Abstract
Long QT syndrome (LQTS) is an inherited disorder characterized by prolonged QT intervals and potentially life-threatening arrhythmias. Mutations in 12 different genes have been associated with LQTS. Here we describe a patient with LQTS who has a mutation in KCNQ1 as well as a polymorphism in KCNH2. The proband (MMRL0362), a 32-year-old female, exhibited multiple ventricular extrasystoles and one syncope. Her ECG (QT interval corrected for heart rate (QTc) = 518ms) showed an LQT2 morphology in leads V4–V6 and LQT1 morphology in leads V1–V2. Genomic DNA was isolated from lymphocytes. All exons and intron borders of 7 LQTS susceptibility genes were amplified and sequenced. Variations were detected predicting a novel missense mutation (V110I) in KCNQ1, as well as a common polymorphism in KCNH2 (K897T). We expressed wild-type (WT) or V110I Kv7.1 channels in CHO-K1 cells cotransfected with KCNE1 and performed patch-clamp analysis. In addition, WT or K897T Kv11.1 were also studied by patch clamp. Current–voltage (I-V) relations for V110I showed a significant reduction in both developing and tail current densities compared to WT at potentials >+20 mV (p < 0.05; n = 8 cells, each group), suggesting a reduction in IKs currents. K897T- Kv11.1 channels displayed a significantly reduced tail current density compared with WT-Kv11.1 at potentials >+10 mV. Interestingly, channel availability assessed using a triple-pulse protocol was slightly greater for K897T compared with WT (V0.5 = −53.1 ± 1.13 mV and −60.7 ± 1.15 mV for K897T and WT, respectively; p < 0.05). Comparison of the fully activated I-V revealed no difference in the rectification properties between WT and K897T channels. We report a patient with a loss-of-function mutation in KCNQ1 and a loss-of-function polymorphism in KCNH2. Our results suggest that a reduction of both IKr and IKs underlies the combined LQT1 and LQT2 phenotype observed in this patient.
Keywords: genetics, arrhythmias, electrophysiology, HERG
Introduction
Long QT syndrome (LQTS) is an inherited disorder characterized by prolonged QT intervals and potentially life-threatening arrhythmias (Splawski et al. 2000; Keating and Sanguinetti 2001). To date, mutations in multiple genes have been identified as contributing to the development of LQTS (Splawski et al. 2000, 2004; Zhang et al. 2005; Medeiros-Domingo et al. 2007; Ueda et al. 2008). Some affected LQTS patients have symptoms ranging from syncope to severe arrhythmias such as torsades de pointes, but in most cases patients are asymptomatic (Zhang et al. 2005; Medeiros-Domingo et al. 2007; Ueda et al. 2008).
Variations of phenotype expression are thought to be attributable to the severity of the disease-causing mutation, as well as the possible co-existence of other genetic variations, including single nucleotide polymorphisms (SNP) that can alter expression and function of other genes. This has been demonstrated with several SNPs, such as D85N (in KCNE1) (Salisbury et al. 2006), K897T (in KCNH2) (Crotti et al. 2005), and H558R (in SCN5A) (Kupershmidt et al. 2002; Westenskow et al. 2004; Poelzing et al. 2006). In all these cases, the patients also had mutations in the same gene, causing the disease phenotype. SNPs have been shown to modify clinical expression either by aggravating the clinical phenotype (Kupershmidt et al. 2002) or by attenuating the clinical phenotype (Yang et al. 2002; Westenskow et al. 2004).
We present an individual who carried an inherited common polymorphism in KCNH2 (the gene encoding the Kv11.1 channel) as well as a mutation in KCNQ1 (the gene encoding the Kv7.1 channel), resulting in LQTS. ECG analysis of the patient showed characteristics of both LQT1 and LQT2. Functional analysis of the changes responsible for the phenotypes was determined in a mammalian heterologous expression system.
Methods
ECG analysis
QT interval was measured and adjusted to heart rate (QTc), according to Bazett’s formula (Bazett 1920). The end of the T wave was defined as the intersection with the isoelectric line of a tangent drawn to the steepest portion (the maximum slope) of the descending part of the T wave. Clinical and genetic studies were performed in accordance with human subject guidelines after written informed consent was obtained according to protocols approved by the local institutional review boards.
Genetic evaluation
After informed consent was obtained, blood was collected from family members. Genomic DNA was extracted from peripheral blood leukocytes and from fresh and frozen tissue using a commercial kit (Puregene, Gentra Systems Inc., Minneapolis, Minn.). The genomic DNA was amplified by PCR on GeneAmp PCR System 9700 (Applied Biosystems, Foster City, Calif.). All exons and intron borders of the KCNQ1, KCNH2, SCN5A, KCNE2, and KCNE2 genes were amplified and analyzed by direct sequencing. PCR products were purified with a commercial reagent (ExoSAP-IT, USB, Cleveland, Ohio) and directly sequenced from both directions using ABI PRISM 3100 Automatic DNA sequencer (Applied Biosystems, Foster City, Calif.). Electropherograms were visually examined for heterozygous peaks and compared with reference sequences for homozygous variations (GenBank accession No. NM_000219) using the CodonCode Aligner Ver. 2.0.4 (CodonCode Corp., Dedham, Mass).
Mutagenesis
KCNH2 cDNA (accession No. NM_000238) in a bicistronic vector encoding green fluorescent protein (GFIrHerg) was a kind gift from Dr. Connie Bezzina. The K897T polymorphism was introduced using the QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, Calif.) and the following primers: sense: GTAGCCGGGGCCGGC-CGGGGGGGGCCGTGGGGGGAGAGCCCGTC; antisense: GACGGGCTCTCCCCCCACGGCCCCCCCCGGCCGGCC-CCGGCTAC.
