Abstract
Excess production of reactive oxygen species in the brain has been implicated as a common underlying risk factor for the pathogenesis of a number of neurodegenerative disorders, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and stroke. In recent years, there is considerable interest concerning investigation of antioxidative and anti-inflammatory effects of phenolic compounds from different botanical sources. In this review, we first describe oxidative mechanisms associated with stroke, AD, and PD, and subsequently, we place emphasis on recent studies implicating neuroprotective effects of resveratrol, a polyphenolic compound derived from grapes and red wine. These studies show that the beneficial effects of resveratrol are not only limited to its antioxidant and anti-inflammatory action but also include activation of sirtuin 1 (SIRT1) and vitagenes, which can prevent the deleterious effects triggered by oxidative stress. In fact, SIRT1 activation by resveratrol is gaining importance in the development of innovative treatment strategies for stroke and other neurodegenerative disorders. The goal here is to provide a better understanding of the mode of action of resveratrol and its possible use as a potential therapeutic agent to ameliorate stroke damage as well as other age-related neurodegenerative disorders.
Keywords: Neurodegenerative disorders, Stroke, Resveratrol, Antioxidant, Anti-inflammatory, SIRT1, Ischemia/reperfusion, Polyphenols, Mitochondria dysfunction, Apoptosis
Introduction
Oxidative damage has been regarded as the underlying cause for a number of neurodegenerative diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD), and stroke [1–3]. Reactive oxygen species (ROS) can damage proteins, nucleic acids, and membrane polyunsaturated fatty acids, causing lipid peroxidation and leading to loss of membrane integrity, reduction of mitochondrial membrane potential, and increased permeability to Ca2+ in plasma membrane [4]. In the central nervous system, neurons are especially vulnerable to insults induced by neurotoxins, ischemia/stroke, and seizure/excitotoxic injury, and oxidative stress is a common underlying factor for these injuries. Many polyphenolic compounds from fruits and vegetables are known for their antioxidant properties and thus have been implicated as therapeutic agents [4, 5]. In this review, we will discuss recent literature pertaining to the beneficial roles of resveratrol for the prevention and treatment of neurodegenerative disorders, including stroke, AD, and PD.
Resveratrol is present in a variety of plants including vegetables, fruits, grains, roots, flowers, seeds, tea, and wine. It has been shown to offer protective effects against a number of cardiovascular and neurodegenerative diseases, and cancer. Although the mechanisms by which resveratrol exerts such a wide range of beneficial effects on these diseases have not yet been clearly elucidated, a number of studies have reported on its antioxidant, anti-inflammatory, and metal-chelating properties [4, 6–8]. There is increasing evidence that besides its antioxidant and anti-inflammatory properties, resveratrol can activate sirtuin 1 (SIRT1), which is a class of deacetylase [9]. SIRT1 has recently emerged as a therapeutic target for the treatment of age-related degenerative diseases. Studies with neuronal cells showed that resveratrol acting as a SIRT1 activator protected SK-N-BE cells from oxidative stress and cytotoxicity by amyloid beta (Aβ) peptide and alpha-synuclein [9]. Caloric restriction has been shown to extend lifespan in mammals, and this effect is attributed to activation of the SIRT1 gene [10, 11]. Resveratrol appears to mimic caloric restriction by increasing SIRT1 activity, proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) deacetylation, and mitochondrial biogenesis [12]. In turn, these enzymes are linked to regulation of the vitagene system, longevity genes which are important in controlling the maintenance and repair processes in the body [4, 13–15]. Taken together, these studies suggest that resveratrol may offer a promising approach for treatment of neurodegenerative disorders [13, 14].
Oxidative Stress and Neurodegenerative Disorders
Stroke
Stroke is the third leading cause of death and the first leading cause of long-lasting disability in aging population. Stroke is associated with loss of brain function(s) due to the disturbance in blood supply to the brain, and can be due to ischemia (lack of blood supply) or a hemorrhage. As a result, the affected areas of the brain are unable to function, leading to the inability to move, understand, or formulate speech or to perceive the visual field. In terms of definitive therapy, immediate attention is to remove the blood blockage. Pharmacologic thrombolysis using the tissue plasminogen activator has been used to dissolve the clot in the artery. Oral anticoagulants such as warfarin have also been used for stroke patients. However, in patients with intracerebral hemorrhage, anticoagulants and antithrombotics may cause excessive bleeding and other dangerous side effects. Indeed, there is no good medication or drug therapy for stroke prevention or treatment.
Over the years, many animal models have been developed for studies to elucidate the pathogenesis and therapeutic management of stroke [4]. Focal cerebral ischemia produced by the occlusion of middle cerebral artery has been a commonly used model because it reflects a similar form of clinical stroke. On the other hand, the global cerebral ischemia model is appropriate for understanding acute brain ischemia such as that following a cardiac arrest [16]. Regardless of the models, oxidative stress is implicated in neuronal damage after ischemia/reperfusion (I/R). This raises attention to the beneficial effects of dietary antioxidants, especially the plant polyphenolic compounds [4, 17–20]. There is strong evidence suggesting that many polyphenols can be preventive and therapeutic agents for stroke, and that these compounds act at multiple cellular levels and influence both the early and late phases of stroke progression [4, 21, 22].
Other Neurodegenerative Diseases
Elevations in ROS and reactive nitrogen species can cause damage to neural membranes and are the major factors associated with the pathogenesis of many neurodegenerative diseases [1]. Indeed, the increase in oxidative and nitrosative stress associated with the concomitant decline in cognitive deficit and motor performance through aging reveals a definite link between aging and neurodegenerative processes.
