Abstract
Obesity and diabetes are major risk factors for the development of vascular disease in the lower limbs. Previous studies have demonstrated reduced nitric oxide (NO)-mediated vasodilation, increased adrenergic constriction and inward, atrophic remodeling in the limb circulation of obese Zucker rats but the component of the “metabolic syndrome” driving these changes is unclear. Because insulin resistance precedes the state of frank diabetes, the current study hypothesized that insulin resistance independent of obesity induced by fructose-feeding would impair microvascular function in the skeletal muscle circulation in Lean Zucker rats (LZR). A 66% fructose diet impaired glucose tolerance and induced moderate insulin resistance with no changes in whole body hemodynamics of anesthetized rats (FF-LZR) compared to control LZR. NO-mediated vasodilation of isolated gracilis arteries, assessed in vitro with acetylcholine and sodium nitroprusside, was reduced ~20% in FF-LZR vs. LZR. NO-independent cGMP-mediated vasodilation was unimpaired. Pre-treatment of isolated vessels with super-oxide scavenger Tempol improved responses to both vasodilators. Reactivity to adrenergic stimulation was unaltered in FF-LZR vs. LZR, through constriction to endothelin was increased. Structural and passive mechanical characteristics of isolated gracilis arteries were similar in both LZR and FF-LZR. Taken together, these findings indicate that moderate insulin resistance is sufficient to impair endothelial function in an oxidant-dependent manner in the rat hindlimb circulation. Other aspects of skeletal muscle vascular function documented in obese models, specifically adrenergic tone and inward remodeling, must reflect either severe insulin resistance or other aspects of obesity. The factors accounting for non-endothelial vasculopathies remain unknown.
Keywords: microcirculation, adrenergic, nitric oxide, super oxide
INTRODUCTION
Obesity is a major risk factor for cardiovascular dysfunction in the United States (2, 3) but the links between obesity and cardiovascular disease remain incompletely understood. In addition to cardiovascular disease, the “obese phenotype” is associated with a greater incidence of metabolic dysfunction, including insulin resistance (IR) (16, 20), adrenal excess (5, 38) and sympathoactivation(1, 4). Because of the close association between obesity-induced metabolic disease and obesity-induced cardiovascular disease, a common speculation has become that metabolic disease causes the cardiovascular disease(6). The extent to which this is true remains unclear.
The primary metabolic disease in obesity is IR and diabetes (20). While experimental diabetes (21, 25) or short-term hyperglycemia (7, 8, 37) are well documented to cause vascular deficits, increasing evidence suggests that the source of vascular dysfunction may lie earlier in the progression of metabolic dysfunction and that IR, prior to diabetes, may be the causal factor. While IR as an independent variable has been shown to impair vascular function in the coronary(26), mesenteric(19, 31) and cerebral beds(12), its impact on the skeletal muscle vasculature remains unknown. Understanding the effects of obesity and IR on the skeletal muscle vasculature is of cardinal importance as peripheral vascular disease, a disease primarily of the limb circulation, is a major source of morbidity in the obese, diabetic population (32).
Previous studies from our lab and others have examined the effect of obesity on skeletal muscle vascular reactivity in the Zucker rat model of obesity and IR. These studies revealed that obesity impairs relaxation to nitric oxide-dependent stimuli, such as acetylcholine and nitroprusside. Indices of oxidant load were elevated in plasma and blood vessels from obese rats and correction of oxidant tone normalized endothelium-dependent vasodilation(15). Adrenergic vasoconstrictor reactivity is augmented (35) and evidence of low-flow remodeling, characterized by smaller lumens and thinner walled microvessels, is present(36). The extent to which IR is the risk factor underlying these deficits and the extent to which these deficits co-segregate is unclear.
In the current study, we hypothesize that IR, independent of obesity, will impair control of skeletal muscle microvascular tone and these changes will parallel previous observations in obesity. IR was induced in lean Zucker rats using a high fructose diet. Metabolic dysfunction was determined using measurements of glucose clearance and indices of plasma chemistry. Baseline hemodynamics were assessed with measurements of blood pressure, heart rate and hindlimb vascular resistance in isoflurane-anesthetized rats. Reactivity to acetylcholine, nitroprusside, endothelin and norepinephrine were assessed in isolated pressurized microvessels. Taken together, these data provide the first examination of the effects of IR as an independent variable on the microcirculation of the lower limb.
METHODS
Animal Model
Male lean Zucker rats were purchased from Harlan at 6 weeks of age and allowed to acclimate for one week before being entered into one of two groups: Control diet-fed lean Zucker rats (LZR) and lean Zucker rats fed a 66% Fructose diet (FF-LZR). Rats were maintained on diet for 8 weeks and then entered into experimental studies. The Zucker rat strain was used to maintain consistency with the obese, insulin resistant strain studied previously. A duration of 8 weeks on diet was selected to mimic the similar period over which Obese Zucker rats develop IR (11).
Hemodynamic assessment
To determine the hemodynamic impact of fructose-induced IR, rats were anesthetized with 1% isoflurane and maintained on a nose cone with isoflurane-supplemented room air. A polyurethane catheter was placed in the carotid artery to monitor arterial pressure and a jugular vein was catheterized to provide supplemental volume as needed. A midline incision was performed and the viscera retracted to expose the abdominal aorta. A 2.0 PRB Transonic flow probe was placed around the aorta just above the iliac bifurcation to measure hindquarters blood flow. Hemodynamics were allowed to stabilize for 30 minutes and were then monitored for 15 minutes. Data are reported as the average values over 15 minutes. Rats were then euthanized with overdose of isoflurane and decapitation. The hindquarters were weighed and blood flow normalized to that weight. Hindquarters vascular resistance was calculated as the quotient of arterial pressure and normalized hindquarters blood flow.
In vitro vascular reactivity
Reactivity of skeletal muscle resistance vessels was assessed in vitro using isolated pressurized branches of the gracilis artery approximate 180 μm i.d. maximum passive diameter. Vessels were dissected free of connective tissues and mounted on glass pipettes with 11.0 ophthalmic suture in physiologic saline solution containing (in mM): 119 NaCl, 4.7 KCl, 1.17 MgSO4, 1.6 CaCl2, 1.18 NaH2PO4, 24 NaHCO3, 0.026 EDTA, and 5.5 glucose. Pressure was increased to 60 mm Hg and maintained at a constant pressure with no flow as described previously (31). Vasoactive agents were added to the bath and internal and external diameters recorded using video calipers. All inhibitors (L-NAME, Tempol) were added to the external bathing medium and allowed to equilibrate for 30 minutes. Vasodilation was assess as diameter as percentage of the maximum diameter under passive conditions. Vasoconstriction was assessed as % reduction from baseline diameter.
