Abstract
Mutualisms between fungi and fungus-growing animals are model systems for studying coevolution and complex interactions between species. Fungal growing behavior has enabled cultivating animals to rise to major ecological importance, but evolution of farming symbioses is thought to be restricted to three terrestrial insect lineages. Surveys along 2,000 km of North America's Atlantic coast documented that the marine snail Littoraria irrorata grazes fungus-infected wounds on live marsh grass throughout its range. Field experiments demonstrate a facultative, farming mutualism between Littoraria and intertidal fungi. Snails graze live grass primarily not to feed but to prepare substrate for fungal growth and consume invasive fungi. Fungal removal experiments show that snails and fungi act synergistically to suppress marsh grass production. These results provide a case of fungus farming in the marine environment and outside the class Insecta and reveal a previously undemonstrated ecological mechanism (i.e., facilitation of fungal invasion) by which grazers can exert top-down control of marine plant production.
Keywords: top-down control, salt marshes, fungi–animal interactions
Relationships between fungus-farming animals and fungi are models of how coevolution can drive positive interactions and establish some species as ecosystem engineers (1–5). These intimate mutualisms can be obligate for both animal and fungal species and are distinguished by elaborate behavioral adaptations of participant animals to a life of fungal cultivation (1–3). Around 40–60 million years ago, three distinct insect lineages, ants, termites, and beetles, independently evolved the characteristic of cultivating fungi for nutrition (6). Fungus-growing behavior evolved only once in ants (7) and termites (8), whereas it evolved at least seven times in beetles (9–11). There has been remarkable radiation within these insect lineages, and evolution of fungus-growing behavior is thought to be a major force driving each radiation (6, 7). Development of fungal cultivation has also enabled these insects to rise to major ecological importance in a variety of terrestrial communities (e.g., forests and grasslands), where they can strongly regulate ecosystem processes and community structure through their farming activities (4, 6, 12, 13). Despite obvious success afforded by fungus-growing behavior, evolution of fungiculture is thought to have occurred only in these three distinct, terrestrial insect lineages (6).
In detritus-based marine systems, fungi that grow on dead plant material are a primary food source for invertebrate grazers (14–17). Fungal supply to grazers is thought to be a bottom-up process largely dependent on the availability of dead plant mass (14, 15). However, significant abrasion by grazers can facilitate both the invasion and growth of nutritious fungi (18). Marine detritivores (e.g., snails and crabs) capable of shredding and cutting live plant material might therefore have the potential to initiate and encourage the growth of fungi on living plant substrates.
In salt marshes along U.S. southeast coasts, the marsh snail Littoraria irrorata is one of the most abundant grazers and commonly occurs at densities ranging from 40 to 500 individuals per m2 (19–21). Long thought to be strictly a detritivore, recent research has shown that Littoraria actively grazes live salt marsh cordgrass, Spartina alterniflora (20). When grazing live plants, however, snails do not consume live tissue directly; instead, they create and maintain longitudinal wounds on the leaf surface with their radulae (typically, wounds eventually go all of the way through the leaf; see Fig. 1) and feed on the senescent material surrounding those wounds [radulations (20)]. Microscopic examination of injured leaves indicates that ascomycete fungi [probably primarily ascomycetes in the genera Phaeosphaeria and Mycosphaerella (22, 23)], the snails' preferred food (16, 17), are more abundant in snail-maintained wounds than on green leaf surfaces and fungi dominate the microbial communities that invade radulated Spartina leaves [bacteria are typically <5%, by mass, of the microbial community on decomposing, standing Spartina leaves (22, 23)]. Field observations also suggest that snails concentrate deposition of nitrogen- and hyphae-rich fecal pellets (24, 25) on fungus-invaded wounds [Littoraria feces are typically dense with fungal hyphae, and snails digest only ≈50% of the mycelium they consume (25)]. Based on these preliminary findings, we hypothesize that (i) Littoraria promote fungal growth on live Spartina plants through grazing activities and direct application of fecal pellets and that this growth promotion has a positive effect on snail growth; and (ii) fungi benefit from snail wound-grazing by gaining access to nutritious and relatively defenseless inner plant tissues and by receiving supplements (potentially nutrients and/or propagules) from snail feces.