Alzheimer’s Disease
AD is a progressive, age-dependent neurodegenerative disorder affecting the cortex and hippocampus, and eventually leading to cognitive impairment. The presence of neurofibrillary tangles and Aβ plaques in these brain regions are the hallmarks of AD [23, 24]. In many familial AD cases, mutations are found in the amyloid precursor protein, presenilin 1, and presenilin 2 genes [24, 25]. However, familial AD constitutes only a small portion of all AD patients [26] and has an early age onset (younger than 65 years). In contrast, the vast majority of AD cases are sporadic and are associated with a late age onset (65 years and older). Although the specific causes of sporadic AD are still unknown, factors under study include aging [25], mitochondrial defects [26], insulin-dependent diabetes [27, 28], environmental conditions [29], and diet [30]. In both familial and sporadic AD, Aβ peptides are regarded as a key factor in the disease pathology [25, 31]. These peptides are cleavage products of amyloid precursor protein through β and γ secretases. More recent studies have pointed to the formation of small diffusible Aβ oligomers as a basis of cytotoxic effects in AD [32]. Studies in our laboratory indicated that oligomeric Aβ can stimulate ROS production through signaling pathways involving NADPH oxidase, and in turn, the ROS result in activation of MAPK and PLA2 [33]. Other studies also demonstrated the role of Aβ to induce oxidative stress in the progression and pathogenesis of AD [34].
Parkinson’s Disease
PD is a chronic and progressive neurodegenerative disease associated with impairment of motor function. PD patients show increasing muscle rigidity, resting tremors, brady-kinesia, and in extreme cases, a nearly complete loss of movement. These symptoms are associated with the loss of dopaminergic neurons in the substantia nigra. Despite numerous hypotheses and continued speculations, the etiology of PD remains unclear. The discovery of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), an environmental toxin, which can selectively damage the substantia nigra and subsequently results in Parkinson-like syndromes in animals and humans, has accelerated the search for neurotoxins as the possible cause of PD [35, 36]. Other environmental toxins such as manganese [37], dimethoxyphenyl-ethylamine [38], and paraquat [39, 40] have also been shown to kill dopamine neurons. Studies with these agents indicate that oxidative damage, mitochondrial and proteasomal dysfunction, and inflammatory changes are the underlying factors for degeneration of dopaminergic neurons in PD patients.
Alcohol-Induced Neurodegenerative Disorder
In our earlier studies, we demonstrated that alcohol can participate in the free radical reaction to form ethoxyl radical. This type of radical has longer half-life than the hydroxyl radical and is just as active as the hydroxyl free radicals [41]. Subsequent studies further demonstrated that ethanol could induce cell death through oxidative mechanism [42]. Recent studies support the hypothesis that chronic alcohol intake can cause oxidative stress and induce neurotoxicity due to its participation in free radical reaction [43, 44]. Our study also demonstrated that chronic alcohol administration induced neurochemical alteration of IP3R1, IP3-kinase, and mGluR-1 in mouse cerebellum [45] and increased COX-2 mRNA in rat brain [46]. Ethanol-induced oxidative stress in the brain has been shown to cause the release of glutamate and proinflammatory cytokines, which are probably an important cause of alcohol-related neurodegeneration (for review, see [47]).
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder characterized by loss of upper and lower motor neurons. There is accumulating evidence indicating that oxidative stress and mitochondrial dysfunction are important risk factors for pathogenesis of ALS [48, 49]. Other factors, including glutamate excitotoxicity, reactive astrogliosis, and mutant superoxide dismutase, may also be involved in the progression of cellular dysfunction in this disease [50, 51].
Huntington’s Disease
Huntington’s disease (HD) is a neurodegenerative disorder that results from the neuronal damage in basal ganglia. There is evidence that administration of 3-nitropropionic acid to experimental animals can induce oxidative and nitrosative stress and cause neurobehavioral and biochemical changes similar to those of HD patients. These studies appear to confirm that excitotoxicity, dopamine toxicity, metabolic impairment, mitochondrial dysfunction, apoptosis, and autophage are involved in the progressive degeneration of this disease [52].
Protective Effect of Resveratrol on Stroke and Alcohol-Induced Neurodegeneration
Epidemiological studies have demonstrated a lower incidence of cardiovascular diseases in populations with moderate wine drinking, despite consuming a fatty diet (the “French paradox”). It is believed that the cardioprotective effects is partially due to the anti-platelet aggregation properties of the polyphenols in red wine and their ability to prevent the development of atherosclerotic plaques [53, 54]. Resveratrol (3,5,4′-trihydroxystilbene) has been identified to be a major compound responsible for these effects. In recent years, there is considerable interest to investigate whether red wine and grape poly-phenols may offer protective effects against neurodegenerative diseases [46, 53, 55]. Besides grapes and berries, resveratrol is also concentrated in some oriental herbal plants, such as Polygonum cuspidatum or Kojokan, which are used to treat fevers, hyperlipidemia, atherosclerosis, and inflammation [4]. Our studies with PC-12 cells showed that resveratrol is more effective in protecting against oxidative damage than vitamins E and C combined [56]. Studies from our laboratory provided evidence that a dietary supplement of polyphenols extracted from grape skin and seeds could ameliorate chronic alcohol-induced oxidative damage in synaptic membranes in the brain [57, 58]. Grape poly-phenols also prevented chronic ethanol-induced increase in COX-2 mRNA expression in the rat brain [46]. Although grapes and berries contain other types of polyphenols, resveratrol is probably the most effective in offering beneficial health results. Although other antioxidants can also protect alcohol-induced brain damage [59, 60], resveratrol is shown to offer neuroprotective effects against the oxidative stress and spatial learning deficit resulted from alcohol consumption [61].
A bioavailability study of resveratrol indicated that this compound is transported to the circulatory system after i.p. administration and can cross the blood–brain barrier and reach the brain tissue rapidly [62]. Using the global cerebral ischemia induced to gerbils, studies from our laboratory demonstrated the ability for resveratrol to protect against delayed neuronal cell death [62]. In this study, resveratrol not only protected neuronal cells against oxidative damage but also suppressed the activation of astrocytes and microglial cells after I/R. Brain response to ischemic injury is also associated with an acute and prolonged inflammatory process characterized by the activation of glial cells and production of inflammatory cytokines. Since cytokines are mediators of inflammatory response that contribute to secondary injury in the ischemic brain, activated glial cells not only secrete proinflammatory cytokines but also other neurotoxic mediators such as nitric oxide, superoxide, and peroxynitrite, and together, these mediators can influence neuronal cell survival [63]. Resveratrol may exert neuroprotective effects by suppressing glial activation.
