Abstract
Lead (Pb2+) is a ubiquitous environmental neurotoxicant that continues to threaten public health on a global scale. Epidemiological studies have demonstrated detrimental effects of Pb2+ on childhood IQ at very low levels of exposure. Recently, a mechanistic understanding of how Pb2+ affects brain development has begun to emerge. The cognitive effects of Pb2+ exposure are believed to be mediated through its selective inhibition of the N-methyl-d-aspartate receptor (NMDAR). Studies in animal models of developmental Pb2+ exposure exhibit altered NMDAR subunit ontogeny and disruption of NMDAR-dependent intracellular signaling. Additional studies have reported that Pb2+ exposure inhibits presynaptic calcium (Ca2+) channels and affects presynaptic neurotransmission, but a mechanistic link between presynaptic and postsynaptic effects has been missing. Recent work has suggested that the presynaptic and postsynaptic effects of Pb2+ exposure are both due to inhibition of the NMDAR by Pb2+, and that the presynaptic effects of Pb2+ may be mediated by disruption of NMDAR activity-dependent signaling of brain-derived neurotrophic factor (BDNF). These findings provide the basis for the first working model to describe the effects of Pb2+ exposure on synaptic function. Here, we review the neurotoxic effects of Pb2+ exposure and discuss the known effects of Pb2+ exposure in light of these recent findings.
Keywords: Lead, Neurotoxicology, NMDA Receptor, BDNF, Synaptogenesis, Neurotransmission
Neurotoxicity of Low-Level Pb2+ Exposure in Children
The neurological effects of Pb2+ have been a driving factor in reducing the level of human Pb2+ exposure from anthropogenic and environmental sources. The first report of the effects of Pb2+ on the cognitive abilities and social behavior in children was in 1943 [1], however, the full implications of these effects were not appreciated until decades later. Studies from the late 1970s to the early 1990s showed effects of Pb2+ on cognitive abilities at progressively lower exposures [2]. In 1991, the CDC lowered the definition of Pb2+ intoxication from 25 µg/dL blood lead level (BLL) to 10 µg/dL BLL, which is the current regulatory level, motivated by evidence that children with BLLs of at least 10 µg/dL had impaired intellectual function [3]. More recently, studies have shown that the dose–response of Pb2+ on IQ in children is non-linear, with lower exposures of Pb2+ resulting in a greater rate of IQ loss than at higher exposures [4–7]. These studies indicate that the majority of the estimated IQ loss in Pb2+-exposed children occurs during the first 10 µg/dL of exposure, and suggest that Pb2+ may be a non-threshold neurotoxicant [4–8].
In addition to the cognitive deficits associated with Pb2+ exposure, children with elevated BLLs have behavioral deficits. Several studies have reported that school children with elevated BLLs are more likely to be disruptive in class, display anti-social behavior, and have attention problems [1, 9–12]. These behavioral effects appear to have an attention-deficit hyperactivity disorder (ADHD) phenotype; in fact, a recent study identified that childhood Pb2+ exposure was positively associated with ADHD diagnosis [13].
One of the most troubling aspects of Pb2+ effects in children is that the cognitive and behavioral effects of Pb2+ are irreversible. Chelation therapy, an intervention that is recommended for children with BLLs >45 µg/dL [14], can reduce the body burden of Pb2+. However, even though the level of Pb2+ is reduced, chelation therapy does not remediate the cognitive or behavioral deficits associated with childhood Pb2+ exposure [15, 16]. This highlights the possibility that Pb2+ exposure induces long-lasting (or permanent) changes in the brain during a critical period of development in childhood.
Perhaps due to these long-lasting changes in the brain, children exposed to Pb2+ experience persistent cognitive and behavioral deficits long after the cessation of Pb2+ exposure [17]. Prenatal Pb2+ exposure has been associated with anti-social and delinquent behavior as adolescents [18], and individuals who had elevated BLLs as children are more likely to be arrested during adolescence [19] and as adults [20]. This predilection towards violent and anti-social behavior is believed by some to underlie trends in violent crime both in the USA and internationally [21–23]. Furthermore, some studies have shown that children who experience elevated BLLs are more likely to have reductions in brain volume as adults [24]. These changes could account for altered behavior in adults exposed to Pb2+ during childhood. Thus, developmental Pb2+ exposure in humans results in long-lasting effects on cognition and behavior even after cessation of exposure that may be mediated by Pb2+-induced cellular changes in the brain.
Pb2+ Effects in the Brain
NMDA Receptor
The N-methyl-d-aspartate receptor (NMDAR) plays an essential role in hippocampus-mediated learning and memory, based on studies showing that intra-ventricular administration of an NMDAR antagonist (aminophosphonovaleric acid (APV)) in rats resulted in spatial learning impairments similar to those encountered with hippocampal lesions [25, 26]. Targeted knockout of the NMDAR in the hippocampus impairs spatial learning [27], lending further support to the role of the NMDAR in hippocampus-mediated learning processes. The major cellular mechanism within the hippocampus believed to be responsible for acquisition of new memories is long-term potentiation (LTP), a cellular phenomenon in which a long-lasting increase in synaptic efficacy follows brief, high-frequency stimulation [28, 29]. LTP is disrupted by inhibition of the NMDAR [28], and mice exhibiting hippocampal LTP deficits perform poorly in spatial memory tasks [28, 30]. Thus, impairment of the NMDAR has been shown to produce learning deficits on both the behavioral and cellular level.
The NMDAR is one of three main types of glutamatergic receptors in the mammalian brain [31] and is heavily expressed in the hippocampus and cerebral cortex [32, 33]. The NMDAR is composed of an obligatory NR1 subunit and one or more accessory subunits from the NR2 or NR3 families. The NR1 gene is alternatively spliced and specific splice variants of NR1 impart different pharmacological characteristics to the NMDAR [34]. The NR2 family consists of NR2A, NR2B, NR2C, and NR2D family members [32, 35, 36]. In the hippocampus, NR2A and NR2B are the most abundant NR2 family members. These two subunits exhibit differential developmental expression, with NR2B subunit expression levels high during fetal development and early postnatal life while NR2A subunit expression increases with postnatal maturation [32]. Besides exhibiting differential developmental expression, NR2A and NR2B subunits also have distinct intracellular protein associations [37] and signaling pathways [38–41], believed to be mediated through protein interactions with the C terminus. The different NR2 family members are linked to differential MAPK signaling [41], pro-death or pro-life signaling [38], and differential induction of nuclear gene expression [40]. Furthermore, NR2A-containing NMDARs (NR2A-NMDARs) are predominately located synaptically, while NR2B-containing NMDARs (NR2B-NMDARs) are expressed both synaptically and extrasynaptically [42]. Together, between NR1 splice variation and NR2 protein associations, NMDARs exhibit an exquisite degree of specialization and play essential roles in central synapses.