At the end of each experiment, the bathing solution was switched to one with the identical composition as above except that calcium was excluded and 2 MM Ethyleneglycol-bis(beta-aminoethyl ether)-N,N,N′,N′-tetraacetic acid was included to chelate any remaining calcium. In this condition, vessels become completely passive and were subject to a series of pressure steps to assess passive mechanical properties of the arteries as described previously (17, 36).
Oxidant measurements
Quantitation of superoxide production was performed via electron paramagnetic resonance spectroscopy in pool hindlimb vessels (femoral branches from both legs from a single animal) in phosphate buffered saline and 25 μM desferrioxamine, homogenized and protein concentrations normalized (2 mg per mL). Samples were then split into two volumes containing polyethylene glycolated superoxide dismutase (100 U per ml) or solvent (vehicle). These mixtures were then incubated with methoxycarbonyl-2,2,5,5-tetramethyl-pyrrolidine (CMH, 20μM) for 45min Samples were analyzed immediately with a MiniScope MS200 EPR (Magnettech, Berlin, Germany) at a microwave power of 40 mW, modulation amplitude of 1,000 mG, and modulation frequency of 100 kHz. EPR spectra were analyzed measured for amplitude using ANALYSIS software (version 2.02; Magnettech).
Localization of oxidant production was performed in hindlimb microvessels flash-frozen and immediately placed in OCT. Vessels were sectioned to 10 m and mounted on Fischer premium glass slides. Each slide was washed in room temperature PBS for 30 minutes, incubated in 200 uM DHE (DMSO, 37 degrees) for 30 minutes, then washed in ice cold PBS (4 degrees) for 1 hour. Slipcovers were then applied onto the slides with a drop of PBS and the vessels were imaged immediately. Microscopy was performed with a Leica DMIL inverted microscope and Leica 320 digital camera at 40X magnification.
Metabolic Assessment
To measure glucose tolerance, rats were isoflurane-anesthetized and a catheter inserted into the jugular vein. After a 15–20 minute stabilization period, baseline blood glucose was measured twice and averaged. A bolus of 50 mg of glucose in 200 μl was injected intravenously and blood glucose measured 5 minutes later. This value was taken as peak glucose and subsequent glucose measurements were expressed as the residual fraction of this value. Data were taken at 5 minute intervals from 5 animals in each group.
Plasma glucose was measured using a Precision XL over-the-counter glucometer. Insulin was assessed using an ELISA from Alpco, Lipid peroxides were determined using a colorimetric kit from Calbiochem. Salem NH and plasma lipids were determined using colorimetric assays from Wako Chemicals USA, Richmond, VA.
Chemicals
Fructose diet was obtained from Harlan Teklad (Catalog #89247). All vasoactive compounds and buffer components were purchased from Sigma Chemical, St. Louis, MO.
Statistics
All data are expressed as mean ± SEM. Comparisons under baseline or maximum were performed by Student’s T-Test. Dose-response curves were analyzed by two-way ANOVA with Fisher’s test for post-hoc analysis. Significance was accepted at p<0.05.
RESULTS
Anatomic and hemodynamic characteristics
The baseline anatomic and anesthetized hemodynamic characteristics of the animals used in this study are shown in Table 1. Fructose-feeding had no effect on body weight or baseline whole-body hemodynamics. This observation is consistent with previous studies in conscious animals indicating the fructose-induced insulin resistant does not increase mean arterial pressure (9, 11). Baseline tone was slightly but significantly reduced in FF-LZR compared to LZR (32±2 vs. 38±2%, p<0.05) and neither L-NAME nor Tempol affected baseline diameter.
Table 1.
Baseline Anatomic, Hemodynamic and Metabolic Characteristics of Fructose-Fed Rats.
| Parameter | LZR | FF-LZR |
|---|---|---|
| Body weight (gms) | 372±8 | 384±7 |
| HQ weight (gms) | 132±4 | 141±11 |
| Aortic Pressure (mm Hg) | 108±8 | 108±7 |
| Heart Rate (mm Hg) | 357±34 | 343±42 |
| HQ blood flow (ml/min/gm tissue) | 0.12±.01 | 0.12±.01 |
| HQ vascular Resistance (ml/min/gm/mm Hg) | 927±47 | 931±55 |
| Plasma Glucose (mg/dl) | 110±14 | 131±18 |
| Plasma Insulin (ng/ml) | 0.52±0.04 | 1.85±0.12* |
| Plasma Cholesterol (mg/dl) | 58±8 | 71±5* |
| Plasma Triglycerides (mg/dl) | 63±5 | 172±14* |
| Plasma Lipid Peroxides (μM) | 7±1 | 10±1* |
n ≥10 in each group.
p>0.05 vs. LZR
Plasma Chemistry
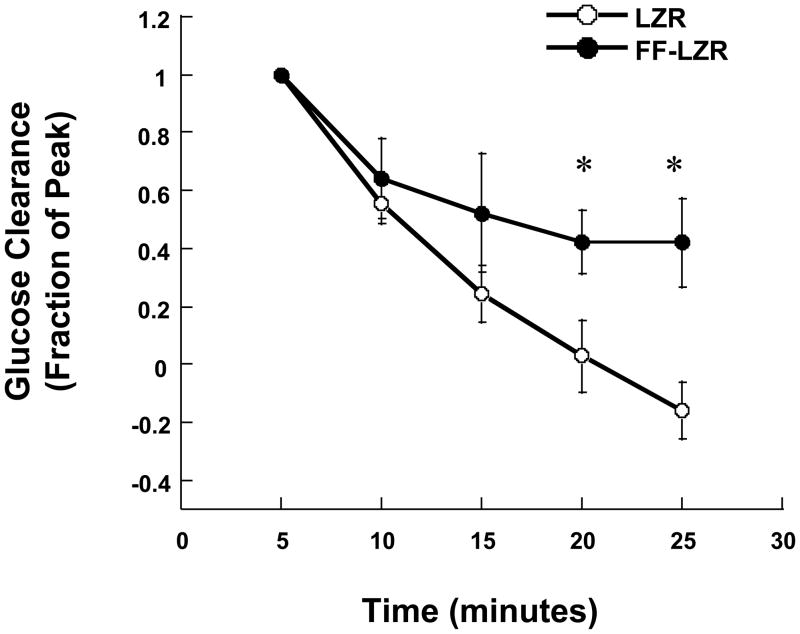
Metabolic profiling of plasma chemistry is shown in Table 1. Consistent with previous reports, fructose-feeding induces a moderate IR (34) and impairs glucose tolerance (Figure 1). Baseline blood glucose was similar between FF-LZR and LZR whereas insulin and triglycerides were modestly increased, consistent with pre-diabetic IR. Cholesterol was similar between both groups. Lipid peroxidation, assessed as the combined concentration of malondialdehydes and hydrononenals, were increased in FF-LZR compared to LZR, indicative of an IR-induced pro-oxidant state as reported previously(29, 31, 33).
Figure 1.