In a study, we fed gerbils with a dietary supplement of grape powder rich in polyphenols for 2 months and found that this feeding regimen could offer neuroprotective effects and ameliorated neuronal damage due to global ischemic insult [64]. Furthermore, oral administration of polyphenols extract from grapes to gerbils could also minimize ischemic damage [65]. Oral administration of grape polyphenols was shown to protect against oxidative damage of DNA (as demonstrated by immunostaining of 6-OHdG in the hippocampal CA1 area) and decreased apoptotic neurons (as demonstrated by the TUNEL assay). Using an open field locomotor activity regimen to assess behavioral deficits of gerbils at 48 h after I/R, results demonstrated the benefit of grape polyphenols treatment in functional outcome after stroke [65]. Results from our study are in agreement with several other studies demonstrating similar beneficial effects of resveratrol and grape polyphenols in animal models of stroke [4, 66].
Protective Effects of Resveratrol on Other Neurodegenerative Disorders
Besides damage due to cerebral ischemia, resveratrol has been shown to exert protective effects on other neurodegenerative diseases, including AD, PD, ALS, and HDs. Chronic administration of resveratrol could also prevent cognitive impairment and oxidative stress induced by intracerebroventricular injection of streptozotocin [67]. Resveratrol was shown to inhibit the formation and extension of Aβ fibrils and destabilize fibrilized Aβ [68, 69]. Furthermore, resveratrol could reduce Aβ secretion from different cell lines [70]. In an animal model of AD and tau pathology, resveratrol was shown to reduce neurodegeneration in the hippocampus and prevented learning deficit [71]. Since red wine is known to have higher concentrations of resveratrol as compared to white wine, its consumption can significantly attenuate deterioration of spatial memory function and Aβ neuropathology in the Tg2576 mice [72].
Resveratrol administration was shown to protect mice from MPTP-induced motor coordination impairment, hydroxyl radical overloading, and neuronal loss [73]. More recently, resveratrol was shown to prevent toxicity triggered by hydrogen peroxide or 6-hydroxydopamine (6-OHDA). This action was attributed to its ability to activate SIRT1, as the protective effect was lost in the presence of the SIRT1 inhibitor, sirtinol [9]. Resveratrol has also been tested to provide beneficial effects in the 6-OHDA-induced PD rat model. This model involves chronic inflammation, mitochondrial dysfunction, and oxidative stress, and loss of dopaminergic neurons in the substantia nigra. Resveratrol treatment significantly decreased the levels of COX-2 and tumor necrosis factor-α mRNA, and COX-2 protein expression in the substantia nigra [74].
Although the involvement of oxidative stress in ALS pathogenesis has been well described, antioxidant treatments generally show poor efficacy in ALS animal models and in human clinical trials. In one study, Barber et al. tested 164 antioxidant molecules and identified three top performing antioxidants, including caffeic acid phenethyl ester, esculetin, and resveratrol [75]. It was report that SIRT1, a human homologue of SIR2, is upregulated in mouse models for AD, ALS, and in primary neurons challenged with neurotoxic insults. Both resveratrol and SIRT1 were able to promote neuronal survival. In the inducible p25 transgenic mouse, which is a model of AD and tauopathies, resveratrol administration was shown to reduce neurodegeneration in the hippocampus, prevent learning impairment, and decrease the acetylation of PGC-1α and p53, which are known substrates for SIRT1 [71]. Repeated treatment with resveratrol also significantly improved the 3-nitropropionic acid-induced motor and cognitive impairment in the experimental Huntington disease animal model [76].
Since oxidative stress is involved in traumatic brain injury and may contribute to some of the pathophysiologic changes, treatment with resveratrol immediately after traumatic brain injury was shown to reduce the oxidative damage and lesion volume [77]. Sonmez et al. have also shown that acute treatment of resveratrol has a neuroprotective role against trauma-induced hippocampal neuronal loss and associated cognitive impairment in rats [78].
Molecular Mechanisms of the Neuroprotective Effects of Resveratrol
Numerous studies have demonstrated the beneficial effects of resveratrol through its antioxidant, anti-inflammatory, and metal-chelating properties [4, 6–8]. However, recent studies started to reveal the ability for resveratrol to exert neuroprotective effects through activation of SIRT1 and production of vitagenes [79]. Resveratrol was shown to confer special effects in increasing the lifespan of Saccharomyces cerevisiae [80], and this effect was attributed to its ability to activate sirtuins, which belong to a conserved family of NAD+-dependent deacetylases [66]. This effect of resveratrol seems to mimic that of dietary caloric restriction, which is also associated with activation of sirtuin proteins [10, 11, 80–83].
Resveratrol’s ability to increase SIRT1 activity is also linked to deacetylation of PGC-1α, a protein factor involved in mitochondrial biogenesis [14, 84, 85]. Another protein, peroxisome proliferator-activated receptor-γ (PPAR-γ), has been proposed as a therapeutic target for neurodegenerative disease due to its ability to protect against mitochondrial damage through upregulation of Bcl-2, an anti-apoptotic protein [86]. There is indication that resveratrol’s ability to attenuate tissue injury in the brain and restore mitochondrial function is partly attributed to its effect on SIRT1-dependent deacetylation of PGC-1α and activation of PPAR-γ [87, 88], and in turn, linking to learning impairment as observed in a well-established animal model [83]. Activation of PPAR-γ may also target the transcription of SOD and catalase genes through increasing the Nrf2/keap 1 pathway [89]. Thus, ability for resveratrol to increase SIRT1 and related enzyme activity could lead to alterations of neuronal transcription profiles and enhanced anti-apoptotic activities [90].
A number of studies demonstrated the ability for resveratrol to reduce Aβ secretion from different cell lines [70] and suppress neuroinflammation by inhibiting NADPH oxidase and attenuating NF-κB-induced expression of iNOS, COX-2, and sPLA2 [4, 65, 91–93]. Resveratrol is shown to activate the hormetic pathway, which involves the induction of SOD and catalase genes through increasing the level of the PI3K/Nrf2/keap 1 pathway [89]. Indeed, dietary administration of resveratrol was shown to increase MnSOD expression and activity in mouse brain [94]. In addition, resveratrol was also shown to upregulate the expression of HO-1 by activating Nrf-2 [95]. This antioxidant pathway is known to protect against ischemia-induced oxidative brain damage [88]. Resveratrol also stimulates mitochondrial biogenesis that has been shown to be dependent on AMP-kinase (AMPK) [84, 85]. The neuronal activation of AMPK by resveratrol could affect neuronal energy homeostasis and contribute to the neuroprotective effects of resveratrol. Taken together, these studies strongly indicate that besides its antioxidant and anti-inflammatory properties, resveratrol also exerts neuroprotective effects through activation of SIRT1 and increased production of vitagenes [96, 97]. This and other evidence can make resveratrol a promising therapeutic candidate for neurodegenerative disorders [96, 97].
