Abstract
In the photosynthetic reaction center from Rhodobacter sphaeroides, the primary (QA) and secondary (QB) electron acceptors are both ubiquinone-10, but with very different properties and functions. To investigate the protein environment that imparts these functional differences, we have applied X-band HYSCORE, a 2D pulsed EPR technique, to characterize the exchangeable protons around the semiquinone (SQ) in the QA and QB sites, using samples of 15N-labeled reaction centers, with the native high spin Fe2+ exchanged for diamagnetic Zn2+, prepared in 1H2O and 2H2O solvent. The powder HYSCORE method is first validated against the orientation-selected Q-band ENDOR study of the QA SQ by Flores et al. (Biophys. J. 2007, 92, 671–682), with good agreement for two exchangeable protons with anisotropic hyperfine tensor components, T, both in the range 4.6–5.4 MHz. HYSCORE was then applied to the QB SQ where we found proton lines corresponding to T~5.2, 3.7 MHz and T~1.9 MHz. Density functional-based quantum mechanics/molecular mechanics (QM/MM) calculations, employing a model of the QB site, were used to assign the observed couplings to specific hydrogen bonding interactions with the QB SQ. These calculations allow us to assign the T=5.2 MHz proton to the His-L190 NδH…O4 (carbonyl) hydrogen bonding interaction. The T =3.7 MHz spectral feature most likely results from hydrogen bonding interactions of O1 (carbonyl) with both Gly-L225 peptide NH and Ser-L223 hydroxyl OH, which possess calculated couplings very close to this value. The smaller 1.9 MHz coupling is assigned to a weakly bound peptide NH proton of Ile-L224. The calculations performed with this structural model of the QB site show less asymmetric distribution of unpaired spin density over the SQ than seen for the QA site, consistent with available experimental data for 13C and 17O carbonyl hyperfine couplings. The implications of these interactions for QB function and comparisons with the QA site are discussed.
Introduction
The primary event in photosynthesis is light-driven charge separation, catalyzed by the reaction center (RC), a (bacterio)chlorophyll-containing protein complex. Light activation results in electron transfer from the primary donor, P, a dimer of (bacterio)chlorophyll, through a series of cofactors of low potential. On time scales longer than a nanosecond, the charge separation in RCs from purple bacteria resides on the primary donor and on the acceptor quinones. The primary quinone, QA, is tightly bound and functions as a one-electron redox species, whereas the secondary quinone, QB, is reversibly bound and can be doubly reduced via QA−, with the uptake of two protons (reviewed in1–3). In spite of these marked functional differences, in Rba. sphaeroides the two quinones are chemically identical ubiquinone-10 molecules,1–4 presenting an ideal opportunity to study the effect of protein structure on cofactor redox properties. The two neutral forms of QB – quinone and quinol – are rapidly exchangeable, but the semiquinone (SQ) intermediate, QB−, is tightly bound and stabilized, contributing to the redox properties of both reduction steps. It can be expected that hydrogen-bonding underlies this stability.
From the known structures of bacterial RCs, QA and QB are positioned symmetrically about an iron-histidine, FeII-(His)4, complex and both quinones are bound by hydrogen bonds to the protein (Figure 1).5,7,8 However, the plethora of X-ray structures now available does not provide unequivocal descriptions of the two quinone sites. The C4 carbonyl of QA is hydrogen bonded to Nδ of His-M219 (an Fe-ligand), while the backbone NH of Ala-M260 is a H-bond donor to the C1 carbonyl.7 On the other hand, the H-bond distances and the torsional angles of the two methoxy group substituents of the ubiquinone ring are quite variable in different structures (reviewed in3).
Figure 1.
Essential features of the QA and QB binding sites, showing putative hydrogen-bond donors to the quinone carbonyls. The structure is from 1dv3.pdb.8 The figure was prepared in VMD. 42
For the QB site, crystal structures show even more diversity, including at least two different positions relative to the FeII-(His)4 complex.7–9 The functional position has been established to be the proximal location, essentially symmetrical to the position of QA. In the proximal position, QB is clearly hydrogen bonded through the C4 carbonyl to Nδ of His-L190 (an Fe-ligand), but a second H-bond to the C1 carbonyl is variously indicated from the backbone NH groups of Ile-L224 and/or Gly-L225 as potential donors (Figure 1).7–9 The hydroxyl from Ser-L223 is also thought to form a hydrogen bond with either the quinone C1 carbonyl - especially in the semiquinone state - or Asp-L213.2,3,6,10 An additional H-bond from the peptide NH of Thr-L226 to one methoxy group was inferred from a recent structure.9 Thus, in addition to uncertainties in the geometry of the H-bonds to QB, even the number is uncertain.
