Abstract
Nur77 orphan steroid receptor and its family member Nor-1 are required for apoptosis of developing T cells. In thymocytes, signals from the TCR complex induce Nur77 and Nor-1 expression followed by translocation from the nucleus to mitochondria. Nur77 and Nor-1 associate with Bcl-2 in the mitochondria, resulting in a conformation change that exposes the Bcl-2 BH3 domain, a presumed pro-apoptotic molecule of Bcl-2. As Nur77 and Nor-1 are heavily phosphorylated, we examined the requirement of Nur77 and Nor-1 phosphorylation in mitochondria translocation and Bcl-2 BH3 exposure. We found that HK434, a PKC agonist, in combination with calcium ionophore, can induce Nur77 and Nor-1 phosphorylation, translocation, Bcl-2 BH3 exposure and thymocyte apoptosis. Inhibitors of both classical and novel forms of PKC were able to block this process. In contrast, only the general but not classical PKC-specific inhibitors were able to block the same process initiated by PMA, a commonly used PKC agonist. These data demonstrate a differential activation of PKC isoforms by PMA and HK434 in thymocytes, and show the importance of PKC in mitochondria translocation of Nur77/Nor-1 and Bcl-2 conformation change during TCR-induced thymocyte apoptosis.
Keywords: Apoptosis, Nor-1, Nur77, PKC, Thymocytes
Introduction
T-cell development is a dynamic process that involves the balance of apoptosis and proliferation [1–5]. Early in development, DN (double negative CD4−CD8−) thymocytes that fail to express pre-TCR complex die through p53-dependent apoptosis. Those that express pre-TCR proliferate and differentiate into DP (double positive CD4+CD8+) thymocytes. DP thymocytes are exquisitely sensitive to apoptosis and survive for only a few days. DP thymocytes that are autoreactive to ubiquitously expressed self-antigen die immediately in a process called negative selection [3]. Only a few DP thymocytes differentiate into SP (single positive CD4+CD8− or CD4−CD8+) cells. Some of these SP cells (semi-mature SP cells) are still subject to negative selection. Those that are reactive to “tissue-specific” antigens expressed under the control of AIRE in medullary thymic epithelial cells die through apoptosis [6]. AIRE deficiency results in the escape of autoreactive semi-mature SP cells, leading to multi-organ autoimmunity. The signal transduction pathways of negative selection are poorly understood although many genes have been implicated, including the Nur77 family of transcription factors and their regulators (e.g. MEK5, HDAC7) [7–9], Bim (and its downstream proteins Bak and Bax) [10, 11], PTEN, a lipid phosphatase [12], E2F1 cell cycle protein [13] and members of the MAP kinase family [4].
As members of the orphan steroid receptor, Nur77 and its family member, Nor-1, are transcription factors that are active without addition of any known ligands [14]. Nur77 and Nor-1 expression is induced by TCR signaling. Expression of a dominant negative Nur77 protein can inhibit negative selection [15, 16]. Some of the Nur77 transcriptional regulated genes include FasL, TRAIL and NDG-1, a novel pro-apoptotic gene that initiates cell death through caspase-8 [17]. However, signaling proteins downstream of FasL, TRAIL and NDG-1 like FADD and caspase-8 are not required for negative selection [18, 19]. Nur77 and Nor-1 can also act through a non-transcriptional manner to initiate apoptosis. We have previously shown that during the early phase of thymocyte apoptosis, Nur77 and Nor-1 translocate from the nucleus to the mitochondria where they bind Bcl-2 [20]. Their association with Bcl-2 exposes the BH3 domain within Bcl-2, converting the protein into a potential killer molecule similar to those found in cancer cells [21, 22]. However, the upstream signals regulating Nur77’s translocation in thymocytes have not been defined. As Nur77 is heavily phosphorylated, it seems plausible that phosphorylation regulates the protein’s subcellular localization, which has been shown in some cell lines. In prostate and lung cancer cell lines, for example, Nur77’s mitochondrial targeting is dependent on both induction of the JNK kinase and inhibition of the Akt kinase [23]. In DO11.10 T-cell hybridomas, expression of a constitutively active Akt protein inhibited Nur77’s transcriptional activities, possibly by stimulating its association with 14–3–3 for nuclear exclusion [24, 25]. Also in DO11.10 cells, RSK, a kinase downstream of the ERK1/2 pathway was shown recently to be responsible for phosphorylation of Nur77 required for mitochondria translocation [26]. The signals mediating Nur77’s localization to mitochondria in primary cells like thymocytes, however, remain unclear.
TCR stimulation during negative selection results in activation of several downstream cascades, involving protein tyrosine kinases, PKC and MAPK [3]. Activation of the protein tyrosine kinases and signaling through the MAP kinase pathway causes activation of ERK1/2, JNK, p38 and ERK5. JNK, p38 and ERK5 have been established as key molecules during negative selection [4] while ERK1/2 are required for positive selection [27]. PKC proteins have also been implicated in negative selection [28].
The PKC family of serine/threonine kinases consists of multiple isozymes involved in a myriad of signal transduction pathways. PKC isozymes are classified into calcium-independent or classical cPKC (α, β and γ), novel nPKC (δ, ε, η and θ) and atypical aPKC (μ and ζ) [29, 30]. In T lymphocytes, PKC isoforms play important roles in facilitating cell survival, activation, differentiation and the induction of cell death [31–33]. PKCθ is a nPKC selectively expressed in T cells and muscle and plays a particularly important role in TCR/CD28 signaling pathways [33]. In mature T cells, PKCθ functions to activate the JNK/AP-1 pathways and participate in IL-2 induction and activation of NF-κB. However, in thymocytes, the induction of NF-κB is independent of PKCθ signaling, as PKCθ−/− thymocytes treated with anti-CD3 and anti-CD4 or TNF show normal activation of NF-κB [34]. Other PKC proteins regulate apoptosis in thymocytes. PKCα, for example, regulates transcription of the pro-apoptotic molecule Bim in anti-CD3/CD28 treated thymocytes [28]. In another study, a general inhibitor of all PKC isoforms was demonstrated to prevent peptide-mediated apoptosis in thymocytes [35]. Additionally, the activation of nPKC was reported to promote a pathway for negative selection [36, 37]. The significance of PKC proteins during clonal deletion is further exemplified by findings showing the block in negative selection observed in Vav−/− mice can be rescued with PKC activation [35]. Thus, the PKC family proteins are crucial prerequisites for negative selection.