Pb2+ is a potent, non-competitive antagonist of the NMDAR [43–46], and has been shown to impair hippocampus-mediated learning in animal models of Pb2+ exposure [47–49]. It is believed to bind at the Zn2+ regulatory site of the NMDAR in a voltage-independent manner [50–52]. The NR2 subunits have different Zn2+ binding sites; the NR2A-NMDAR binds Zn2+ at a high-affinity site (nM affinity) while the NR2B-NMDAR binds Zn2+ with lower affinity (µM range) [45, 46, 53]. Recombinant NR2A- and NR2B-NMDARs containing mutated Zn2+ binding sites exhibit decreased affinity for Pb2+. The Pb2+ IC50 for wild type NR2A-NMDARs was reported to be 1.3 µM, while it increased to 11.3 µM in mutant NR2A-NMDARs which exhibit reduced Zn2+ sensitivity. Similarly, the Pb2+ IC50 of wild type NR2B-NMDARs was 1.2 µM but increased to 6.9 µM in mutant NR2B-NMDARs [50]. Furthermore, this study observed evidence of competitive inhibition of Pb2+ with the NR2A-NMDAR Zn2+ binding site, but did not observe competitive inhibition with the NR2B-NMDAR Zn2+ site [50]. While several studies also observed evidence of competitive inhibition of Pb2+ for the Zn2+ binding site, one study observed evidence of non-competitive inhibition. The work of Lasley and Gilbert used cortical preparations from adult rats to observed some evidence consistent with the above studies [54]. However, they also observed that in the presence of Zn2+, the IC50 of Pb2+ was decreased and the inhibition curve was shifted to the left, indicating non-competitive inhibition [54]. Thus, some disagreement regarding the site of Pb2+ action remains.
Regardless of the above disagreement there is other evidence that may support the hypothesis that Pb2+ interacts with the Zn2+ binding site. Since Zn2+ binds with very high affinity (nanomolar) at a regulatory site on the NR2A subunit [53], but with lower affinity to the NR2B subunit [45], this may indicate a preferential sensitivity of NR2A-NMDARs for Pb2+ [50, 52]. In support of this hypothesis, electrophysiological studies with recombinant receptors demonstrate that Pb2+ more potently inhibits NR2A-NMDARs (IC50=0.87 µM) than NR2B-NMDARs (IC50= 1.21 µM) [51, 55] or the tri-heteromeric form, NR1/NR2A/NR2B-NMDAR (IC50=6.1 µM) [55].
In addition to acting as an NMDAR antagonist, Pb2+ exposure also disrupts normal NMDAR ontogeny. Chronic developmental Pb2+ exposure results in decreased NR2A content in the hippocampus [56–59] and altered expression of NR1 splice variants [59–61]. In contrast, NR2B mRNA levels either remained unchanged or exhibited a slight increase in rats developmentally exposed to Pb2+ [56–59]. NR2B-specific radioligand binding is increased in the hippocampus and cerebral cortex of adults rats after developmental Pb2+ exposure [62]. Together, these data suggest that Pb2+ delays the developmental switch of increased NR2A incorporation with synapse maturation [62, 63]. Similar trends have also been observed in cultured neuron systems ([64] and Neal et al., unpublished data) and suggest that Pb2+ exposure may cause lasting changes in NMDAR subunit composition and expression. We hypothesize that long-term inhibition of the NMDAR by Pb2+ results in altered NMDAR targeting and expression. In support of this hypothesis, other paradigms of NMDAR inhibition have shown increased NR2B and decreased NR2A surface expression in response to decreased NMDAR activity [65, 66], which is very similar to what is observed during chronic or prolonged Pb2+ exposure.
Changes in NMDAR subunit composition can result in altered NMDAR-dependent signaling. As described above, many signaling pathways are dependent on NMDAR subunit composition and/or localization. Thus, Pb2+-induced alterations in NMDAR subunit composition could result in changes in downstream signaling. In support of this hypothesis, chronic developmental Pb2+ exposure results in altered MAPK signaling [67], calcium/calmodulin kinase II (CaMKII) activity [68], and altered cyclic AMP response element binding protein (CREB) phosphorylation status and binding affinity [62, 69]. CREB is a transcription factor for many immediate early genes (IEGs), which play an essential role in memory consolidation and are expressed as a result of NMDAR activity [70, 71]. Altered IEG expression in animals exposed to Pb2+ has been observed [72], indicating that altered CREB activity due to Pb2+-mediated disruption of NMDAR signaling may result in impaired cellular learning and memory processes.
Presynaptic Function
Chronic developmental Pb2+ exposure also results in impaired neurotransmission. In vivo, rats chronically exposed to low levels of Pb2+ have reduced Ca2+-dependent glutamate and γ-aminobutyric acid (GABA) release in the hippocampus [73–75]. In vitro, Pb2+ exposure impairs excitatory postsynaptic currents (EPSCs) and inhibitory postsynaptic currents (IPSCs) in cultured hippocampal neurons [76] and brain slices [75]. EPSCs and IPSCs are dependent upon neurotransmitter release from the presynaptic neuron, thus, reductions in EPSCs and IPSCs indicate a deficit in neurotransmission in both the glutamatergic and GABAergic systems as a result of Pb2+ exposure.
A recent study from our laboratory has shown that prolonged (5-day) Pb2+ exposure in cultured hippocampal neurons resulted in altered presynaptic protein expression and deficits in vesicular neurotransmitter release [77]. Pb2+ exposure reduced the expression of key presynaptic proteins involved in vesicular release, such as synaptophysin (Syn) and synaptobrevin (Syb). Reductions of vesicular release proteins were associated with both glutamatergic and GABAergic synapses, consistent with electrophysiological observations regarding EPSC and IPSC generation during Pb2+ exposure [75, 76]. Vesicular release in Pb2+-exposed neurons was significantly slower than under control conditions, as determined by live-imaging studies using the synaptic vesicle dye FM 1–43 [77]. These studies revealed that Pb2+-exposed neurons exhibited a specific loss of fast-releasing sites. Deficits in vesicular release were likely due to a reduction in presynaptic proteins involved in the release process, and not a reduction in the total vesicle pool, since there was no significant difference in FM 1–43 loading between control and Pb2+-treated neurons [77]. These findings are consistent with electrophysiological studies in which the effects of Pb2+ on IPSCs and EPSCs in cultured hippocampal neurons were not due to vesicle pool size, as treatment with 4-aminopyridine, an agent which forces exocytosis of the vesicle pool, abolished the effects of Pb2+ on postsynaptic currents [76]. Thus, similar effects of Pb2+ on neurotransmission have been observed in a range of studies using different techniques.
Several hypotheses for the effects of Pb2+ on neurotransmission have been suggested. One hypothesis is that Pb2+ interacts with presynaptic intracellular targets and has the ability to modulate presynaptic neurotransmission. Pb2+ has been shown to interact with synaptotagmin I (Syt) in vitro [78]. Syt is a Ca2+-sensing protein found in neurotransmitter vesicles and is responsible for promoting vesicular fusion in the presence of Ca2+ signaling [79]. Pb2+ bound Syt with 1000-fold higher affinity than Ca2+, which may prevent detection of Ca2+ signaling essential to neurotransmission [78]. Although Pb2+ exposure did not affect Syt protein expression in cultured hippocampal neurons [77], it is possible that Pb2+ may interfere with the Ca2+-sensing ability of Syt in neurons, thus masking the cellular signal for Ca2+-dependent vesicular release.