The mutated plasmid was sequenced to ensure the presence of the K897T polymorphism, as well as the absence of other substitutions introduced by the DNA polymerase.
The wild-type (WT) KCNQ1 and KCNE1 cDNAs were generated as described previously (Aizawa et al. 2007). The substitution of isoleucine at position 110 was introduced to WT-KCNQ1 cDNA by site-directed mutagenesis. The V110I-KCNQ1 clone was confirmed by sequencing.
Transient expression in CHO-K1 cells
Chinese hamster ovary (CHO-K1) cells were grown in GIBCO F-12 nutrient mixture (GIBCO, Invitrogen, Carlsbad, Calif.) in 35 mm culture dishes and placed in a 5% CO2 incubator at 37 °C. The cells were transfected using FuGENE6 (Roche Diagnostics, Indianapolis, Ind.) and electrophysiological studies were carried out 48 to 72 h after transfection on cells expressing fluorescence.
Electrophysiology
Voltage clamp recordings were made as previously described (Cordeiro et al. 2005), using patch pipettes fabricated from borosilicate glass capillaries (1.5 mm O.D., Fisher Scientific, Pittsburgh, Penn). The pipettes were pulled using a gravity puller (NARISHIGE Corp., East Meadow, N.Y.) and filled with pipette solution of the following composition (mmol/L): 10 KCl, 125 K aspartate, 1.0 MgCl2, 10 HEPES, 10 NaCl, 5 MgATP, and 10 EGTA; pH 7.2 (KOH). The pipette resistance ranged from 1 to 4 MΩ when filled with the internal solution. The perfusion solution contained (mmol/L): 130 NaCl, 5 KCl, 1.8 CaCl2, 1. MgCl2, 2.8 Na acetate, 10 HEPES; pH 7.4 with NaOH. Current signals were recorded using a MultiClamp 700A amplifier (Molecular Devices, Sunnyvale, Calif.) and series resistance errors were reduced by about 60%–70% with electronic compensation. Signals were acquired at 5–50 kHz (Digidata 1322, Molecular Devices) and analyzed using a microcomputer running pCLAMP 9 software (Molecular Devices). All recordings were made at room temperature.
Biotinylation of cell surface proteins
Two days after transfection, cells with cDNA encoding either WT or mutant channels were washed twice with PBS. Nontransfected cells were used as negative control. Membrane-impermeable sulfosuccinimidyl-2-(biotinamido)-ethyl-1,3-dithiopropionate (EZ-Link NHS-SS-Biotin; Pierce Protein Research Products, Rocford, Ill.) was freshly dissolved in PBS++ (PBS containing 1 mmol/L CaCl2 and 1 mmol/L MgCl2) to a final concentration of 0.25 mg/mL. All steps were performed at 4 °C and solutions were ice cold. Cells were incubated for 30 min in 4 mL biotin solution, and subsequently washed with and then incubated for 20 min in PBS++ containing 100 mmol/L glycine to quench the residual biotin. Cells were washed with PBS and scraped into an Eppendorf tube. Samples were centrifuged for 2 min at 5000g and pellets were resuspended in lysis buffer (50 mmol/L Tris–HCl, pH 7.4; 10 mmol/L NaCl; 0.5% DOC, 1% Triton X-100, protease inhibitors). After sonication on ice at low power to prevent foaming, samples were incubated on ice in lysis buffer for 30 min with agitation, then centrifuged for 10 min at 10 000g. The supernatants were transferred to fresh tubes, an aliquot taken to determine protein concentration, and 125 μg protein lysate used for isolation of biotinylated proteins using streptavidin-coupled beads (Dynabeads MyOne Streptavidin T1, Invitrogen) according to the manufacturer’s instructions. Briefly, beads were washed in Tris-buffered saline, added to the samples, and incubated overnight at 4 °C with gentle rotation. Protein beads were separated using a magnet, and supernatant transferred to a new tube for analysis of streptavidin bead unbound proteins. After repeated washings with Tris-buffered saline, the biotinylated proteins bound to the beads were eluted by boiling in SDS–PAGE sample buffer containing 100 mmol/L dithiothreitol. Beads were again separated using a magnet, and the biotinylated proteins containing supernatants were transferred to new tubes. Total cell lysates and streptavidin precipitates were analyzed by Western blotting.
Western blotting
Total lysate and CHO biotinylated and unbound fractions were separated on 4%–15% SDS–PAGE via a minigel system (Bio-Rad Biosciences, Copenhagen, Denmark). Proteins were transferred onto nitrocellulose transfer membranes (Bio-Rad; 0.2 μm), and membranes were blocked in 5% nonfat milk in PBS solution for 2 h at room temperature and then incubated with primary antibody against Kv7.1 (Calloe et al. 2007) or calnexin (1:2000; Nordic Biosite, Copenhagen, Denmark) overnight. Proteins were detected with HRP-conjugated donkey anti-mouse and anti-rabbit secondary antibodies (1:10 000; Jackson ImmunoResearch Laboratories, Newmarket, Suffolk, UK). Enhanced chemiluminescence incubation was performed using standard procedures, and immunoblots were exposed on Hyperfilm ECL (GE Healthcare, Piscataway, N.J.).