Equivalent knowledge of the oxidized and reduced (SQ) states is necessary to understand the chemical reactivities and kinetic pathways of QA− and QB−, but the H-bond environment around the semiquinone states is not indicated by the crystal structures. This, however, can be addressed through the application of pulsed EPR methods,3,11 and the QA- and QB-sites have been amongst the most thoroughly explored by EPR techniques.10–16 To observe the SQ signals directly by these techniques it is necessary to replace the native high spin Fe2+ with Zn2+, which does not perturb the function of the acceptor quinones.11,12
Structural information about the SQs and their interactions with protein and solvent has mainly been obtained from proton ENDOR spectra in frozen solutions. Three classes of protons can contribute to the spectra: (i) non-exchangeable protons of substituents (isoprene chain, two methoxy groups, and a methyl group), (ii) exchangeable protons forming H-bonds to the quinone oxygens, and (iii) protons associated with the protein or solvent in the immediate vicinity. The major limitation of one-dimensional ENDOR spectroscopy, particularly for ubiquinones, is the large number of protons contributing to the spectra, with fully or partially overlapping frequencies11, and the lack of an effective analysis of one-dimensional powder spectra in this situation. To address this, elaborate sample preparations have been required, involving combinations of fully protonated/deuterated protein with protonated/deuterated quinone. As a result, the hyperfine (and nuclear quadrupole) tensors in Rba. sphaeroides have been determined only for two protons (deuterons) H-bonded with the carbonyl oxygens of the QA SQ, using orientation-selected 1H and 2H Q-band ENDOR.12
An alternative approach is the recently developed two-dimensional (2D) ESEEM spectroscopy, also called HYSCORE.17 1H HYSCORE spectroscopy allows additional resolution by spreading out peaks that overlap in 1D spectra into 2D in the form of off-diagonal cross-peaks. This technique effectively resolves protons with substantially different anisotropic tensors, although complications remain for protons with close hyperfine couplings in X-band powder spectra.
We have previously used X-band HYSCORE to explore the structural neighborhood of the ubisemiquinones stabilized at the Qi-site of the bc1 complex of Rba. sphaeroides18 and the QH-site of the E. coli cytochrome bo3 ubiquinol oxidase.19 These studies have shown that X-band 1H HYSCORE spectra together with solvent deuterium exchange readily separate the cross-peaks of exchangeable hydrogen bonded protons from the lines of non-exchangeable protons, and allow quantitative analysis for determinations of the isotropic and anisotropic components of the hyperfine tensors. These parameters, especially when combined with couplings with nitrogen donors, can be used for the computational construction of structural models.20
In this article we describe 1D and 2D ESEEM studies of the QA and QB SQs in reaction centers from Rba. sphaeroides. The work has three aims:
First, we demonstrate the resolution of powder 1H HYSCORE spectra by analysing model spectral simulations for two protons hydrogen bonded to the QA SQ possessing anisotropic components of the hyperfine tensor that differ by only ~10%, using the Q-band ENDOR derived tensors12. We then show the consistency between data for the QA SQ from orientation-selected Q-band ENDOR12 and from powder X-band 2D ESEEM used in this work, despite partial overlap of individual cross-features from the two protons.
Second, we provide a quantitative characterization of the hyperfine tensors of exchangeable protons around the SQ in the QB site of the reaction center. The structural symmetry between the quinone sites allows the findings in the model QA SQ study to guide the analysis of the QB site, where a larger number of exchangeable protons contributes to the spectra.
Third, we utilize QM/MM calculations on a QB site model to assign the observed hyperfine tensors to specific hydrogen bond interactions in the QB site, and we compare this with the situation in the QA site. The QM/MM simulations also show a difference in the asymmetry of unpaired spin density distribution between the QA and QB sites, in agreement with available experimental data.
The hyperfine tensors for the exchangeable proton(s) provide a conclusive picture of the hydrogen bond network with the carbonyls of the SQ in the QB site. Previously, even the number of hydrogen bonds and the strength of coupling to the QB SQ were uncertain, which has frustrated the critical analysis of the spin density distribution and simulations exploiting different structural models.
