Abstract
Fms-like tyrosine kinase 1 (Flt1)/vascular endothelial growth factor (VEGF) receptor 1, a receptor for VEGF-A and placental growth factor, is expressed in the spongiotrophoblast layer that segregates the maternal and fetal vasculature in the mouse placenta. A soluble form of Flt1 (sFlt1) produced in the mouse and human placenta can also be detected in the maternal blood. Levels of maternal sFlt1 are elevated in preeclampsia, suggesting that placental sFlt1 plays roles in regulating the maternal vasculature during pregnancy. However, it remains to be determined whether placental Flt1/sFlt1 serves as a regulator of VEGF-A activity in the placenta per se. Here, we investigated the placental development in Flt1-deficient mice. Flt1 is expressed in a subpopulation of ectoplacental cone cells and later marks the spongiotrophoblast cells, peri/endovascular trophoblast cells, and trophoblast glycogen cells. The labyrinth of Flt1lacZ/lacZ placentae lacked the fetal capillary network because of a defect in allantoic mesoderm invasion. To address whether the absence of Flt1 in the trophoblast alone affects placental development, we investigated chimeric placentae comprised of Flt1lacZ/lacZ trophoblast and Flt1+/+ mesoderm, generated by tetraploid aggregation. Fetal growth was supported normally, and no defect in the formation of placental circulation into the maternal spiral artery or invasion of peri/endovascular trophoblast was detected. These findings indicate that trophoblast-derived Flt1/sFlt1 is dispensable for the initial establishment of the maternal–fetal interface in the mouse placenta. Targeting maternal sFlt1 levels for treatment of preeclampsia may thus be possible without affecting the proper formation of the placenta.
In the mammalian placenta, respiratory gases, nutrients, and wastes are exchanged between the fetal and maternal circulatory systems (1, 2). In the mouse, the exchange takes place in the labyrinth layer between fetal blood vessels lined by endothelial cells (ECs) and maternal blood sinuses lined by trophoblast cells. Fetal ECs within the labyrinth layer form a vascular network connected to the umbilical vessels, whereas maternal ECs of the incoming spiral arteries are replaced by peri/endovascular trophoblasts at the proximal deciduas. Maternal blood sinuses in the spongiotrophoblast and labyrinth layers are thus lined by trophoblast cells (3). The spongiotrophoblast layer is in intimate contact with the labyrinth layer and is a key cellular structure that secures the maternal–fetal exchange in the mouse placenta. Establishment of interactions between maternal and fetal vascular beds in the placenta is key to placental function and also has systemic effects on the maternal circulatory system. Shallow trophoblast invasion and remodeling of the maternal spiral arteries are associated with preeclampsia, a severe complication of pregnancy involving maternal hypertension, proteinuria, and glomerular malfunction. Understanding the regulation of placental vascular development is thus important for identifying target pathways for therapeutic intervention to prevent pregnancy complications.
Vascular endothelial growth factor (VEGF)-A is a major player in all aspects of vascular development, including proliferation, migration, survival, and regulation of vascular permeability (4–6). Targeted disruption of the VEGF-A gene in mice demonstrated that heterozygous mutant embryos die (7, 8). A hypermorphic allele of the VEGF-A gene, producing a higher amount of VEGF-A, led to severe abnormalities in heart development and embryonic lethality (9). These studies indicated that the concentration of VEGF-A needs to be tightly regulated for the normal development of the circulatory system.
VEGF-A binds to two receptor tyrosine kinases, fetal liver kinase 1 (Flk1)/VEGF receptor 2 and fms-like tyrosine kinase 1 (Flt1)/VEGF receptor 1 (10, 11). Both are strongly expressed in ECs during mouse embryogenesis (12–14). Previous genetargeting studies showed distinct roles for these VEGF-A receptors in vascular development. Flk1 is essential for the development of ECs and hematopoietic cells during embryogenesis in a cell autonomous manner (15, 16). Several lines of evidence suggest that Flk1 is the receptor responsible for most VEGF-A signaling activity. Targeted disruption of the Flt1 gene also resulted in embryonic lethality with severe defects in the embryonic vasculature (17). Flt1 lacking the tyrosine kinase domain is, however, sufficient for normal vascular development in mice (18), indicating that Flt1 function in embryogenesis does not depend on its signaling activity. Further analysis of the Flt1 mutants suggested that the lethal phenotype resulted from excess of endothelial and perhaps hemangioblast precursors (19). These studies indicated that a major role for Flt1 during the development of the vascular system is to regulate Flk1 signaling negatively by sequestering VEGF-A rather than transducing the Flt1 signal itself. In other words, Flt1 seems to work mainly as a “decoy” receptor for the VEGF-A agonist during mouse embryogenesis (4, 6).