Action potential simulations
Left ventricular epicardial action potentials were simulated using a modified Luo-Rudy 2 model (Luo and Rudy 1994; Zeng et al. 1995), which includes the transient outward current (Ito) as described previously (Dumaine et al. 1999). Maximal conductance of L-type Ca2+ current (ICaL) was decreased by 50% to obtain a realistic peak of this current (Dumaine et al. 1999). The effect of V110I mutation in Kv7.1 current (IKs) was simulated by decreasing maximal conductance of the current to 42% of the normal value, in agreement with experimental data. The effect of K897T polymorphism in Kv11.1 current (IKr) was simulated by decreasing maximal conductance of the current to 59% of the normal value. A train of 10 stimuli with BCL of 1000 ms was applied, and the last action potentials are presented in Fig. 3B. Differential equations were solved numerically using an adaptive second-order Runge-Kutta method (time steps <10 μs).
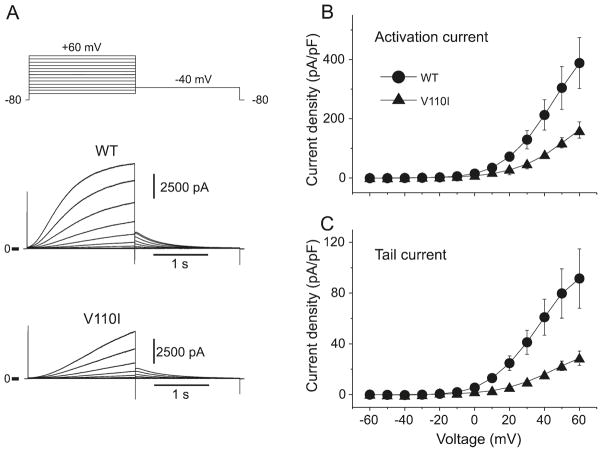
Fig. 3.
V110I mutation in KCNQ1 causes a reduction in Kv7.1 (IKs). Representative current traces recorded from wild-type (WT) and V110I expressed in CHO-K1 cells (A). Both developing current (B) and tail current (C) amplitudes were significantly reduced with the V110I mutant, compared with WT.
Statistical analysis
Membrane currents were analyzed with Clampfit 9 software (Molecular Devices). Results from pooled data are presented as mean ± SEM and n represents the number of cells in each experiment. Statistical analysis was performed using an ANOVA followed by a Student Newman–Keuls test using SigmaStat ver. 3.5 software (Systat Software Inc., Chicago, Ill.). p < 0.05 was considered to indicate statistical significance.
Results
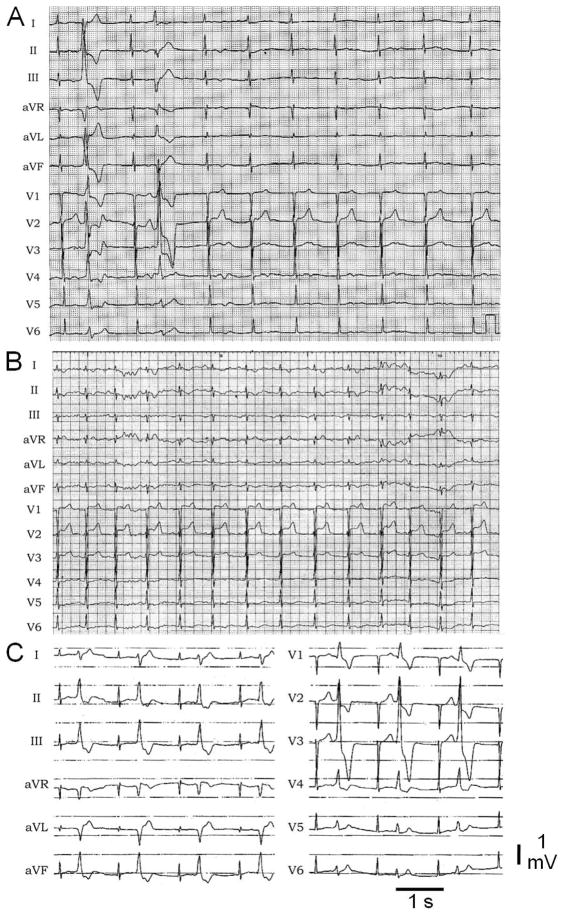
The proband (MMRL0362), a 32-year-old female, experienced multiple ventricular extrasystoles (premature ventricular contraction, PVC) and 1 episode of syncope in 2006. The syncopal episode occurred immediately after the proband was rushing up the stairs and was startled by a person she was not expecting. A preliminary ECG showed a LQT2 morphology in leads V2–V6 and an LQT1 morphology in leads V1–V2 with a QTc = 518 ms (Figs. 1A, 1B). Beta blockers were started in 2006 (metoprolol 50 mg bid), but the medication had to be stopped due to significant presyncope. ECG and repetitive Holter recordings showed episodic polymorphic ventricular extrasystoles at a rate of up to 4000/day (Fig. 1A). The mechanism of the PVC is still not clear. Two cardiac MRIs during follow-up did not reveal a structural abnormality in the patient, and especially no late enhancement. An ajmaline challenge was negative, ruling out the possibility of drug-induced Brugada syndrome. Even though the PVCs were polymorphic in nature, the decision was made to try to reduce the PVC burden. A preceding Purkinje spike was not seen. Therefore, a radiofrequency ablation of a left inferolateral focus was performed in 2008. During a follow-up in 2010, the patient still presented with recurrence of PVCs at a rate of up to 1000/day. The patient declined reablation. The patient presented with no other symptoms. Family history was negative for palpitations or syncope. The only remarkable case is the maternal grandfather, who experienced sudden cardiac death at the age of 59.
Fig. 1.