Pb2+ interactions with Syt may be related to the ability of Pb2+ to mimic Ca2+. Pb2+ has an ionic radius of 1.2 Å, which is similar to the ionic radius of Ca2+ (0.99 Å) [80, 81]. The positive charges and high electronegativity (2.33 on the Pauling scale) of Pb2+ may allow it to interact with the same residues on Ca2+ binding sites that interact with Ca2+ ions [81]. Pb2+ has been shown to interact with several neuronal intracellular Ca2+-binding proteins in addition to Syt (described above), such as the Ca2+-binding protein calmodulin (CaM) [80, 82, 83], the CaM/Ca2+-dependent phosphatase calcineurin [84], CaMKII [68], and protein kinase C [85–88], suggesting that Ca2+ mimicry may be a common characteristic of Pb2+ toxicity [89–91]. Thus, the ability of Pb2+ to mimic Ca2+ may interfere with normal synaptic signaling events.
Another hypothesis regarding the disruption of neurotransmission is that Pb2+ may interfere with Ca2+ signals by inhibiting Ca2+ channels [75, 76, 92]. Neurotransmission relies on the influx of Ca2+ from P/Q-, N-, and to some extent R-type voltage-gated Ca2+ channels (VGCCs) [93]. Pb2+ has been shown to inhibit VGCCs in recombinant systems with high affinity [92]. Furthermore, removal of extracellular Ca2+ resulted in identical effects on IPSC frequency as Pb2+ exposure, suggesting that the Pb2+-induced inhibition of IPSC frequency is via reduction of Ca2+ influx through VGCCs [75]. Inhibition of presynaptic VGCCs may prevent the necessary rise in internal Ca2+ required for fast, Ca2+-dependent vesicular release, thus interfering with neurotransmission.
Inhibition of presynaptic VGCCs by Pb2+ is not the only mechanism underlying the effects of chronic Pb2+ exposure on neurotransmitter release. Exposure to APV, an NMDAR antagonist that does not inhibit presynaptic VGCCs, resulted in nearly identical effects on presynaptic and postsynaptic proteins as Pb2+ exposure (Neal et al., unpublished data and [77]). This suggests that NMDAR inhibition independent of VGCC inhibition can mediate presynaptic changes during Pb2+ exposure.
Disruption of NMDAR Activity-Dependent BDNF Signaling by Pb2+
As an alternative hypothesis for the presynaptic effects of Pb2+ exposure, we have advanced the possibility that inhibition of synaptic NMDARs by Pb2+ results in altered NMDAR-dependent retrograde signaling, particularly of brain-derived neurotrophic factor (BDNF). We demonstrated that incubating hippocampal neurons with exogenous BDNF for the last 24 h of prolonged (5-day) Pb2+ exposure resulted in complete recovery of Pb2+-induced changes in presynaptic protein levels and vesicular neurotransmitter release [77]. This indicates that disruption of NMDAR-dependent retrograde signaling of BDNF may be impaired during Pb2+ exposure.
In developing neurons, the stabilization of functional presynaptic release sites is controlled by retrograde signals from the postsynaptic side [94–96]. One of these retrograde signals, BDNF, has been implicated in axon morphology, synaptic connectivity, and synaptic ultrastructure [97]. NMDAR activation can result in the generation and release of BDNF [98–100], which can be released from both axon and dendrite [101]. BDNF has been shown in live-imaging studies to be secreted postsynaptically from hippocampal neuron cultures when stimulated by NMDA [102]. This NMDAR-dependent release of BDNF may be essential to the generation or un-masking of presynaptic neurotransmitter release sites [98]. Interestingly, BDNF signaling can stimulate further glutamate release by increasing glutamate release probability [103], resulting in a positive feedback cycle.
In addition to exhibiting activity-dependent release, BDNF exhibits activity-dependent gene transcription. The BDNF gene is unique in that it contains eight different 5′ non-coding exons with separate promoters and one coding 3′ exon (exon IX) [104]. Differential transcription of promoters results in distinct splice variant transcripts, but all give rise to an identical BDNF protein. In particular, synaptic activity-dependent BDNF upregulation occurs after stimulation with depolarizing conditions, kainate treatment, and NMDAR activation [100, 105–108]. BDNF activity-dependent gene expression in hippocampal neurons has been linked to NR2A-NMDAR activation, and may be inhibited by NR2B-NMDAR activity [109].
BDNF signaling results in changes in gene expression of both pre- and postsynaptic proteins. Exogenous BDNF can increase the expression of Syn, Syt, and Syb in hippocampal slices [110], and may enhance NR2A but not NR2B subunit expression [111, 112]. Conversely, BDNF knockout mice exhibit reduced expression of the NR2A but not the NR2B subunit [113] and reduced Syn and Syb expression [114]. Therefore, it appears that NR2A-NMDARs may be preferentially linked to BDNF activating pathways and that BDNF can modulate presynaptic plasticity through changes in protein and/or gene expression.
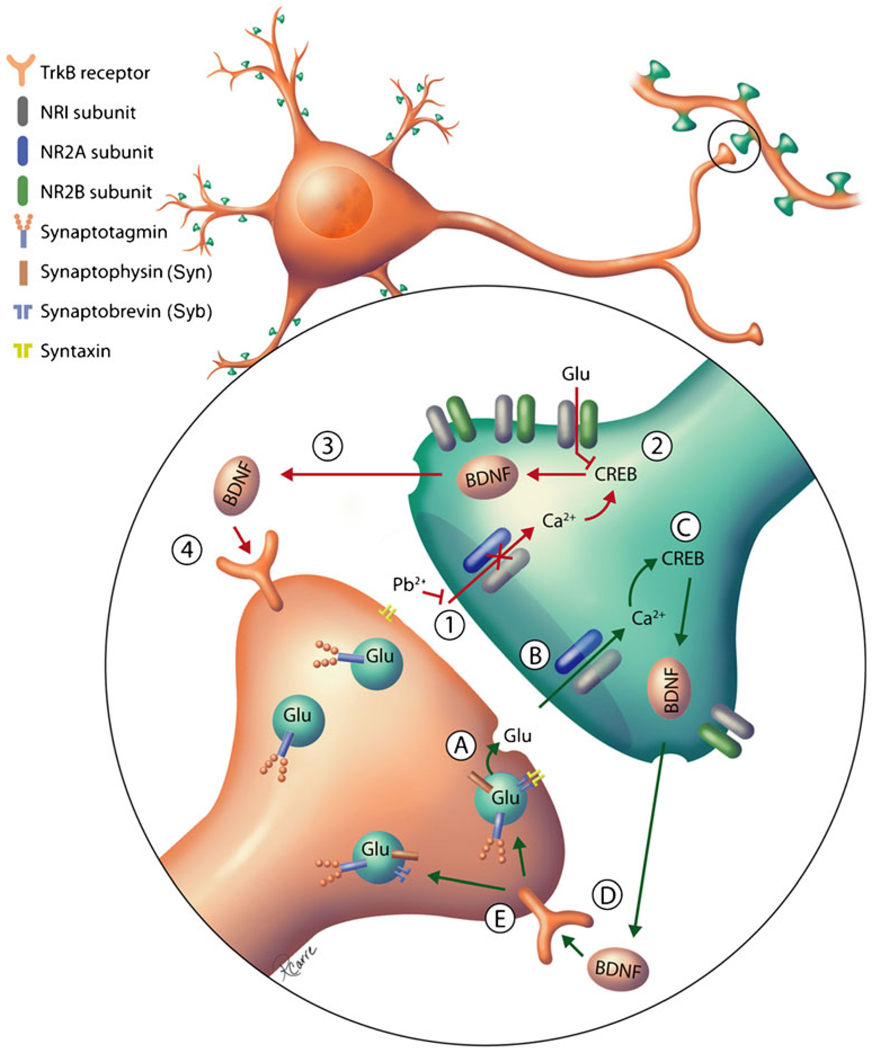
Pb2+ exposure during hippocampal neuron synapto-genesis decreases the number of synapses expressing NR2A-NMDARs (Neal et al., unpublished data), suggesting that the production and release of BDNF may be impaired. Indeed, Pb2+-exposed hippocampal neurons exhibit reduced proBDNF expression and BDNF release [77]. The functional outcome of disrupting BDNF signaling on NR2A and NR2B subunit expression as seen in BDNF knockout mice is similar to the phenotype observed in Pb2+-exposed hippocampal cultures (Neal et al., unpublished data) and in the hippocampus of developing animals exposed to Pb2+ [57, 62]. Furthermore, BDNF knockout animals exhibit deficits in vesicular release that can be alleviated by exogenous BDNF [114] in a similar manner to what has been observed with Pb2+ exposure [77]. Together, these data strongly suggest a role for the disruption of NMDAR activity-dependent BDNF signaling in the mechanism of Pb2+ toxicity in synapses. We have summarized the effects of Pb2+ on this signaling pathway in a working model of Pb2+ molecular neurotoxicity in Scheme 1.