During placental development, the expression of Flt1 is not only detected in ECs but also in the developing trophoblast (20, 21). As well as binding VEGF-A, Flt1 can be activated by another ligand of the VEGF family, placental growth factor (PlGF), which is highly produced by the placenta. Most of the Flt1 produced in the mouse and human placenta during later stages of gestation is a soluble form (sFlt1) generated by alternative splicing, leading to a premature termination after the sixth Ig-like domain (22, 23). sFlt1 binds both VEGF-A and PlGF and works as a soluble antagonist of their action. A recent report (24) showed that levels of maternal sFlt1 were elevated in preeclampsia and that administration of sFlt1 to pregnant rats can cause symptoms of preeclampsia with glomerular endotheliosis. It was proposed that the source of sFlt1 is the placental trophoblasts. This idea clearly suggests that placental Flt1 can play roles in regulating maternal vasculature during pregnancy. However, it is not clear whether Flt1 also plays a role in the development of the placenta per se.
Here, we investigate the importance of Flt1 for direct regulation of the mouse placental vascular bed development. A placental expression study showed that Flt1 is expressed in a subpopulation of ectoplacental cone cells and their derivatives and in fetal ECs. In Flt1 homozygous mutant placentae from Flt1+/lacZ intercrosses, the fetal capillary network was absent in the labyrinth because of a defect in allantoic mesoderm invasion. To address whether Flt1 in the trophoblast is required for placental circulation systems, we next generated chimeric embryos by aggregating Flt1+/+ embryonic stem (ES) cells with tetraploid embryos from Flt1+/lacZ intercrosses (25). The structure of Flt1 homozygous mutant placentae was completely rescued by the Flt1+/+ allantoic mesoderm. This finding suggests that the major role for trophoblast-derived Flt1 is likely to be via systemic effects of sFlt1 on the maternal circulation, rather than locally on the placental vasculature.
Materials and Methods
Mice. A colony of outbred mice heterozygous for a null allele of Flt1 (Flt1+/lacZ) (17) or Flk1 (Flk1+/lacZ) (15) was maintained for these studies. Heterozygous males were crossed to ICR (Harland Sprague–Dawley) random outbred females to generate stock for timed matings. Strategies for genotyping of these mice have been described (15, 19). Noon of the day on which the vaginal plug was detected was considered as embryonic day (E) 0.5. Embryos were genotyped by PCR analysis using either the embryo proper or yolk sac as a DNA source.
Tetraploid Aggregations. Two-cell stage embryos were collected from Flt1+/lacZ intercrosses, and the blastomeres were electrically fused to generate tetraploid embryos. Conceptuses in which trophoblast and primitive endoderm derivatives were Flt1+/+, Flt1+/lacZ, or Flt1lacZ/lacZ but the embryos were WT generated by aggregating enhanced yellow fluorescent protein (EYFP)+ YV1 ES cells (kindly provided by Andras Nagy, Mount Sinai Hospital) with individual tetraploid embryos. Aggregates were transferred into WT recipient mothers (26). Chimeric embryos were dissected at E12.5 or E14.5 and divided into three tissues, the embryo proper, placenta, and yolk sac. Both embryo proper and a half piece of placenta were checked for EYFP under a dissecting microscope with an enhanced GFP filter and were eventually fixed and stained with 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal). The other half of the placenta was fixed in 4% paraformaldehyde at 4°C for 3 h, dehydrated in alcohol, embedded in paraffin, and cut into 5-μm sections for histological analysis. After the yolk sac was treated with PBS containing 2.5% pancreatin (Sigma) and 0.5% trypsin (Sigma) for 1 h at 4°C, pieces of the visceral endoderm were peeled off from the yolk sac mesoderm. The visceral endoderm was used as a DNA source for genotyping of tetraploid embryos.
X-Gal Staining of the Placenta. After the uterine and ovarian vessels were tied, the uterus was harvested and fixed in 100 mM sodium phosphate (pH 7.3) containing 0.2% glutaraldehyde, 2 mM MgCl2, and 5 mM EGTA at room temperature for 1 h, and then placentae were dissected out. In some cases, placentae were cut into two pieces and fixed again in the same fixative at room temperature for 30–60 min depending on size of tissue. The rest of the procedure for X-Gal staining has been described (27). Background staining was minimal in WT control placentae at the stages examined (data not shown).
Histological Analysis of the Placenta. Hematoxylin and eosin staining was performed to observe general morphology. To observe the distribution of trophoblast cells and ECs in the chimeric placentae, immunohistochemistry with rabbit polyclonal antibody for cytokeratin (anti-Keratin, WSS, DAKO; 1:1,200) or rat mAb for platelet-endothelial cell adhesion molecule 1 (PECAM-1) (Mec13.3, Pharmingen; 1:200) was performed on adjacent sections, as described (3).