Fig. 1.
(A) Snail fecal pellets at high density (>40 pellets; average densities were found to be 22.75 pellets per 10 cm of radulation; see Results) concentrated on a snail-induced wound on a live Spartina leaf. Note the fungal concentration (dark area) along the radulation edges. A leaf with fecal pellets was collected at night and photographed the next morning. (B) Littoraria on a Spartina leaf grazing a radulation. The majority (>80%) of snail-induced wounds on cordgrass extend through the leaf, as is the case in this picture.
Removal experiments demonstrate that Littoraria's unique grazing behavior suppresses Spartina growth and that snail consumption accounts for <5% of biomass reductions (18, 19). Given these findings and the observation that potentially growth-suppressing fungi are abundant on snail-induced wounds, we further hypothesize that (iii) the primary mechanism of snail control of plant growth is tissue death caused by the facilitation of fungal invasion.
Methods
Patterns in Snail Grazing. To examine the geographical extent of Littoraria wound-grazing, we surveyed 16 marshes (haphazardly chosen on a map) along 2,000 km of southeast shoreline in eight different states in September 2002 (Table 1). Ten 0.25-m2 quadrats in each marsh were randomly placed in the short-form Spartina zone, and the total length of the radulations per stem was measured (18).
Table 1. Snail grazing intensity on green leaves of salt marsh cordgrass in southeast salt marshes.
| State | Radulations, cm per stem |
|---|---|
| Delaware | 8.6 ± 1.8 |
| Maryland | 10.3 ± 1.4 |
| Virginia | 16.7 ± 2.3 |
| North Carolina | 12.8 ± 1.6 |
| South Carolina | 9.3 ± 2.8 |
| Georgia | 28.3 ± 2.3 |
| Florida | 13.6 ± 3.5 |
| Louisiana | 9.8 ± 2.7 |
Data are means ± SE and represent pooled data from two marshes in each state.
Based on these observations from the southeast coast, we surveyed and conducted experiments in marshes on Sapelo Island, Georgia, at the University of Georgia Marine Institute. Initially, we quantified our preliminary observations that fungi are abundant on radulations and that snails concentrate fecal deposition on grazer-induced wounds. In May 2000, we sampled 16 uninjured (i.e., completely green with no abrasions) and 16 radulated [i.e., completely green except for snail grazing scars (Fig. 1)] Spartina leaves and analyzed them for fungal biomass by using ergosterol-proxy techniques (23). Each leaf was collected from a different Spartina stem, and stems were located at least 3 m apart. We sampled the first 16 leaves of each leaf type we encountered while walking a 200-m line transect through the short-form Spartina zone. Leaf sections (5 cm in length) were cut from each sample, all fecal pellets were removed, and, because of logistical constraints (i.e., the constraint of having to use short leaf sections with available lab equipment combined with the difficulty of detecting ergosterol concentrations in small samples), four sections of each leaf type, uninjured or radulated, were pooled for analysis (n = 4 per treatment). We also quantified fecal pellet density on 10-cm-long midleaf sections of 300 uninjured and 300 radulated green leaves sampled from independent stems. We counted pellets on the 10-cm-long radulations themselves, not on green tissue surrounding the area (see Fig. 1B). On undamaged leaves, we counted fecal pellets on similarly sized and oriented areas. We counted fecal pellets on the first 300 leaves of each leaf type we encountered while walking a 1,000-m transect through the short-form Spartina zone. We conducted this survey at night during ebb and flood tides, when snails are most actively grazing (17). These field surveys (i.e., fungal biomass in radulations and fecal pellet counts) were conducted to establish initial patterns of interactions between fungi and snail grazing activities. The following experiments were designed to elucidate potential causal processes underlying those patterns.