Experimental Section
Sample preparations
In order to isolate SQ EPR signals, the native, high spin Fe2+ must be replaced by diamagnetic Zn2+. Procedures for biochemical metal exchange, along with the methods of bacterial cell growth and RC isolation, were as previously described.21 To eliminate cross suppression effects in the HYSCORE spectra and to facilitate resolution of the proton signals (see also Results), RCs were uniformly 15N labeled. 15N enrichment of RCs was accomplished during cell growth by using 15N-labeled ammonium sulfate (Cambridge Isotopes) in the growth medium. Prior to EPR sample generation, the detergent, LDAO, used in RC isolation, was exchanged for Triton X-100 by diluting approximately 100-fold and reconcentrating. Samples with QA− were made by chemical reduction with 8 mM Na-dithionite. For samples with QB− the RCs were combined with a 3-fold excess of both cytochrome c and ubiquinone-10. To trap the QB SQ, the sample was illuminated by a single laser flash at 532 nm and immediately frozen in liquid nitrogen. Routine optical assay of Fe/Zn-exchanged RC preparations showed a minimum of 80% reconstitution of QB activity. However, pulse EPR measurements showed no sign of QA SQ signals in QB SQ samples in either 1H-HYSCORE (this work) or in 14N- or 15N-HYSCORE21, indicating that the functional reconstitution was complete, probably because of the much higher concentrations involved.
EPR and ESEEM experiments
The instrumentation for X-band and Q-band CW EPR measurements was as previously described.21 The instrumentation, pulse sequences and spectral processing for X-band 1D 4-pulse ESEEM (π/2-τ-π/2-t-π-t-π/2-echo) and 2D 4-pulse ESEEM (HYSCORE) (π/2-τ-π/2-t1-π-t2-π/2-echo) were also as described.18,19,21 Spectral simulations were carried out using PC software developed by Dr. Alexei Tyryshkin (now at Princeton University).21
Computational Studies
Starting with the Rba. sphaeroides structure of Axelrod et al.8 (PDB file 1dv3), we created a model of the QB site. This model consisted of L subunit residues 177–242, and M subunit residues 218–220, 233–235 and 265–267 plus the non-heme Fe2+. Hydrogens were added and the native Fe2+ ion was replaced by Zn2+. The ubiquinone isoprene chain was reduced to CH2CHCH2. For the optimisation studies, two-layer ONIOM calculations ONIOM(B3LYP/6–31G(d):UFF) were performed. The QM layer contained the ubisemiquinone QB, His-L190, Gly-L225, Ile-L224, Ser-L223, Zn and its other ligands. The remaining atoms formed the MM layer. Linking between the QM and MM layers was achieved using hydrogen link atoms. Keeping all heavy atoms except the semiquinone fixed, we optimised the semiquinone geometry within the site. All hydrogen atom positions were optimised. Charges for the MM layer were generated using the qEq method and electrostatic embedding, i.e., ONIOM-EE was employed.22 This geometry was then used in a further single point ONIOM(B3LYP/EPR-II:UFF) calculation to obtain spin densities and hyperfine couplings. For the Zn atom the 6-31G(d) basis set was used. All calculations were performed using Gaussian 03 software.23
Spectroscopic Background
Orientationally disordered 1H HYSCORE spectra and their analysis
In this work, the proton environment of the SQs is best probed by the 2D ESEEM (HYSCORE) experiment.17 The essential advantage of the HYSCORE technique is the creation, in 2D spectra, of off-diagonal cross-peaks whose coordinates are nuclear frequencies from opposite electron spin manifolds. The cross-peaks significantly simplify the analysis of congested spectra by correlating the nuclear frequencies of individual nuclei. In addition, HYSCORE separates overlapping peaks along a second dimension and enhances the signal-to-noise ratio through a second Fourier transform. HYSCORE is also very valuable for the detection of extended, anisotropic, “ridge-like” features of low intensity, which are not seen in 1D ESEEM spectra. 24–26
A nucleus with I = ½, such as 1H, has two hyperfine nuclear frequencies, να and νβ, corresponding to two states ms = ±1/2 of the electron spin system in the applied magnetic field. In HYSCORE, these may produce a pair of cross-features, (να, νβ) and (νβ, να).