Results
Flt1 and Flk1 Expression in the Mature Mouse Placenta. Previous reports on RNA in situ hybridization analysis demonstrated that Flt1 and Flk1 are expressed in the mouse placenta (20, 22). We observed the expression pattern of Flt1 and Flk1 in the E12.5 placenta by detecting a lacZ reporter knocked into the Flt1 or Flk1 locus (15, 17). Whole-mount X-Gal staining of heterozygous conceptuses from heterozygous intercrosses showed that Flt1 was detected in spongiotrophoblast cells and the fetal and maternal ECs (Fig. 1A), whereas Flk1 was detected only in ECs (Fig. 1B). Although previous reports demonstrated that Flk1 expression declined in most organs at the later stage of mouse embryogenesis (10, 12), Flk1 was strongly expressed in the placenta at E12.5. In the labyrinth layer, staining for Flt1 showed more homogenous intensity than that for Flk1, probably because Flt1 was also detected in the mesenchymal cells (data not shown). In placentae of WT conceptuses in the heterozygous mothers, maternal ECs positive for Flt1 or Flk1 were absent from the spongiotrophoblast and labyrinth layer where the maternal blood directly contacts with the trophoblast (Fig. 1 C and D). Flk1-lacZ expression was also missing from the maternal spiral arteries in the proximal decidua (Fig. 1 B and D). Spiral arteries are characterized by their spiral nature, central location, and large diameter. These results were consistent with a previous report that maternal ECs are replaced by peri/endovascular trophoblasts in the proximal decidua (3), in a similar manner to trophoblast invasion of the spiral arteries in the human placenta.
Fig. 1.
Whole-mount analysis of Flt1 and Flk1 expression in mouse placenta at E12.5. A lacZ reporter knocked into the Flt1 or Flk1 locus was detected by whole-mount X-Gal staining of heterozygous or WT bisected conceptuses from heterozygous intercrosses. Note that Flk1 expression was absent in the spiral arteries of the proximal decidua (arrowheads). lb, labyrinth layer; sp, spongiotrophoblast layer.
Flt1 Expression in Conceptus-Derived Tissues During Placental Development. As the placenta is comprised of maternal-derived and conceptus-derived cells, it was difficult to see which tissues expressed Flt1, especially at the interface between them. We took advantage of the lacZ reporter at the Flt1 locus to distinguish the Flt1 expression in the trophoblast from that in maternal tissues during placental development. In Flt1+/lacZ conceptuses from crosses between Flt1+/lacZ males and WT females, we detected Flt1 expression by X-Gal staining in the conceptus-derived cells only (Fig. 2). At all of the stages examined, Flt1 was detected in ECs and mesenchymal cells derived from the allantoic mesoderm in the labyrinth layer. The expression of Flt1 was also detected in the trophoblast. At E8.5, Flt1 was expressed in a subpopulation of ectoplacental cone cells near the maternal decidua and in a few isolated cells in the maternal decidua (Fig. 2 A and B). After E10.5, Flt1 was detected in the spongiotrophoblast cells and in the peri/endovascular trophoblast cells around spiral arteries (Fig. 2 C–H). By E14.5, highly vacuolated cells positive for Flt1 were apparent in the spongiotrophoblast layer and maternal decidua (Fig. 2 G and H). Their foamy appearance and diffuse interstitial invasion of the deciduas suggested that these cells are trophoblast glycogen cells. Flt1 expression was not detected in trophoblast giant cells or trophoblast cells lining maternal blood sinuses in the labyrinth at all stages examined. These results showed that Flt1 marks a subpopulation of ectoplacental cone cells and later the spongiotrophoblast cells, peri/endovascular trophoblast cells, and trophoblast glycogen cells.
Fig. 2.
Whole-mount and histological analyses of Flt1 expression in conceptus-derived tissues during placental development. A lacZ reporter knocked into the Flt1 locus was detected by whole-mount X-Gal staining of Flt1+/lacZ bisected conceptuses from crosses between Flt1+/lacZ males and WT females. These X-Gal-stained placentae were sagittally sectioned for histological analysis. An arrow indicates an isolated cell in the maternal decidua; arrowheads indicate peri/endovascular trophoblast cells; asterisks indicate trophoblast glycogen cells. al, allantois; ch, chorion; epc, ectoplacental cone; lb, labyrinth layer; sa, spiral artery; sp, spongiotrophoblast layer. (Scale bars, 200 μm.)