Effects of Snail Grazing Activities on Fungal Biomass. To test the hypothesis that snails facilitate fungal invasion through grazing activities, we measured the effects of snail presence and simulated snail grazing (i.e., razorblade cuts) on fungal growth in green Spartina leaves in a 2-month caging experiment. In February 2001, before snails began to graze live marsh grass (20, 21), in the intermediate height-form Spartina zone, we established replicated (n = 6) 1-m2 galvanized mesh cages (20) assigned to the following treatments: control (≈220 snails per m2), snail removal, and snail removal plus simulated grazing. We simulated snail grazing on uninjured green Spartina by using razorblades to make longitudinal cuts that went all of the way through the leaves. Every week, the average total length of radulations per stem in the control snail treatments was quantified. We then replicated those radulation distributions (i.e., the same average number of radulations) on all stems in simulated grazing treatments. After 2 months, we measured fungal biomass on respective green leaf types (uninjured, radulated, and simulated scar) from each treatment (n = 2 leaves per leaf type per replicate) by using the ergosterol methods described above (23). We randomly pooled the two leaves from each of our six replicates into three groups of four leaves each for analysis because of logistical constraints (see above) of the ergosterol extraction technique. For statistical analysis, then, n = 3 per treatment. Snail densities were monitored weekly (20).
To test the hypothesis that snail deposition of fecal pellets on exposed wounds stimulates fungal growth, we added fecal pellets to artificially induced wounds on green Spartina leaves in a 2-week field experiment. In May 2001, in an intermediate height-form Spartina zone naturally devoid of snails, we applied the following treatments (n = 6) to 1-m2, staked areas: simulated snail grazing and simulated snail grazing plus fecal pellets. We simulated natural snail grazing intensity (17–19) by cutting 10-cm longitudinal wounds on three green leaves on one-third of the stems in each plot. Fecal pellets were collected from a snail-holding container in an outside flow tank that housed snails, fresh Spartina stems (changed every 3 days), and seawater and were applied directly on simulated grazer scars (very similar in appearance to the radulation in Fig. 1B) at a rate of 22 pellets per scar per day, a natural deposition rate as determined by our field survey (see Results). After 2 weeks, we measured fungal biomass on artificially injured leaves from each treatment (n = 4 leaves per replicate) by using methods described above (23). We randomly pooled replicates into pairs for analysis because of logistical constraints (n = 3 per treatment).
Effects of Fungus Production on Snail Growth. To test the prediction that the extent of fungal biomass in a green-leaf food source positively affects snail growth, substrate-specific growth rates for juvenile snails (shell height = 3 mm) were determined for three food items: unwounded green leaves, green leaves with radulations, and green leaves with 10-cm long, 1- to 2-month-old razorblade cuts. Leaves were collected from Spartina plants housed in mesh cages in the marsh at equal tidal elevations (see above) and exposed to the following treatments (n = 6): control snails (≈220 individuals per m2), snail removal, and snail removal plus simulated grazing. We used this experimental design to control as much as possible for potential differences in plant quality other than fungal content [e.g., differential flooding frequencies leading to differences in plant nitrogen content (21)]. Snails were housed in 473-ml glass jars in the laboratory. Each treatment had 16 replicates. Each jar housed four juvenile snails and four, 20-cm-long leaf segments of the designated food item. Seawater (60 mm) was added to each jar to maintain a hydrated environment for both plants and snails, and leaves and water were changed every 4 days to ensure treatment integrity. Change in shell length was measured after 5 months. We used mean change in shell length in each replicate as a single datum per replicate.