The X-band (~9.7 GHz) EPR spectrum of a SQ is usually a single line with width ~0.8 mT.11 This width is comparable with the excitation width of the microwave pulses, and the spectra obtained can be considered to be powder-type 1D or 2D ESEEM spectra because the resulting pattern contains contributions from all possible orientations of the SQ relative to the applied magnetic field. Orientationally disordered (i.e., powder) HYSCORE spectra of I = ½ nuclei reveal, in the form of cross-ridge projections, the interdependence of να and νβ belonging to the same orientations, and analysis of the ridges allows for direct, simultaneous determination of the nuclear isotropic and anisotropic components of the hyperfine tensor.27
The contour lineshape of the cross-ridge in the powder 2D spectrum of a 1H nucleus, for hyperfine interactions with the axially symmetric tensor (a−T, a−T, a+2T), is described27 by Equation 1:
| [1] |
where and and νH is the 1H Zeeman frequency. For each cross-feature, the frequency values along the ridge can be plotted as να2 versus νβ2, transforming the contour lineshape into a straight line segment whose slope and intercept are proportional to Qα and Gα, respectively. These values can then be used to obtain two possible solutions of isotropic (a) and anisotropic (T) couplings with the same value of |2a+T| and interchanged A⊥=|a-T| and A||=|a+2T|.27
Analysis of the HYSCORE spectra simulated using ENDOR data for the QA semiquinone
Orientation-selected Q-band ENDOR experiments with fully deuterated RC from Rba. sphaeroides in 1H2O buffer have provided the hyperfine tensors for two protons participating in H-bonds with O1 and O4 of the QA semiquinone.12 The principal values of the anisotropic hyperfine tensors and isotropic couplings determined for these protons are summarized in Table 1. It is clear that the ENDOR data show one proton (H1) to possess a purely axial anisotropic tensor. For the second proton (H2), the rhombicity is only ≈4% and it, too, can be considered axial for spectral estimates. Table 2 shows the hyperfine tensors and nuclear frequencies for α and β manifolds assuming axial symmetry for both H1 and H2. These tensors support the previous assignment of the three couplings A1=4.7 MHz, A2=6.3 MHz, and A3=9.0 MHz (±0.1 MHz) observed in powder ENDOR spectra, to two A⊥ and one A||.11 The latter represents the overlap of two lines, with the predicted difference between A|| for the two protons on the order of ~0.2 MHz (Table 2). Monitoring the time dependence of the ENDOR line intensities, following substitution of 1H2O by 2H2O with fully protonated and fully deuterated RCs, confirmed that the three observed couplings belong to two protons. The 1H/2H exchange times for the protons of the QA SQ were found to be τ1~50 min and τ2~1200 min in protonated RCs and τ1~10 min and τ2~90 min in fully deuterated RCs.13
Table 1.
ENDOR determined hyperfine tensors (MHz) for the hydrogen bonded protons H1 and H2 in the QA-site of the reaction center from Rba. sphaeroides R-26 (from refs.12,13)
| Proton | H-bond | T33 | T22 | T11 | a | Exchange rate |
|---|---|---|---|---|---|---|
| H1 | O4-His (M219) | 10.43 | −5.23 | −5.20 | −1.28 | slow |
| H2 | O1–Ala (M260) | 9.12 | −4.75 | −4.37 | −0.17 | fast |
Table 2.
ENDOR derived hyperfine frequencies in the axial approximation (MHz) for protons H1 and H2a (from ref.12)
| Proton | H-bond | A⊥ =a-T | A||=a+2T | να|| | νβ|| | να⊥ | νβ⊥ |
|---|---|---|---|---|---|---|---|
| H1 | O4-His (M219) | −6.50 | 9.15 | 19.29 | 10.15 | 11.47 | 17.97 |
| H2 | O1–Ala (M260) | −4.73 | 8.95 | 19.2 | 10.24 | 12.36 | 17.08 |
hyperfine frequencies were calculated for proton Zeeman frequency νH=14.73 MHz
On the other hand, for the QB SQ, couplings assigned to three exchangeable protons were detected by ENDOR, but their hyperfine tensors were not characterized.10
To illustrate the characteristics of powder 1H HYSCORE and to identify the complications that arise when the spectra result from more than one nucleus (especially with similar hyperfine couplings) we use the ENDOR data for the QA SQ to generate simulated HYSCORE spectra for model analysis. Figure 2 shows, for two protons with principal values of the hyperfine tensors and corresponding nuclear frequencies provided in Tables 1 and 2, an idealized contour presentation of the powder HYSCORE spectra (A,C,E), and the corresponding calculated HYSCORE spectra, with time τ=136 ns between first and second microwave pulses (B,D,F). The idealized presentation just shows the location of full cross-ridges determined by the hyperfine parameters, as described by Eq. (1). The calculated spectra take into account suppression effects and show the distribution of intensity along the ridges, which depends on the selected time τ as determined by the intensity coefficient sin(πνα τ)•sin(πνβ τ).28,29 For each proton (H1, H2) there are two cross-ridges with permutated coordinates, (να, νβ) or (νβ, να), extending between (να(β)||, νβ(α)||) and (να(β)⊥, ν β (α)⊥). These are located symmetrically on both sides of the diagonal of the 2D spectrum and pass through the diagonal in the idealized presentation. The branches of the two ridges on the same side of the diagonal are located close to each other. In the calculated spectra, the cross-ridges are suppressed in the region of the diagonal. This effectively leads to the appearance of two isolated sub-ridges, 1αβ and 1′αβ, and 2αβ and 2′αβ, for H1 and H2, respectively. These sub-ridges can be correlated to each other using a linear regression approach based on Eq. (1) and illustrated below. Also shown is a superposition of the spectra from two protons in both presentations (Fig. 2E,F). The να|| and νβ|| frequencies are very close for both protons (Table 2). As a result, the upper parts of the ridges 1αβ(βα) and 2αβ(βα) are practically indistinguishable in the spectrum and are seen as one ridge (1+2)αβ(βα). However, the presence of two protons can be recognized from the sub-ridges 1′αβ(βα) and 2′αβ(βα) located near the antidiagonal and corresponding to orientations approaching 90° between the magnetic field and the unique axis of the hyperfine tensor.