Clearance of a glucose bolus in LZR and FF-LZR. Data collection begins at the peak glucose concentration 5 minutes post-injection (Fraction 1.0) and continues at 5 minute intervals. Data represent the mean and SEM of 5 rats in each group. * indicates p<0.05 vs. LZR.
Endothelial Vasodilation
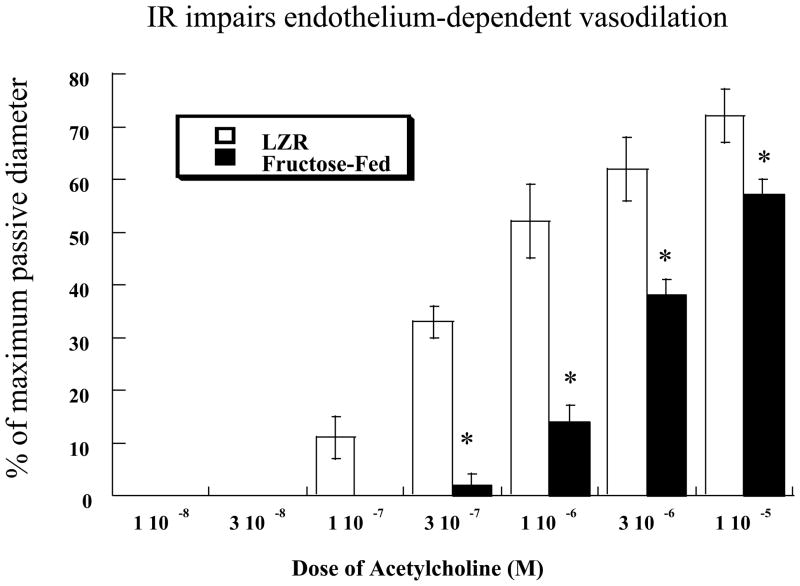
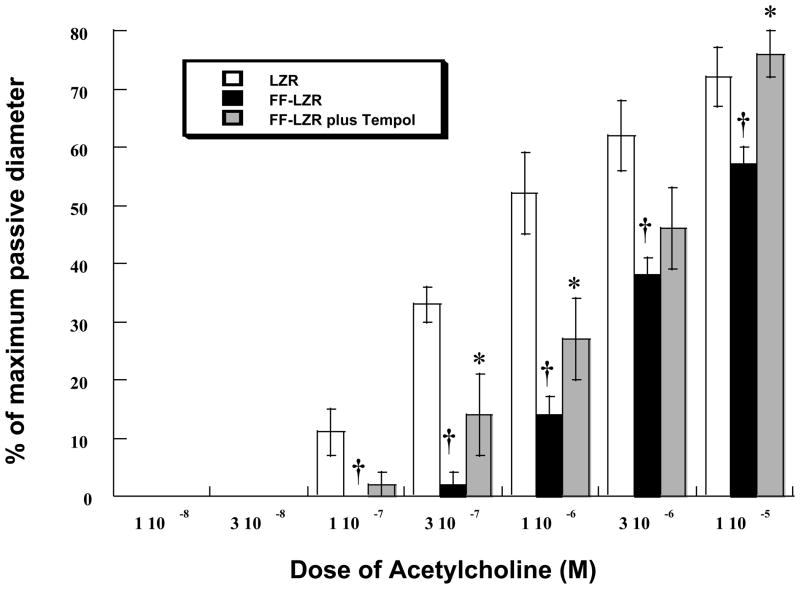
To assess endothelial function, serial doses of acetylcholine were added to the bath and increases in diameter measured. Results are shown in Figure 2. Acetylcholine produced a dose-dependent increase in diameter that was significantly reduced in FF-LZR compared to LZR (maximum response = 57±3 vs. 72±5 % of passive diameter at 60 mm Hg, n= 6, p<0.05). Treatment with 500 μM L-NAME reduced dilation to minimal levels in both groups (maximum response = 12±2 vs. 17±2 % of passive diameter at 60 mm Hg, LZR vs. FF-LZR, n= 6, p=NS). Treatment with Tempol improved vasodilation in arteries from FF-LZR to a level no different than those from LZR at the maximum concentration (Figure 2). Treatment of arteries from LZR with Tempol had no effect on vasodilation to acetylcholine (maximum response = 76±4 vs. 72±5 % of passive diameter at 60 mm Hg, n= 6, p=NS)
Figure 2.
Effects of insulin resistance on microvascular endothelium dependent dilation to acetylcholine. Data collected represents the mean and SEM of individual gracilis arteries taken 1/rat from 6 rats (n=6). † indicates p<0.05 of FF-LZR vs. LZR. * indicates p<0.05 Tempol-treated vs. untreated FF-LZR.
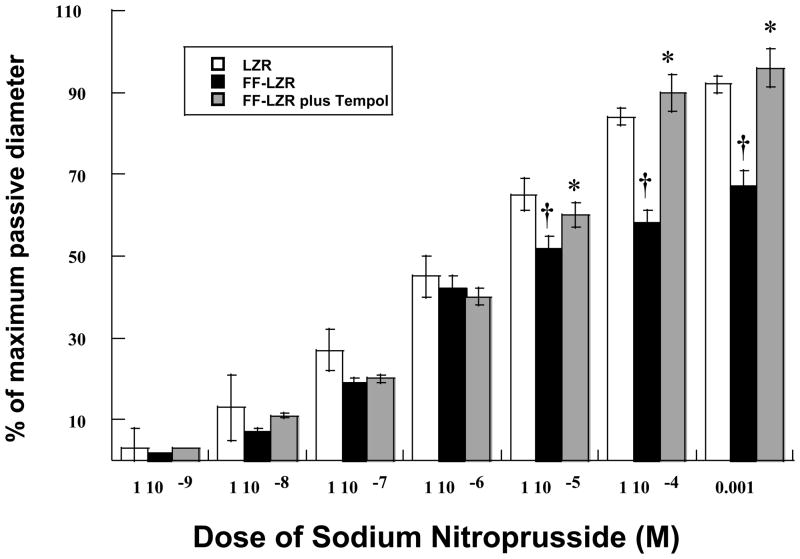
Because the primary vasodilator pathway activated by acetylcholine in the gracilis artery of the Zucker strain is NO (15), we assessed the reactivity of NO directly by measuring vasodilation to sodium nitroprusside (SNP). Results are depicted in Figure 3. SNP produced a dose-dependent increase in diameter that was significantly reduced in FF-LZR compared to LZR (maximum response = 78±4 vs. 92±3% of passive diameter at 60 mm Hg, n= 7, p<0.05). Scavenging of superoxide with Tempol increased vasodilation in arteries from FF-LZR to a level no different than those from LZR at the maximum concentration (Figure 3). Treatment of arteries from LZR with Tempol had no effect on vasodilation to SNP (maximum response = 98±3 vs. 92±3 % of passive diameter at 60 mm Hg, n= 7, p=NS).
Figure 3.