Activation of the PKC isozymes depends on the binding of phorbol ester tumor promoters or diacylglycerol (DAG) to the regulatory domain of the kinase. PMA is widely used as a PKC activator. However, PMA induces pleiotropic effects as it activates “non-kinase” proteins in addition to PKC isozymes [38]. To this end, potent PKC ligands have been synthesized based on the constrained structure of DAG. These DAG-lactones bind to the regulatory domain of PKCα with high affinity. However, the biological activity of these DAG-lactones in thymocytes has never been investigated [39–41]. Here, we show that PKC and Ca2+ signals induced by the DAG-lactone HK434 and ionomycin, respectively, can induce the mitochondrial targeting of Nur77 and Nor-1 to promote their association with Bcl-2. PKC is crucial for Nur77/Nor-1 mitochondrial targeting, apoptosis and exposure of the Bcl-2 BH3 domain in DP thymocytes.
Results
Chemical inhibitors for PKC can block Nur77 and Nor-1 mitochondria translocation
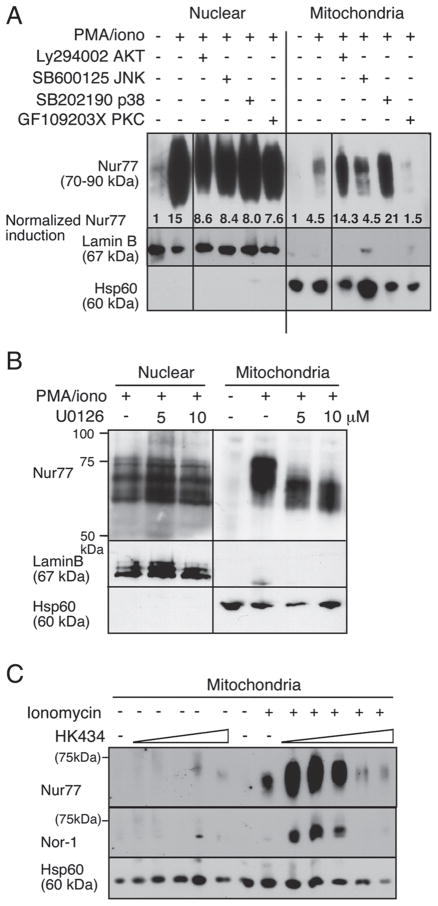
In TCR-stimulated thymocytes, slower migrating forms of Nur77 were seen at the mitochondria. These have been previously shown as heavily phosphorylated Nur77 [42]. We stimulated thymocytes with PMA/ionomycin in the presence of numerous kinase inhibitors, including LY294002 for Akt, GF109203X for PKC, SB202190 for p38, SP600125 for JNK and U0126 for the ERK1/2 pathways. We found that only with inhibition of the PKC family was Nur77’s translocation to the mitochondria greatly reduced (Fig. 1A). Inhibition of Akt, p38 or JNK had no effect or even led to increased levels of Nur77 at the mitochondria. In contrast to the requirement of the ERK1/2 pathway in DO11.10 T-cell hybridoma, Nur77 mitochondria localization was still seen in thymocytes treated with the ERK1/2 inhibitor U0126 (Fig. 1B). Even though reduced Nur77 phosphorylation by U0126 was evident, Nur77 could nevertheless be seen in the mitochondria fraction (Fig. 1B). No effects on the levels of nuclear Nur77 were seen with these inhibitors, including GF109203X, the PKC inhibitor.
Figure 1.
PKC proteins regulate Nur77/Nor-1 mitochondrial targeting. (A) Thymocytes were treated with 20 μM LY294002, 20 μM SB600125, 10 μM SB202190 or 1 μM GF109203X for 1 h followed by stimulation with 2.5 ng/mL PMA/0.5 μM ionomycin for 2 h and fractionated into nuclear and mitochondrial fractions. Western blot analysis was carried out with antibodies specific for Nur77, lamin B or Hsp60. Quantitation of Nur77 was performed using Adobe Photoshop CS3 as outlined in the methods. The absolute intensity of Nur77 was normalized with that of either Lamin B, for the nuclear fraction, or Hsp60 for the mitochondrial fraction. Fold difference is shown relative to the no treatment control. (B) Same as (A) except U0126 was used. (C) Mitochondrial fraction of thymocytes cultured with increasing concentrations of the PKC activator, HK434, in the absence or presence of ionomycin. Mitochondrial fractions were run on an SDS-PAGE gel and blotted with either anti-Nur77, anti-Nor-1, anti-Lamin B or anti-HSP60 antibodies. Data in (A) and (B) are representative of three independent experiments; (C) is representative of two independent experiments.
To show that the PKC family is indeed responsible for targeting Nur77 to the mitochondria, we used a specific PKC agonist, termed HK434 [39]. HK434 treatment alone could not induce expression of Nur77 (Fig. 1C). This is in line with work by our lab and other groups showing that treatment with the PKC activator, PMA alone, could not induce Nur77 protein levels in thymocytes or T-cell hybridomas [42, 43]. Treatment with ionomycin alone could induce a small amount of Nur77 expression and target the protein to the mitochondria (Fig. 1C). This is most likely due to the ability of ionomycin to weakly activate the PKC pathway [44]. However, Nur77 levels were significantly enhanced when PMA or the DAG-lactone, HK434, were added (Fig. 1C and data not shown). Nur77 levels dropped at the highest HK434 concentrations, presumably due to extensive apoptosis. The same results were found with Nor-1 mitochondria translocation (data not shown and Fig. 1C). We conclude that Nur77 and Nor-1 induction and mitochondrial targeting are dependent on two intracellular signals, the PKC and the calcium pathways.
Differential effect of a PKC inhibitor on PMA versus HK434-induced Nur77 mitochondria translocation
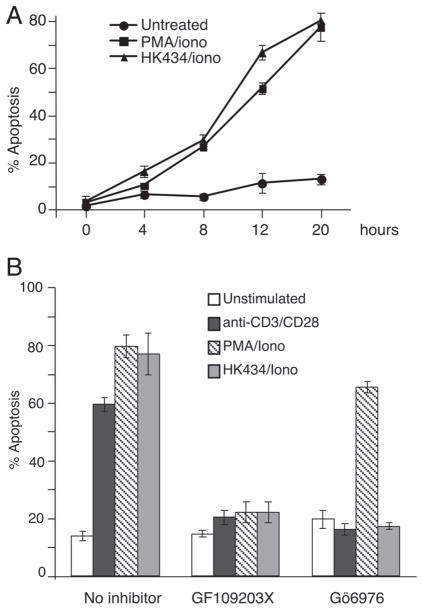
It is well established that activation of PKC by phorbol esters such as PMA triggers an apoptotic response in thymocytes [35, 45, 46]. In LNCaP cells, the PKC activator, HK434, was shown to mimic the action of PMA with respect to apoptosis. In thymocytes, the level and kinetics of apoptosis induced by HK434 and ionomycin were similar to that induced by PMA and ionomycin (Fig. 2A). To confirm that the apoptotic effect of PMA and the DAG-lactone in thymocytes is mediated by activation of PKC, we assessed the affect of HK434 and PMA in the presence of pharmacological inhibitors that specifically block classical or novel PKC isoforms. The classical PKC inhibitor, Gö6976 sufficiently abrogated HK434-induced death (Fig. 2B) as well as the cytotoxic affects of anti-CD3/CD28 antibody treatment (Fig. 2B).