Electrocardiograms of the patient. Twelve-lead ECG showing prolonged QT intervals with 2 ventricular premature beats (A). The first (left axis deviation, right bundle branch block) was the dominant type in sequential Holter ECG recordings. (A and B) Low voltage bifurcated T waves characteristic of LQT2 are observed in most leads, whereas broad-based tall T waves characteristic of LQT1 are present in leads V2 and V3 (C).
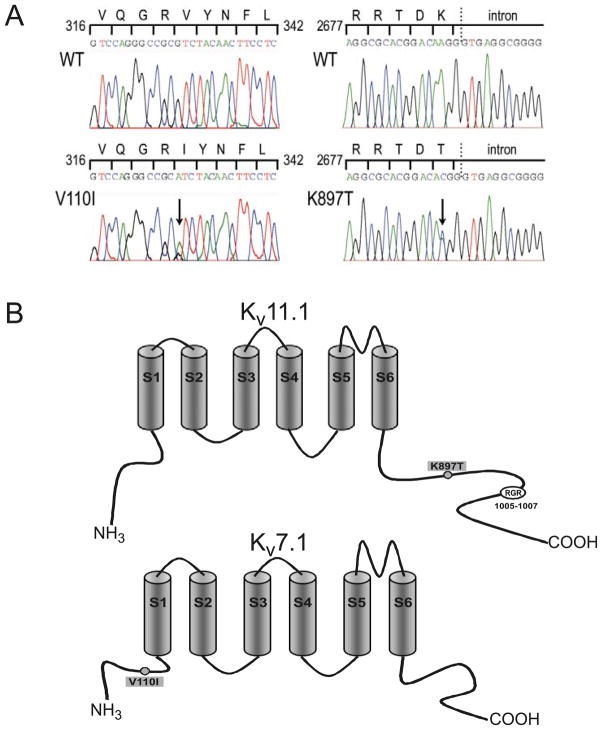
Analysis of the patient’s DNA showed multiple ion channel variations. One variation, a novel missense mutation in KCNQ1, involved a C to T substitution in exon 1, predicting a substitution from valine (V) to isoleucine (I) at codon 110 (V110I) located in the N-terminus of the Kv7.1 channel. In addition, a common polymorphism was found in KCNH2, predicting amino acid substitution of threonine (T) for lysine (K) at codon 897 (K897T) located in the C-terminus of the Kv11.1 channel (Fig. 2).
Fig. 2.
(A) DNA sequence chromatogram showing C to T substitution in exon 1 of KCNQ1 (left side). Chromatogram showing heterozygous nucleotide change from A to C at position 2690 of KCNH2 (right side). (B) The polymorphism resulted in an amino acid change from lysine (K) to threonine (T) at codon 897 (K897T) located in the C-terminus of the Kv11.1 channel (top). RGR is an endoplasmic reticulum
We first assessed the effect of V110I mutation on Kv7.1 currents. Conventional whole-cell patch-clamp experiments were conducted on cells transfected with WT and (or) mutant KCNQ1. Representative current traces are shown in Fig. 3A. Cells transfected with WT-KCNQ1 together with KCNE1 exhibited slowly activating outward currents similar to IKs recorded from native cardiac myocytes (Fig. 3A), whereas cells transfected with the V110I mutant + KCNE1 produced activating and tail current of reduced amplitudes (Figs. 3B, 3C).
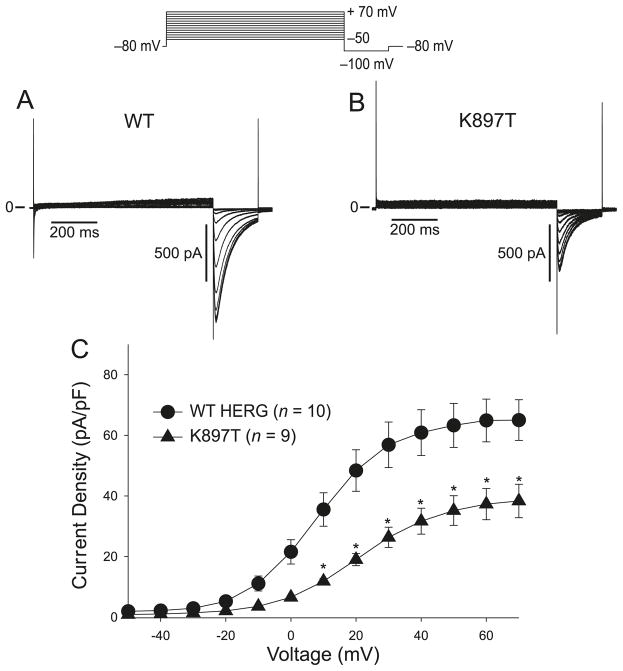
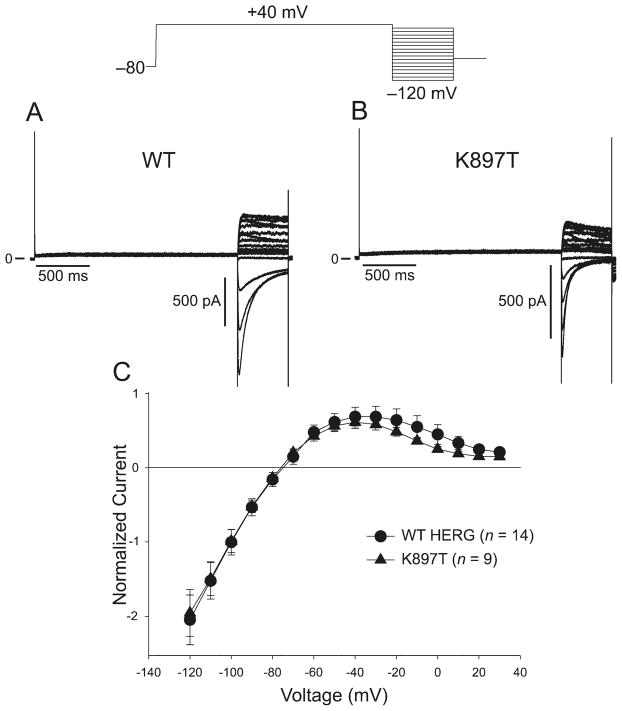
Compared with WT Kv11.1 current, the K897T polymorphism in KCNH2 has been shown to alter the biophysical properties of Kv11.1 current (Bezzina et al. 2003; Anson et al. 2004; Crotti et al. 2005), but with conflicting results. As an initial basis of comparison, we determined how K897T altered the biophysical properties of Kv11.1 current. We first compared the wild-type (WT) and K897T currents elicited by 800 ms depolarizing pulses in transfected CHO-K1 cells. Both WT and K897T currents activated rapidly during step depolarizations to positive potentials and displayed the characteristic tail currents upon repolarization (Fig. 4A). The current–voltage relationship showed that both WT and K897T currents began to plateau at about +20 mV, but the magnitude of K897T currents were reduced compared with WT (Fig. 4C).