Figure 2.
The HYSCORE spectra for protons H1 and H2 identified by ENDOR. Left column (A,C,E): contour presentation of the full cross-peaks. Right column B,D,F: calculated HYSCORE spectra using Q-band ENDOR derived hyperfine tensors shown in Tables 1 and 2, and time between first and second microwave pulses τ=136 ns. 1H Zeeman frequency is 14.73 MHz (see also text). The spectra qualitatively demonstrate the relative intensity of different ridges. The wider and more extended ridges possess greater intensity.
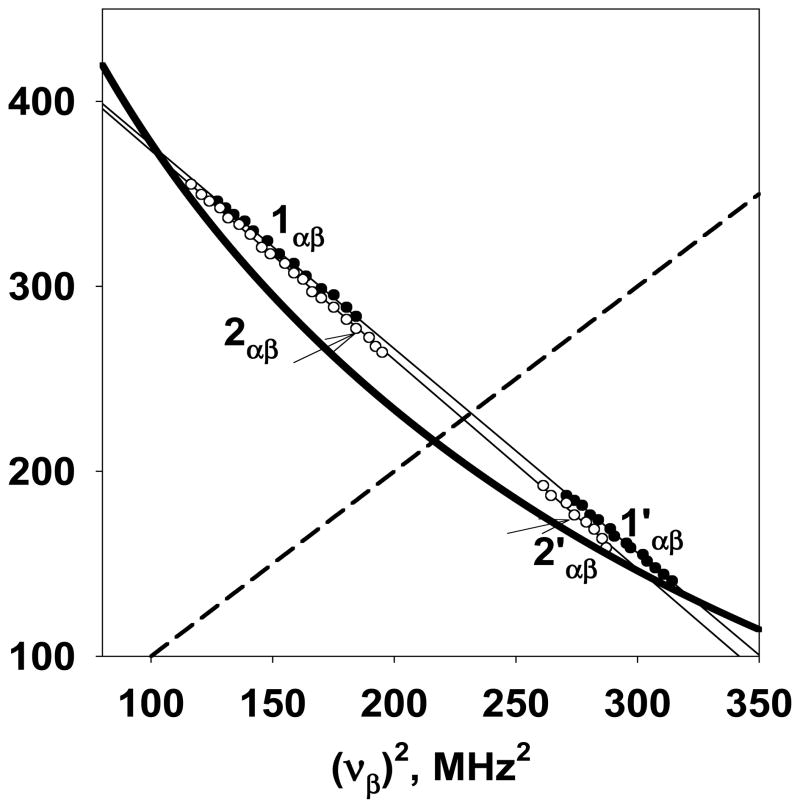
We illustrate the approach for determining the hyperfine tensor components by exploring the simulated HYSCORE spectra of Figure 2. The coordinates ν1 and ν2 of arbitrary points along cross-ridges were measured from the HYSCORE spectra calculated separately for H1 and H2 (Fig. 2B, D) and plotted as sets of values in να2 vs. νβ2. Figure 3 shows the plots where the larger frequency ν1 of each point from sub-ridges 1αβ and 2αβ was arbitrarily selected as να, and the smaller frequency ν2 as νβ. In contrast, for sub-ridges 1′αβ and 2′αβ, on the opposite side of the diagonal, the smaller coordinates should be assigned to να and the larger ones to νβ. This selection places the cross-ridges 1αβ,2αβ and 1′αβ,2′αβ on opposite sides of the graph relative to the dashed line corresponding to the diagonal in the spectra in Figure 2. In such a presentation, the points from 1αβ and 1′αβ fit the linear regression well, thus confirming that they are two parts of the same ridge from proton H1. The same is true for sub-ridges 2αβ and 2′αβ from proton H2. The slopes and intercepts for the linear regressions shown in Figure 3 are presented in Table 3, together with two possible sets of (a, T) satisfying Eq.(1) for protons H1 and H2, and A⊥ and A|| values. The curve |να + νβ| = 2νH (with νH =14.73 MHz, corresponding to the proton Zeeman frequency in a field of 346 mT) is also plotted in Figure 3 to explain the nature of the two solutions determined by Eq.(1). The points at which this curve crosses each extrapolated straight line correspond to the nuclear frequencies (να||, νβ||) or (να⊥, νβ⊥). There are two possible assignments of the parallel or perpendicular orientations for each point and, consequently, two sets of hyperfine tensors, one for each assignment. In this approach, the hyperfine couplings are identical to those determined from the slope and intercept. From the two solutions, one (marked by bold font in Table 3) gives a and T values close to the ENDOR derived values. In addition, Table S1 in Supporting Info shows that accurate estimates of the hyperfine tensors for H1 and H2 can be obtained from linear fitting of the 1′αβ and 2′αβ sub-ridges only. This is especially important when the extended sub-ridges 1αβ and 2αβ are not individually resolved.