Resveratrol is regarded as a cancer chemopreventive agent. While studies have demonstrated the antioxidant effect of resveratrol and its ability to counteract ROS production, and thus inhibit oxidative DNA damage, there is growing evidence that resveratrol may also act as a pro-oxidant and thus causes induction of apoptosis of cancer cells [98]. The antioxidant and pro-oxidant activities of resveratrol may induce a multitude of effects depending on the cell type (“normal” and cancer cells), cellular conditions (normal, stressed, or malignant), and concentrations present. Because neoplastic cells contain elevated levels of copper, they are more sensitive to electron transfer with resveratrol to generate ROS (strong pro-oxidant properties). Although the exact mechanism of chemopreventive effect of resveratrol is still not clear and under investigation, the DNA damage induced by resveratrol in the presence of Cu(II) may be an important mechanism through which preneoplastic cells and neoplastic cells can be killed while maintaining survival of normal cells. Therefore, the ability to regulate antioxidant and pro-oxidant activities of resveratrol due to the presence of Cu(II) may be a new strategy for modulating cytotoxicity and induce apoptotic activities in neoplastic cell system [99].
Concluding Remarks
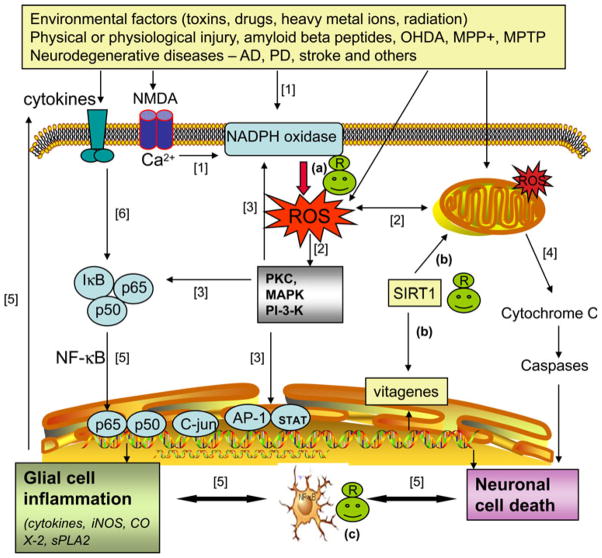
Many environmental factors including neurotoxins, drugs, heavy metal ions, and radiation have been shown to cause neurodegeneration through activation of oxidative pathways (Fig. 1). Activation of NADPH oxidase and mitochondrial dysfunction are two major mechanisms for production of ROS in relation to neuronal excitotoxicity and glial cell activation. ROS from NADPH oxidase can activate signaling molecules including PKC and MAPK. In turn, the kinase pathways can further activate NADPH oxidase, NF-κB, transcription factors, and mitochondria, forming a vicious cycle for more oxidative stress and leading to neuron cell death and glial cell inflammation. On one hand, resveratrol can exert a wide range of beneficial effects through suppressing the oxidative pathways [100]. On the other hand, recent studies show the ability for resveratrol to exert neuroprotective effects through activation of SIRT1 and production of vitagenes.
Fig. 1.
Oxidative pathways leading to neuronal cell death and glial cell inflammation: protective effects of resveratrol [1]. Various endogenous and exogenous factors may stimulate reactive oxygen species (ROS) production directly or indirectly through NADPH oxidase and mitochondria [2]; ROS can cause damage to mitochondrial membrane and activate protein kinases, e.g., PKC, p38, MAPK, ERK1/2, and PI3 kinase [3]; protein kinases target multiple proteins, including regulation of the NF-κB transcription pathway, phosphorylation of NADPH oxidase subunits, and increase in the expression of protective enzymes and anti-apoptotic genes [4]; mitochondrial dysfunction leads to increase in pro-apoptotic proteins and neuron cell death [5]; neuron cell death leads to activation of microglial cells and production of ROS and proinflammatory cytokines [6]; cytokines induce NF-κB pathway leading to glial cell inflammatory responses, including induction of iNOS, sPLA2, and COX-2, and thus forming a vicious cycle of chronic inflammation and cell death. In the above scheme, resveratrol (R) may exert protective effects through (a) scavenge ROS produced by NADPH oxidase, (a) activate SIRT1 which leads to restoration of mitochondrial function and biogenesis, and stimulation of biosynthesis of vitagenes, and (c) inhibit microglial activation
Experimental and epidemiological evidence further demonstrate that resveratrol, together with other flavonoids, may have synergistic effects to improve age-related cognitive decline in PD, AD, and stroke [4]. In particular, synergistic effects were observed when resveratrol was administered in combination with catechin, a polyphenolic compound enriched in green tea [101, 102]. Based on these multiple factors, it is reasonable that dietary consumption rich in flavonoids can offer benefits to limit neurodegeneration and to preserve cognitive functions in aging and age-related neurodegenerative diseases. These studies also provide strong support for resveratrol as a therapeutic agent for neurodegenerative diseases.
Acknowledgments
This work was supported in part by NIH grant 2P01 AG018357.
Contributor Information
Albert Y. Sun, Email: suna@health.missouri.edu, Department of Medical Pharmacology and Physiology, University of Missouri, Columbia, MO 65212, USA. Department of Pathology and Anatomical Sciences, University of Missouri School of Medicine, 1 Hospital Drive, Columbia, MO 65212, USA
Qun Wang, Department of Medical Pharmacology and Physiology, University of Missouri, Columbia, MO 65212, USA.
Agnes Simonyi, Department of Biochemistry, University of Missouri, Columbia, MO 65212, USA.