Figure 2.
Thymocyte apoptosis induced by DAG-lactone, HK434/ionomycin and PMA/ionomycin. (A) Thymocytes were stimulated with 1 μM DAG-lactone, HK434 or 0.26 ng/mL PMA with the time course indicated. Cells were stained for Annexin V and PI. Apoptotic cells are defined as Annexin V+ PI− cells. (B) Thymocytes were treated in the presence or absence of 1 μM Gö6976 or 1 μM GF109203X for 1 h followed by stimulation with 1 μM DAG-lactone, HK434 or 0.26 ng/mL PMA or 10 μg/mL anti-CD3/2 μg/mL CD28 (plate-bound). Twenty hours following stimulation, cells were stained for Annexin V and PI to assess apoptosis. Results shown in (A) and (B) represent four independent experiments with mean±SD indicated.
The inhibitory effect of Gö6976 on PMA/ionomycin-induced thymocyte cell death is controversial. One group found that it could block PMA/ionomycin death although the effect was modest at best [28] while another group could not see any effect [46]. In our hands, Gö6976 could not block thymocyte death induced by PMA, even at subnanomolar concentrations of the phorbol ester. However, the classical and novel PKC isoform inhibitor, GF109203X, almost completely blocked cell death induced by all treatments (Fig. 2B). Pre-treatment with GF109203X effectively blocked activation induced by all stimulation conditions, as assessed by CD69 staining (data not shown). Interestingly, though 1 μM Gö6976 had no affect on PMA-induced thymocyte apoptosis; the inhibitor was sufficient in blocking thymocyte activation mediated by PMA as assessed by CD69 staining. These results suggest that cPKC isozymes are responsible for the death induced by the PKC ligand, HK434 and anti-CD3/CD28 antibodies. Yet, nPKC but not cPKC isoforms play a role in thymocyte apoptosis induced by PMA.
PKC inhibitors can block Nur77/Nor-1 association with Bcl-2 in stimulated thymocytes
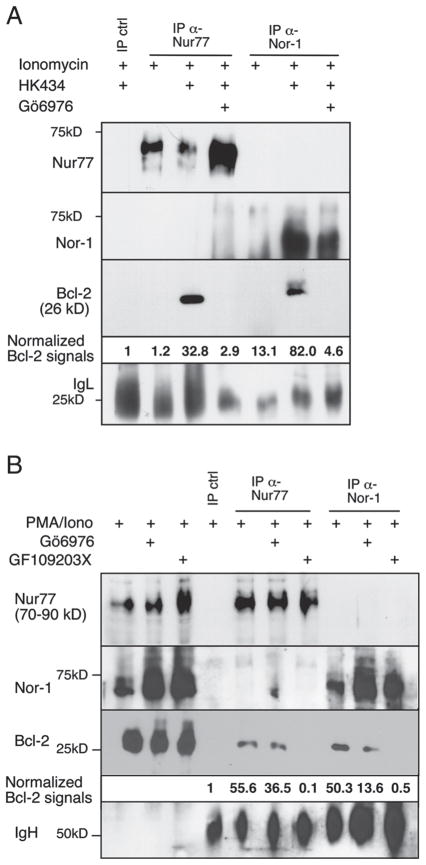
Inhibition of conventional PKC isozymes with Gö6976 was effective in blocking cell death induced by HK434/ionomycin but not PMA/ionomycin signals; therefore, we wanted to examine Nur77 localization in the presence of this cPKC-specific inhibitor as well as the PKC general inhibitor. Inhibition of cPKC with Gö6976 is sufficient in blocking Nur77 and Nor-1 translocation to the mitochondria mediated by HK434/ionomycin (Fig. 3A). The same inhibitor had no effect in Nur77/Nor-1 translocation stimulated by PMA/ionomycin (Fig. 3B). GF109203X, an inhibitor of both classical and novel PKC isoforms, could prevent Nur77 and Nor-1 nuclear/cytoplasmic shuttling in PMA/or HK434/ionomycin stimulated thymocytes (Fig. 3B and data not shown). We have previously shown that PMA/ionomycin signals target Nur77 to the mitochondria, where the protein binds to Bcl-2 in thymocytes [20]. To determine if specific activation of PKC could induce Nur77/Bcl-2 association, we treated thymocytes with ionomycin in the absence and presence of PKC ligand, HK434 or PMA. Figure 4A shows that treatment of thymocytes with ionomycin alone cannot induce Nur77/Bcl-2 or Nor-1/Bcl-2 association. Yet, when thymocytes were stimulated with HK434/ionomycin, anti-Nur77 and anti-Nor-1 but not control antibodies could pull down Bcl-2. The HK434-induced association of Nur77 and Bcl-2 could be interrupted when cells were stimulated in the presence of PKC inhibitor, Gö6976 (Fig. 4A). It should be noted that the Nur77 and Nor-1 being pulled down in the presence of the PKC inhibitors represents the nuclear localized form of these proteins, as Nur77 and Nor-1 are unable to target the mitochondria when PKC proteins are inhibited. The PMA/ionomycin induced Nur77/Bcl-2 association could only be disrupted with GF109203X pre-treatment. Thymocytes stimulated with PMA/ionomycin in the presence of classical PKC inhibitor, Gö6976 show similar levels of Bcl-2 association with Nur77 as compared to thymocytes stimulated in the absence of inhibitor (Fig. 4B). Similarly, the association between Nor-1 and Bcl-2 induced by PMA/ionomycin is disrupted only when nPKC in addition to cPKC isoforms are inhibited by GF 109203X (Fig. 4B).
Figure 3.