Fig. 4.
(A) Wild type (WT) KCNH2 currents elicited by 800 ms depolarizing pulses in CHO-K1 transfected cells. Currents were activated by depolarizing pulses in +10 mV increments. A large inward tail current was observed upon repolarization to −100 mV. (B) The same protocol as in (A) applied to K897T. Tail currents were reduced by the polymorphism. (C) Tail current–voltage relationship as a function of the activating step potential.
We next examined the rectification properties of WT and K897T-Kv11.1 channels by activating channels at +40 mV and measuring the instantaneous current upon return to membrane potentials between −120 and −40 mV (full I-V relationship; Fig. 5). Peak tail current during the recovering pulse is proportional to the maximum number of channels available at each voltage, and data were normalized to −100 mV to account for differences in current density (shown in Fig. 4). WT peak tail currents exhibited strong inward rectification characterized by a region of negative slope conductance for membrane potentials above −40 mV (Fig. 5A, 5C), as previously described (Spector et al. 1996). The full I-V relation of K897T was similar to WT Kv11.1 channels, demonstrating that rectification was not affected by the K897T polymorphism.
Fig. 5.
Inward rectification is similar for wild-type (WT) and K897T currents. Representative traces of WT HERG (A) and K897T (B) fully activated currents. (C) Peak tail current amplitude was plotted as a function of the test potential. The current–voltage (I-V) relationship for both channels showed the hallmark rectification and region of negative slope conductance.
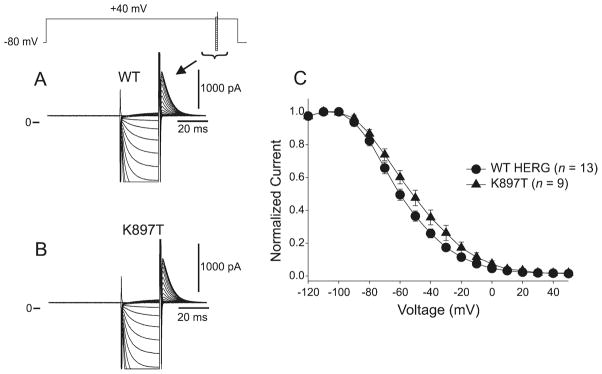
We assessed the voltage dependence of WT and K897T channel availability using a standard triple-pulse protocol (Fig. 6A, 6B). Channel availability was estimated by plotting the normalized tail current amplitude (to peak) following the conditioning potential against the voltage of their respective recovery pulse (Fig. 6C). A Boltzmann distribution function fitted to the normalized amplitudes yielded mid-inactivation values of −60.7 ± 1.15 mV (n = 13) and −53.1 ± 1.13mV (n = 9) for WT and K897T, respectively (p < 0.05).
Fig. 6.
Inactivation of K897T-KCNH2 channels occurs at voltages slightly more positive than wild-type (WT) HERG. (A) Representative current recordings from WT channels recovered using a standard triple pulse protocol. During the 25 ms pulse, the potentials were varied between −130 and +50 mV. (B) Representative recordings from K897T channels with recovery potentials between −130 and +50 mV. (C) Average steady-state inactivation curves for WT and K897T. Mid-inactivation potential of K897T channels occurred at slightly more depolarized potentials than in WT.