Effects of insulin resistance on microvascular endothelium independent dilation to sodium nitroprusside. Data collected represents the mean and SEM of individual gracilis arteries taken 1/rat from 7 rats (n=7). † indicates p<0.05 of FF-LZR vs. LZR. * indicates p<0.05 Tempol-treated vs. untreated FF-LZR.
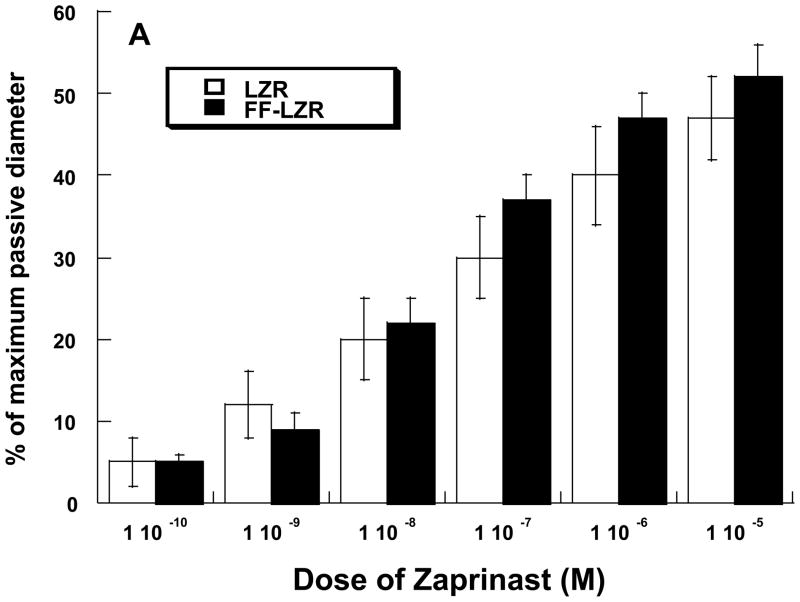
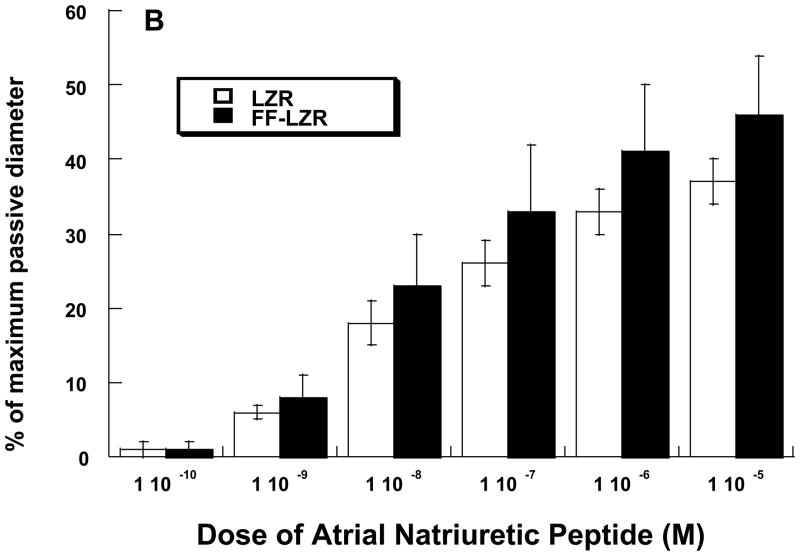
Because both endothelium-dependent- and independent- vasodilation to NO is impaired, we examined the prospect of a smooth muscle deficit in cGMP-mediated dilation as a potential contributor the observed impairments. To do this, we assessed vasodilation to Zaprinast, an inhibitor of cGMP phosphodiesterase and Atrial Natriuretic Peptide (ANP), a stimulant of the particulate guanylate cyclase. Data are shown in Figure 4. In either case, accumulation of cGMP in response to each agent produced a dose-dependent vasodilation and the response was similar in both LZR and FF-LZR.
Figure 4.
Effects of insulin resistance on vasodilation to zaprinast (A) and atrial natriuretic peptide (B). Data collected represents the mean and SEM of individual gracilis arteries taken 1/rat from 7 rats (n>4). No significant differences were observed.
Oxidant status
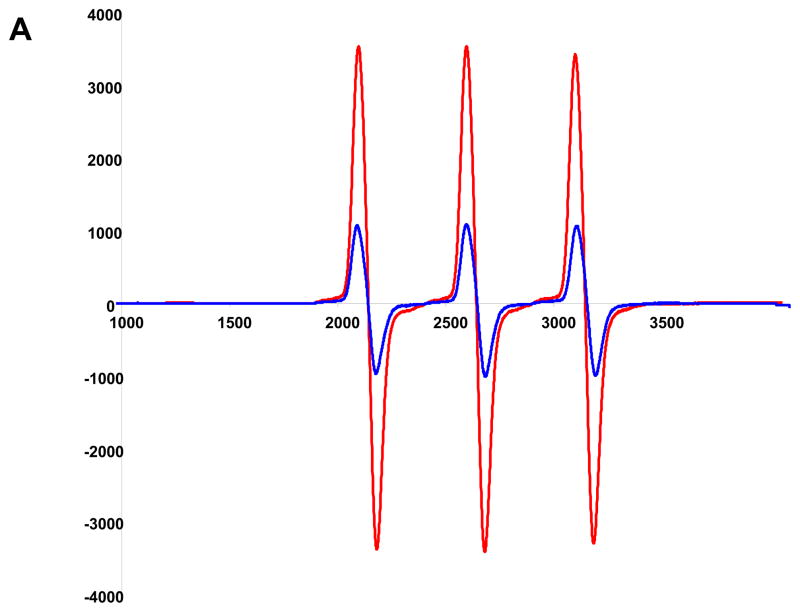
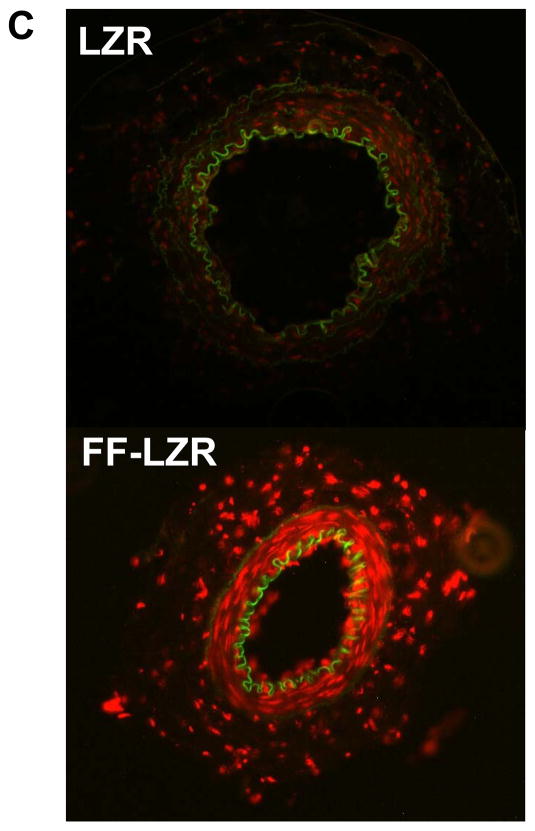
Given the improvement in vasodilator function observed with superoxide scavenging, we determined superoxide production via two methods – electronic paramagnetic resonance and localization with DHE staining. Results are shown in Figure 5. In Figure 5A, a representative EPR amplitude tracing is shown for one LZR and One FF-LZR sample Parallel samples were treated PEG-SOD to scavenge super-oxide and provide a background subtraction. Summary data of background-subtracted amplitudes is shown in Figure 5B, indicating an ~2.5 fold increase in superoxide abundance in hindlimb small arteries in insulin-resistant rats. When superoxide production was localized with DHE staining, a diffuse pattern was observed indicating superoxide production in both the endothelial and medial layer. Taken together, these data indicate that superoxide production is locally increased in the vasculature of FF-LZR, consistent with the mechanistic effects observed with Tempol.