Scheme 1.
Working model of molecular mechanism(s) by which chronic Pb2+ exposure disrupts synapse formation and plasticity in developing hippocampal neurons. Green arrows indicate normal processes; red arrows indicate Pb2+-impaired processes. In normal glutamatergic synapses, glutamatergic vesicles undergo Ca2+-dependent fusion with the plasma membrane in response to presynaptic signals (a). This process is mediated by the interaction with the v-SNARE Synaptobrevin and the t-SNAREs Syntaxin or SNAP-25. As a result of vesicular fusion, glutamate (Glu) is released from presynaptic terminals, resulting in NMDAR activation (b). Ca2+ enters the postsynaptic cell through synaptic NMDARs, activating downstream intracellular pathways. Under normal conditions, the predominant NMDARs at mature synapses are NR2A-NMDARs, which are linked to CREB phosphorylation (c). Activity-dependent CREB activation results in transcription of BDNF, which is transported and released in an activity-dependent retrograde fashion. Secreted BDNF interacts with the tropomyosin-related kinase receptor B (TrkB) (d). In the presynaptic neuron, BDNF activation of TrkB increases the number of docked vesicles and enhances vesicular release, as well as increases the incorporation of Syn and Syb into synaptic vesicles (e). During chronic Pb2+ exposure, however, Ca2+ influx through synaptic NMDAR is inhibited [1]. This causes a reduction in NR2A-NMDARs while NR2B-NMDARs are increased, possibly at extrasynaptic sites. As a result of reduced NR2A-NMDAR activation, NR2B-NMDARs are operational and are coupled to a CREB shut-off pathway. Reduced CREB signaling during Pb2+ exposure may result in decreased BDNF protein levels [2]. BDNF secretion is impaired during Pb2+ exposure [3], resulting in reduced activation of presynaptic TrkB receptors by BDNF [4]. Without positive feedback, there is reduced incorporation of synaptic vesicle proteins Syn and Syb and impaired vesicular release that can be rescued by exogenous addition of BDNF
Discussion
Until now, the postsynaptic and presynaptic effects of Pb2+ exposure have been hypothesized to occur through different mechanisms. Studies in animal models and hippocampal cultures have revealed that Pb2+ exposure results in altered NMDAR mRNA and protein expression, delaying or preventing the developmental switch from NR2B-NMDARs to NR2A-NMDARs [56, 57, 59, 62]. It has been hypothesized that these postsynaptic effects are due to dampened NMDAR activity, as Pb2+ is a potent NMDAR antagonist [43, 50, 52, 55] and activity-dependent alterations in NMDAR subunit trafficking and expression have been reported in other models of NMDAR inhibition [66, 115–117]. In addition to these postsynaptic effects, other studies have reported that both glutamatergic and GABAergic neurotransmission are impaired after Pb2+ exposure [73, 76]. These effects, for lack of a mechanistic link between the presynaptic and postsynaptic zones, have been postulated to be a result of inhibition of presynaptic Ca2+ channels. While Pb2+ does inhibit Ca2+ channels [92], loss of NMDAR-dependent retrograde signaling may be a viable alternate mechanism underlying the presynaptic effects of Pb2+ exposure [77].
This working model may provide insight to possible remediation of Pb2+ neurotoxicity. Children exposed to Pb2+ exhibit cognitive and behavioral deficits long after the cessation of elevated exposure [17] that are unresponsive to chelation therapy [15, 16]. In the laboratory setting, rats exposed to Pb2+ from conception to weaning exhibit impaired learning ability in adulthood [48]. However, placing Pb2+-exposed rats in an enriched environment completely mitigates the effects of Pb2+ [49]. A key finding from the latter study is that BDNF gene expression levels are increased in the enriched, Pb2+-exposed rats, suggesting that the reversal of learning deficits by the enriched environment may be due to upregulation of BDNF [49]. Thus, not only does our model suggest a mechanistic role for impaired retrograde signaling in Pb2+ toxicity mediated by NMDAR inhibition, it also suggests that the effects of Pb2+ exposure are at least partially reversible with interventions that result in increased BDNF levels.
NMDAR activity-dependent retrograde signaling appears to be a global mechanism governing central synapse development [98, 118, 119]. Thus, it is not surprising that other exposure paradigms involving NMDAR inhibition result in similar presynaptic and postsynaptic effects as observed with Pb2+ exposure. For example, ethanol, another developmental neurotoxicant that inhibits the NMDAR [120, 121], can cause similar effects on NMDAR targeting that have been observed with Pb2+ exposure. Neurons exposed to ethanol in chronic or intermittent exposure paradigms exhibit altered synaptic NMDAR targeting, with increased levels of NR2B-NDMARs [122, 123], suggesting a specific increase in NR2B-NMDARs similar to what has been observed with Pb2+ exposure in both cell and animal models ([62] and Neal et al., unpublished data). Furthermore, several other groups have reported effects on presynaptic neurotransmission after ethanol exposure [124]. Thus, it may be that disruption of NMDAR activity-dependent retrograde signaling is a general mechanism underlying neurodevelopmental disorders with an environmental etiology.
Acknowledgements
Work performed in the laboratory of TRG is funded by NIEHS grant ES006189. APN is currently funded by R01-ES03299.
Contributor Information
April P. Neal, Email: Nealap@msu.edu, Department of Pharmacology and Toxicology, Michigan State University, B307 Life Sciences Building, East Lansing, MI 48824, USA.
Tomás R. Guilarte, Email: trguilarte@columbia.edu, Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University, 600 Haven Avenue, B1-108, New York, NY 10032, USA.