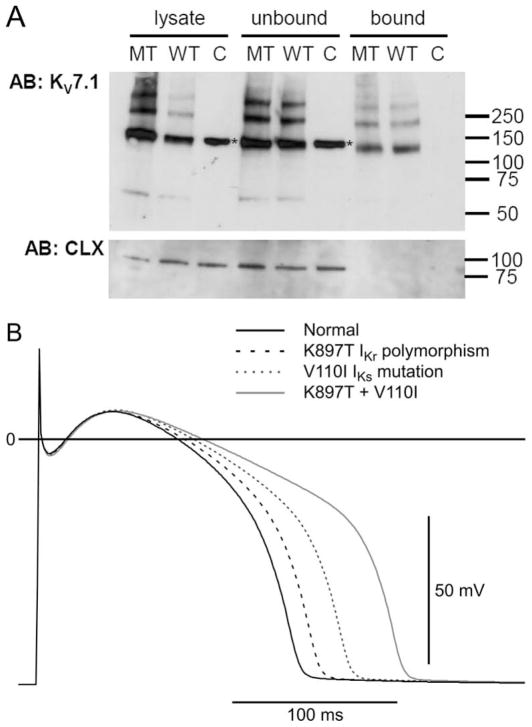
For KV11.1 K897T, it has been shown that surface expression of K897T is reduced compared with WT in human embryonic kidney cells (Paavonen et al. 2003), consistent with the reduction in current densities observed in this study (Fig. 4). To analyze whether the mutation V110I in KV7.1 caused changes in protein levels or expression at the cell surface, we performed biotinylation experiments. Cell surface and intracellular KV7.1 protein pools were analyzed by Western blotting of CHO cells expressing with WT and V110I channels (Fig. 7A). The absence of the endoplasmatic reticulum resident calnexin in the biotinylated (bound) samples indicated that biotin specifically labeled proteins in the plasma membrane and did not leak into the cells. The KV7.1 antibody recognized a weak immunoreactive band at the expected molecular weight of a KV7.1 monomer. The antibody also recognized several strongly immunoreactive bands at higher apparent molecular weight, probably representing different multimeric states of the KV7.1 channel protein, as described previously (Calloe et al. 2007). The plasma membrane and intracellular expression level mutant channels were similar to that of WT, indicating that current reduction is not due to trafficking effects.
Fig. 7.
(A) Cell surface expression of KV7.1 wild-type (WT) and mutant. Human KV7.1 WT or V110I (MT) cDNA were transfected into CHO cells and 2 days after transfection, total membrane proteins were labeled with membrane impermeable biotinylation reagent. The labeled proteins were isolated with streptavidin beads and analyzed by Western blotting. Upper panel: Immunostaining of KV7.1 protein using total cell lysates, unbound fraction, and streptavidin precipitates. Lower panel: calnexin, an endoplasmic reticulum resident protein, served as control for selective biotinylation of plasma membrane proteins. Molecular marker in kDa; asterisks indicate unspecific band in mock-transfected cells that have a higher apparent molecular weight than the putative dimer of KV7.1; C = nontransfected cells. (B) Effect of reducing IKr and IKs magnitude on simulated left ventricular epicardial APs (see Methods section for details). Both the K897T polymorphism (dashed line) and the V110I mutation (dotted line) lengthened action potential duration (APD). The presence of both mutation and polymorphism (gray trace) produced a marked prolongation in APD.
To explore the effects of altering the magnitude of K+ channels (IKr and IKs) on action potential waveform, we simulated action potentials from the epicardial region of the left ventricle, which is known to exhibit a prominent transient outward current (Ito) in several species, including human (Wettwer et al. 1994; Akar et al. 2004). Either the V110I mutation in Kv7.1 (IKs) or the K897T polymorphism in Kv11.1 (IKr) caused a prolongation of the APD. However, the presence of both the mutation and the polymorphism caused a marked prolongation of the APD (Fig. 7B).
Discussion
LQT1 and LQT2 are caused by gene mutations in KCNQ1 and KCNH2, respectively, and mutations in these 2 genes are responsible for about 85% of LQTS-linked mutations. KCNQ1 encodes Kv7.1, the pore-forming α-subunit of the IKs potassium channel, whereas KCNH2 encodes Kv11.1, the α-subunit of the IKr channel. A reduction in either repolarizing current contributes to a prolongation of action potential duration (APD) and prolongation of the QT interval. In the present study, we identify a novel KCNQ1 mutation (V110I), as well as a polymorphism in KCNH2. Patch-clamp analysis of the mutated IKs channel showed a significant loss of function. In addition, analysis of the K897T polymorphism also showed a reduction in current density, suggesting that the K897T polymorphism reduces IKr and contributes to the more severe LQTS phenotype. The reduction in both IKs and IKr is consistent with the overlapping LQT1 and LQT2 clinical phenotype observed in this patient.
Common SNPs have previously been implicated in accentuating the electrocardiographic and arrhythmic phenotype of both acquired and congenital forms of the long QT syndrome (Yang et al. 2002; Crotti et al. 2005). Crotti et al. (2005) and Westenskow and co-workers (Westenskow et al. 2004) were the first to present evidence that a common SNP (KCNE1-D85N and HERG-K897T, respectively) can modify the disease phenotype of congenital LQTS. The role of K897T has been a matter of some debate. In large-scale population studies, the 897T allele has been associated with a shorter QTc interval compared with the 897K allele (Laitinen et al. 2000; Bezzina et al. 2003; Gouas et al. 2005; Pfeufer et al. 2005; Newton-Cheh et al. 2007). In one study (Pietilä et al. 2002), Finnish women with K897T had a longer QTc than those with K897K. In contrast to the population studies, functional studies involving expressing K897T-HERG channels in heterologous expression systems have, in most cases, reported that K897T alters the channel biophysical properties, leading to a reduction in HERG current (Anson et al. 2004; Crotti et al. 2005). An increase in repolarizing current resulting from alteration in K897T channel kinetics has also been observed (Bezzina et al. 2003). Our study provides further support for the hypothesis that K897T polymorphism contributes to a mild loss of function of IKr capable of accentuating the electrocardiographic and arrhythmic manifestation of other long QT mutations.
The 32-year-old female suffered from episodes of dizziness, possibly due to paroxysms of PVCs. The syncopal episode occurred immediately after the patient was rushing up the stairs and was startled by a person she was not expecting. Repetitive ECG recordings revealed fluctuations of re-polarization with significant alteration, including bifid T waves in all precordial leads (Figs. 1A–1C). Under continuous drug therapy with beta blocker (metroprolol, 50 mg/day), no further episodes of syncope could be documented. During a follow-up at 4 years, only mild symptoms due to episodic palpitations persisted.