Grace Y. Sun, Department of Pathology and Anatomical Sciences, University of Missouri School of Medicine, 1 Hospital Drive, Columbia, MO 65212, USA. Department of Biochemistry, University of Missouri, Columbia, MO 65212, USA
References
- 1.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 2.Harman AW, Maxwell MJ. An evaluation of the role of calcium in cell injury. Annu Rev Pharmacol Toxicol. 1995;35:129–144. doi: 10.1146/annurev.pa.35.040195.001021. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Campo R, Lopez-Torres M, Cadenas S, Rojas C, Barja G. The rate of free radical production as a determinant of the rate of aging: evidence from the comparative approach. J Comp Physiol [B] 1998;168:149–158. doi: 10.1007/s003600050131. [DOI] [PubMed] [Google Scholar]
- 4.Sun AY, Wang Q, Simonyi A, Sun GY. Botanical phenolics and brain health. Neuromolecular Med. 2008;10:259–274. doi: 10.1007/s12017-008-8052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farooqui T, Farooqui AA. Aging: an important factor for the pathogenesis of neurodegenerative diseases. Mech Ageing Dev. 2009;130:203–215. doi: 10.1016/j.mad.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Rice-Evans C, Miller N. Measurement of the antioxidant status of dietary constituents, low density lipoproteins and plasma. Prostaglandins Leukot Essent Fatty Acids. 1997;57:499–505. doi: 10.1016/s0952-3278(97)90435-x. [DOI] [PubMed] [Google Scholar]
- 7.Martin S, Andriambeloson E, Takeda K, Andriantsitohaina R. Red wine polyphenols increase calcium in bovine aortic endothelial cells: a basis to elucidate signalling pathways leading to nitric oxide production. Br J Pharmacol. 2002;135:1579–1587. doi: 10.1038/sj.bjp.0704603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ndiaye M, Chataigneau M, Lobysheva I, Chataigneau T, Schini-Kerth VB. Red wine polyphenol-induced, endothelium-dependent NO-mediated relaxation is due to the redox-sensitive PI3-kinase/Akt-dependent phosphorylation of endothelial NO-synthase in the isolated porcine coronary artery. Faseb J. 2005;19:455–457. doi: 10.1096/fj.04-2146fje. [DOI] [PubMed] [Google Scholar]
- 9.Albani D, Polito L, Batelli S, De Mauro S, Fracasso C, Martelli G, Colombo L, Manzoni C, Salmona M, Caccia S, Negro A, Forloni G. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1-42) peptide. J Neurochem. 2009;110:1445–1456. doi: 10.1111/j.1471-4159.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- 10.Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Allard JS, Perez E, Zou S, de Cabo R. Dietary activators of Sirt1. Mol Cell Endocrinol. 2009;299:58–63. doi: 10.1016/j.mce.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson R, Prolla T. PGC-1alpha in aging and anti-aging interventions. Biochim Biophys Acta. 2009;1790:1059–1066. doi: 10.1016/j.bbagen.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, Ungvari Z. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calabrese V, Cornelius C, Mancuso C, Barone E, Calafato S, Bates T, Rizzarelli E, Kostova AT. Vitagenes, dietary antioxidants and neuroprotection in neurodegenerative diseases. Front Biosci. 2009;14:376–397. doi: 10.2741/3250. [DOI] [PubMed] [Google Scholar]
- 15.Calabrese V, Cornelius C, Mancuso C, Pennisi G, Calafato S, Bellia F, Bates TE, Giuffrida Stella AM, Schapira T, Dinkova Kostova AT, Rizzarelli E. Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res. 2008;33:2444–2471. doi: 10.1007/s11064-008-9775-9. [DOI] [PubMed] [Google Scholar]
- 16.Traystman RJ. Animal models of focal and global cerebral ischemia. ILAR J. 2003;44:85–95. doi: 10.1093/ilar.44.2.85. [DOI] [PubMed] [Google Scholar]
- 17.Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 18.Voko Z, Hollander M, Hofman A, Koudstaal PJ, Breteler MM. Dietary antioxidants and the risk of ischemic stroke: the Rotterdam Study. Neurology. 2003;61:1273–1275. doi: 10.1212/01.wnl.0000090458.67821.a3. [DOI] [PubMed] [Google Scholar]
- 19.Youdim KA, Joseph JA. A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: a multiplicity of effects. Free Radic Biol Med. 2001;30:583–594. doi: 10.1016/s0891-5849(00)00510-4. [DOI] [PubMed] [Google Scholar]
- 20.Deschamps V, Barberger-Gateau P, Peuchant E, Orgogozo JM. Nutritional factors in cerebral aging and dementia: epidemiological arguments for a role of oxidative stress. Neuroepidemiology. 2001;20:7–15. doi: 10.1159/000054752. [DOI] [PubMed] [Google Scholar]
- 21.Simonyi A, Wang Q, Miller RL, Yusof M, Shelat PB, Sun AY, Sun GY. Polyphenols in cerebral ischemia: novel targets for neuroprotection. Mol Neurobiol. 2005;31:135–147. doi: 10.1385/MN:31:1-3:135. [DOI] [PubMed] [Google Scholar]
- 22.Curin Y, Ritz MF, Andriantsitohaina R. Cellular mechanisms of the protective effect of polyphenols on the neurovascular unit in strokes. Cardiovasc Hematol Agents Med Chem. 2006;4:277–288. doi: 10.2174/187152506778520691. [DOI] [PubMed] [Google Scholar]
- 23.Selkoe DJ, Podlisny MB. Deciphering the genetic basis of Alzheimer’s disease. Annu Rev Genom Hum Genet. 2002;3:67–99. doi: 10.1146/annurev.genom.3.022502.103022. [DOI] [PubMed] [Google Scholar]
- 24.Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Selkoe DJ. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001;3:75–80. doi: 10.3233/jad-2001-3111. [DOI] [PubMed] [Google Scholar]