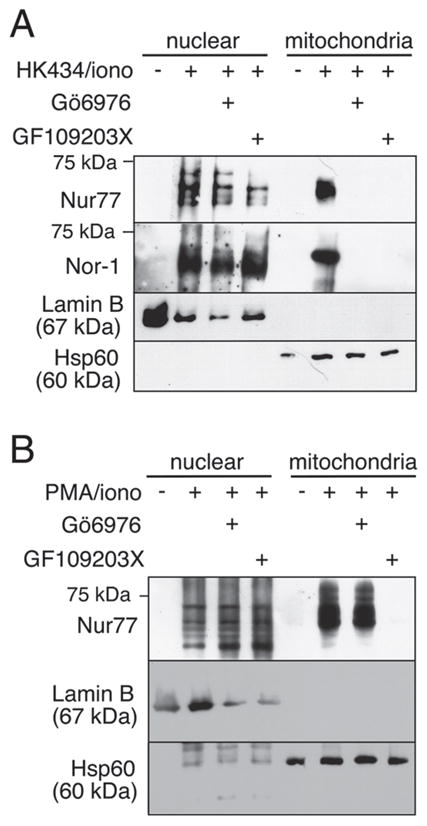
Inhibition of classical PKC blocks Nur77/Nor-1 mitochondrial targeting in HK434/ionomycin- but not PMA/ionomycin-treated thymocytes. (A) Thymocytes were treated in the presence or absence of 1 μM Gö6976 or 1 μM GF109203X for 1 h followed by stimulation with 1 μM HK434/0.5 μM ionomycin. (B) Thymocytes were treated in the presence or absence of 1 μM Gö6976 or 1 μM GF 109203X for 1 h followed by stimulation with 2.5 ng/mL PMA/0.5 μM ionomycin. Data in this figure are representative of three independent experiments.
Figure 4.
Nur77 family proteins are prevented from associating with Bcl-2 in the presence of PKC inhibitors. (A) Cell lysates from lck-Bcl-2 [59] thymocytes cultured in the presence or absence of 1 μM Gö6976 for 1 h followed by stimulation with 0.5 μM ionomycin with or without 1 μM HK434 were immunoprecipitated (IP) with anti-rabbit IgG (IP crtl), anti-Nur77 and anti-Nor-1 antibodies, followed by blotting with anti-Nur77, anti-Nor-1 or anti-Bcl-2 antibody. (B) Cell lysates from lck-Bcl-2 thymocytes cultured in the presence or absence of 1 μM Gö6976 or 1 μM GF109203X for 1 h followed by stimulation with 2.5 ng/mL PMA/0.5 μM ionomycin were immunoprecipitated as in (A). Quantitation of Bcl-2 was performed using Adobe Photoshop CS3 as outlined in the Materials and methods section. The absolute intensity of Bcl-2 was normalized with that of IgL (A) or IgH (B). Fold difference is shown relative to the control. Data in this figure are representative of three independent experiments.
Nur77’s targeting of Bcl-2 induces a conformational change in which the buried BH3 domain of Bcl-2 is exposed [20–22, 47]. Similar to anti-CD3/CD28 and PMA/ionomycin treatment, stimulation with HK434/ionomycin induces a Bcl-2 conformational change in stimulated thymocytes (Fig. 5A). This Bcl-2 conformational change was blocked in thymocytes pre-incubated with Gö6976 and GF109203X. The cPKC inhibitor was also effective in blocking the conversion of Bcl-2 induced by anti-CD3/CD28 antibody treatment. In contrast, only the inhibitor of both classical and novel PKC could block the Bcl-2/BH3 exposure in PMA/ionomycin stimulated thymocytes. The exposure of Bcl-2 is restricted to DP thymocytes. There was no conversion of Bcl-2 observed in DN, CD4+ SP or CD8+ SP cells (Fig. 5B). Ionomycin treatment alone is unable to induce the BH3 conformational change within Bcl-2 (Fig. 5B). These data combined suggest that cPKC isoenzymes are responsible for Nur77/Nor-1 mitochondrial targeting and the subsequent conversion of Bcl-2 into a killer molecule in HK434/ionomycin- and anti-CD3/CD28-treated thymocytes. Yet, nPKC proteins regulate Nur77 and Nor-1 subcellular localization following PMA/ionomycin stimulation. These results not only demonstrate the significance of PKC in Nur77/Nor-1 regulation but also confirm the importance of PKC proteins in thymocyte apoptosis mimicking negative selection.
Figure 5.
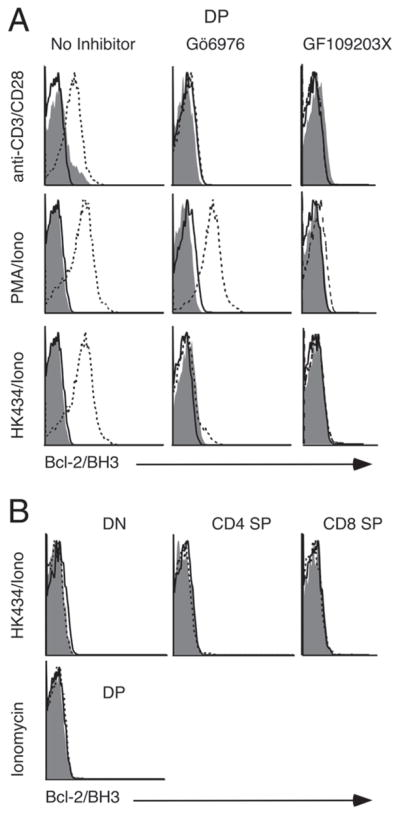
Bcl-2/BH3 exposure of stimulated thymocytes is inhibited in the presence of PKC inhibitors. Thymocytes were treated in the presence or absence of 1 μM Gö6976 or 1 μM GF109203X for 1 h followed by stimulation with (plate bound) anti-CD3/CD28 antibodies or 1 μM HK434/0.5 μM ionomycin or 2.5 ng/mL PMA/0.5 μM ionomycin or ionomycin alone. Here, shaded area represents isotype control, solid line represents untreated thymocytes and dotted line represents stimulated thymocytes in the absence of PKC inhibitors (left panels in A and all the panels in part B), in the presence of 1 μM Gö6976 or 1 μM GF109203X. Data in this figure are representative of three independent experiments.
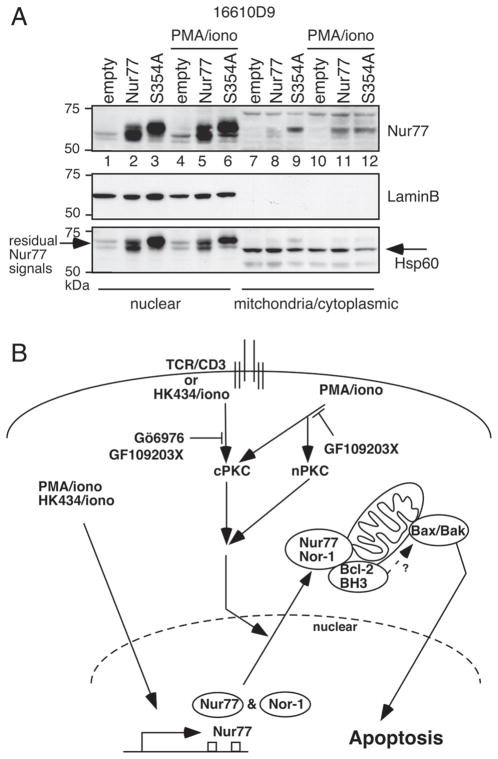
In DO11.10 T-cell hybridoma, ERK1/2-RSK pathway was shown to phosphorylate Nur77 at residue 354. An alanine substitution at this site impairs Nur77 nuclear export and apoptosis [26]. To see if this might be true in DP cells, we used 16610D9 cells, a CD4+CD8+ thymoma cell line that possesses many characteristics of primary DP thymocytes [48]. As shown in Fig. 6A, 16610D9 cells express very little endogenous Nur77 (lanes 1, 4, 7 and 10). Infection of these cells with Nur77 retrovirus led to expression of Nur77 in the nuclear compartment (lanes 2 and 5). However, very little Nur77 was found in the mitochondria/cytoplasmic fractions unless PMA/ionomycin were added (see lane 11 versus lane 8). Interestingly, expression of Nur77(354A) mutant (mutation verified by sequencing) led to constitutive translocation of Nur77 to the mitochondria/cytoplasmic fraction (lane 9). These data show that phosphorylation of Nur77 at residue 354 might have a different effect in DP cells from DO11.10 cells and that regulation of Nur77 nuclear transport and its association with Bcl-2 is more complicated than initially thought.