Figure 3.
Plots of cross-peaks from the calculated HYSCORE spectra of Figure 2 in the να2 vs. νβ2 coordinate system (see text for detailed explanations). The straight lines show the linear fit of plotted data points. The thick curved line is defined by (να+νβ)=2νH with proton Zeeman frequency 14.73 MHz. The dashed line corresponds to the diagonal line in the spectra.
Table 3.
Parameters derived from contour lineshape analysis of the simulated HYSCORE spectrum in Figure 2
| Proton | Qα | Gα, MHz2 | (a,T)1, MHz | A⊥=a–T, MHz | A||=a+2T, MHz |
|---|---|---|---|---|---|
| H1 (ridges 1αβ,1′αβ) | −1.10 (0.002) | 487.0 (0.5) | ∓4.04, ±5.17 ∓1.13, ±5.17 |
∓9.2 ∓6.3 |
± 6.3 ±9.2 |
| H2 (ridges 2αβ,2′αβ) | −1.13 (0.004) | 486.4 (0.8) | ∓4.13, ±4.65 ∓0.52, ±4.65 |
∓8.8 ∓5.2 |
± 5.2 ± 8.8 |
Results
Quantitative information about the proton environment around the SQ in the QA and QB sites of RC was obtained from 2D ESEEM (HYSCORE) experiments, because the simpler 1D approaches do not provide the necessary resolution of the multinuclear contributions from protons. In our studies of protons we used uniformly 15N labeled protein, because the 14N nuclei of atoms hydrogen bonded with carbonyl oxygens of the SQs produce deep ESEEM, especially for the QA SQ (Figure S1). 14N ESEEM completely suppressed the proton peaks in HYSCORE spectra of the QA SQ due to the cross-suppression effect.30,31 The 15N nucleus, with spin I = ½ and no nuclear quadrupole moment, produces a modulation of the echo amplitude that is shallower than the 14N isotope. This allows the appearance of the proton peaks in HYSCORE spectra of the QA SQ. For the QB SQ in the RC sample with natural abundance 14N, the proton HYSCORE peaks are observed, but 15N labeling further improves the intensity and resolution of the proton spectra. The 14N and 15N X-band HYSCORE spectra of the QA and QB semiquinones are clearly different (see Figure S2, and ref.21) allowing one to draw convincing conclusions about the purity of the preparations containing QA or QB SQ.
QA semiquinone 1H HYSCORE
The time, τ, which is kept constant in each individual experiment, influences the HYSCORE spectrum by modulating the intensity of cross-peaks. Therefore, measurements were performed at several values of τ to ensure that all cross-peaks were observed. Figure 4 shows the contour and 3D-like stacked presentations of the 1H spectra recorded with τ=136 ns for the QA SQ prepared in 1H2O and 2H2O buffer. To provide a better view of the different spectral features, in this figure (and others discussed below) only the half of the contour spectrum above the diagonal is shown. In addition, the sum of three spectra recorded with τ=136 ns, 200 ns and 400 ns in similar presentations is shown in Figure S3 to ensure adequate representation of all peaks. No new peaks appear in the sum spectrum although the relative intensities of the different peaks are changed. (Other views and presentations of the QA SQ spectra are provided in Supplemental Information, Figure S4.)
Figure 4.
Contour (A,B) and stacked (C,D) presentations of the experimental 1H HYSCORE spectra of the QA SQ of Rba. sphaeroides reaction centers prepared in 1H2O and 2H2O (magnetic field 345.9 mT (1H2O) and 346.0 mT (2H2O), time between first and second pulses τ=136 ns, microwave frequency 9.702 GHz (1H2O) and 9.707 GHz (1H2O)). In the stacked presentation, in 1H2O, peak 3A is hidden between ridges 1A and 4A.