- 26.Reddy PH, Beal MF. Are mitochondria critical in the pathogenesis of Alzheimer’s disease? Brain Res Brain Res Rev. 2005;49:618–632. doi: 10.1016/j.brainresrev.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 27.de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. J Alzheimers Dis. 2005;7:45–61. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- 28.Qiu WQ, Folstein MF. Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer’s disease: review and hypothesis. Neurobiol Aging. 2006;27:190–198. doi: 10.1016/j.neurobiolaging.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Tchantchou F, Chan A, Kifle L, Ortiz D, Shea TB. Apple juice concentrate prevents oxidative damage and impaired maze performance in aged mice. J Alzheimers Dis. 2005;8:283–287. doi: 10.3233/jad-2005-8306. [DOI] [PubMed] [Google Scholar]
- 31.Selkoe DJ. Alzheimer disease: mechanistic understanding predicts novel therapies. Ann Intern Med. 2004;140:627–638. doi: 10.7326/0003-4819-140-8-200404200-00047. [DOI] [PubMed] [Google Scholar]
- 32.Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 33.Shelat PB, Chalimoniuk M, Wang JH, Strosznajder JB, Lee JC, Sun AY, Simonyi A, Sun GY. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J Neurochem. 2008;106:45–55. doi: 10.1111/j.1471-4159.2008.05347.x. [DOI] [PubMed] [Google Scholar]
- 34.Butterfield DA, Reed T, Newman SF, Sultana R. Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer’s disease and mild cognitive impairment. Free Radic Biol Med. 2007;43:658–677. doi: 10.1016/j.freeradbiomed.2007.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langston JW. MPTP: insights into the etiology of Parkinson’s disease. Eur Neurol. 1987;26(Suppl 1):2–10. doi: 10.1159/000116349. [DOI] [PubMed] [Google Scholar]
- 36.Tipton KF, Singer TP. Advances in our understanding of the mechanisms of the neurotoxicity of MPTP and related compounds. J Neurochem. 1993;61:1191–1206. doi: 10.1111/j.1471-4159.1993.tb13610.x. [DOI] [PubMed] [Google Scholar]
- 37.Sun AY, Yang WL, Kim HD. Free radical and lipid peroxidation in manganese-induced neuronal cell injury. Ann N Y Acad Sci. 1993;679:358–363. doi: 10.1111/j.1749-6632.1993.tb18322.x. [DOI] [PubMed] [Google Scholar]
- 38.Koshimura I, Imai H, Hidano T, Endo K, Mochizuki H, Kondo T, Mizuno Y. Dimethoxyphenylethylamine and tetrahydropapaverine are toxic to the nigrostriatal system. Brain Res. 1997;773:108–116. doi: 10.1016/s0006-8993(97)00922-0. [DOI] [PubMed] [Google Scholar]
- 39.Miller RL, James-Kracke M, Sun GY, Sun AY. Oxidative and inflammatory pathways in Parkinson’s disease. Neurochem Res. 2009;34:55–65. doi: 10.1007/s11064-008-9656-2. [DOI] [PubMed] [Google Scholar]
- 40.Miller RL, Sun GY, Sun AY. Cytotoxicity of paraquat in microglial cells: involvement of PKCdelta- and ERK1/2-dependent NADPH oxidase. Brain Res. 2007;1167:129–139. doi: 10.1016/j.brainres.2007.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oldfield FF, Cowan DL, Sun AY. The involvement of ethanol in the free radical reaction of 6-hydroxydopamine. Neurochem Res. 1991;16:83–87. doi: 10.1007/BF00965833. [DOI] [PubMed] [Google Scholar]
- 42.Sun AY, Chen YM, James-Kracke M, Wixom P, Cheng Y. Ethanol-induced cell death by lipid peroxidation in PC12 cells. Neurochem Res. 1997;22:1187–1192. doi: 10.1023/a:1021968526696. [DOI] [PubMed] [Google Scholar]
- 43.Loeber S, Duka T, Welzel H, Nakovics H, Heinz A, Flor H, Mann K. Impairment of cognitive abilities and decision making after chronic use of alcohol: the impact of multiple detoxifications. Alcohol Alcohol. 2009;44:372–381. doi: 10.1093/alcalc/agp030. [DOI] [PubMed] [Google Scholar]
- 44.Moselhy HF, Georgiou G, Kahn A. Frontal lobe changes in alcoholism: a review of the literature. Alcohol Alcohol. 2001;36:357–368. doi: 10.1093/alcalc/36.5.357. [DOI] [PubMed] [Google Scholar]
- 45.Simonyi A, Zhang JP, Sun AY, Sun GY. Chronic ethanol on mRNA levels of IP3R1, IP3 3-kinase and mGluR1 in mouse Purkinje neurons. NeuroReport. 1996;7:2115–2118. doi: 10.1097/00001756-199609020-00010. [DOI] [PubMed] [Google Scholar]
- 46.Simonyi A, Woods D, Sun AY, Sun GY. Grape polyphenols inhibit chronic ethanol-induced COX-2 mRNA expression in rat brain. Alcohol Clin Exp Res. 2002;26:352–357. [PubMed] [Google Scholar]
- 47.Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lev N, Ickowicz D, Barhum Y, Melamed E, Offen D. DJ-1 changes in G93A-SOD1 transgenic mice: implications for oxidative stress in ALS. J Mol Neurosci. 2009;38:94–102. doi: 10.1007/s12031-008-9138-7. [DOI] [PubMed] [Google Scholar]
- 49.Muyderman H, Hutson PG, Matusica D, Rogers ML, Rush RA. The human G93A-superoxide dismutase-1 mutation, mitochondrial glutathione and apoptotic cell death. Neurochem Res. 2009;34:1847–1856. doi: 10.1007/s11064-009-9974-z. [DOI] [PubMed] [Google Scholar]
- 50.Lunn JS, Hefferan MP, Marsala M, Feldman EL. Stem cells: comprehensive treatments for amyotrophic lateral sclerosis in conjunction with growth factor delivery. Growth Factors. 2009;27:133–140. doi: 10.1080/08977190902814855. [DOI] [PubMed] [Google Scholar]
- 51.Zagami CJ, Beart PM, Wallis N, Nagley P, O’Shea RD. Oxidative and excitotoxic insults exert differential effects on spinal motoneurons and astrocytic glutamate transporters: implications for the role of astrogliosis in amyotrophic lateral sclerosis. Glia. 2009;57:119–135. doi: 10.1002/glia.20739. [DOI] [PubMed] [Google Scholar]
- 52.Gil JM, Rego AC. Mechanisms of neurodegeneration in Huntington’s disease. Eur J NeuroSci. 2008;27:2803–2820. doi: 10.1111/j.1460-9568.2008.06310.x. [DOI] [PubMed] [Google Scholar]