Figure 6.
The role of Nur77 serine 354 in a thymoma cell line and a schematic diagram of the PMA/HK434 differential effects on Nur77/Nor-1 localization. (A) 16610D9 cells were infected with MSCV empty virus, MSCV-Nur77 or MSCV-Nur77 (354A) retroviruses. Forty-eight hours after infection, cells were stimulated with PMA/ionomycin for 2 h where indicated. They were then lysed and fractionated into nuclear or mitochondria/cytoplasmic fractions (the fragile nature of mitochondria in this cell line made it impossible to isolate the pure mitochondrial fraction). Western blot analysis with Nur77-, lamin-B- or Hsp60-specific antibodies was then carried out. Please note the residual Nur77 signals that could not be stripped away in the Hsp60 blot. Data are representative of three independent experiments. (B) A schematic diagram of HK434/ionomycin (iono)-, PMA/iono- or TCR-induced Nur77/Nor-1 pathways as discussed in this study. Arrows denote stimulation, whereas T arrows denote inhibition.
Discussion
Nur77 has been reported as the target of numerous kinases including protein kinase A, PKC, Akt, JNK, ERK5 and p90 ribosomal S6 kinase [23, 24, 49–51]. Phosphorylation of Nur77 by these proteins was demonstrated in various in vitro and in vivo experiments. However, the functional consequence of Nur77 phosphorylation remains controversial. Here, we report that the PKC proteins regulate Nur77 phosphorylation and nuclear/cytoplasmic translocation in thymocytes during apoptosis that mimics negative selection. Chemical inhibition of PKC proteins prevented Nur77 and family member Nor-1 from targeting the mitochondria and their targeting of Bcl-2. In contrast, inhibition of AKT, JNK, ERK1/2 and p38 did not affect the subcellular localization of Nur77 family proteins in thymocytes. These results are different from mitochondria translocation of Nur77 induced by the retinoid analog CD437, which requires activation of the JNK and inhibition of the AKT pathways [23]. Inhibition of ERK1/2 was also recently reported to block Nur77 mitochondria translocation in DO11.10 T-cell hybridoma cells [26]. The discrepancy with our results is most likely due to the differences in the cells used. Consistent with this, we found that alanine mutation at Nur77 residue 354, which impairs mitochondrial translocation in DO11.10 cells, causes constitutive translocation of Nur77 in 16610D9 CD4+ CD8+ cells. Thus, the involvement of kinase pathway(s) in Nur77 mitochondria translocation is cell type and stimulus specific. Though calcium signals alone were adequate in causing Nur77 to be localized to the mitochondria, these levels may be inadequate for binding Bcl-2, as no Bcl-2/Nur77 interaction could be detected in ionomycin treated thymocytes. In addition, ionomycin could not induce Nor-1 to any appreciable levels. In line with these findings, ionomycin-stimulated thymocytes did not show BH3 exposure of Bcl-2. Therefore, we believe that calcium and PKC signals are required for sufficient Nur77/Nor-1 mitochondrial localization and reversal of Bcl-2 pro-survival function.
In this study, we report the biological activity of a synthetic DAG-lactone, HK434, in thymocytes. HK434, like the other synthesized DAG analogs, binds with high potency to the phorbol ester/DAG binding site within the C1 domain of PKC [52]. Using the crystal structure of the PKCδ C1b domain with pharmacophore and receptor-guided approaches, structurally primitive DAG-lactone ligands were designed with binding affinities for PKCα in the low nanomolar range [39]. These DAG-lactones exhibit 3–4 orders of magnitude higher affinity for PKC isozymes than natural DAG and phorbol esters. They have been characterized in other cell types and have phorbol ester-like effects [39, 53–56]. Here, we report that DAG-lactone, HK434 and ionomycin signals are sufficient to induce Nur77/Nor-1 mitochondrial targeting in thymocytes. Furthermore, HK434, like phorbol esters can induce apoptosis in thymocytes. An interesting finding is that HK434 and PMA exert their regulation of Nur77 and their apoptotic activities through activation of different subsets of PKC isoforms (Fig. 6B). While the classical PKC isoform inhibitor Gö6976 is sufficient in blocking HK434/ionomycin-induced Nur77 mitochondrial targeting and thymocyte apoptosis, no effect was observed with PMA/ionomycin-stimulated thymocytes. A correlation was found between PKC activation, induction of thymocyte apoptosis, Nur77/Nor-1 phosphorylation, mitochondria translocation and exposure of the Bcl-2 BH3 epitope in stimulated thymocytes, further confirming the important role of Nur77/Nor-1 mitochondria translocation in TCR-induced thymocyte apoptosis. It is not clear if PKC acts directly or indirectly on Nur77/Nor-1. An interaction between PKC and Nur77 has been reported before [57]. Ser350 within the DNA binding domain of Nur77 was previously shown to be phosphorylated by protein kinase A and PKC in an in vitro kinase assay of stimulated PC12 neuronal cells [49]. However, in another study, the association of Nur77 and PKCθ in T-cell hybridomas did not induce Nur77 phosphorylation [57]. It is possible that a direct PKC regulation of Nur77 might be unique to immature T cells. Alternatively, phosphorylation of Nur77 may be indirectly regulated by PKC proteins.