Defect in Allantoic Mesoderm Invasion into the Chorionic Plate in Flt1lacZ/lacZ Placenta. To investigate the structure of Flt1 homozygous mutant placentae, conceptuses from Flt1+/lacZ intercrosses were dissected. Before chorioallantoic fusion ≈E8.5, the structure of the ectoplacental cone and chorion in the homozygous mutant was comparable to that in WT and heterozygous littermates (data not shown). At E9.5, the branching morphogenesis of the chorioallantoic interface and invasion of underlying allantoic mesoderm were apparent in Flt1+/lacZ conceptuses but not in Flt1lacZ/lacZ littermates (Fig. 3 A and B). The existence of X-Gal-stained cells on the surface of the chorion in Flt1lacZ/lacZ conceptus suggested that the allantois attached to the chorion but the allantoic mesoderm did not intrude into the chorionic plate (Fig. 3B). Further observation through serial sections of placentae revealed that homozygous mutant placentae were comparable to WT and heterozygous littermates in size and fundamental structure except for a defect in allantoic mesoderm invasion at this stage (Fig. 3 C and D). When we dissected conceptuses from Flt1+/lacZ intercrosses from E10.5 onward, we still detected, at a normal Mendelian frequency, homozygous mutant placentae, although they were smaller than WT and heterozygous placentae (data not shown). In some (≈30%) cases, a few blood vessels were observed within the Flt1lacZ/lacZ labyrinthine trophoblast, although the vasculature was minimal and nonfunctional (data not shown). Flt1lacZ/lacZ embryos die around the time of chorioallantoic fusion with excess blood vessel formation (17). However, loss of Flt1 in the placenta did not seem to promote excess vessel formation or invasion in the placenta; rather, fetal vascular invasion was reduced, suggesting that Flt1 signaling might be positively required for placental vascularization.
Fig. 3.
Whole-mount and histological analyses of Flt1 mutant placentae at E9.5. (A and B) Whole-mount X-Gal staining of bisected conceptuses from Flt1+/lacZ intercrosses. The branching morphogenesis of the chorioallantoic interface and invasion of underlying allantoic mesoderm were apparent in Flt1+/lacZ conceptuses, but not in Flt1lacZ/lacZ littermates. (C and D) Hematoxylin and eosin staining of conceptuses from Flt1+/lacZ intercrosses. Flt1lacZ/lacZ conceptus was comparable to Flt1+/+ littermates in size and fundamental structure except for a defect in allantoic mesoderm. al, allantois; ch, chorion; lb, labyrinth layer; sp, spongiotrophoblast layer; ys, yolk sac. (Scale bars, 100 μm.)
Flt1lacZ/lacZ Tetraploid Chimeric Embryos Generated by Aggregation with WT ES Cells. To address the role for Flt1 in the trophoblast, we investigated chimeric placentae comprised of Flt1 mutant trophoblast and WT allantoic mesoderm and maternal cells. For this purpose, we generated chimeric embryos by aggregating Flt1+/+ EYFP+ ES cells with individual tetraploid embryos from Flt1+/lacZ intercrosses and transferred them into WT recipient mothers (Fig. 4A). In these chimeric embryos, the embryo proper and extraembryonic mesoderm were totally derived from ES cells, whereas the trophoblast and visceral endoderm were derived from tetraploid embryos.
Fig. 4.
Tetraploid embryo aggregations and chimeric analysis. (A) Chimeric placentae were generated by aggregating Flt1+/+ EYFP+ ES cells with individual tetraploid embryos from Flt1+/lacZ intercrosses and transferred into WT recipient mothers. In these chimeric embryos, the embryo proper, extraembryonic mesoderm, and maternal tissue were of Flt1+/+ origin, whereas the trophoblast and the visceral endoderm of the yolk sac were derived from tetraploid embryos. The visceral endoderm was peeled off from the mesoderm for genotyping tetraploid embryos by PCR. (B–E) Fluorescent whole-mount images of tetraploid chimeric embryos at E12.5. (B and C) Bisected placentae. Dashed lines show the region of the fetal placenta. Flt1lacZ/lacZ chimeric placentae experienced invasion of the EYFP+ allantoic mesoderm in the labyrinth layer similar to Flt1+/+ chimeric placentae. (D and E) The fetal growth supported by Flt1lacZ/lacZ chimeric placentae was comparable in size and morphology to that supported by Flt1+/+ chimeric placentae.
