, Basland MC, Badawi AF, Rogan EG. Catechol estrogen metabolites and conjugates in different regions of the prostate of Noble rats treated with 4-hydroxyestradiol: implications for estrogen-induced initiation of prostate cancer. Carcinogenesis. 2002;23:329–333. doi: 10.1093/carcin/23.2.329. [DOI] [PubMed] [Google Scholar]
- Chapman GA, Denton D, Lazorchak JM. In: Short-term methods for estimating the chronic toxicity of effluents and receiving waters to West Coast marine and estuarine organisms. Agency EP, editor. 1995a. pp. 321–388. [Google Scholar]
- Chapman GA, Denton D, Lazorchak JM. In: Short-term methods for estimating the chronic toxicity of effluents and receiving waters to West Coast marine and estuarine organisms. Agency EP, editor. 1995b. pp. 389–465. [Google Scholar]
- Choi J, Liu RM, Forman HJ. Adaptation to oxidative stress: quinone-mediated protection of signaling in rat lung epithelial L2 cells. Biochem Pharmacol. 1997;53:987–993. doi: 10.1016/s0006-2952(96)00867-2. [DOI] [PubMed] [Google Scholar]
- Das SK, Tan J, Johnson DC, Dey SK. Differential spatiotemporal regulation of lactoferrin and progesterone receptor genes in the mouse uterus by primary estrogen, catechol estrogen, and xenoestrogen. Endocrinol. 1998;139:2905–2915. doi: 10.1210/endo.139.6.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich GJ, Szpyrka A, Wojtczak M, Dobosz S, Goryczko K, Żakowski Ł, et al. Effects of UV irradiation and hydrogen peroxide on DNA fragmentation motility, and fertilizing ability of rainbow trout (Oncorhynchus mykiss) spermatozoa. Theriogenology. 2005;64:1809–1822. doi: 10.1016/j.theriogenology.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Hong HZ, Cao HC, Wang YS. Identification and quantification of a guanine-thymine intrastrand cross-link lesion induced by Cu(II)/H2O2/ascorbate. Chem Res Toxicol. 2006;19:614–621. doi: 10.1021/tx060025x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapshina EA, Zavodnik IB, Labieniec M, Rękawieka K, Bryszewska M. Cytotoxic and genotoxic effects of tert-butyl hydroperoxide on Chinese hamster B14 cells. Mut Res. 2005;583:189–197. doi: 10.1016/j.mrgentox.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Latour I, Demoulin JB, Buc-Calderon P. Oxidative DNA damage by t-butyl hydroperoxide causes DNA single strand breaks which is not linked to cell lysis. A mechanistic study in freshly isolated rat hepatocytes. FEBS Lett. 1995;373:299–302. doi: 10.1016/0014-5793(95)01065-m. [DOI] [PubMed] [Google Scholar]
- Lazzé MC, Pizzala R, Savio M, Stivala LA, Prosperi E, Bianchi L. Anthocyanins protect against DNA damage induced by tert-butyl-hydroperoxide in rat smooth muscle and hepatoma cells. Mut Res. 2003;535:103–115. [PubMed] [Google Scholar]
- Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT. Characterization of the oxidative metabolites of 17B-estradiol and estrone formed by 15 selectively expressed human cytochrome P450 isoforms. Endocrinol. 2003;144:3382–3398. doi: 10.1210/en.2003-0192. [DOI] [PubMed] [Google Scholar]
- Lewis SEM, Aitken RJ. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 2005;322:33–41. doi: 10.1007/s00441-005-1097-5. [DOI] [PubMed] [Google Scholar]
- Lu XY, Wu RSS. Ultraviolet damages sperm mitochondrial function and membrane integrity in the sea urchin Anthocidaris crassispina. Ecotoxicol Environ Safety. 2005a;61:53–59. doi: 10.1016/j.ecoenv.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Lu XY, Wu RSS. UV induces reactive oxygen species, damages sperm, and impairs fertilisation in the sea urchin Anthocidaris crassispina. Mar Biol. 2005b;148:51–57. [Google Scholar]
- Luconi M, Muratori M, Forti G, Baldi E. Identification and characterisation of a novel functional estrogen receptor on human sperm membrane that interferes with progesterone effects. J Clin Endocrinol Metab. 1999;85:1670–1678. doi: 10.1210/jcem.84.5.5670. [DOI] [PubMed] [Google Scholar]
- Luconi M, Francavilla F, Porazzi I, Macerola B, Forti G, Baldi E. Human spermatozoa as a model for studying membrane receptors mediating rapid nongenomic effects of progesterone and estrogens. Steroids. 2004;69:553–559. doi: 10.1016/j.steroids.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Markides CS, Liehr JG. Specific binding of 4-hydroxyestradiol to mouse uterine protein: evidence of a physiological role for 4-hydroxyestradiol. J Endocrinol. 2005;185:235–242. doi: 10.1677/joe.1.06014. [DOI] [PubMed] [Google Scholar]