- 53.Sun AY, Simonyi A, Sun GY. The “French Paradox” and beyond: neuroprotective effects of polyphenols. Free Radic Biol Med. 2002;32:314–318. doi: 10.1016/s0891-5849(01)00803-6. [DOI] [PubMed] [Google Scholar]
- 54.Sun AY, Sun GY. Ethanol and oxidative mechanisms in the brain. J Biomed Sci. 2001;8:37–43. doi: 10.1007/BF02255969. [DOI] [PubMed] [Google Scholar]
- 55.Esposito E, Rotilio D, Di Matteo V, Di Giulio C, Cacchio M, Algeri S. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol Aging. 2002;23:719–735. doi: 10.1016/s0197-4580(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 56.Chanvitayapongs S, Draczynska-Lusiak B, Sun AY. Amelioration of oxidative stress by antioxidants and resveratrol in PC12 cells. NeuroReport. 1997;8:1499–1502. doi: 10.1097/00001756-199704140-00035. [DOI] [PubMed] [Google Scholar]
- 57.Sun GY, Xia J, Draczynska-Lusiak B, Simonyi A, Sun AY. Grape polyphenols protect neurodegenerative changes induced by chronic ethanol administration. NeuroReport. 1999;10:93–96. doi: 10.1097/00001756-199901180-00018. [DOI] [PubMed] [Google Scholar]
- 58.Sun GY, Xia J, Xu J, Allenbrand B, Simonyi A, Rudeen PK, Sun AY. Dietary supplementation of grape polyphenols to rats ameliorates chronic ethanol-induced changes in hepatic morphology without altering changes in hepatic lipids. J Nutr. 1999;129:1814–1819. doi: 10.1093/jn/129.10.1814. [DOI] [PubMed] [Google Scholar]
- 59.Collins MA, Zou JY, Neafsey EJ. Brain damage due to episodic alcohol exposure in vivo and in vitro: furosemide neuroprotection implicates edema-based mechanism. FASEB J. 1998;12:221–230. doi: 10.1096/fasebj.12.2.221. [DOI] [PubMed] [Google Scholar]
- 60.Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J. Cytokines and alcohol. Alcohol Clin Exp Res. 2006;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- 61.Ranney A, Petro MS. Resveratrol protects spatial learning in middle-aged C57BL/6 mice from effects of ethanol. Behav Pharmacol. 2009;20:330–336. doi: 10.1097/FBP.0b013e32832f0193. [DOI] [PubMed] [Google Scholar]
- 62.Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, Sun AY. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002;958:439–447. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- 63.Kriz J, Lalancette-Hebert M. Inflammation, plasticity and real-time imaging after cerebral ischemia. Acta Neuropathol. 2009;117:497–509. doi: 10.1007/s00401-009-0496-1. [DOI] [PubMed] [Google Scholar]
- 64.Wang Q, Simonyi A, Li W, Sisk BA, Miller RL, Macdonald RS, Lubahn DE, Sun GY, Sun AY. Dietary grape supplement ameliorates cerebral ischemia-induced neuronal death in gerbils. Mol Nutr Food Res. 2005;49:443–451. doi: 10.1002/mnfr.200500019. [DOI] [PubMed] [Google Scholar]
- 65.Wang Q, Sun AY, Simonyi A, Miller DK, Smith RE, Luchtefeld RG, Korthuis RJ, Sun GY. Oral administration of grape polyphenol extract ameliorates cerebral ischemia/reperfusion-induced neuronal damage and behavioral deficits in gerbils: comparison of pre- and post-ischemic administration. J Nutr Biochem. 2009;20:369–377. doi: 10.1016/j.jnutbio.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 67.Sharma M, Gupta YK. Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci. 2002;71:2489–2498. doi: 10.1016/s0024-3205(02)02083-0. [DOI] [PubMed] [Google Scholar]
- 68.Ono K, Naiki H, Yamada M. The development of preventives and therapeutics for Alzheimer’s disease that inhibit the formation of beta-amyloid fibrils (fAbeta), as well as destabilize preformed fAbeta. Curr Pharm Des. 2006;12:4357–4375. doi: 10.2174/138161206778793010. [DOI] [PubMed] [Google Scholar]
- 69.Ono K, Yamada M. Antioxidant compounds have potent anti-fibrillogenic and fibril-destabilizing effects for alpha-synuclein fibrils in vitro. J Neurochem. 2006;97:105–115. doi: 10.1111/j.1471-4159.2006.03707.x. [DOI] [PubMed] [Google Scholar]
- 70.Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 71.Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SM, Puigserver P, Sinclair DA, Tsai LH. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007;26:3169–3179. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J, Ho L, Zhao Z, Seror I, Humala N, Dickstein DL, Thiyagarajan M, Percival SS, Talcott ST, Pasinetti GM. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s disease. Faseb J. 2006;20:2313–2320. doi: 10.1096/fj.06-6281com. [DOI] [PubMed] [Google Scholar]
- 73.Lu KT, Ko MC, Chen BY, Huang JC, Hsieh CW, Lee MC, Chiou RY, Wung BS, Peng CH, Yang YL. Neuroprotective effects of resveratrol on MPTP-induced neuron loss mediated by free radical scavenging. J Agric Food Chem. 2008;56:6910–6913. doi: 10.1021/jf8007212. [DOI] [PubMed] [Google Scholar]
- 74.Jin F, Wu Q, Lu YF, Gong QH, Shi JS. Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson’s disease in rats. Eur J Pharmacol. 2008;600:78–82. doi: 10.1016/j.ejphar.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 75.Barber SC, Higginbottom A, Mead RJ, Barber S, Shaw PJ. An in vitro screening cascade to identify neuroprotective antioxidants in ALS. Free Radic Biol Med. 2009;46:1127–1138. doi: 10.1016/j.freeradbiomed.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumar P, Padi SS, Naidu PS, Kumar A. Effect of resveratrol on 3-nitropropionic acid-induced biochemical and behavioural changes: possible neuroprotective mechanisms. Behav Pharmacol. 2006;17:485–492. doi: 10.1097/00008877-200609000-00014. [DOI] [PubMed] [Google Scholar]
- 77.Ates O, Cayli S, Altinoz E, Gurses I, Yucel N, Sener M, Kocak A, Yologlu S. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol Cell Biochem. 2007;294:137–144. doi: 10.1007/s11010-006-9253-0. [DOI] [PubMed] [Google Scholar]