Flt1 mutant tetraploid chimeric embryos were dissected at E12.5 or E14.5. At the both stages, we obtained Flt1 WT, heterozygous, and homozygous chimeric embryos (Table 1). Observation of chimeric embryos under fluorescent microscopy confirmed that the embryos and the mesodermal components in the labyrinth layer of the placenta were positive for EYFP (Fig. 4 B–E). ECs derived from tetraploid embryos were not found in either embryo or placentae after X-Gal staining (data not shown). Chimeric placentae comprised of the Flt1lacZ/lacZ trophoblast and Flt1+/+ allantoic mesoderm (Flt1lacZ/lacZ chimeric placentae) showed invasion of the EYFP+ allantoic mesoderm in the labyrinth layer similar to WT chimeric placentae at E12.5 (Fig. 4 B and C) and E14.5 (data not shown). Fetal development supported by Flt1lacZ/lacZ chimeric placentae was comparable in size and morphology to that supported by Flt1+/+ chimeric placentae at E12.5 (Fig. 4 D and E) and E14.5 (data not shown). These results showed that absence of Flt1 in the trophoblast alone had no apparent effect on development of the placenta or the fetus.
Table 1. Generation and genotype of chimeric embryos between WT ES cells and tetraploid embryos from Flt1+/lacZ intercrosses.
| Flt1
|
|||||
|---|---|---|---|---|---|
| Stage | No. transferred | No. recovered | +/+ | +/lacZ | lacZ/lacZ |
| E12.5 | 105 | 16 | 2 | 10 | 4 |
| E14.5 | 186 | 16 | 6 | 9 | 1 |
| Total | 291 | 32 | 8 | 19 | 5 |
| 25% | 59% | 16% | |||
Tetraploid chimeric embryos were generated and genotyped as described in Materials and Methods (Fig. 4). The numbers of chimeric embryos transferred into recipient mothers and that of EYFP+ normal embryos recovered at dissection are indicated. No abnormal EYFP+ fetuses were found.
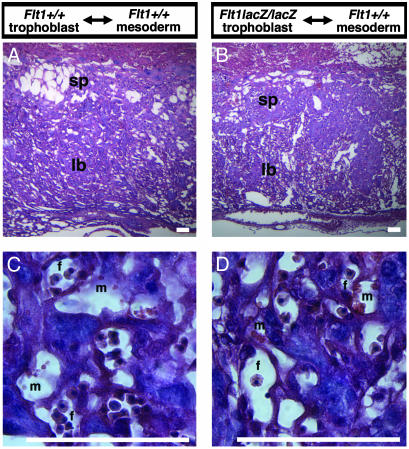
No Alteration of Morphogenesis and Maternal–Fetal Interface in Flt1lacZ/lacZ Chimeric Placentae. We investigated the chimeric placentae in histological sections. Hematoxylin and eosin staining showed that the fundamental structure of the placenta was comparable between Flt1+/+ and Flt1lacZ/lacZ chimeric placentae (Fig. 5 A and B). Observation at higher magnification did not reveal any abnormality in the distribution of fetal (nucleated) and maternal (enucleated) red blood cells in the labyrinth layer of Flt1+/+ and Flt1lacZ/lacZ chimeric placentae (Fig. 5 C and D), indicating that fetal and maternal circulation separately developed in Flt1lacZ/lacZ chimeric placentae as in WT placentae. These results indicated that the absence of Flt1 in the trophoblast did not affect either the allantoic mesoderm invasion or the formation of maternal or fetal circulation in the labyrinth layer.
Fig. 5.
Hematoxylin and eosin staining of Flt1 chimeric placentae. The fundamental structure of the placenta was comparable between Flt1+/+ and Flt1lacZ/lacZ chimeric placentae. Observation at higher magnification did not reveal any abnormality in the distribution of fetal (f; nucleated) and maternal (m; enucleated) red blood cells in the labyrinth layer. lb, labyrinth layer; sp, spongiotrophoblast layer. (Scale bars, 100 μm.)
We performed immunohistochemistry on adjacent sections for PECAM-1, a marker for ECs, and for cytokeratin, a marker for trophoblast cells, to obtain a more detailed view of the endothelial/trophoblast interface in the chimeric placentae. Consistent with the distribution of EYFP+ mesodermal cells (Fig. 4 B and C), the localization of fetal ECs were comparable between Flt1+/lacZ and Flt1lacZ/lacZ chimeric placentae (Fig. 6 A and B). The spongiotrophoblast layer was comprised of cytokeratin+ trophoblast cells in Flt1+/lacZ and Flt1lacZ/lacZ chimeric placentae (Fig. 6 C and D). We did not detect PECAM-1+ cytokeratin– cells in the spongiotrophoblast layer, suggesting that the distribution of maternal ECs was not affected. We detected cytokeratin-positive peri/endovascular trophoblast cells associated with maternal spiral arteries in Flt1lacZ/lacZ chimeric placentae (Fig. 6D), indicating that trophoblast invasion and vascular remodeling was not affected by the loss of Flt1.
Fig. 6.