Mechanisms of Top-Down Control. To test the hypothesis that facilitation of microbial invasion is a primary mechanism by which snails control Spartina growth (i.e., fungal invasion in snail-induced wounds, and not the wounds themselves, are the primary cause of decreased growth), we experimentally separated the negative effects of snail consumption and fungal invasion on marsh grass growth by using fungal removal techniques (i.e., application of fungicide). In the intermediate-form Spartina zone, we established replicated (n = 6) 1-m2 cages assigned to the following treatments: snail exclusion, snail exclusion plus fungal removal, control snails (≈220 individuals per m2), and control snails plus fungal removal. To exclude fungi from plant tissue, we sprayed Spartina stems in fungal removal treatments once every 5 days with the systemic fungicide Daconil Ultrex Turf Care with Chlorothanlo (Zeneca, Wilmington, DE). This fungicide seemed ideal for marsh use, because plants take it up within 2 h and it is an effective killer of terrestrial fungi (G. Gilbert, University of California, Santa Cruz, personal communication) taxonomically similar to the dominant marsh fungi Phaeosphaeria and Mycosphaerella, which are typically >90% of marsh fungal biomass (22). Preliminary marsh experiments showed that Daconil application does not affect growth of uninjured Spartina plants (see Fig. 4C) or Littoraria grazing preferences (when offered a choice between leaves with and without Daconil; 2.4- ± 0.7-cm radulations per leaf for nonfungicide treatments; 3.1- ± 1.1-cm radulations per leaf for fungicide treatments; P > 0.30, paired t test). Daconil was sprayed for 30 seconds on each plot during a rainless day at low tide, when snails are inactive for at least 6 h, and water was similarly sprayed on nonfungicide treatments as a disturbance control. Snail-grazing was simulated, the cages were constructed, and the snail densities were monitored as described above. After 4 months, we quantified aboveground plant biomass in a 25-cm2 quadrat by using destructive techniques (20) and recorded the total length of radulations on 15 randomly selected plants (20). We also measured fungal biomass on green leaf types from each treatment (n = 4 leaves per replicate) by using methods described above (23). We randomly pooled replicates into pairs for analysis because of logistical constraints (n = 3 per treatment).
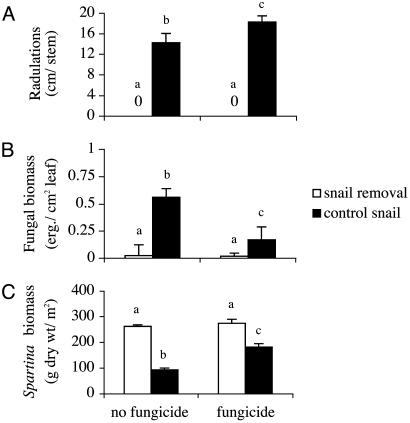
Fig. 4.
Interactive and separate effects of snail presence and fungicide on the total length of grazer-induced wounds per stem (A), fungal biomass [μg of ergosterol (erg.) per cm2 of leaf blade] on green leaves (B), and Spartina aboveground biomass (C). Different letters denote significant pairwise differences at P < 0.05 in mean values as determined from Tukey's post hoc test. Error bars represent ± SE.
Statistics. Treatment differences were assessed by using one- and two-way ANOVA followed by Tukey's post hoc test. Data either exhibited homogeneity of variance and were normally distributed or were log transformed to meet ANOVA assumptions. Transformations produced data that met ANOVA assumptions.
Results
Patterns in Snail Grazing. Extensive survey of southeast and gulf coast salt marshes showed that wound-grazing by snails is widespread throughout its range (Table 1). Analysis of Spartina leaves collected from our initial marsh survey in Georgia revealed that mean fungal biomass was significantly higher on radulated green leaves compared with uninjured green leaves (Fig. 2A). Fungal biomass was >15-fold higher on leaves with snail-induced wounds, and nearly undetectable on green leaves (Fig. 2A). Our initial marsh survey in Georgia also showed that mean density of snail fecal pellets was >4-fold greater on wounds on radulated green leaves compared with uninjured green plant surfaces (P < 0.01, paired t test; pellets per radulation = 22.75 ± 6.45; pellets per green leaf surface = 5.41 ± 4.35).
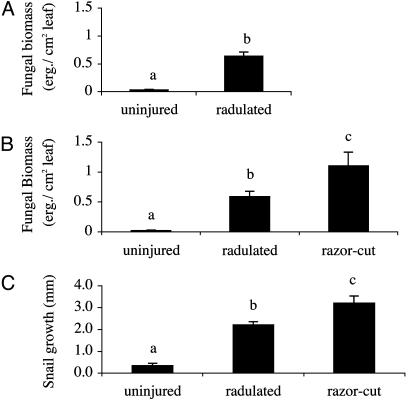
Fig. 2.
(A and B) Fungal abundance [μg of ergosterol (erg.) per cm2 of leaf blade] in naturally occurring uninjured and radulated green Spartina leaves (A) and experimentally generated uninjured (snail removal treatments), snail-grazed (control snail treatments), and razor-cut (simulated snail grazing treatments) green leaves (B). (C) Juvenile snail growth rates on each experimentally generated green leaf type. Different letters denote significant pairwise differences at P < 0.05 in mean values as determined from Tukey's post hoc test. Error bars represent ± SE.