- 78.Sonmez U, Sonmez A, Erbil G, Tekmen I, Baykara B. Neuroprotective effects of resveratrol against traumatic brain injury in immature rats. Neurosci Lett. 2007;420:133–137. doi: 10.1016/j.neulet.2007.04.070. [DOI] [PubMed] [Google Scholar]
- 79.Rasouri S, Lagouge M, Auwerx J. SIRT1/PGC-1: a neuroprotective axis? Med Sci (Paris) 2007;23:840–844. doi: 10.1051/medsci/20072310840. [DOI] [PubMed] [Google Scholar]
- 80.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 81.Lamming DW, Wood JG, Sinclair DA. Small molecules that regulate lifespan: evidence for xenohormesis. Mol Microbiol. 2004;53:1003–1009. doi: 10.1111/j.1365-2958.2004.04209.x. [DOI] [PubMed] [Google Scholar]
- 82.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 83.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci USA. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pallas M, Casadesus G, Smith MA, Coto-Montes A, Pelegri C, Vilaplana J, Camins A. Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr Neurovasc Res. 2009;6:70–81. doi: 10.2174/156720209787466019. [DOI] [PubMed] [Google Scholar]
- 86.Fuenzalida K, Quintanilla R, Ramos P, Piderit D, Fuentealba RA, Martinez G, Inestrosa NC, Bronfman M. Peroxisome proliferator-activated receptor gamma up-regulates the Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial stabilization and protection against oxidative stress and apoptosis. J Biol Chem. 2007;282:37006–37015. doi: 10.1074/jbc.M700447200. [DOI] [PubMed] [Google Scholar]
- 87.Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130:685–696. doi: 10.1016/j.neuroscience.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 88.Wu JS, Cheung WM, Tsai YS, Chen YT, Fong WH, Tsai HD, Chen YC, Liou JY, Shyue SK, Chen JJ, Chen YE, Maeda N, Wu KK, Lin TN. Ligand-activated peroxisome proliferator-activated receptor-gamma protects against ischemic cerebral infarction and neuronal apoptosis by 14-3-3 epsilon upregulation. Circulation. 2009;119:1124–1134. doi: 10.1161/CIRCULATIONAHA.108.812537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rubiolo JA, Mithieux G, Vega FV. Resveratrol protects primary rat hepatocytes against oxidative stress damage: activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur J Pharmacol. 2008;591:66–72. doi: 10.1016/j.ejphar.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 90.Tang BL, Chua CE. SIRT1 and neuronal diseases. Mol Aspects Med. 2008;29:187–200. doi: 10.1016/j.mam.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 91.Lin TN, Wang Q, Simonyi A, Chen JJ, Cheung WM, He YY, Xu J, Sun AY, Hsu CY, Sun GY. Induction of secretory phospholipase A2 in reactive astrocytes in response to transient focal cerebral ischemia in the rat brain. J Neurochem. 2004;90:637–645. doi: 10.1111/j.1471-4159.2004.02540.x. [DOI] [PubMed] [Google Scholar]
- 92.Bi XL, Yang JY, Dong YX, Wang JM, Cui YH, Ikeshima T, Zhao YQ, Wu CF. Resveratrol inhibits nitric oxide and TNF-alpha production by lipopolysaccharide-activated microglia. International immunopharmacology. 2005;5:185–193. doi: 10.1016/j.intimp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 93.Kim YA, Lim SY, Rhee SH, Park KY, Kim CH, Choi BT, Lee SJ, Park YM, Choi YH. Resveratrol inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression in beta-amyloid-treated C6 glioma cells. Int J Mol Med. 2006;17:1069–1075. [PubMed] [Google Scholar]
- 94.Robb EL, Winkelmolen L, Visanji N, Brotchie J, Stuart JA. Dietary resveratrol administration increases MnSOD expression and activity in mouse brain. Biochem Biophys Res Commun. 2008;372:254–259. doi: 10.1016/j.bbrc.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 95.Chen CY, Jang JH, Li MH, Surh YJ. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem Biophys Res Commun. 2005;331:993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- 96.Anekonda TS. Resveratrol—a boon for treating Alzheimer’s disease? Brain Res Rev. 2006;52:316–326. doi: 10.1016/j.brainresrev.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 97.Mancuso C, Bates TE, Butterfield DA, Calafato S, Cornelius C, De Lorenzo A, Dinkova Kostova AT, Calabrese V. Natural antioxidants in Alzheimer’s disease. Expert Opin Investig Drugs. 2007;16:1921–1931. doi: 10.1517/13543784.16.12.1921. [DOI] [PubMed] [Google Scholar]
- 98.de la Lastra CA, Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem Soc Trans. 2007;35:1156–1160. doi: 10.1042/BST0351156. [DOI] [PubMed] [Google Scholar]
- 99.Qian YP, Cai YJ, Fan GJ, Wei QY, Yang J, Zheng LF, Li XZ, Fang JG, Zhou B. Antioxidant-based lead discovery for cancer chemoprevention: the case of resveratrol. J Med Chem. 2009;52:1963–1974. doi: 10.1021/jm8015415. [DOI] [PubMed] [Google Scholar]
- 100.Saiko P, Szakmary A, Jaeger W, Szekeres T. Resveratrol and its analogs: defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat Res. 2008;658:68–94. doi: 10.1016/j.mrrev.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 101.Conte A, Pellegrini S, Tagliazucchi D. Effect of resveratrol and catechin on PC12 tyrosine kinase activities and their synergistic protection from beta-amyloid toxicity. Drugs Exp Clin Res. 2003;29:243–255. [PubMed] [Google Scholar]
- 102.Conte A, Pellegrini S, Tagliazucchi D. Synergistic protection of PC12 cells from beta-amyloid toxicity by resveratrol and catechin. Brain Res Bull. 2003;62:29–38. doi: 10.1016/j.brainresbull.2003.08.001. [DOI] [PubMed] [Google Scholar]