Acknowledgments
We gratefully acknowledge the assistance of our molecular genetics database manager, Susan Bartkowiak, as well as excellent technical assistance from Nancy Mutsaers. Supported by a grant from the American Health Assistance Foundation (Cordeiro), and grants HL-47678 (Antzelevitch) and 5K01HL073161 (Perez) from the National Heart, Lung and Blood Institute.
Footnotes
No conflict of interest.
Contributor Information
Jonathan M. Cordeiro, Masonic Medical Research Laboratory, Utica, New York 13501, USA.
Guillermo J. Perez, Masonic Medical Research Laboratory, Utica, New York 13501, USA
Nicole Schmitt, Danish Arrhythmia Research Centre, Faculty of Health Sciences, University of Copenhagen, Blegdamsvej 3B, DK-2200 Copenhagen, Denmark.
Ryan Pfeiffer, Masonic Medical Research Laboratory, Utica, New York 13501, USA.
Vladislav V. Nesterenko, Masonic Medical Research Laboratory, Utica, New York 13501, USA
Elena Burashnikov, Masonic Medical Research Laboratory, Utica, New York 13501, USA.
Christian Veltmann, Department of Medicine, University Hospital Mannheim, University of Heidelberg, Mannheim, Germany.
Martin Borggrefe, Department of Medicine, University Hospital Mannheim, University of Heidelberg, Mannheim, Germany.
Christian Wolpert, Department of Medicine, University Hospital Mannheim, University of Heidelberg, Mannheim, Germany.
Rainer Schimpf, Department of Medicine, University Hospital Mannheim, University of Heidelberg, Mannheim, Germany.
Charles Antzelevitch, Masonic Medical Research Laboratory, Utica, New York 13501, USA.
References
- Aizawa Y, Ueda K, Scornik F, Cordeiro JM, Wu Y, Desai M, et al. A novel mutation in KCNQ1 associated with a potent dominant negative effect as the basis for the LQT1 form of the long QT syndrome. J Cardiovasc Electrophysiol. 2007;18(9):972–977. doi: 10.1111/j.1540-8167.2007.00889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akar FG, Wu RC, Deschênes I, Armoundas AA, Piacentino V, 3rd, Houser SR, Tomaselli GF. Phenotypic differences in transient outward K+ current of human and canine ventricular myocytes: insights into molecular composition of ventricular Ito. Am J Physiol Heart Circ Physiol. 2004;286(2):H602–H609. doi: 10.1152/ajpheart.00673.2003. [DOI] [PubMed] [Google Scholar]
- Anson BD, Ackerman MJ, Tester DJ, Will ML, Delisle BP, Anderson CL, January CT. Molecular and functional characterization of common polymorphisms in HERG (KCNH2) potassium channels. Am J Physiol Heart Circ Physiol. 2004;286(6):H2434–H2441. doi: 10.1152/ajpheart.00891.2003. [DOI] [PubMed] [Google Scholar]
- Bazett HC. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- Bezzina CR, Verkerk AO, Busjahn A, Jeron A, Erdmann J, Koopmann TT, et al. A common polymorphism in KCNH2 (HERG) hastens cardiac repolarization. Cardiovasc Res. 2003;59(1):27–36. doi: 10.1016/S0008-6363(03)00342-0. [DOI] [PubMed] [Google Scholar]
- Calloe K, Nielsen MS, Grunnet M, Schmitt N, Jorgensen NK. KCNQ channels are involved in the regulatory volume decrease response in primary neonatal rat cardiomyocytes. Biochim Biophys Acta. 2007;1773(6):764–773. doi: 10.1016/j.bbamcr.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Cordeiro JM, Brugada R, Wu YS, Hong K, Dumaine R. Modulation of IKr inactivation by mutation N588K in KCNH2: a link to arrhythmogenesis in short QT syndrome. Cardiovasc Res. 2005;67(3):498–509. doi: 10.1016/j.cardiores.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Crotti L, Lundquist AL, Insolia R, Pedrazzini M, Ferrandi C, De Ferrari GM, et al. KCNH2-K897T is a genetic modifier of latent congenital long-QT syndrome. Circulation. 2005;112(9):1251–1258. doi: 10.1161/CIRCULATIONAHA.105.549071. [DOI] [PubMed] [Google Scholar]
- Dumaine R, Towbin JA, Brugada P, Vatta M, Nesterenko DV, Nesterenko VV, et al. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res. 1999;85(9):803–809. doi: 10.1161/01.res.85.9.803. [DOI] [PubMed] [Google Scholar]
- Gouas L, Nicaud V, Berthet M, Forhan A, Tiret L, Balkau B, Guicheney P D.E.S.I.R. Study Group. Association of KCNQ1, KCNE1, KCNH2 and SCN5A polymorphisms with QTc interval length in a healthy population. Eur J Hum Genet. 2005;13(11):1213–1222. doi: 10.1038/sj.ejhg.5201489. [DOI] [PubMed] [Google Scholar]
- Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104(4):569–580. doi: 10.1016/S0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Kupershmidt S, Yang T, Chanthaphaychith S, Wang Z, Towbin JA, Roden DM. Defective human Ether-à-go-go-related gene trafficking linked to an endoplasmic reticulum retention signal in the C terminus. J Biol Chem. 2002;277(30):27442–27448. doi: 10.1074/jbc.M112375200. [DOI] [PubMed] [Google Scholar]