PKCθ has been initially suggested to be the PKC isoform crucial for negative selection. This notion was based on findings that during negative selection, PKCθ, but not other PKC isoenzymes, is recruited to the site of TCR aggregation [35]. However, PKCθ−/− mice show no defects in negative selection [58]. This suggests some functional redundancy among PKC family members and that a PKC isoenzyme distinct from PKCθ is involved in TCR signaling events in thymocytes. Interestingly, thymocytes are protected from apoptosis induced by TCR cross-linking in the absence of nPKCδ [46]. Given our findings, it seems classical, as well as novel PKC isoenzymes, may be capable of regulating thymocyte apoptosis in the absence of PKCθ. The association of Nur77 and PKC further exemplifies the significance of how these molecules act in concert to mediate a crucial component of thymocyte development. Cante-Barret et al. [28] have shown that PKC regulates Bim transcription during negative selection; thus, PKC can activate at least two apoptotic pathways converging at mitochondria. Further studies are necessary to more clearly elucidate their role in negative selection.
Materials and methods
Antibodies and reagents
The PKCα and -θ antibodies were provided by Cell Signaling and Santa Cruz, respectively. The anti-CD3 (clone 2C11) and anti-CD28 (clone PV-1) antibodies were purchased from the University of California, San Francisco, Hybridoma Facility. All other antibodies and reagents have been described previously [20]. Bcl-2 BH3 intracellular staining was done as described [20]. The Nur77 Serine-354-Alanine (S354A) mutant in the pSG5 vector backbone was generously provided by Dr. Lester Lau (University of Chicago) through Dr. Philippa Melamed. Nur77 and the Nur77(S354A) mutant were cloned into the MSCV 2.2-ires-GFP retroviral vector, a gift from Dr. William Sha (Berkeley). The VSV-G and a gag-pol helper plasmid for retroviral transduction were from the Nolan laboratory (Stanford).
Thymocyte culture
Thymocytes were stimulated with PMA or 1 μM HK434 plus ionomycin or plate-bound anti-CD3 (10 μg/mL) anti-CD28 (2 μg/mL). One-hour pre-treatment with 1 μM Gö6976 or GF109203X or 10 μM SB 203580 or U0126 or 50 μM LY294002 or 20 μM SB600125 was used where indicated. All animal-related experiments have been approved by the Berkeley Animal Use and Care Committee.
16610D9 transduction
Phoenix cells were transfected with MSCV, VSV-G and gag-pol helper plasmids by Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Five hours after transfection, the media was changed to Opti-MEM supplemented with 10% FCS, penicillin/streptomycin and α-mercaptoethanol (16610D9 media). Two days after transfection, the viral supernatant was syringe filtered (0.45 μm), supplemented with 4 μg/mL polybrene and added to 2.5 × 106 16610D9 cells. The cells were spun at 2500 rpm for 1 h and cultured for 2 days, with fresh 16610D9 media added 24 h after infection, before cell fractionation.
16610D9 cell fractionation
Retrovirally transduced 16610D9 cells were stimulated with 2.5 ng PMA/0.5 μM ionomycin for 2 h. After washing 1.5 × 107 16610D9s with PBS, cells were resuspended in 200 μL Solution A (10 mM HEPES-KOH [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 0.2 mM PMSF, 1 mM DTT and 0.5–0.6% Nonidet P40). They were then incubated on ice for 10 min and spun down briefly. The nuclear pellet was washed three times with PBS and resuspended in 40 μL 16610D9s of Solution B (20 mM HEPES-KOH [pH 7.9], 400 mM NaCl 20% glycerol, 0.2 mM EDTA, 0.2 mM PMSF, 1 mM DTT and 0.5–0.6% Nonidet P40). The supernatant, containing the mitochondria/cytoplasmic fraction, was then transferred to a fresh tube and centrifuged twice at 700 ×g for 5 min each. The resulting supernatant was resuspended in 10 μL of Solution A. The protein concentration of the nuclear and mitochondria/cytoplasm fractions was determined using the Biorad Protein Assay.
Immunoprecipitation, immunoblotting and thymocyte fractionation
These procedures were done as previously described [20]. Quantitation of the Western blots was performed using Adobe Photoshop CS3 as described (http://lukemiller.org/journal/2007/08/quantifying-western-blots-without.html). Briefly, the Adobe Photoshop lasso tool was used to outline each protein band and a background region on the membrane. The mean gray value and the pixel value were multiplied to determine the absolute intensity of the band. When no band was visible, the outlined region was made equal in pixel number to that of the background region. The background to be subtracted from a given band was determined by multiplying the mean gray value of the outlined background region by the pixel measurement for the corresponding band.
Acknowledgments
The authors thank Victor E. Marquez for his generous gift of HK434 and Yuefang Sun for taking care of the mouse colonies. This study was supported by a grant from the National Institute of Health (to A. W.) and the Research Supplement for underrepresented minorities from the National Cancer Institute (to J. T.).
Abbreviations
- DAG
diacylglycerol
- DN
double negative CD4−CD8−
- DP
double positive CD4+CD8+
- SP
single positive (CD4+CD8− or CD4−CD8+)
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Palmer E. Cell death and immunity: negative selection – clearing out the bad apples from the T-cell repertoire. Nat Rev Immunol. 2003;3:383–391. doi: 10.1038/nri1085. [DOI] [PubMed] [Google Scholar]
- 2.von Boehmer H, Aifantis I, Gounari F, Azogui O, Haughn L, Apostolou I, Jaeckel E, et al. Thymic selection revisited: how essential is it? Immunol Rev. 2003;191:62–78. doi: 10.1034/j.1600-065x.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 3.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 4.Sohn SJ, Thompson J, Winoto A. Apoptosis during negative selection of autoreactive thymocytes. Curr Opin Immunol. 2007;19:510–515. doi: 10.1016/j.coi.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Strasser A, Puthalakath H, O’Reilly LA, Bouillet P. What do we know about the mechanisms of elimination of autoreactive T and B cells and what challenges remain. Immunol Cell Biol. 2008;86:57–66. doi: 10.1038/sj.icb.7100141. [DOI] [PubMed] [Google Scholar]
- 6.Mathis D, Benoist C. A decade of AIRE. Nat Rev Immunol. 2007;7:645–650. doi: 10.1038/nri2136. [DOI] [PubMed] [Google Scholar]