References
- 1.Byers RK, Lord EE. Late effects of lead poisoning on mental development. Am J Dis Child. 1943;66:471–494. [Google Scholar]
- 2.Gilbert SG, Weiss B. A rationale for lowering the blood lead action level from 10 to 2 microg/dl. Neurotoxicology. 2006;27:693–701. doi: 10.1016/j.neuro.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Needleman HL, Gatsonis CA. Low-level lead exposure and the IQ of children. A meta-analysis of modern studies. JAMA. 1990;263:673–678. [PubMed] [Google Scholar]
- 4.Canfield RL, Henderson CR, Jr, Cory-Slechta D, Cox C, Jusko TA, Lanphear BP. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med. 2003;348:1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jusko TA, Henderson CR, Lanphear BP, Cory-Slechta DA, Parsons PJ, Canfield RL. Blood lead concentrations <10 microg/dl and child intelligence at 6 years of age. Environ Health Perspect. 2008;116:243–248. doi: 10.1289/ehp.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu H, Tellez-Rojo MM, Bellinger D, Smith D, Ettinger AS. Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development. Environ Health Perspect. 2006;114:1730–1735. doi: 10.1289/ehp.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miranda ML, Kim D, Galeano MA, Paul CJ, Hull AP, Morgan SP. The relationship between early childhood blood lead levels and performance on end-of-grade tests. Environ Health Perspect. 2007;115:1242–1247. doi: 10.1289/ehp.9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Needleman HL, Riess JA, Tobin MJ, Biesecker GE, Greenhouse JB. Bone lead levels and delinquent behavior. JAMA. 1996;275:363–369. [PubMed] [Google Scholar]
- 10.Leviton A, Bellinger D, Allred EH, Rabinowitz M, Needleman H, Schoenbaum S. Pre- and postnatal low-level lead exposure and children’s dysfunction in school. Environ Res. 1993;60:30–43. doi: 10.1006/enrs.1993.1003. [DOI] [PubMed] [Google Scholar]
- 11.Roy A, Bellinger D, Hu H, Schwartz J, Ettinger AS, Wright RO, Bouchard M, Palaniappan K, Balakrishnan K. Lead exposure and behavior among young children in Chennai, India. Environ Health Perspect. 2009;117:1607–1611. doi: 10.1289/ehp.0900625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellinger D, Leviton A, Allred E, Rabinowitz M. Pre- and postnatal lead exposure and behavior problems in school-aged children. Env Res. 1994;66:12–30. doi: 10.1006/enrs.1994.1041. [DOI] [PubMed] [Google Scholar]
- 13.Froehlich TE, Lanphear BP, Auinger P, Hornung R, Epstein JN, Braun J, Kahn RS. Association of tobacco and lead exposures with attention-deficit/hyperactivity disorder. Pediatrics. 2009;124:E1054–E1063. doi: 10.1542/peds.2009-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.United States Centers for Disease Control and Prevention. Managing elevated blood lead levels among young children: recommendations from the advisory committee on childhood lead poisoning prevention. Atlanta: CDC; 2002
- 15.Rogan WJ, Dietrich KN, Ware JH, Dockery DW, Salganik M, Radcliffe J, Jones RL, Ragan NB, Chisolm JJ, Rhoads GG. The effect of chelation therapy with succimer on neuropsyhological development in children exposed to lead. N Engl J Med. 2001;344:1421–1426. doi: 10.1056/NEJM200105103441902. [DOI] [PubMed] [Google Scholar]
- 16.Dietrich KN, Ware JH, Salganik M, Radcliffe J, Rogan WJ, Rhoads GG, Fay ME, Davoli CT, Denckla MB, Bornschein RL, Schwarz D, Dockery DW, Adubato S, Jones RL. Effect of chelation therapy on the neuropsychological and behavioral development of lead-exposed children after school entry. Pediatrics. 2004;114:19–26. doi: 10.1542/peds.114.1.19. [DOI] [PubMed] [Google Scholar]
- 17.White LD, Cory-Slechta DA, Gilbert ME, Tiffany-Castiglioni E, Zawia NH, Virgolini M, Rossi-George A, Lasley SM, Qian YC, Basha R. New and evolving concepts in the neuro-toxicology of lead. Toxicol Appl Pharmacol. 2007;225:1–27. doi: 10.1016/j.taap.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich KN, Ris MD, Succop PA, Berger OG, Bornschein RL. Early exposure to lead and juvenile delinquency. Neurotoxicol Teratol. 2001;23:511–518. doi: 10.1016/s0892-0362(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 19.Needleman HL, McFarland C, Ness RB, Fienberg SE, Tobin MJ. Bone lead levels in adjudicated delinquents. A case control study. Neurotoxicol Teratol. 2002;24:711–717. doi: 10.1016/s0892-0362(02)00269-6. [DOI] [PubMed] [Google Scholar]
- 20.Wright JP, Dietrich KN, Ris MD, Hornung RW, Wessel SD, Lanphear BP, Ho M, Rae MN. Association of prenatal and childhood blood lead concentrations with criminal arrests in early adulthood. PLoS Med. 2008;5:0732–0740. doi: 10.1371/journal.pmed.0050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nevin R. How lead exposure relates to temporal changes in IQ, violent crime, and unwed pregancy. Environ Res. 2000;83:1–22. doi: 10.1006/enrs.1999.4045. [DOI] [PubMed] [Google Scholar]
- 22.Nevin R. Understanding international crime trends: the legacy of preschool lead exposure. Environ Res. 2007;104:315–336. doi: 10.1016/j.envres.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Stretesky PB, Lynch MJ. The relationship between lead exposure and homicide. Arch Pediatr Adolesc Med. 2001;155:579–582. doi: 10.1001/archpedi.155.5.579. [DOI] [PubMed] [Google Scholar]
- 24.Cecil KM, Brubaker CJ, Adler CM, Dietrich KN, Altaye M, Egelhoff JC, Wessel S, Elangovan I, Hornung R, Jarvis K, Lanphear BP. Decreased brain volume in adults with childhood lead exposure. PLoS Med. 2008;5:0741–0749. doi: 10.1371/journal.pmed.0050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 26.Morris RGM, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 27.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 28.Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- 29.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 30.Chen C, Tonegawa S. Molecular genetic analysis of synaptic plasticity, activity-dependent neural development, learning, and memory in the mammalian brain. Annu Rev Neurosci. 1997;20:157–184. doi: 10.1146/annurev.neuro.20.1.157. [DOI] [PubMed] [Google Scholar]
- 31.Monaghan DT, Holets VR, Toy DW, Cotman CW. Anatomical distributions of four pharmacologically distinct 3H-l-glutamate binding sites. Nature. 1983;306:176–179. doi: 10.1038/306176a0. [DOI] [PubMed] [Google Scholar]