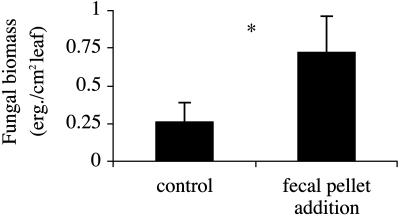
Effects of Snail Grazing Activities on Fungal Biomass. Removal of snails from the marsh reduced fungal biomass on green leaves to low levels (Fig. 2B). In contrast, snail removal plus simulated snail grazing significantly facilitated fungal invasion, because fungal biomass increased ≈21-fold relative to removals and 76% relative to control snail treatments. Grazing by control snails produced fungal growth on green leaves markedly similar to that on radulated green leaves collected in the survey (Fig. 2) but lower than simulated grazing scars. This difference in fungal biomass likely occurred because snails are cropping fungi at the same time they are facilitating it, unlike simulated grazing treatments, which only facilitate fungal growth. Field experiments indicated snails further enhance fungal growth through deposition of feces. Addition of fecal pellets at natural deposition rates to simulated grazer scars on green leaves increased fungal biomass by 171% (Fig. 3).
Fig. 3.
Response of fungal growth [μg of ergosterol (erg.) per cm2 of leaf blade] to snail fecal pellet addition on simulated grazing scars on green Spartina leaves. Error bars represent ± SE. *, P < 0.05, paired t test.
Effects of Fungus Production on Snail Growth. Snail growth rates mirrored fungal availability on each leaf type: Mean growth rates were greatest on razor-cut leaves, less on radulated, and least on green leaves (Fig. 2C). Snail growth on green leaves, like fungal availability, was negligible, and 48% of juveniles in uninjured, green-leaf treatments died (<3% for other treatments).
Mechanisms of Top-Down Control. Survey of Spartina stems in the fungicide × snail experiment revealed that snail presence resulted in substantial scarring of green leaves and that grazing intensity depended on fungicide (Fig. 4A). With fungicide application, the total length of radulations per stem increased by 29% (Fig. 4A), a pattern possibly indicating snails compensate for decreased fungal availability with intensified grazing.
Analysis of Spartina leaves confirmed our initial findings that snail grazing facilitates fungal invasion: Fungal biomass was negligible on green leaves in snail removal treatments but high in treatments with Littoraria (Fig. 4B). Snails differentially affected fungal biomass depending on the presence/absence of fungicide. In nonfungicide treatments, snail grazing increased fungal biomass by ≈17-fold, whereas in fungicide treatments this facilitative effect was dampened markedly (≈4-fold). The effect of fungicide application on the fungal biomass on green leaves depended on snail level, as fungicide had no significant effect in snail removals where fungal invasion was not facilitated but strongly suppressed (71%) fungal growth in treatments where snail grazing promoted invasion (Fig. 4B).
Coincident with fungal invasion of grazer-induced wounds on live Spartina were dramatic decreases in plant growth (Fig. 4C). The magnitude of this top-down effect, however, like fungal biomass, depended on fungicide level, because snails exhibited relatively less control of Spartina in plots with fungicide (Fig. 4C). In nonfungicide treatments, snail grazing reduced standing biomass by 65% relative to removals, whereas in fungicide addition plots, grazing by Littoraria decreased growth by only 31% (Fig. 4C). The effect of fungicide on Spartina biomass depended on snail presence; fungicide had no effect in snail removals where fungal invasion was not facilitated but increased Spartina growth in treatments where snail grazing promoted invasion (Fig. 4 B and C).
Discussion
Fungal Farming in Ants, Beetles, Termites, and Snails. Evolutionary biologists have recently suggested that fungus-growing animals, like human agriculturists (26), use a range of cultivation strategies, varying from “low-” to “high-level food production” (27). Low-level promotion of fungal growth includes cases where animals modify local ecosystems to encourage or protect fungal growth, provide substrate to promote growth, and consume the cultivated fungi (27). At the other extreme, in high-level fungus cultivation, animals additionally vertically transmit cultivar inocula, inoculate substrate with propagules, fertilize the crop, employ physical and/or chemical means to exclude competitors and pathogens, and harvest and consume the cultivated fungi (27).