Figure 5.
Effects of insulin resistance on superoxide production. A representative EPR amplitude tracing for 1 LZR in blue and 1 FFLZR in red (A) is shown with summary data background subtracted with SOD (B). Localization of superoxide production with DHE (C) is shown with one LZR and one FF-LZR representative of three rats in each group.
Vasoconstriction
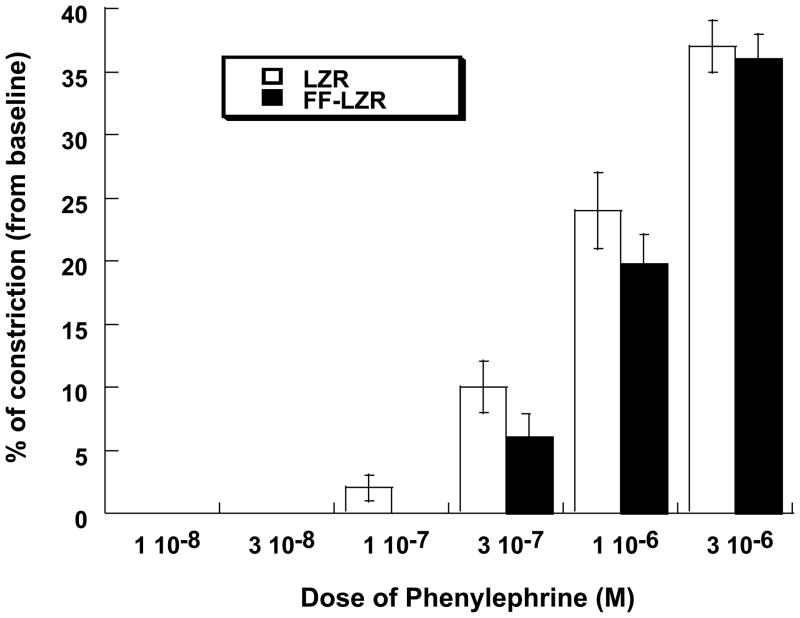
To asses the effects of insulin resistance on increases in resistance, reactivity to two independent constrictors, phenylephrine and endothelin, were examined. Adrenergic reactivity in LZR and FF-LZR is shown in Figure 6. The α1-agonist phenylephrine causes a dose dependent decrease in diameter to a maximum value ~40% of its basal value. Reactivity to phenylephrine was similar in LZR and FF-LZR.
Figure 6.
Effects of insulin resistance on vasoconstriction to phenylephrine. Data collected represents the mean and SEM of individual gracilis arteries taken 1/rat from 7 rats (n=7). No significant differences were observed.
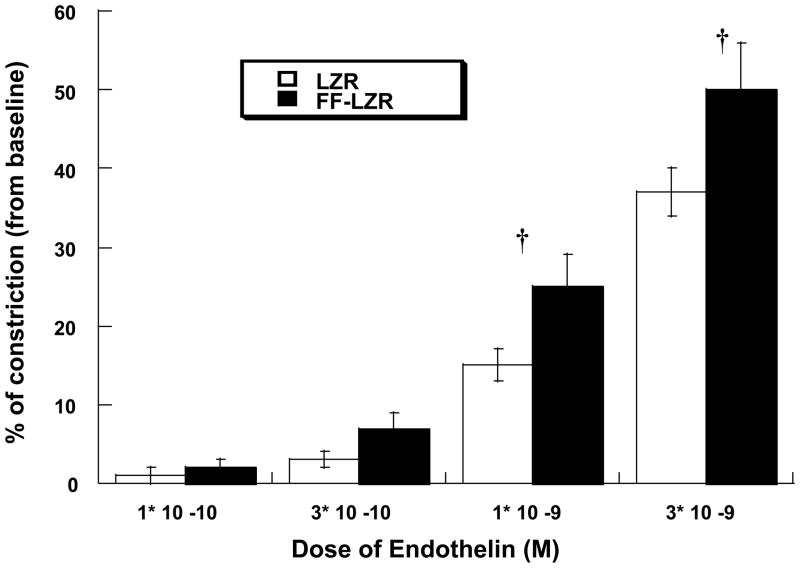
Reactivity to serial doses of endothelin is shown in Figure 7. Endothelin produced a dose-dependent reduction in diameter to a maximum value of 37±3% (n=7) in LZR. Vascular reactivity to endothelin in FF-LZR was markedly increased, reaching a maximum constriction on 50±6 (n=7, p<0.05 vs. LZR).
Figure 7.
Effects of insulin resistance on vasoconstriction to endothelin. Data collected represents the mean and SEM of individual gracilis arteries taken 1/rat from 7 rats (n=7). † indicates p<0.05 of FF-LZR vs. LZR.