- 32.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 33.Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- 34.Durand GM, Gregor P, Zheng Z, Bennett MVL, Uhl GR, Zukin RS. Cloning of an apparent splice variant of the rat N-methyl-d-aspartate receptor NMDAR1 with altered sensitivity to polyamines and activators of protein kinase C. Proc Natl Acad Sci USA. 1992;89:9359–9363. doi: 10.1073/pnas.89.19.9359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seebur PH. Heteromeric NMDA receptors: Molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 36.Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M, Nakanishi S. Molecular characterization of the family of the N-methyl-d-aspartate receptor subunits. J Biol Chem. 1993;268:2836–2843. [PubMed] [Google Scholar]
- 37.Al Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ. NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci. 2007;27:8334–8343. doi: 10.1523/JNEUROSCI.2155-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soriano FX, Martel MA, Papadia S, Vaslin A, Baxter P, Rickman C, Forder J, Tymianski M, Duncan R, Aarts M, Clarke PGH, Wyllie DJA, Hardingham GE. Specific targeting of pro-death NMDA receptor signals with differing reliance on the NR2B PDZ ligand. J Neurosci. 2008;28:10696–10710. doi: 10.1523/JNEUROSCI.1207-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hardingham GE. 2B synaptic or extrasynaptic determines signaling from the NMDA receptor. J Physiol. 2006;572:614–615. doi: 10.1113/jphysiol.2006.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 41.Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A and NR2B containing NMDA receptors in RAS-ERD signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 42.Tovar K, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alkondon M, Alberto CS, Radhakrishnan V, Aronstam RS, Albuquerque EX. Selective blockade of NMDA-activated channel currents may be implicated in learning deficits caused by lead. FEBS Lett. 1990;261:124–130. doi: 10.1016/0014-5793(90)80652-y. [DOI] [PubMed] [Google Scholar]
- 44.Guilarte TR, Miceli RC. Age-dependent effects of lead on [3H]MK-801 binding to the NMDA receptor-gated ionophore: in vitro and in vivo studies. Neurosci Lett. 1992;148:27–30. doi: 10.1016/0304-3940(92)90796-a. [DOI] [PubMed] [Google Scholar]
- 45.Rachline J, Perin-Dureau F, Le Goff A, Neyton J, Paoletti P. The micromolar zinc-binding domain on the NMDA receptor subunit NR2B. J Neurosci. 2005;25:308–317. doi: 10.1523/JNEUROSCI.3967-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paoletti P, Perin-Dureau F, Fayyazuddin A, Le Goff A, Callebaut I, Neyton J. Molecular organization of a zinc binding N-terminal modulatory domain in a NMDA receptor subunit. Neuron. 2000;28:911–925. doi: 10.1016/s0896-6273(00)00163-x. [DOI] [PubMed] [Google Scholar]
- 47.Jett DA, Kuhlmann A, Guilarte TR. Intrahippocampal administration of lead (Pb) impairs performance of rats in the Morris water maze. Pharmacol Biochem Behav. 1996;57:263–269. doi: 10.1016/s0091-3057(96)00349-8. [DOI] [PubMed] [Google Scholar]
- 48.Kuhlmann AC, McGlothan JL, Guilarte TR. Developmental lead exposure causes spatial learning deficits in adult rats. Neurosci Lett. 1997;233:101–104. doi: 10.1016/s0304-3940(97)00633-2. [DOI] [PubMed] [Google Scholar]
- 49.Guilarte TR, Toscano CD, McGlothan JL, Weaver SA. Environmental enrichment reverses cognitive and molecular deficits induced by developmental lead exposure. Ann Neurol. 2003;53:50–56. doi: 10.1002/ana.10399. [DOI] [PubMed] [Google Scholar]
- 50.Gavazzo P, Zanardi I, Baranowska-Bosiacka I, Marchetti C. Molecular determinants of Pb2+ interaction with NMDA receptor channels. Neurochem Int. 2008;52:329–337. doi: 10.1016/j.neuint.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Yamada Y, Ujihara H, Sada H, Ban T. Pb2+ reduces the current from NMDA receptors expressed in Xenopus oocytes. FEBS Lett. 1995;377:390–392. doi: 10.1016/0014-5793(95)01402-0. [DOI] [PubMed] [Google Scholar]
- 52.Guilarte TR, Miceli RC, Jett DA. Biochemical evidence of an interaction of lead at the zinc allosteric sites of the NMDA receptor complex: effects of neuronal development. Neurotoxicology. 1995;16:63–71. [PubMed] [Google Scholar]
- 53.Fayyazuddin A, Villarroel A, Le Goff A, Lerma J, Neyton J. Four residues of the extracellular N-terminal domain of the NR2A subunit control high-affinity Zn2+ binding to NMDA receptors. Neuron. 2000;25:685–694. doi: 10.1016/s0896-6273(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 54.Lasley SM, Gilbert ME. Lead inhibits the rat N-methyl-d-aspartate receptor channel by binding to a site distinct from the zinc allosteric site. Toxicol Appl Pharmacol. 1999;159:224–233. doi: 10.1006/taap.1999.8743. [DOI] [PubMed] [Google Scholar]
- 55.Omelchenko IA, Nelson CS, Marino JL, Allen CN. The sensitivity of N-methyl-d-aspartate receptors to lead inhibition is dependent on the receptor subunit composition. J Pharmacol Exp Ther. 1996;278:15–20. [PubMed] [Google Scholar]
- 56.Nihei MK, Desmond NL, McGlothan JL, Kuhlmann AC, Guilarte TR. N-methyl-d-aspartate receptor subunit changes are associated with lead-induced deficits of long-term potentiation and spatial learning. Neuroscience. 2000;99:233–242. doi: 10.1016/s0306-4522(00)00192-5. [DOI] [PubMed] [Google Scholar]
- 57.Nihei MK, Guilarte TR. NMDAR-2A subunit protein expression is reduced in the hippocampus of rats exposed to Pb2+ during development. Mol Brain Res. 1999;66:42–49. doi: 10.1016/s0169-328x(99)00005-4. [DOI] [PubMed] [Google Scholar]
- 58.Guilarte TR, McGlothan JL. Hippocampal NMDA receptor mRNA undergoes subunit specific changes during developmental lead exposure. Brain Res. 1998;790:98–107. doi: 10.1016/s0006-8993(98)00054-7. [DOI] [PubMed] [Google Scholar]
- 59.Xy Z, Liu AP, Ruan DY, Liu J. Effect of developmental lead exposure on the expression of specific NMDA receptor subunit mRNAs in the hippocampus of neonatal rats by digoxigenin-labeled in situ hybridization histochemistry. Neurotoxicol Teratol. 2002;24:149–160. doi: 10.1016/s0892-0362(01)00210-0. [DOI] [PubMed] [Google Scholar]
- 60.Guilarte TR, McGlothan JL, Nihei MK. Hippocampal expression of N-methyl-d-aspartate receptor (NMDAR1) subunit splice variant mRNA is altered by developmental exposure to Pb2+ Mol Brain Res. 2000;76:299–305. doi: 10.1016/s0169-328x(00)00010-3. [DOI] [PubMed] [Google Scholar]
- 61.Guilarte TR, McGlothan JL. Selective decrease in NR1 subunit splice variant mRNA in the hippocampus of Pb2+-exposed rats: implications for synaptic targeting and cell surface expression of NMDAR complexes. Mol Brain Res. 2003;113:37–43. doi: 10.1016/s0169-328x(03)00083-4. [DOI] [PubMed] [Google Scholar]
- 62.Toscano CD, Hashemzadeh-Gargari H, McGlothan JL, Guilarte TR. Developmental Pb2+ exposure alters NMDAR subtypes and reduces CREB phosphorylation in the rat brain. Dev Brain Res. 2002;139:217–226. doi: 10.1016/s0165-3806(02)00569-2. [DOI] [PubMed] [Google Scholar]