Histological analysis of ECs and trophoblast cells in Flt1 chimeric placentae. Immunostaining for PECAM-1 and for cytokeratin on adjacent sections of Flt1+/lacZ and Flt1lacZ/lacZ chimeric placentae. Note the peri/endovascular trophoblast cells (arrowheads) associated with maternal blood vessels. lb, labyrinth layer; sp, spongiotrophoblast layer. (Scale bars, 300 μm.)
Discussion
Here, we investigated the role of Flt1 in mouse placental development. Flt1 is not only expressed in ECs and mesenchymal cells but in some populations of the trophoblast such as the ectoplacental cone cells, spongiotrophoblast cells, peri/endovascular trophoblast cells, and trophoblast glycogen cells. Flt1lacZ/lacZ placentae lack a fetal capillary network in the labyrinth because of a defect in allantoic mesoderm invasion. Chimeric placentae comprised of Flt1lacZ/lacZ trophoblast and Flt1+/+ allantoic mesoderm are morphologically and functionally comparable, however, showing that the placental defects observed in Flt1lacZ/lacZ conceptuses are related to fetal endothelial defects and that there is no clear role for trophoblast-expressed Flt1 in regulating placental vascular architecture.
Flt1 Expression in the Mouse Placenta. Flt1 was previously shown to be expressed in the spongiotrophoblast in mice (20) and in the extravillous trophoblast in human placentae (21). By using a lacZ reporter knocked into the Flt1 locus, we showed that Flt1 is expressed in a subpopulation of ectoplacental cone cells near the decidua basalis and later in the spongiotrophoblast, peri/endovascular trophoblast cells, and trophoblast glycogen cells. Previous studies (28, 29) showed that the trophoblast glycogen cell expresses 4311/Tpbp, a well known marker for spongiotrophoblast cells (30), indicating that this cell type is derived from the spongiotrophoblast. Our expression study on Flt1 also supports this idea. At all stages examined, the expression pattern of Flt1 in the trophoblast is actually similar to that of 4311. Flt1 will be a useful marker to characterize differentiated trophoblast cells in vivo or derivatives from trophoblast stem cells (31) in vitro.
Formation of Circulatory Systems in Flt1-Deficient Placentae. Flt1lacZ/lacZ placentae showed defects in allantoic mesoderm invasion into the chorionic plate and lacked the fetal capillary network at later stages of development. Chorioallantoic fusion did occur because fetal blood vessels were occasionally detected within the trophoblast even after the death of the mutant embryos. Given that the embryo dies because of vascular failure around the time of chorioallantoic fusion, it is possible that placental vascular defects are secondary to the death of the embryo. However, it is striking that no excess vessel formation occurs in the placenta as is observed in the embryo itself (17). It has recently been shown that activation of Flt1 by placental growth factor (PlGF) results in intermolecular transphosphorylation of Flk1, thus amplifying the effects of Flk1 signaling (32). Although no placental defect was reported in Flt1-deficient mice (18), loss of endothelial Flt1 might reduce rather than enhance VEGF-A-driven angiogenesis in the placenta where PlGF is highly expressed. On the other hand, Flt1 expression in the trophoblast must play a different role because Flk1 is not coexpressed. This notion is confirmed by the analysis of tetraploid chimeric embryos in which allantoic mesoderm invasion was not affected by the absence of Flt1 in the trophoblast.
Placentae from tetraploid aggregates in which the trophoblast was Flt1lacZ/lacZ and the maternal and fetal components were Flt1+/+, showed no morphological defects and supported normal fetal growth by E14.5. The absence of Flt1 in the trophoblast did not obviously affect the formation of fetal and maternal circulatory systems in the undisturbed state. Because the placental labyrinth reaches its definitive features ≈E14 (33), our results indicate that Flt1 in the trophoblast is not necessary for placental development per se. Even when we examined in more detail the arrangement of fetal and maternal blood vessels and the trophoblast invasion of the spiral arteries, they were normal. Thus, although Flt1 is required for placental development, it is not required in the trophoblast for the development of either the fetal or maternal vascular bed.
What Is the Role for Flt1/sFlt1 Produced by the Trophoblast? It has been shown in both mouse and human that the placenta produces much of its Flt1 protein in the truncated sFlt1 form, that acts as an antagonist of VEGF-A action and can be secreted into the bloodstream. Thus, trophoblast-derived Flt1 could play a role as a modulator of angiogenic influences on the placenta, perhaps acting as an insurance if VEGF-A levels in the placenta rise anomalously. He et al. (22) reported that the treatment of pregnant mice with exogenous VEGF-A resulted in an increase in the number of resorption sites. In the placenta of ongoing pregnancies, fibrin deposition was apparent within the labyrinth layer, suggesting that the excess amount of VEGF-A injected into the mother went across the spongiotrophoblast layer to the labyrinth layer. In the Flt1lacZ/lacZ chimeric placentae, we did not detect abnormal fibrin deposition by Martius Scarlet Blue staining (data not shown), suggesting that VEGF-A activity was not aberrantly high in the absence of sFlt1 produced by the trophoblast. Of note is that both fetal and maternal ECs were of Flt1+/+ origin in the Flt1lacZ/lacZ chimeric placentae. The Flt1 protein on their own surface may be enough to tightly regulate Flk1 signaling by sequestering VEGF-A.