Vascular architecture and mechanics
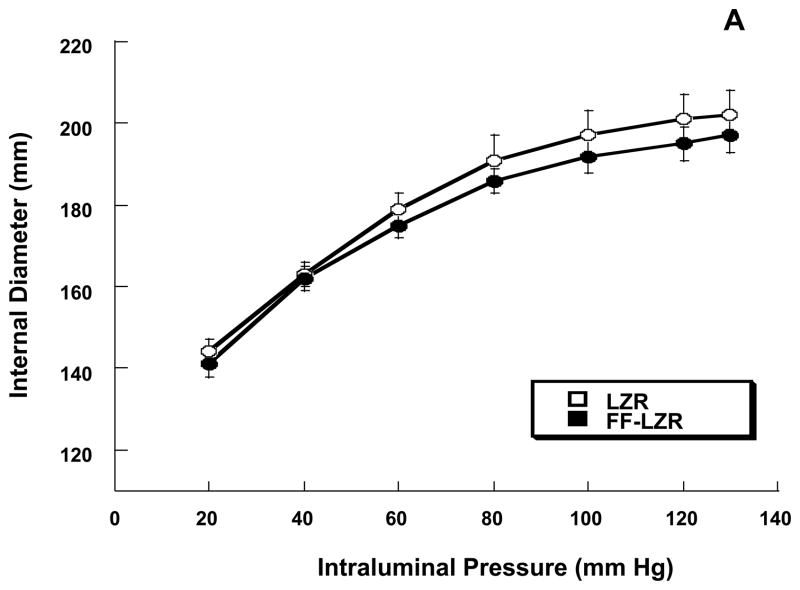
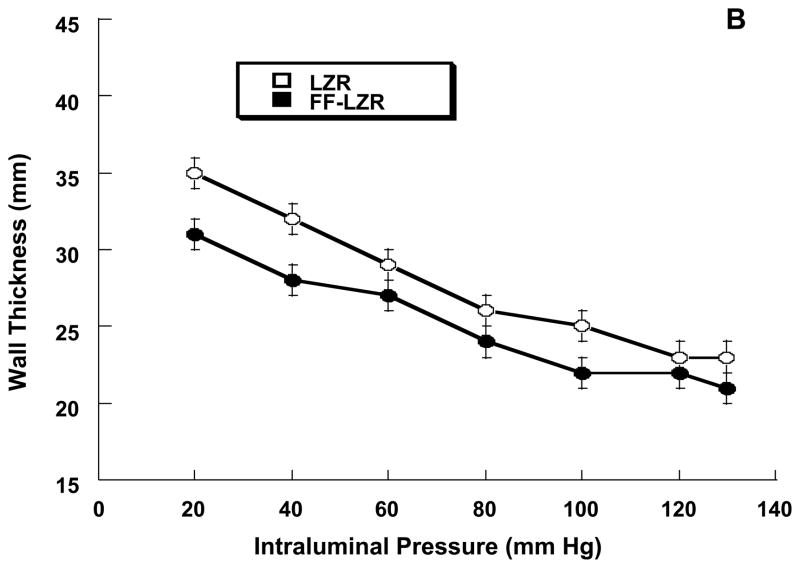
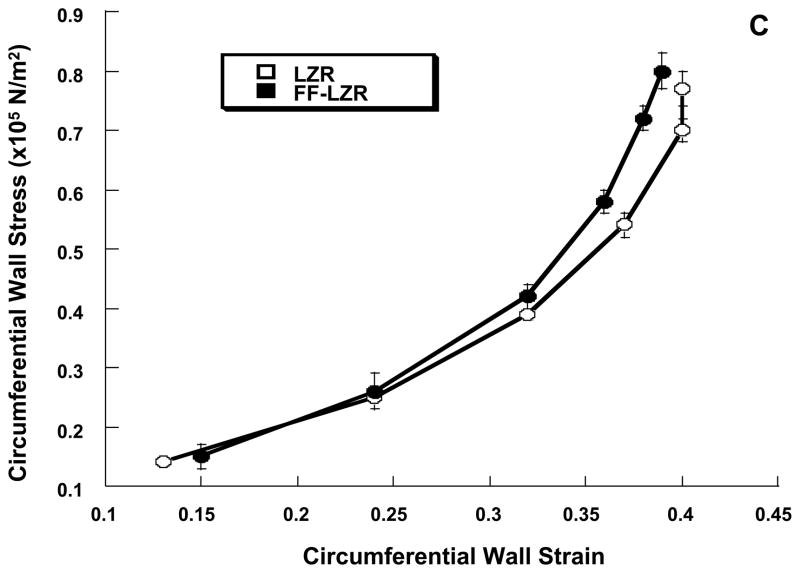
Passive properties of skeletal muscle resistance arteries are shown in Figure 8. At a maximum distending pressure of 130 mm Hg, maximum passive internal diameter (6A, 202±6 vs.197±4), wall thickness (6B, 23±1 vs. 21±1) and wall to lumen ratio (0.23±0.01 vs.0.22±0.01) were similar resistance arteries from FF-LZR compared to LZR (n=10). As shown in Figure 6C, the stress-strain relationship was similar between LZR and FF-LZR (β-coefficient or exponential slope = 4.30±0.7 vs. 4.45±0.9, n=10, p=NS).
Figure 8.
Effects of insulin resistance on lumen diameter (A), wall thickness (B) and compliance assessed by the stress-strain relationship (C). Data collected represents the mean and SEM of individual gracilis arteries taken 1/rat from 10 rats (n=10). No significant differences were observed.
DISCUSSION
The goal of the current study was to determine the extent to which insulin resistance, independent of obesity, impairs skeletal muscle microvascular function. The key observations of the current study are that 1) insulin resistance specifically impairs NO-mediated dilation in a super-oxide dependent manner, 2) the insulin resistance does not affect adrenergic vasoconstriction outside the context of obesity and 3) loss of endothelial dilation due to insulin resistance does not alter the vascular architecture of the skeletal muscle microcirculation.
Endothelial dysfunction is considered a hallmark of vascular disease. Previous studies have documented impaired NO-mediated dilation in the hindlimb circulation of the Obese Zucker rat, a model of the metabolic syndrome which is insulin resistant, hypertensive and obese(15). A similar impairment is also evident in the mesenteric (31), renal (18) and cerebral circulations (30). The ubiquity of this observation has led to the suggestion that a change in the composition of the plasma, which touches all compartments, if a likely culprit. In obesity, the most profound alteration in plasma composition is caused by insulin resistance, which causes periodic hyperglycemia, hyperinsulinemia and triglyceride dyslipidemia. Using the fructose-diet model, one can model insulin resistance as an isolated variable as it lacks obesity or it’s associated hypertension (11). Previous studies with this model have documented reduced endothelial vasodilation in the coronary(26), mesenteric (19, 31) and cerebral circulations(12). The current study extends the observation that insulin resistance, independent of obesity, impairs NO-mediated dilation into the skeletal muscle circulation. Moreover, the current study documents that the loss of NO-mediated dilation is at least in part due to the excess production of super-oxide. Insulin resistance induced by fructose feeding results in increases in both tissue and plasma markers of oxidant load and results in impairment of NO-mediated dilation that is corrected when the super-oxide scavenger Tempol is added to the bathing medium. Moreover, this impairment appears restricted to NO-mediated dilation as non-NO-mediated, cGMP-mediated vasodilation is unimpaired. This observation is consistent with previous studies in Obese Zucker Rats in the gracilis circulation (15) and in the fructose-fed rat in the mesenteric circulation (31). Taken together, these data offer further compelling evidence that insulin resistance is the risk factor in obesity that contributes to superoxide-induced corruption of NO-signaling.