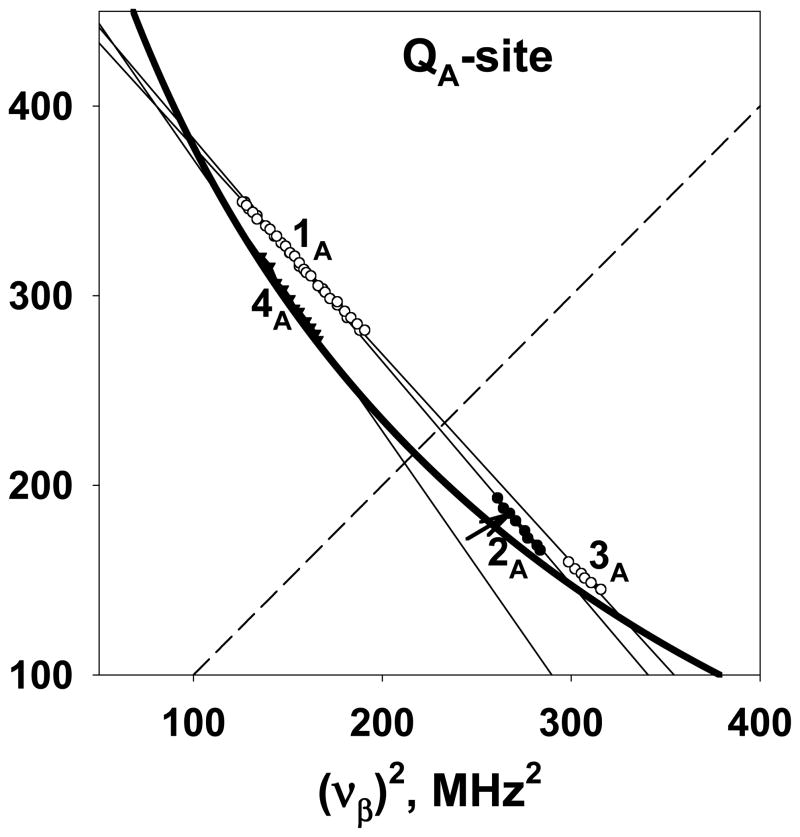
The spectrum in Figure 4 shows up to four pairs of resolved cross-ridges with different lengths and intensities located symmetrically relative to the diagonal. They are designated 1A, 2A, 3A, and 4A. The peaks or ridges of 1A, 2A, and 3A deviate from the antidiagonal, indicating a significant anisotropic component (see full spectrum in Figure S3). In contrast, cross-peaks 4A are approximately normal to the diagonal, suggesting smaller anisotropy.24,25,,27
The intensity of cross-ridges 1A and 3A (Figure 4) is substantially decreased in the HYSCORE spectra obtained under the same conditions but with the sample incubated in 2H2O. The remaining ridge 1A is also significantly narrower. Cross-peak 2A (Figure 4) completely disappeared in this HYSCORE spectrum showing that it is produced by exchangeable proton(s). The same conclusion is probably applicable to cross-features 1A and 3A, taking into account the significantly different exchange rates for the two exchangeable protons H1 and H2 obtained in ENDOR experiments on the QA SQ. The remaining signals, therefore, could be assigned to the residue of slowly exchanging proton H1. The intensities of the diagonal peak and its shoulders spread along the antidiagonal (Figure 4C,D) are also partially suppressed in this spectrum, indicating contributions from weaker coupled exchangeable protons. Nevertheless, cross-peak 4A and the diagonal peak, with its shoulders, still appear in all spectra, indicating contribution from protons of the SQ substituents and nonexchangeable protons of protein environment.
The HYSCORE spectra simulated using the ENDOR data (Figure 2) suggest that cross-ridge 1A is the overlap of two cross-ridges, 1αβ and 2αβ, from protons H1 and H2, i.e., (1+2)αβ. Then cross-peaks 2A and 3A correspond to cross-features 2′βα and 1′βα, respectively. These cross-features, defined by local maxima along the ridge, are plotted in ν2 coordinates according to Eq. 1 (Figure 5). This presentation clearly shows that the points for 1A and 3A as well as 1A and 2A fall along two straight lines, indicating the presence of two protons contributing to these three cross-features. Permutation of the larger and smaller coordinates was performed for cross-peaks 2A and 3A, corresponding to the transformation of the sub-ridges 2′βα and 1′βα to 2′αβ and 1′αβ, for correlation with (1+2)αβ (see Fig. 2F). The cross-features 4A, produced by nonexchangeable protons, possess much smaller hyperfine anisotropy than protons responsible for cross-peaks 1A-3A and do not cross the diagonal. As a result only one cross-feature is used in the linear regression analysis of these points in Figure 5.
Figure 5.