The second possibility is that trophoblast-derived sFlt1 acts systemically to modulate the maternal angiogenic changes, which are quite profound during pregnancy. It has been shown that elevated levels of circulating sFlt1 are associated with preeclampsia in humans, and that administration of sFlt1 to pregnant rats causes preeclamptic symptoms (24). Loss of placental Flt1 did not produce any obvious deleterious effects on the development of other fetuses in the pregnancies of the Flt1+/lacZ heterozygous crosses. However, multiple conceptuses existed in a single pregnant female mouse, with only a few placentae comprised of Flt1lacZ/lacZ trophoblast. Although local levels of VEGF-A may be affected, this loss may not induce a dramatic change of VEGF-A concentration in the maternal blood. The mouse is not therefore an easy system to use for analysis of effects of fetal genotype on maternal environment. It is clear, however, from the current study, that trophoblast-derived Flt1/sFlt1 is dispensable for the initial establishment of maternal–fetal interface in the mouse placenta. If reduction of maternal sFlt1 is developed as a therapy for preeclampsia, our results suggest that it will not have any deleterious effects on the placenta itself.
Acknowledgments
We thank Dr. Andras Nagy and Marina Gertsentein for the YV1 ES cell line; Dr. Pantelis Georgiades for helpful discussion; Qiang Xu, Ken Harpal, Heather Hill, Lily Morikawa, and Jorge Cabezas for technical assistance; and Drs. Lee Adamson and Tilo Kunath for a critical reading of this manuscript. This work was supported by grants from the Canadian Institutes of Health Research and the National Cancer Institute of Canada. M.H. is a recipient of The Samuel Lunenfeld Research Institute/Ontario Research and Development Challenge Fund Fellowship. J.R. is a Canadian Institutes of Health Research Distinguished Investigator.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: En, embryonic day n; EC, endothelial cell; ES, embryonic stem; EYFP, enhanced yellow fluorescent protein; Flk1, fetal liver kinase 1; Flt1, fms-like tyrosine kinase 1; PECAM-1, platelet-endothelial cell adhesion molecule 1; sFlt1, soluble Flt1; VEGF, vascular endothelial growth factor; X-Gal, 5-bromo-4-chloro-3-indolyl β-d-galactoside.
References
- 1.Cross, J. C., Werb, Z. & Fisher, S. J. (1994) Science 266, 1508–1518. [DOI] [PubMed] [Google Scholar]
- 2.Georgiades, P., Ferguson-Smith, A. C. & Burton, G. J. (2002) Placenta 23, 3–19. [DOI] [PubMed] [Google Scholar]
- 3.Adamson, S. L., Lu, Y., Whiteley, K. J., Holmyard, D., Hemberger, M., Pfarrer, C. & Cross, J. C. (2002) Dev. Biol. 250, 358–373. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara, N. (1999) J. Mol. Med. 77, 527–543. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet, P. & Collen, D. (2000) Ann. N.Y. Acad. Sci. 902, 249–262; discussion 262–264. [DOI] [PubMed] [Google Scholar]
- 6.Yancopoulos, G. D., Davis, S., Gale, N. W., Rudge, J. S., Wiegand, S. J. & Holash, J. (2000) Nature 407, 242–248. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet, P., Ferreira, V., Breier, G., Pollefeyt, S., Kieckens, L., Gertsenstein, M., Fahrig, M., Vandenhoeck, A., Harpal, K., Eberhardt, C., et al. (1996) Nature 380, 435–439. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara, N., Carver Moore, K., Chen, H., Dowd, M., Lu, L., O'Shea, K. S., Powell Braxton, L., Hillan, K. J. & Moore, M. W. (1996) Nature 380, 439–442. [DOI] [PubMed] [Google Scholar]
- 9.Miquerol, L., Langille, B. L. & Nagy, A. (2000) Development (Cambridge, U.K.) 127, 3941–3946. [DOI] [PubMed] [Google Scholar]