Another major alteration observed in the hindlimb circulation of Obese Zucker rats is altered reactivity to vasoconstrictors. Using the gracilis artery model, it has been observed that adrenergic reactivity is augmented in the obese Zucker rats(22, 27, 35) and this adrenergic reactivity likely contributes to increased basal vascular tone (28) and limited reactivity to ischemic or pharmacologic vasodilation (13, 14). Whether adrenergic tone limits functional hyperemia remains controversial (13, 28) and the underlying mechanisms driving the increased adrenergic tone in obese rodents is unclear. In the current study, it was observed that induction of insulin resistance, independent of obesity neither increased nor decreased reactivity to adrenergic tone. Thus, it must be concluded that 1) loss of endothelial function is not sufficient to produce increased constrictor reactivity to norepinephrine and 2) other aspects of obesity, more severe insulin resistance or some combination of the two is required to recapitulate the adrenergic phenotype observed in obese Zucker rats. Interestingly, where as endothelin reactivity has not been observed to be increased in gracilis arteries for Obese Zucker Rats, an elevated responsiveness was observed in gracilis arteries from Fructose-Fed Zucker rats. The mechanism for this is unclear but may be related to the loss of endothelial function as NO and endothelin counter-regulate each other in endothelial control of vascular tone as shown in animal (22) and human studies of obese and insulin resistant individuals (10, 23, 24).
While acute adjustments in flow are regulated by vasodilation and constriction, maximum perfusion is set by the size and distensibility of the resistance vessels. In gracilis arteries from Obese Zucker rats, an inward remodeling is evident resulting in a thinner wall, smaller lumen and greater stiffness of isolated microvessels (36), likely reflecting the altered hemodynamics of skeletal muscle blood flow in this obese model. The extent to which insulin resistance determines this remodeling and whether impaired dilation or augmented constriction play a greater role in it’s development are unclear. In the current study were observed that fructose-feeding induced a moderate insulin resistance that produced no substantive changes in the architecture or passive mechanics of gracilis arteries. There are two key conclusions from this. First, as with adrenergic tone, it appears that other aspects of obesity are required to recapitulate the phenotype observed in obese Zucker rats. Second, the observation that microvascular remodeling appears to be unchanged despite endothelial dysfunction would argue that, in the context of obesity, endothelial dysfunction is probably not the mechanism driving microvascular remodeling. While adrenergic tone is not directly indicted in the current studies, it seems plausible that the chronic constriction present on Obese Zucker rats but not Fructose-Fed Zucker rats explains the microvascular remodeling presented in Obese Zucker rats but not Fructose-Fed Zucker rats.
In summary, we have provided the first examination of skeletal muscle microvascular function in the fructose-fed model of insulin resistance. We document oxidant-induced endothelial dysfunction similar to that observed in Zucker rats in which insulin-resistance is induced by obesity. Hyperreactivity to endothelin was also observed in this model. Other hallmarks of impaired skeletal muscle blood flow observed in Obese Zucker rats, increased adrenergic reactivity and microvascular remodeling, are absent. Taken together, these studies suggest that insulin resistance is a likely culprit for microvascular dysfunction in the metabolic syndrome. Risk factors driving other cardiovascular dysfunctions in obesity remain to be determined.
Acknowledgments
The authors wish to thank Julie Campbell for her expert technical assistance. This work was supported by NIH R01 76533 to DWS.
CITED REFERENCES
- 1.Abate NI, Mansour YH, Tuncel M, Arbique D, Chavoshan B, Kizilbash A, Howell-Stampley T, Vongpatanasin W, Victor RG. Overweight and sympathetic overactivity in black Americans. Hypertension. 2001;38:379–383. doi: 10.1161/01.hyp.38.3.379. [DOI] [PubMed] [Google Scholar]
- 2.Agapitov AV, Correia ML, Sinkey CA, Dopp JM, Haynes WG. Impaired skeletal muscle and skin microcirculatory function in human obesity. Journal of hypertension. 2002;20:1401–1405. doi: 10.1097/00004872-200207000-00027. [DOI] [PubMed] [Google Scholar]
- 3.Al Suwaidi J, Higano ST, Holmes DR, Jr, Lennon R, Lerman A. Obesity is independently associated with coronary endothelial dysfunction in patients with normal or mildly diseased coronary arteries. J Am Coll Cardiol. 2001;37:1523–1528. doi: 10.1016/s0735-1097(01)01212-8. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533–2536. doi: 10.1161/01.cir.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- 5.Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci (Lond) 1999;96:513–523. doi: 10.1042/cs0960513. [DOI] [PubMed] [Google Scholar]
- 6.Baron AD. Insulin resistance and vascular function. J Diabetes Complications. 2002;16:92–102. doi: 10.1016/s1056-8727(01)00209-4. [DOI] [PubMed] [Google Scholar]
- 7.Bohlen HG, Nase GP. Arteriolar nitric oxide concentration is decreased during hyperglycemia-induced betaII PKC activation. American journal of physiology. 2001;280:H621–627. doi: 10.1152/ajpheart.2001.280.2.H621. [DOI] [PubMed] [Google Scholar]
- 8.Bohlen HG, Nase GP. Obesity lowers hyperglycemic threshold for impaired in vivo endothelial nitric oxide function. American journal of physiology. 2002;283:H391–397. doi: 10.1152/ajpheart.00019.2002. [DOI] [PubMed] [Google Scholar]
- 9.Brands MW, Garrity CA, Holman MG, Keen HL, Alonso-Galicia M, Hall JE. High-fructose diet does not raise 24-hour mean arterial pressure in rats. Am J Hypertens. 1994;7:104–109. doi: 10.1093/ajh/7.1.104. [DOI] [PubMed] [Google Scholar]
- 10.Cardillo C, Campia U, Iantorno M, Panza JA. Enhanced vascular activity of endogenous endothelin-1 in obese hypertensive patients. Hypertension. 2004;43:36–40. doi: 10.1161/01.HYP.0000103868.45064.81. [DOI] [PubMed] [Google Scholar]
- 11.D’Angelo G, Elmarakby AA, Pollock DM, Stepp DW. Fructose feeding increases insulin resistance but not blood pressure in Sprague-Dawley rats. Hypertension. 2005;46:806–811. doi: 10.1161/01.HYP.0000182697.39687.34. [DOI] [PubMed] [Google Scholar]
- 12.Erdos B, Miller AW, Busija DW. Alterations in KATP and KCa channel function in cerebral arteries of insulin-resistant rats. American journal of physiology. 2002;283:H2472–2477. doi: 10.1152/ajpheart.00516.2002. [DOI] [PubMed] [Google Scholar]