In terrestrial systems, instances of high-level fungal cultivation have been extensively documented and studied (1–11, 27). Fungus-growing attine ants and termites, for example, exhibit high-level food production (1, 4). Their farming strategies include evolved mechanisms of cultivar transmission from one parent to offspring, preparation of substrate, collection and concentration of growth medium, inoculation with propagules, fertilization of crop with fecal material or oral exudates, chemical and physical weeding, and harvesting and consumption of fungi. Many beetle species also farm fungi, but do so in bored-out holes in live trees, where cultivated hyphae provide food for adults and developing pupae (12, 13). Many of their fungal production strategies also fit the high-level category. Examples of low-level fungal production have not been experimentally demonstrated (27), although evolution of this strategy could be common, given its relative engineering simplicity.
We argue that our experimental and large-scale survey results reveal a low-level, facultative farming mutualism§ between the marine snail Littoraria irrorata and intertidal marsh fungi that may occur over the entire extent of Littoraria's 2,000-km range (Table 1 and refs. 19–21). When grazing live marsh grass, Littoraria create and maintain wounds on leaf blades with their radulae, and the opening of live plant tissue results in microbial invasion, significantly increasing availability of their preferred food (16, 17), leaf material containing ascomycete fungi. Simulation of snail grazing demonstrates that the simple mechanical opening of grass tissue is ample engineering to promote invasion and growth of marine fungi (Fig. 2), whose spores are ubiquitous across the marsh surface (22). Seeding and/or propagule transplantation is therefore not necessary for snail promotion of fungal growth. Nonetheless, our field experiments and night-time marsh survey showed that snails concentrate deposition of nitrogen- and hyphae-rich fecal pellets¶ on radulations and that this activity enhances fungal growth. Potential mechanisms of fungal growth-enhancement by means of pellet deposition include fertilization [growth of marsh fungi is limited by nitrogen (23)] and/or propagule enhancement (it remains to be determined whether undigested mycelia in fecal pellets are viable). Laboratory studies indicate snail success is intrinsically linked to fungal availability in green Spartina leaves, because the growth of juveniles increased with increasing fungal biomass. Most revealing was that juvenile snails did not grow and experienced 48% mortality when fed uninjured, green leaves, a finding consistent with controlled lab experiments (28), showing that snails can grow only on fungus-colonized Spartina or pure mycelium and not on sterile leaves or ones colonized by bacteria. These growth-study results indicate that Littoraria obligately employs fungus-promoting feeding strategies to benefit from grazing live Spartina. In other words, the primary purpose of grazing live grass is likely not feeding, but preparation of substrate for growth of nutritious fungi and consumption of facilitated invasive fungi.
Together, these experimental findings reveal that Littoraria promotes fungal growth on live Spartina through a combination of relatively nonnutritive grazing (Fig. 2 and ref. 28) and fecal pellet deposition, and that this fungal production has a positive effect on snail growth. In turn, fungi benefit from wound-grazing by gaining access to inner plant tissues and by receiving supplements∥ from snail feces. Littoraria thus employs a low-level food production strategy whereby it prepares a favorable environment for fungal growth, provides substrate to promote growth, adds supplemental nutrients and/or propagules, and consumes fungus.
Unlike ants and termites, snails do not seem to inoculate prepared substrate to initiate fungal growth, weed their crops, or obligately rely on one fungal species for farming. If there is an important message to be learned from Littoraria's distinct lower-level fungal production strategy, it may be that evolutionary success of fungal farmers may not depend on intricate pest management and inoculation techniques as long as cultivated fungi naturally occur and are successful even without farmer's care (i.e., fungi are effective dispersers and have strong pathogen and competitor resistance).