- Laitinen P, Fodstad H, Piippo K, Swan H, Toivonen L, Viitasalo M, et al. Survey of the coding region of the HERG gene in long QT syndrome reveals six novel mutations and an amino acid polymorphism with possible phenotypic effects. Hum Mutat. 2000;15(6):580–581. doi: 10.1002/1098-1004(200006)15:6<580::AID-HUMU16>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Luo CH, Rudy Y. A dynamic model of the cardiac ventricular action potential. I. Simulations of ionic currents and concentration changes. Circ Res. 1994;74(6):1071–1096. doi: 10.1161/01.res.74.6.1071. [DOI] [PubMed] [Google Scholar]
- Medeiros-Domingo A, Kaku T, Tester DJ, Iturralde-Torres P, Itty A, Ye B, et al. SCN4B-encoded sodium channel β4 subunit in congenital long-QT syndrome. Circulation. 2007;116(2):134–142. doi: 10.1161/CIRCULATIONAHA.106.659086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton-Cheh C, Guo CY, Larson MG, Musone SL, Surti A, Camargo AL, et al. Common genetic variation in KCNH2 is associated with QT interval duration: the Framingham Heart Study. Circulation. 2007;116(10):1128–1136. doi: 10.1161/CIRCULATIONAHA.107.710780. [DOI] [PubMed] [Google Scholar]
- Paavonen KJ, Chapman H, Laitinen PJ, Fodstad H, Piippo K, Swan H, et al. Functional characterization of the common amino acid 897 polymorphism of the cardiac potassium channel KCNH2 (HERG) Cardiovasc Res. 2003;59(3):603–611. doi: 10.1016/S0008-6363(03)00458-9. [DOI] [PubMed] [Google Scholar]
- Pfeufer A, Jalilzadeh S, Perz S, Mueller JC, Hinterseer M, Illig T, et al. Common variants in myocardial ion channel genes modify the QT interval in the general population: results from the KORA study. Circ Res. 2005;96(6):693–701. doi: 10.1161/01.RES.0000161077.53751.e6. [DOI] [PubMed] [Google Scholar]
- Pietilä E, Fodstad H, Niskasaari E, Laitinen PPJ, Swan H, Savolainen M, et al. Association between HERG K897T polymorphism and QT interval in middle-aged Finnish women. J Am Coll Cardiol. 2002;40(3):511–514. doi: 10.1016/S0735-1097(02)01979-4. [DOI] [PubMed] [Google Scholar]
- Poelzing S, Forleo C, Samodell M, Dudash L, Sorrentino S, Anaclerio M, et al. SCN5A polymorphism restores trafficking of a Brugada syndrome mutation on a separate gene. Circulation. 2006;114(5):368–376. doi: 10.1161/CIRCULATIONAHA.105.601294. [DOI] [PubMed] [Google Scholar]
- Salisbury BA, Judson RS, Pungliya M, Carr J, Qi M, Zareba W, et al. The single nucleotide polymorphism D85N-KCNE1 is associated with both congenital and drug-induced long QT. Heart Rhythm. 2006;3(5):S98. doi: 10.1016/j.hrthm. 2006.02.297. [DOI] [Google Scholar]
- Spector PS, Curran ME, Zou AR, Keating MT, Sanguinetti MC. Fast inactivation causes rectification of the IKr channel. J Gen Physiol. 1996;107(5):611–619. doi: 10.1085/jgp.107.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splawski I, Shen J, Timothy KW, Lehmann MH, Priori SG, Robinson JL, et al. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation. 2000;102(10):1178–1185. doi: 10.1161/01.cir.102.10.1178. [DOI] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, et al. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119(1):19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Ueda K, Valdivia C, Medeiros-Domingo A, Tester DJ, Vatta M, Farrugia G, et al. Syntrophin mutation associated with long QT syndrome through activation of the nNOS-SCN5A macromolecular complex. Proc Natl Acad Sci USA. 2008;105(27):9355–9360. doi: 10.1073/pnas.0801294105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenskow P, Splawski I, Timothy KW, Keating MT, Sanguinetti MC. Compound mutations: a common cause of severe long-QT syndrome. Circulation. 2004;109(15):1834–1841. doi: 10.1161/01.CIR.0000125524.34234.13. [DOI] [PubMed] [Google Scholar]
- Wettwer E, Amos GJ, Posival H, Ravens U. Transient outward current in human ventricular myocytes of subepicardial and subendocardial origin. Circ Res. 1994;75(3):473–482. doi: 10.1161/01.res.75.3.473. [DOI] [PubMed] [Google Scholar]
- Yang P, Kanki H, Drolet B, Yang T, Wei J, Viswanathan PC, et al. Allelic variants in long-QT disease genes in patients with drug-associated torsades de pointes. Circulation. 2002;105(16):1943–1948. doi: 10.1161/01.CIR.0000014448.19052.4C. [DOI] [PubMed] [Google Scholar]
- Zeng J, Laurita KR, Rosenbaum DS, Rudy Y. Two components of the delayed rectifier K+ current in ventricular myocytes of the guinea pig type. Theoretical formulation and their role in repolarization. Circ Res. 1995;77(1):140–152. doi: 10.1161/01.res.77.1.140. [DOI] [PubMed] [Google Scholar]
- Zhang L, Benson DW, Tristani-Firouzi M, Ptacek LJ, Tawil R, Schwartz PJ, et al. Electrocardiographic features in Andersen-Tawil syndrome patients with KCNJ2 mutations: characteristic T-U-wave patterns predict the KCNJ2 genotype. Circulation. 2005;111(21):2720–2726. doi: 10.1161/CIRCULATIONAHA.104.472498. [DOI] [PubMed] [Google Scholar]