- 7.Parra M, Mahmoudi T, Verdin E. Myosin phosphatase dephosphorylates HDAC7, controls its nucleocytoplasmic shuttling, and inhibits apoptosis in thymocytes. Genes Dev. 2007;21:638–643. doi: 10.1101/gad.1513107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sohn SJ, Lewis GM, Winoto A. Non-redundant function of the MEK5-ERK5 pathway in thymocyte apoptosis. EMBO J. 2008;27:1896–1906. doi: 10.1038/emboj.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldwin TA, Hogquist KA. Transcriptional analysis of clonal deletion in vivo. J Immunol. 2007;179:837–844. doi: 10.4049/jimmunol.179.2.837. [DOI] [PubMed] [Google Scholar]
- 10.Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 11.Rathmell JC, Lindsten T, Zong WX, Cinalli RM, Thompson CB. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol. 2002;3:932–939. doi: 10.1038/ni834. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, Yoshida R, et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 13.Zhu JW, DeRyckere D, Li FX, Wan YY, DeGregori J. A role for E2F1 in the induction of ARF, p53, and apoptosis during thymic negative selection. Cell Growth Differ. 1999;10:829–838. [PubMed] [Google Scholar]
- 14.Flaig R, Greschik H, Peluso-Iltis C, Moras D. Structural basis for the cell-specific activities of the NGFI-B and the Nurr1 ligand-binding domain. J Biol Chem. 2005;280:19250–19258. doi: 10.1074/jbc.M413175200. [DOI] [PubMed] [Google Scholar]
- 15.Calnan B, Szychowski S, Chan FKM, Cado D, Winoto A. A role of the orphan steroid receptor Nur77 in apoptosis accompaying antigen-induced negative selection. Immunity. 1995;3:273–282. doi: 10.1016/1074-7613(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 16.Zhou T, Cheng J, Yang P, Wang Z, Liu C, Su X, Bluethmann H, et al. Inhibition of Nur77/Nurr1 leads to inefficient clonal deletion of self-reactive T cells. J Exp Med. 1996;183:1879–1892. doi: 10.1084/jem.183.4.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajpal A, Cho YA, Yelent B, Koza-Taylor PH, Li D, Chen E, Whang M, et al. Transcriptional activation of known and novel apoptotic pathways by Nur77 orphan steroid receptor. EMBO J. 2003;22:6526–6536. doi: 10.1093/emboj/cdg620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newton K, Harris AW, Bath ML, Smith KGC, Strasser A. A dominant interfering mutant of FADD/MORT1 enhance deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J. 1998;17:706–718. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au PY, Berry DM, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17:883–895. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson J, Winoto A. During negative selection, Nur77 family proteins translocate to mitochondria where they associate with Bcl-2 and expose its proapoptotic BH3 domain. J Exp Med. 2008;205:1029–1036. doi: 10.1084/jem.20080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, Dawson MI, et al. Conversion of Bcl-2 from protection to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- 22.Kolluri SK, Zhu X, Zhou X, Lin B, Chen Y, Sun K, Tian X, et al. A short Nur77-derived peptide converts Bcl-2 from a protector to a killer. Cancer Cell. 2008;14:285–298. doi: 10.1016/j.ccr.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han YH, Cao X, Lin B, Lin F, Kolluri SK, Stebbins J, Reed JC, et al. Regulation of Nur77 nuclear export by c-Jun N-terminal kinase and Akt. Oncogene. 2006;25:2974–2986. doi: 10.1038/sj.onc.1209358. [DOI] [PubMed] [Google Scholar]
- 24.Pekarsky Y, Hallas C, Palamarchuk A, Koval A, Bullrich F, Hirata Y, Bichi R, et al. Akt phosphorylates and regulates the orphan nuclear receptor Nur77. Proc Natl Acad Sci USA. 2001;98:3690–3694. doi: 10.1073/pnas.051003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masuyama N, Oishi K, Mori Y, Ueno T, Takahama Y, Gotoh Y. Akt inhibits the orphan nuclear receptor Nur77 and T-cell apoptosis. J Biol Chem. 2001;276:32799–32805. doi: 10.1074/jbc.M105431200. [DOI] [PubMed] [Google Scholar]
- 26.Wang A, Rud J, Olson CM, Jr, Anguita J, Osborne BA. Phosphorylation of Nur77 by the MEK-ERK-RSK cascade induces mitochondrial translocation and apoptosis in T cells. J Immunol. 2009;183:3268–3277. doi: 10.4049/jimmunol.0900894. [DOI] [PubMed] [Google Scholar]
- 27.Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of Erk1 and Erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Cante-Barrett K, Gallo EM, Winslow MM, Crabtree GR. Thymocyte negative selection is mediated by protein kinase C- and Ca2+-dependent transcriptional induction of bim [corrected] J Immunol. 2006;176:2299–2306. doi: 10.4049/jimmunol.176.4.2299. [DOI] [PubMed] [Google Scholar]
- 29.Baier G. The PKC gene module: molecular biosystematics to resolve its T cell functions. Immunol Rev. 2003;192:64–79. doi: 10.1034/j.1600-065x.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 30.Tan SL, Parker PJ. Emerging and diverse roles of protein kinase C in immune cell signalling. Biochem J. 2003;376:545–552. doi: 10.1042/BJ20031406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sedwick CE, Altman A. Perspectives on PKCtheta in T cell activation. Mol Immunol. 2004;41:675–686. doi: 10.1016/j.molimm.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Altman A, Isakov N, Baier G. Protein kinase Ctheta: a new essential superstar on the T-cell stage. Immunol Today. 2000;21:567–573. doi: 10.1016/s0167-5699(00)01749-7. [DOI] [PubMed] [Google Scholar]
- 33.Altman A, Villalba M. Protein kinase C-theta (PKCtheta): it’s all about location, location, location. Immunol Rev. 2003;192:53–63. doi: 10.1034/j.1600-065x.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 34.Morley SC, Weber KS, Kao H, Allen PM. Protein kinase C-theta is required for efficient positive selection. J Immunol. 2008;181:4696–4708. doi: 10.4049/jimmunol.181.7.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong YY, Fischer KD, Bachmann MF, Mariathasan S, Kozieradzki I, Nghiem MP, Bouchard D, et al. Vav regulates peptide-specific apoptosis in thymocytes. J Exp Med. 1998;188:2099–2111. doi: 10.1084/jem.188.11.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asada A, Zhao Y, Kondo S, Iwata M. Induction of thymocyte apoptosis by Ca2+-independent protein kinase C (nPKC) activation and its regulation by calcineurin activation. J Biol Chem. 1998;273:28392–28398. doi: 10.1074/jbc.273.43.28392. [DOI] [PubMed] [Google Scholar]