- 13.Frisbee JC. Enhanced arteriolar alpha-adrenergic constriction impairs dilator responses and skeletal muscle perfusion in obese Zucker rats. J Appl Physiol. 2004;97:764–772. doi: 10.1152/japplphysiol.01216.2003. [DOI] [PubMed] [Google Scholar]
- 14.Frisbee JC. Vascular adrenergic tone and structural narrowing constrain reactive hyperemia in skeletal muscle of obese Zucker rats. American journal of physiology. 2006;290:H2066–2074. doi: 10.1152/ajpheart.01251.2005. [DOI] [PubMed] [Google Scholar]
- 15.Frisbee JC, Stepp DW. Impaired NO-dependent dilation of skeletal muscle arterioles in hypertensive diabetic obese Zucker rats. American journal of physiology. 2001;281:H1304–1311. doi: 10.1152/ajpheart.2001.281.3.H1304. [DOI] [PubMed] [Google Scholar]
- 16.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nature reviews. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajdu MA, Heistad DD, Siems JE, Baumbach GL. Effects of aging on mechanics and composition of cerebral arterioles in rats. Circ Res. 1990;66:1747–1754. doi: 10.1161/01.res.66.6.1747. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi K, Kanda T, Homma K, Tokuyama H, Okubo K, Takamatsu I, Tatematsu S, Kumagai H, Saruta T. Altered renal microvascular response in Zucker obese rats. Metabolism: clinical and experimental. 2002;51:1553–1561. doi: 10.1053/meta.2002.36311. [DOI] [PubMed] [Google Scholar]
- 19.Katakam PV, Ujhelyi MR, Hoenig ME, Miller AW. Endothelial dysfunction precedes hypertension in diet-induced insulin resistance. The American journal of physiology. 1998;275:R788–792. doi: 10.1152/ajpregu.1998.275.3.R788. [DOI] [PubMed] [Google Scholar]
- 20.Keller KB, Lemberg L. Obesity and the metabolic syndrome. Am J Crit Care. 2003;12:167–170. [PubMed] [Google Scholar]
- 21.Kiff RJ, Gardiner SM, Compton AM, Bennett T. Selective impairment of hindquarters vasodilator responses to bradykinin in conscious Wistar rats with streptozotocin-induced diabetes mellitus. British journal of pharmacology. 1991;103:1357–1362. doi: 10.1111/j.1476-5381.1991.tb09793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lesniewski LA, Donato AJ, Behnke BJ, Woodman CR, Laughlin MH, Ray CA, Delp MD. Decreased NO signaling leads to enhanced vasoconstrictor responsiveness in skeletal muscle arterioles of the ZDF rat prior to overt diabetes and hypertension. American journal of physiology. 2008;294:H1840–1850. doi: 10.1152/ajpheart.00692.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mather KJ, Lteif A, Steinberg HO, Baron AD. Interactions between endothelin and nitric oxide in the regulation of vascular tone in obesity and diabetes. Diabetes. 2004;53:2060–2066. doi: 10.2337/diabetes.53.8.2060. [DOI] [PubMed] [Google Scholar]
- 24.Mather KJ, Mirzamohammadi B, Lteif A, Steinberg HO, Baron AD. Endothelin contributes to basal vascular tone and endothelial dysfunction in human obesity and type 2 diabetes. Diabetes. 2002;51:3517–3523. doi: 10.2337/diabetes.51.12.3517. [DOI] [PubMed] [Google Scholar]
- 25.Mayhan WG, Simmons LK, Sharpe GM. Mechanism of impaired responses of cerebral arterioles during diabetes mellitus. The American journal of physiology. 1991;260:H319–326. doi: 10.1152/ajpheart.1991.260.2.H319. [DOI] [PubMed] [Google Scholar]
- 26.Miller AW, Katakam PV, Ujhelyi MR. Impaired endothelium-mediated relaxation in coronary arteries from insulin-resistant rats. J Vasc Res. 1999;36:385–392. doi: 10.1159/000025678. [DOI] [PubMed] [Google Scholar]
- 27.Naik JS, Xiang L, Hester RL. Enhanced role for RhoA-associated kinase in adrenergic-mediated vasoconstriction in gracilis arteries from obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R154–161. doi: 10.1152/ajpregu.00245.2005. [DOI] [PubMed] [Google Scholar]
- 28.Naik JS, Xiang L, Hodnett BL, Hester RL. Alpha-adrenoceptor-mediated vasoconstriction is not involved in impaired functional vasodilation in the obese Zucker rat. Clin Exp Pharmacol Physiol. 2008;35:611–616. doi: 10.1111/j.1440-1681.2007.04849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onuma S, Nakanishi K. Superoxide dismustase mimetic tempol decreases blood pressure by increasing renal medullary blood flow in hyperinsulinemic-hypertensive rats. Metabolism: clinical and experimental. 2004;53:1305–1308. doi: 10.1016/j.metabol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Phillips SA, Sylvester FA, Frisbee JC. Oxidant stress and constrictor reactivity impair cerebral artery dilation in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R522–530. doi: 10.1152/ajpregu.00655.2004. [DOI] [PubMed] [Google Scholar]
- 31.Romanko OP, Stepp DW. Reduced constrictor reactivity balances impaired vasodilation in the mesenteric circulation of the obese Zucker rat. American journal of physiology. 2005;289:H2097–2102. doi: 10.1152/ajpheart.00213.2005. [DOI] [PubMed] [Google Scholar]
- 32.Schaper NC, Nabuurs-Franssen MH, Huijberts MS. Peripheral vascular disease and type 2 diabetes mellitus. Diabetes Metab Res Rev. 2000;16 (Suppl 1):S11–15. doi: 10.1002/1520-7560(200009/10)16:1+<::aid-dmrr112>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 33.Shinozaki K, Kashiwagi A, Nishio Y, Okamura T, Yoshida Y, Masada M, Toda N, Kikkawa R. Abnormal biopterin metabolism is a major cause of impaired endothelium-dependent relaxation through nitric oxide/O2- imbalance in insulin-resistant rat aorta. Diabetes. 1999;48:2437–2445. doi: 10.2337/diabetes.48.12.2437. [DOI] [PubMed] [Google Scholar]
- 34.Sleder J, Chen YD, Cully MD, Reaven GM. Hyperinsulinemia in fructose-induced hypertriglyceridemia in the rat. Metabolism: clinical and experimental. 1980;29:303–305. doi: 10.1016/0026-0495(80)90001-3. [DOI] [PubMed] [Google Scholar]
- 35.Stepp DW, Frisbee JC. Augmented adrenergic vasoconstriction in hypertensive diabetic obese Zucker rats. American journal of physiology. 2002;282:H816–820. doi: 10.1152/ajpheart.00695.2001. [DOI] [PubMed] [Google Scholar]
- 36.Stepp DW, Pollock DM, Frisbee JC. Low-flow vascular remodeling in the metabolic syndrome X. American journal of physiology. 2004;286:H964–970. doi: 10.1152/ajpheart.00836.2003. [DOI] [PubMed] [Google Scholar]
- 37.Tesfamariam B, Brown ML, Cohen RA. Elevated glucose impairs endothelium-dependent relaxation by activating protein kinase C. The Journal of clinical investigation. 1991;87:1643–1648. doi: 10.1172/JCI115179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker BR. Glucocorticoids and cardiovascular disease. European journal of endocrinology/European Federation of Endocrine Societies. 2007;157:545–559. doi: 10.1530/EJE-07-0455. [DOI] [PubMed] [Google Scholar]