- 63.Toscano CD, Guilarte TR. Lead neurotoxicity: from exposure to molecular effects. Brain Res Rev. 2005;49:529–555. doi: 10.1016/j.brainresrev.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 64.Xu SZ, Rajanna B. Glutamic acid reverses Pb2+-induced reductions of NMDAR receptor subunits in vitro. Neurotoxicology. 2006;27:169–175. doi: 10.1016/j.neuro.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 65.Chung HJ, Huang YH, Lau LF, Huganir RL. Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J Neurosci. 2004;24:10248–10259. doi: 10.1523/JNEUROSCI.0546-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee MC, Yasuda R, Ehlers MD. Metaplasticity at single glutamatergic synapses. Neuron. 2010;66:859–870. doi: 10.1016/j.neuron.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cordova FM, Rodrigues LS, Giocomelli MBO, Oliveira CS, Posser T, Dunkley PR, Leal RB. Lead stimulates ERK1/2 and p38MAPK phosphorylation in the hippocampus of immature rats. Brain Res. 2004;998:65–72. doi: 10.1016/j.brainres.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 68.Toscano CD, O’Callaghan JP, Guilarte TR. Calcium/calmodulin-dependent protein kinase II activity and expression are altered in the hippocampus of Pb2+-exposed rats. Brain Res. 2005;1044:51–58. doi: 10.1016/j.brainres.2005.02.076. [DOI] [PubMed] [Google Scholar]
- 69.Toscano CD, McGlothan JL, Guilarte TR. Lead exposure alters cyclic-AMP response element binding protein phosphorylation and binding activity in the developing rat brain. Dev Brain Res. 2003;145:219–228. doi: 10.1016/j.devbrainres.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 70.Athos J, Impey S, Pineda VV, Chen X, Storm DR. Hippocampal CRE-mediated gene expression is required for contextual memory formation. Nat Neurosci. 2002;5:1119–1120. doi: 10.1038/nn951. [DOI] [PubMed] [Google Scholar]
- 71.Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schultz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 72.Kim KA, Chakraborti T, Golstein G, Johnston M, Bressler J. Exposure to lead elevates induction of zif268 and ARC mRNA in rats after electroconvulsive shock: the involvement of protein kinase C. J Neurosci Res. 2002;69:268–277. doi: 10.1002/jnr.10296. [DOI] [PubMed] [Google Scholar]
- 73.Lasley SM, Gilbert ME. Rat hippocampal glutamate and GABA release exhibit biphasic effects as a function of chronic lead exposure level. Toxicol Sci. 2002;66:139–147. doi: 10.1093/toxsci/66.1.139. [DOI] [PubMed] [Google Scholar]
- 74.Lasley SM, Gilbert ME. Presynaptic glutamatergic function in dentate gyrus in vivo is diminished by chronic exposure to inorganic lead. Brain Res. 1996;736:125–134. doi: 10.1016/0006-8993(96)00666-x. [DOI] [PubMed] [Google Scholar]
- 75.Xiao C, Gu Y, Zhou CY, Wang L, Zhang MM, Ruan DY. Pb2+ impairs GABAergic synaptic transmission in rat hippocampal slices: a possible involvement of presynaptic calcium channels. Brain Res. 2006;1088:93–100. doi: 10.1016/j.brainres.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 76.Braga MFM, Pereira EFR, Albuquerque EX. Nanomolar concentrations of lead inhibit glutamatergic and GABAergic transmission in hippocampal neurons. Brain Res. 1999;826:22–34. doi: 10.1016/s0006-8993(99)01194-4. [DOI] [PubMed] [Google Scholar]
- 77.Neal AP, Stansfield KH, Worley PF, Thompson RE, Guilarte TR. Lead exposure during synaptogenesis alters vesicular proteins and impairs vesicular release: potential role of NMDA receptor-dependent BDNF signaling. Toxicol Sci. 2010;116:249–263. doi: 10.1093/toxsci/kfq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bouton CMLS, Frelin LP, Forde CE, Godwin HA, Pevsner J. Synaptotagmin i is a molecular target for lead. J Neurochem. 2001;76:1724–1735. doi: 10.1046/j.1471-4159.2001.00168.x. [DOI] [PubMed] [Google Scholar]
- 79.Chicka MC, Hui E, Lui H, Chapman ER. Synaptotagmin arrests the snare complex before triggering fast, efficient membrane fusion in response to Ca2+ Nat Struct Mol Biol. 2008;15:827–835. doi: 10.1038/nsmb.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chao SH, Suzuki Y, Zysk JR, Cheung WY. Activation of calmodulin by various metal cations as a function of ionic radius. Mol Pharmacol. 1984;26:75–82. [PubMed] [Google Scholar]
- 81.Garza A, Vega R, Soto E. Cellular mechanisms of lead neurotoxicity. Med Sci Monit. 2006;12:RA57–RA65. [PubMed] [Google Scholar]
- 82.Habermann E, Crowell K, Janicki P. Lead and other metals can substitute for Ca2+ in calmodulin. Arch Toxicol. 1983;54:61–70. doi: 10.1007/BF00277816. [DOI] [PubMed] [Google Scholar]
- 83.Kern M, Wisniewski M, Cabell L, Audesirk G. Inorganic lead and calcium interact positively in activation of calmodulin. Neurotoxicology. 2000;3:353–363. [PubMed] [Google Scholar]
- 84.Kern M, Audesirk G. Stimulatory and inhibitory effects of inorganic lead on calcineurin. Toxicology. 2000;150:171–178. doi: 10.1016/s0300-483x(00)00258-4. [DOI] [PubMed] [Google Scholar]
- 85.Simons TJB. Lead-calcium interactions in cellular lead toxicity. Neurotoxicology. 1993;14:77–86. [PubMed] [Google Scholar]
- 86.Sun X, Tian X, Tomsig JL, Suszkiw JB. Analysis of differential effects of Pb2+ on protein kinase C isozymes. Toxicol Appl Pharmacol. 1999;156:40–45. doi: 10.1006/taap.1999.8622. [DOI] [PubMed] [Google Scholar]
- 87.Toscano CD, Schanne FAX. Lead-induced activation of protein kinase C in rat brain cortical synaptosomes. Ann NY Acad Sci. 2000;919:307–311. doi: 10.1111/j.1749-6632.2000.tb06892.x. [DOI] [PubMed] [Google Scholar]
- 88.Long GJ, Rosen JF, Schanne FAX. Lead activation of protein kinase C from rat brain. J Biol Chem. 1994;269:834–837. [PubMed] [Google Scholar]
- 89.Bressler J, Kim KA, Chakraborti T, Goldstein G. Molecular mechanisms of lead neurotoxicity. Neurochem Res. 1999;24:595–600. doi: 10.1023/a:1022596115897. [DOI] [PubMed] [Google Scholar]
- 90.Marchetti C. Molecular targets of lead in brain neurotoxicity. Neurotox Res. 2003;5:221–236. doi: 10.1007/BF03033142. [DOI] [PubMed] [Google Scholar]
- 91.Richardt G, Federolf G, Habermann E. Affinity of heavy metal ions to intracellular Ca2+-binding proteins. Biochem Pharmacol. 1986;35:1331–1335. doi: 10.1016/0006-2952(86)90278-9. [DOI] [PubMed] [Google Scholar]
- 92.Peng S, Hajela RK, Atchison WD. Characteristics of block by Pb2+ of function of human neuronal L-, N-, and R-type Ca2+ channels transiently expressed in human embryonic kidney 293 cells. Mol Pharmacol. 2002;62:1418–1430. doi: 10.1124/mol.62.6.1418. [DOI] [PubMed] [Google Scholar]