- Markides CS, Roy D, Liehr JG. Concentration dependence of prooxidant and antioxidant properties of catecholestrogens. Arch Biochem Biophys. 1998;360:105–112. doi: 10.1006/abbi.1998.0934. [DOI] [PubMed] [Google Scholar]
- Mwatibo JM, Green JD. Estradiol disrupts sea urchin embryogenesis differently from methoxychlor. Bull Environ Cont Toxicol. 1998;61:577–582. doi: 10.1007/pl00002974. [DOI] [PubMed] [Google Scholar]
- Pagano G, de Biase A, Deeva IB, Degan P, Doronin YK, Iaccarino M, et al. The role of oxidative stress in developmental and reproductive toxicity of tamoxifen. Life Sci. 2001;68:1735–1749. doi: 10.1016/s0024-3205(01)00969-9. [DOI] [PubMed] [Google Scholar]
- Rempel MA, Armstrong J, Hong HZ, Wang YS, Schlenk D. Uptake of estradiol from sediment by hornyhead turbot (Pleuronichthys verticalis) and effects on oxidative DNA damage in male gonads. Mar Environ Res. 2008;66:111–112. doi: 10.1016/j.marenvres.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roepke TA, Snyder MJ, Cherr GN. Estradiol and endocrine disrupting compounds adversely affect development of sea urchin embryos at environmentally relevant concentrations. Aquat Toxicol. 2005;71:155–173. doi: 10.1016/j.aquatox.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Schutze N, Vollmer G, Wunsche W, Grote A, Feit B, Knuppen R. Binding of 2-hydroxyestradiol and 4-hydroxyestradiol to the estrogen receptor of MCF-7 cells in cytosolic extracts and in nuclei of intact cells. Exper Clin Endocrinol. 1994;102:399–408. doi: 10.1055/s-0029-1211311. [DOI] [PubMed] [Google Scholar]
- Sestili P, Brambilla L, Cantoni O. Rotenone and pyruvate prevent the tert-butylhydroperoxide-induced necrosis of U937 cells and allow them to proliferate. FEBS Lett. 1999;457:139–143. doi: 10.1016/s0014-5793(99)01027-3. [DOI] [PubMed] [Google Scholar]
- Sharma RK, Said T, Agarwal A. Sperm DNA damage and its clinical relevance in assessing reproductive outcome. Asian J Androl. 2004;6:139–148. [PubMed] [Google Scholar]
- Valentine MR, Rodriguez H, Termini J. Mutagenesis by peroxy radical is dominated by transversions at deoxyguanosine: evidence for the lack of involvement of 8-oxo-dG1 and/or abasic site formation. Biochem. 1998;37:7030–7038. doi: 10.1021/bi973132m. [DOI] [PubMed] [Google Scholar]
- Vernet P, Aitken RJ, Drevet JR. Antioxidant strategies in the epididymis. Mol Cell Endocrinol. 2004;216:31–39. doi: 10.1016/j.mce.2003.10.069. [DOI] [PubMed] [Google Scholar]
- Wellejus A, Bornholdt J, Vogel UB, Risom L, Wiger R, Loft S. Cell-specific oxidative DNA damage induced by estrogen in rat testicular cells in vitro. Toxicol Lett. 2004;150:317–323. doi: 10.1016/j.toxlet.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Wellejus A, Loft S. Receptor-mediated ethinylestradiol-induced oxidative DNA damage in rat testicular cells. FASEB J. 2002;16:195–201. doi: 10.1096/fj.01-0385com. [DOI] [PubMed] [Google Scholar]
- Wu C, Patiño R, Davis KB, Chang X. Localization of estrogen receptor α and β RNA in germinal and nongerminal epithelia of the channel catfish testis. Gen Comp Endocrinol. 2001;124:12–20. doi: 10.1006/gcen.2001.7668. [DOI] [PubMed] [Google Scholar]
- Yao J, Chang M, Li Y, Pisha E, Liu X, Yao D, et al. Inhibition of cellular enzymes by equine catechol estrogens in human breast cancer cells: Specificity for glutathione S-transferase P1-1. Chem Res Toxicol. 2002;14:935–942. doi: 10.1021/tx020018i. [DOI] [PubMed] [Google Scholar]
- Zahid M, Kohli E, Saeed M, Rogan EG, Cavalieri EL. The greater reactivity of estradiol-3,4-quinone vs estradiol-2,3-quinone with DNA in the formation of depurinating adducts: implications for tumor-initiating activity. Chem Res Toxicol. 2006;19:164–172. doi: 10.1021/tx050229y. [DOI] [PubMed] [Google Scholar]
- Zhou B, Liu W, Siu WHL, O'Toole D, Lam PKS, Wu RSS. Exposure of spermatozoa to duroquinone may impair reproduction of the common carp (Cyprinus carpio) through oxidative stress. Aquat Toxicol. 2006;77:136–142. doi: 10.1016/j.aquatox.2005.11.006. [DOI] [PubMed] [Google Scholar]