- 10.Millauer, B., Wizigmann Voos, S., Schnurch, H., Martinez, R., Moller, N. P., Risau, W. & Ullrich, A. (1993) Cell 72, 835–846. [DOI] [PubMed] [Google Scholar]
- 11.de Vries, C., Escobedo, J. A., Ueno, H., Houck, K., Ferrara, N. & Williams, L. T. (1992) Science 255, 989–991. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi, T. P., Dumont, D. J., Conlon, R. A., Breitman, M. L. & Rossant, J. (1993) Development (Cambridge, U.K.) 118, 489–498. [DOI] [PubMed] [Google Scholar]
- 13.Breier, G., Clauss, M. & Risau, W. (1995) Dev. Dyn. 204, 228–239. [DOI] [PubMed] [Google Scholar]
- 14.Fong, G. H., Klingensmith, J., Wood, C. R., Rossant, J. & Breitman, M. L. (1996) Dev. Dyn. 207, 1–10. [DOI] [PubMed] [Google Scholar]
- 15.Shalaby, F., Rossant, J., Yamaguchi, T. P., Gertsenstein, M., Wu, X. F., Breitman, M. L. & Schuh, A. C. (1995) Nature 376, 62–66. [DOI] [PubMed] [Google Scholar]
- 16.Shalaby, F., Ho, J., Stanford, W. L., Fischer, K. D., Schuh, A. C., Schwartz, L., Bernstein, A. & Rossant, J. (1997) Cell 89, 981–990. [DOI] [PubMed] [Google Scholar]
- 17.Fong, G. H., Rossant, J., Gertsenstein, M. & Breitman, M. L. (1995) Nature 376, 66–70. [DOI] [PubMed] [Google Scholar]
- 18.Hiratsuka, S., Minowa, O., Kuno, J., Noda, T. & Shibuya, M. (1998) Proc. Natl. Acad. Sci. USA 95, 9349–9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fong, G. H., Zhang, L., Bryce, D. M. & Peng, J. (1999) Development (Cambridge, U.K.) 126, 3015–3025. [DOI] [PubMed] [Google Scholar]
- 20.Dumont, D. J., Fong, G. H., Puri, M. C., Gradwohl, G., Alitalo, K. & Breitman, M. L. (1995) Dev. Dyn. 203, 80–92. [DOI] [PubMed] [Google Scholar]
- 21.Clark, D. E., Smith, S. K., Sharkey, A. M. & Charnock-Jones, D. S. (1996) Hum. Reprod. 11, 1090–1098. [DOI] [PubMed] [Google Scholar]
- 22.He, Y., Smith, S. K., Day, K. A., Clark, D. E., Licence, D. R. & Charnock-Jones, D. S. (1999) Mol. Endocrinol. 13, 537–545. [DOI] [PubMed] [Google Scholar]
- 23.Clark, D. E., Smith, S. K., He, Y., Day, K. A., Licence, D. R., Corps, A. N., Lammoglia, R. & Charnock-Jones, D. S. (1998) Biol. Reprod. 59, 1540–1548. [DOI] [PubMed] [Google Scholar]
- 24.Maynard, S. E., Min, J. Y., Merchan, J., Lim, K. H., Li, J., Mondal, S., Libermann, T. A., Morgan, J. P., Sellke, F. W., Stillman, I. E., et al. (2003) J. Clin. Invest. 111, 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagy, A., Rossant, J., Nagy, R., Abramow Newerly, W. & Roder, J. C. (1993) Proc. Natl. Acad. Sci. USA 90, 8424–8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagy, A. & Rossant, J. (2000) in Gene Targeting: A Practical Approach, ed. Joyner, A. L. (Oxford Univ. Press, New York), pp. 177–206.
- 27.Ciruna, B. G., Schwartz, L., Harpal, K., Yamaguchi, T. P. & Rossant, J. (1997) Development (Cambridge, U.K.) 124, 2829–2841. [DOI] [PubMed] [Google Scholar]
- 28.Redline, R. W., Chernicky, C. L., Tan, H. Q. & Ilan, J. (1993) Mol. Reprod. Dev. 36, 121–129. [DOI] [PubMed] [Google Scholar]
- 29.Teesalu, T., Blasi, F. & Talarico, D. (1998) Dev. Dyn. 213, 27–38. [DOI] [PubMed] [Google Scholar]
- 30.Lescisin, K. R., Varmuza, S. & Rossant, J. (1988) Genes Dev. 2, 1639–1646. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka, S., Kunath, T., Hadjantonakis, A. K., Nagy, A. & Rossant, J. (1998) Science 282, 2072–2075. [DOI] [PubMed] [Google Scholar]
- 32.Autiero, M., Waltenberger, J., Communi, D., Kranz, A., Moons, L., Lambrechts, D., Kroll, J., Plaisance, S., De Mol, M., Bono, F., et al. (2003) Nat. Med. 9, 936–943. [DOI] [PubMed] [Google Scholar]
- 33.Muntener, M. & Hsu, Y. C. (1977) Acta Anat. 98, 241–252. [PubMed] [Google Scholar]