- 93.Xu J, He L, Wu LG. Role of Ca2+ channels in short-term synaptic plasticity. Curr Opin Neurobiol. 2007;17:352–359. doi: 10.1016/j.conb.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 94.Bacci A, Coco S, Pravettoni E, Schenk U, Armano S, Frassoni C, Verderio C, De Camilli P, Matteoli M. Chronic blockade of glutamate receptors enhances presynaptic release and downregulates the interaction between synaptophysin-synaptobrevin-vesicle-associated-membrane protein 2. J Neurosci. 2001;21:6588–6596. doi: 10.1523/JNEUROSCI.21-17-06588.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Verderio C, Coco S, Pravettoni E, Bacci A, Matteoli M. Synaptogenesis in hippocampal cultures. Cell Mol Life Sci. 1999;55:1448–1462. doi: 10.1007/s000180050384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fitzsimonds RM, Poo MM. Retrograde signaling in the development and modification of synapses. Physiol Rev. 1998;78:143–170. doi: 10.1152/physrev.1998.78.1.143. [DOI] [PubMed] [Google Scholar]
- 97.Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Nev Neurobiol. 2010;70:271–288. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Walz C, Jungling KL, Gottmann K. Presynaptic plasticity in an immature neocortical network requires NMDA receptor activation and BDNF release. J Neurophysiol. 2006;96:3512–3516. doi: 10.1152/jn.00018.2006. [DOI] [PubMed] [Google Scholar]
- 99.Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J. 2001;20:5887–5897. doi: 10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jiang X, Tian F, Mearow K, Okagaki P, Lipsky RH, Marini AM. The excitoprotective effect of N-methyl-d-aspartate receptors is mediated by a brain-derived neurotrophic factor autocrine loop in cultured hippocampal neurons. J Neurochem. 2005;94:713–722. doi: 10.1111/j.1471-4159.2005.03200.x. [DOI] [PubMed] [Google Scholar]
- 101.Matsuda N, Lu H, Fukata Y, Noritake J, Gao H, Mukherjee S, Nemoto T, Fukata M, Mm P. Differential activity-dependent secretion of brain-derived neurotrophic factor from axon to dendrite. J Neurosci. 2009;29:14185–14198. doi: 10.1523/JNEUROSCI.1863-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kolarow R, Brigadski T, Lessmann V. Postsynaptic secretion of BDNF and NT-3 from hippocampal neurons depends on calcium-calmodulin kinase II signaling and proceeds via delayed fusion pore opening. J Neurosci. 2007;27:10350–10364. doi: 10.1523/JNEUROSCI.0692-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Madara JC, Levine ES. Presynaptic and postsynaptic NMDA receptors mediate distinct effects of brain-derived neurotrophic factor on synaptic transmission. J Neurosci. 2008;100:3175–3184. doi: 10.1152/jn.90880.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vanhoutte P, Bading H. Opposing roles of synaptic and extrasynaptic NMDA receptors in neuronal calcium signaling and BDNF gene regulation. Curr Opin Neurobiol. 2003;13:366–371. doi: 10.1016/s0959-4388(03)00073-4. [DOI] [PubMed] [Google Scholar]
- 106.Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 107.Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 108.Metsis M, Timmusk T, Arenas E, Persson H. Differential usage of multiple brain-derived neurotrophic factor promoters in the rat brain following neuronal activation. Proc Natl Acad Sci USA. 1993;90:8802–8806. doi: 10.1073/pnas.90.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen Q, He S, Hu XL, Yu J, Zhou Y, Zheng J, Zhang S, Zhang C, Duan WH, Xiong ZQ. Differential roles of NR2A- and NR2N-containing NMDA receptors in activity-dependent brain-derived neurotrophic factor gene regulation and limbic epileptogenesis. J Neurosci. 2007;27:542–552. doi: 10.1523/JNEUROSCI.3607-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tartaglia N, Du J, Tyler WJ, Neale E, Pozzo-Miller L, Lu B. Protein synthesis-dependent and -independent regulation of hippocampal synapses by brain-derived neurotrophic factor. J Biol Chem. 2001;276:37585–37593. doi: 10.1074/jbc.M101683200. [DOI] [PubMed] [Google Scholar]
- 111.Caldeira MV, Melo CV, Pereira DB, Carvalho RF, Carvalho AL, Duarte CB. BDNF regulates the expression and traffic of NMDA receptors in cultured hippocampal neurons. Mol Cell Neurosci. 2007;35:208–219. doi: 10.1016/j.mcn.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 112.Small DL, Murray CL, Mealing GA, Poulter MO, Buchan AM, Morley P. Brain derived neurotrophic factor induction of N-methyl-d-aspartate receptor subunit NR2A expression in cultured rat cortical neurons. Neurosci Lett. 1998;252:211–214. doi: 10.1016/s0304-3940(98)00587-4. [DOI] [PubMed] [Google Scholar]
- 113.Margottil E, Domenici L. NR2A but not NR2B n-methyl-d-aspartate receptor subunit is altered in the visual cortex of BDNF-knock-out mice. Cell Mol Neurobiol. 2003;23:165–174. doi: 10.1023/A:1022945821455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci. 1999;19:4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- 116.Perez-Otano I, Ehlers MD. Homeostatic plasticity and NMDA receptor trafficking. Trends Neurosci. 2005;28:229–238. doi: 10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 117.Rao A, Craig AM. Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron. 1997;19:801–812. doi: 10.1016/s0896-6273(00)80962-9. [DOI] [PubMed] [Google Scholar]
- 118.Hu B, Nikolakopoulou AM, Cohen-Cory S. BDNF stabilizes synapses and maintains the structural complexity of optic axons in vivo. Development. 2005;132:4285–4298. doi: 10.1242/dev.02017. [DOI] [PubMed] [Google Scholar]
- 119.Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 120.Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1983;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- 121.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans to animal models. Environ Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. J Neurosci. 2004;24:7859–7868. doi: 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qiang M, Denny AD, Ticku MK. Chronic intermittent ethanol treatment selectively alters N-methyl-d-aspartate receptor subunit surface expression in cultured cortical neurons. Mol Pharmacol. 2007;72:95–102. doi: 10.1124/mol.106.033043. [DOI] [PubMed] [Google Scholar]
- 124.Roberto M, Treistman SN, Pietrzykowski AZ, Weiner J, Galindo R, Mameli M, Valenzuela F, Zhu PJ, Lovinger D, Zhang TA, Hendricson AH, Morrisett R, Siggins GR. Actions of acute and chronic ethanol on presynaptic terminals. Alcohol Clin Exp Res. 2006;30:222–232. doi: 10.1111/j.1530-0277.2006.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]