Is Fungal Farming Common but Overlooked? This study demonstrates fungus-growing behavior in the marine environment and outside the class Insecta. However, given the relative engineering simplicity of low-level fungal production, the benefits of having predictable food supply and the fact that many detritivores have been shown to stimulate secondary respiration through foraging activities (refs. 16 and 17 and references therein), fungal farming on live, senescing, and/or dead plants may be more geographically and phylogenetically widespread than presently envisioned, especially in systems where fungal spores are abundant, grazers can manipulate fungus-growing media, and fungus is a major diet component of consumers. Many grasses and grass-like macrophytes other than Spartina exhibit standing decaying shoots (i.e., senescing live leaves invaded by fungi) in both marine and freshwater ecosystems (23), and these plant communities may very well meet the criteria for potentially harboring fungal farmers (high fungal production and mycophagous and shredding invertebrates).
Top-Down Control of Aquatic Plants by Means of the Facilitation of Fungal Invasion. If animals farm fungi on live or senescing plants in other aquatic systems, both fungi and grazers may evolve in the same direction, one that favors fungal growth at the expense of the invaded host. In terrestrial systems, it is well established that the impact of insect fungal farming can be devastating to plants used as growth media (1–13, 16). For example, fungus-farming activities by beetles in bored-out holes of tree trunks can cause massive die-off of forests, because facilitated, pathogenic fungi seriously damage their hosts (6, 12–13). Likewise, snail radular activities promote growth of fungi on green Spartina leaves, and our fungal removal experiments demonstrate that this invasion can account for at least 60% of the negative effects of snail grazing on marsh grass growth [probably more because fungicide was not completely effective in suppressing fungal growth (Fig. 4)]. Snail farming activities thus favor fungal growth at great expense to the infected host plant [i.e., snail grazing activities commonly reduce marsh plant growth 40–100% (19–21)]. These results, combined with the finding that snails consume small amounts of live plant tissue when wound-grazing (20), show that snails exert control of marine plant production by facilitating growth-suppressing fungi and that this top-down effect is disproportionate to grazer consumption capabilities. This study demonstrates top-down control by means of the facilitation of fungal invasion in a marine system; however, if grazer promotion of fungal invasion is not limited to salt marshes (as suggested above), then mesograzers could be exerting similarly strong but undetected control over primary production and community structure in many other aquatic systems.
Acknowledgments
We thank Sarah Lee, Ryan Harlick, and Tracy Buck for their dedicated work and C. Layman, M. Bertness, U. Mueller, T. Schultz, R. Paine, and two anonymous reviewers for their comments on the manuscript, including the suggestion of the word “protofarming” by one anonymous reviewer. This work was supported by the National Science Foundation (Dissertation Improvement Grant and Biological Oceanography), the Environmental Protection Agency (Science to Achieve Results Fellowship), and the National Oceanic and Atmospheric Association (National Estuarine Research Reserve System) Narragansett Bay Fellowship.
This paper was submitted directly (Track II) to the PNAS office.
Footnotes
Strict criteria for “low,” “medium,” and “high” levels of fungal farming have not been established and have only recently been suggested (27). Although we argue that snail fungus-growing behavior represents low-level farming, this could be revised based on future results. Our findings are relatively silent as to whether growth-promotional effects of snails are adaptations or mere by-products. If they are by-products, then this snail–fungus interaction may be more accurately classified as protofarming behavior. In addition, because we have no evidence that either fungus or snails have undergone selection for participation in this interaction and both organisms survive independent of the relationship, we have classified this positive interaction as a facultative mutualism.
We did not determine whether snails purposefully deposited fecal pellets on grazer-induced wounds or, rather, that pellets were simply concentrated on wounds because of increased time spent by snails feeding in those areas. If the former is found to be true, then a stronger case for higher-level fungal cultivation [given its current definition (27)] can be made, because snail behavior would then seem to have evolved for “planting” or “manuring” by means of feces.
Mechanisms of growth enhancement are not exactly known, but nutrient transfer from fecal pellets is likely, given that snail pellets are high in nitrogen content and fungal growth on cordgrass is nitrogen-limited (17, 18, 22, 23). Pellets could additionally provide fungal propagule supplements, given that 50% of mycelium in snail feces typically remains undigested and intact (25).
References
- 1.Wilson, E. O. (1971) The Insect Societies (Belknap, Cambridge, MA).