- 37.Asada A, Zhao Y, Komano H, Kuwata T, Mukai M, Fujita K, Tozawa Y, et al. The calcium-independent protein kinase C participates in an early process of CD3/CD28-mediated induction of thymocyte apoptosis. Immunology. 2000;101:309–315. doi: 10.1046/j.1365-2567.2000.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pu Y, Perry NA, Yang D, Lewin NE, Kedei N, Braun DC, Choi SH, et al. A novel diacylglycerol-lactone shows marked selectivity in vitro among C1 domains of protein kinase C (PKC) isoforms alpha and delta as well as selectivity for RasGRP compared with PKCalpha. J Biol Chem. 2005;280:27329–27338. doi: 10.1074/jbc.M414132200. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Bermejo ML, Leskow FC, Fujii T, Wang Q, Blumberg PM, Ohba M, Kuroki T, et al. Diacylglycerol (DAG)-lactones, a new class of protein kinase C (PKC) agonists, induce apoptosis in LNCaP prostate cancer cells by selective activation of PKCalpha. J Biol Chem. 2002;277:645–655. doi: 10.1074/jbc.M107639200. [DOI] [PubMed] [Google Scholar]
- 40.Truman JP, Rotenberg SA, Kang JH, Lerman G, Fuks Z, Kolesnick R, Marquez VE, et al. PKCalpha activation downregulates ATM and radio-sensitizes androgen-sensitive human prostate cancer cells in vitro and in vivo. Cancer Biol Ther. 2009;8:54–63. doi: 10.4161/cbt.8.1.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang JH, Peach ML, Pu Y, Lewin NE, Nicklaus MC, Blumberg PM, Marquez VE. Conformationally constrained analogues of diacylglycerol (DAG). 25. Exploration of the sn-1 and sn-2 carbonyl functionality reveals the essential role of the sn-1 carbonyl at the lipid interface in the binding of DAG-lactones to protein kinase C. J Med Chem. 2005;48:5738–5748. doi: 10.1021/jm050352m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woronicz JD, Lina A, Calnan BJ, Szychowski S, Cheng L, Winoto A. Regulation of the Nur77 orphan steroid receptor in activation-induced apoptosis. Mol Cell Biol. 1995;15:6364–6376. doi: 10.1128/mcb.15.11.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim H, Lee JE, Kim BY, Cho EJ, Kim ST, Youn HD. Menin represses JunD transcriptional activity in protein kinase C theta-mediated Nur77 expression. Exp Mol Med. 2005;37:466–475. doi: 10.1038/emm.2005.57. [DOI] [PubMed] [Google Scholar]
- 44.Chatila T, Silverman L, Miller R, Geha R. Mechanisms of T cell activation by the calcium ionophore ionomycin. J Immunol. 1989;143:1283–1289. [PubMed] [Google Scholar]
- 45.Nakamura K, Sasada T, Sono H, Yodoi J. Inhibition of protein kinase C-mediated CD4 down-regulation by oxidative stress in T lymphocytes. J Immunol. 1996;157:5339–5349. [PubMed] [Google Scholar]
- 46.Lutz-Nicoladoni C, Letschka T, Leitgaes M, Villunger A, Baier-Bitterlich G. Essential role of PKC [delta] in apoptosis induction of mouse thymocytes. Am J Immunol. 2005;1:14–20. [Google Scholar]
- 47.Luciano F, Krajewska M, Ortiz-Rubio P, Krajewski S, Zhai D, Faustin B, Bruey JM, et al. Nur77 converts phenotype of Bcl-B, an anti-apoptotic protein expressed in plasma cells and myeloma. Blood. 2007;109:3849–3855. doi: 10.1182/blood-2006-11-056879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bain G, Quong MW, Soloff RS, Hedrick SM, Murre C. Thymocyte maturation is regulated by the activity of the helix-loop-helix protein, E47. J Exp Med. 1999;190:1605–1616. doi: 10.1084/jem.190.11.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirata Y, Kiuchi K, Chen HC, Milbrandt J, Guroff G. The phosphorylation and DNA binding of the DNA-binding domain of the orphan nuclear receptor NGFI-B. J Biol Chem. 1993;268:24808–24812. [PubMed] [Google Scholar]
- 50.Fujii Y, Matsuda S, Takayama G, Koyasu S. ERK5 is involved in TCR-induced apoptosis through the modification of Nur77. Genes Cells. 2008;13:411–419. doi: 10.1111/j.1365-2443.2008.01177.x. [DOI] [PubMed] [Google Scholar]
- 51.Wingate AD, Campbell DG, Peggie M, Arthur JS. Nur77 is phosphorylated in cells by RSK in response to mitogenic stimulation. Biochem J. 2006;393:715–724. doi: 10.1042/BJ20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia-Bermejo ML, Leskow FC, Fujii T, Wang Q, Blumberg PM, Ohba M, Kuroki T, et al. Diacylglycerol (DAG)-lactones, a new class of protein kinase C (PKC) agonists, induce apoptosis in LNCaP prostate cancer cells by selective activation of PKCalpha. J Biol Chem. 2002;277:645–655. doi: 10.1074/jbc.M107639200. [DOI] [PubMed] [Google Scholar]
- 53.Benzaria S, Bienfait B, Nacro K, Wang S, Lewin NE, Beheshti M, Blumberg PM, et al. Conformationally constrained analogues of diacylglycerol (DAG), 15. The indispensable role of the sn-1 and sn-2 carbonyls in the binding of DAG-lactones to protein kinase C (PK-C) Bioorg Med Chem Lett. 1998;8:3403–3408. doi: 10.1016/s0960-894x(98)00614-3. [DOI] [PubMed] [Google Scholar]
- 54.Marquez VE, Nacro K, Benzaria S, Lee J, Sharma R, Teng K, Milne GW, et al. The transition from a pharmacophore-guided approach to a receptor-guided approach in the design of potent protein kinase C ligands. Pharmacol Ther. 1999;82:251–261. doi: 10.1016/s0163-7258(98)00048-5. [DOI] [PubMed] [Google Scholar]
- 55.Tamamura H, Bienfait B, Nacro K, Lewin NE, Blumberg PM, Marquez VE. Conformationally constrained analogues of diacylglycerol (DAG), 17. Contrast between sn-1 and sn-2 DAG lactones in binding to protein kinase C. J Med Chem. 2000;43:3209–3217. doi: 10.1021/jm990613q. [DOI] [PubMed] [Google Scholar]
- 56.Nacro K, Bienfait B, Lee J, Han KC, Kang JH, Benzaria S, Lewin NE, et al. Conformationally constrained analogues of diacylglycerol (DAG), 16, How much structural complexity is necessary for recognition and high binding affinity to protein kinase C? J Med Chem. 2000;43:921–944. doi: 10.1021/jm9904607. [DOI] [PubMed] [Google Scholar]
- 57.Kim H, Kim BY, Soh JW, Cho EJ, Liu JO, Youn HD. A novel function of Nur77: physical and functional association with protein kinase C. Biochem Biophys Res Commun. 2006;348:950–956. doi: 10.1016/j.bbrc.2006.07.167. [DOI] [PubMed] [Google Scholar]
- 58.Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, Annes J, et al. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 59.Linette GP, Grusby MJ, Hedrick SM, Hansen TH, Glimcher LH, Korsmeyer SJ. Bcl-2 is upregulated at the CD4+CD8+ stages during positive selection and promotes thymocyte differentiation at several control points. Immunity. 1994;1:197–205. doi: 10.1016/1074-7613(94)90098-1. [DOI] [PubMed] [Google Scholar]