- 2.Chapela, I. H., Rehner, S. A., Schultz, T. R. & Mueller, U. G. (1994) Science 266, 1691–1694. [DOI] [PubMed] [Google Scholar]
- 3.Mueller, U. G., Rehner, S. A. & Schultz, T. R. (1998) Science 281, 2034–2038. [DOI] [PubMed] [Google Scholar]
- 4.Hölldobler, B. & Wilson, E. O. (1990) The Ants (Belknap, Cambridge, MA).
- 5.Shellman-Reeve, J. S. (1997) in Social Competition and Cooperation in Insects and Arachnids, eds. Choe, J. C. & Crespi, B. J. (Cambridge Univ. Press, Cambridge, U.K.), pp. 52–93.
- 6.Mueller, U. G. & Gerardo, N. (2002) Proc. Natl. Acad. Sci. USA 99, 15247–15249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz, T. R. & Meier, R. (1995) Syst. Entomologist 20, 337–370. [Google Scholar]
- 8.Aanen, D. K., Eggleton, P., Rouland-Lefèvre, C., Gulderg-Frøslev, T., Rosendahl, S. & Boomsma, J. J. (2002) Proc. Natl. Acad. Sci. USA 99, 14887–14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassar, S. & Blackwell, M. (1998) Mycologia 88, 596–601. [Google Scholar]
- 10.Jones, K. G. & Blackwell, M. (1998) Mycol. Res. 102, 661–665. [Google Scholar]
- 11.Farrell, B. D., Sequeira, A. S., O'Meara, B. C., Normark, B. B., Chung, J. H. & Jordal, B. H. (2001) Evolution 55, 2011–2027. [DOI] [PubMed] [Google Scholar]
- 12.Batra, L. R. (1966) Science 153, 193–195. [DOI] [PubMed] [Google Scholar]
- 13.Beaver, R. A. (1989) in Insect–Fungus Interactions, eds. Wilding, N., Collins, N. M., Hammond, P. M. & Webber, J. F. (Academic, New York), pp. 121–143.
- 14.Odum, W. E., McIvor, C. C. & Smith, T. J., III (1982) The Ecology of the Mangroves of South Florida: A Community Profile (U.S. Fish and Wildlife Service, Washington, D.C.), FWS/OBS-87/17.
- 15.Zieman, J. C. (1981) The Foodwebs Within Seagrass Beds and Their Relationship to Adjacent Habitats (U.S. Fish and Wildlife Service, Washington, D.C.), FWS/OBS-80/59.
- 16.Newell, S. Y. & Barlocher, F. (1993) J. Exp. Mar. Biol. Ecol. 171, 39–49. [Google Scholar]
- 17.Graca, M. A., Newell, S. Y. & Kneib, R. T. (2000) Mar. Biol. (Berlin) 136, 281–289. [Google Scholar]
- 18.Isaac, S. (1992) Fungal–Plant Interactions (Chapman & Hall, London).
- 19.Silliman, B. R. & Bortolus, A. (2003) Oikos 143, 549–555. [Google Scholar]
- 20.Silliman, B. R. & Zieman, J. C. (2001) Ecology 82, 2830–2843. [Google Scholar]
- 21.Silliman, B. R. & Bertness, M. D. (2002) Proc. Natl. Acad. Sci. USA 99, 10500–10505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newell, S. Y. (2001) Bot. Mar. 44, 277–285. [Google Scholar]
- 23.Newell, S. Y. (2002) in The Encyclopedia of Environmental Microbiology, ed. Bitton, G. (Wiley, New York), pp. 1394–1400.
- 24.Daiber, F. C. (1982) Animals of the Tidal Marsh (Van Nostrand Reinhold, New York).
- 25.Bebout, B. M. (1988) M.S. thesis. (Univ. of North Carolina, Chapel Hill).
- 26.Diamond, J. (1999) Guns, Germs and Steel (Norton, New York).
- 27.Schultz, T. R., Mueller, U. G., Currie, C. R. & Rehner, S. A. in Ecological and Evolutionary Advances in Insect–Fungal Associations, eds. Fernando, V. & Meredith, B. (Oxford Univ. Press, New York), in press.
- 28.Barlocher, F. & Newell, S. Y. (1994) Mar. Biol. (Berlin) 118, 109–114. [Google Scholar]