Cross-peaks 1A-4A from 1H HYSCORE spectrum in Figure 4 plotted in the να2 vs. νβ2 coordinate system (see text for detailed explanations). The straight lines show the linear fit of plotted data points. The thick curved line is defined by (να+νβ)=2νH with proton Zeeman frequency 14.73 MHz. The dashed line corresponds to the diagonal line in the spectra.
Quantitative analysis of the cross-peak contours provides two sets of a and T for exchangeable H1A (ridges 1A,3A) and H2A (ridges 1A,2A), and nonexchangeable H3A (ridge 4A) protons (see note to Table 4 regarding subscripts A). The full results of analysis are summarized in Table S2. The preferred set (Table 4) was selected on the basis of the ENDOR data, computational works and numerical simulations of HYSCORE spectra (see also Discussion). Particularly, the HYSCORE spectra simulated for H1A and H2A using the second solution, with large isotropic constants (Table S2), contain only sub-ridges similar to 1αβ(βα) and 2αβ(βα) in Figure 2 while sub-ridges 1′αβ(βα) and 2′αβ(βα) are absent, in contrast to the experimental spectra and the spectra simulated with the preferred parameter set.
Table 4.
Characteristics of hyperfine tensors of the protons H1A-H3A (MHz) derived from experimental HYSCORE spectra of the QA SQ.a,b
| Proton(s) | a, T | A⊥=a–T | A||=a+2T |
|---|---|---|---|
| H1A (ridges 1A,3A) | −1.38±0.1, 5.41±0.17 | −6.8 | 9.6 |
| H2A (ridges 1A,2A) | −0.17±0.2, 5.07±0.35 | −5.2 | 10 |
| H3A (ridge 4A) | 4.39±0.3, 1.71±0.5 | 2.7 | 7.8 |
| H3A (ridge 4A)c | 4.0±0.3, 1.6±0.5 | 2.4 | 7.2 |
The subscript A indicates tensor assignments, and the corresponding protons, from experimental HYSCORE spectra of the QA semiquinone, in contrast to the ENDOR-derived tensors for H1 and H2 in Tables 1 and 2;
errors for a and T correspond to 95% prediction intervals for the linear regression results shown in Table S2;
sample in 2H2O.
QB semiquinone 1H HYSCORE
Figure 6 shows contour (A) and stacked (C) presentations of the 1H HYSCORE spectrum of the QB SQ prepared in 1H2O buffer, obtained as the sum of three spectra recorded with τ=136 ns, 200 ns and 400 ns. We use this presentation because not all cross-peaks possess observable intensity in the spectra at a single value of τ (see Figure S4 for comparison).
Figure 6.
Contour (A,B) and stacked (C,D) presentations of the experimental 1H HYSCORE spectra of the QB SQ of Rba. sphaeroides reaction centers prepared in 1H2O and 2H2O. Spectra were obtained as a sum of three individual spectra recorded with time between first and second pulses τ=136, 200, and 400 ns, (magnetic field 345.1 mT (1H2O) and 344.6 mT (2H2O), microwave frequency 9.680 GHz (1H2O) and 9.666 GHz (2H2O)).
Six pairs of cross-features 1B-6B can be identified in the spectrum. The cross-ridge 1B shows similar length and deviation from the antidiagonal as cross-peak 1A in the spectrum of the QA SQ although the width and intensity of the ridge are smaller than in the QA SQ spectrum. Using both contour and stacked spectra, careful examination of the line-shape for the cross-feature most closely approaching 1B indicates that it is formed by the overlap of two ridges, marked 2B and 3B, from two different nuclei (this is also suggested by the 4-pulse ESEEM spectra discussed below). All three cross-features 1B-3B completely disappeared in the spectrum of the sample prepared in 2H2O buffer, showing their relation to exchangeable protons. The spectrum of the QB SQ also shows two cross-peaks 4B and 5B, for which there are no counterparts in the spectrum of the QA SQ. These are also absent in the spectrum after 1H/2H exchange. Only peak 6B (similar to peak 4A in the QA SQ spectrum) is still present in the spectrum after 1H/2H exchange, together with the shoulders of the diagonal 1H matrix line.
The να2 vs. νβ2 plot of the cross-features from the QB SQ 1H HYSCORE spectrum (Figure 7) suggests that 1B and 2B, and 3B and 4B are the sub-ridges of cross-features belonging to two different protons. In terms of the designations used in Figure 2, 1B and 2B are similar to sub-ridges 1αβ and 1′βα from proton H1B, and 3B and 4B are sub-ridges 2αβ and 2′βα from proton H2B. The subscript B indicates assignments from experimental HYSCORE spectra of the QB semiquinone. In agreement with this suggestion 1B and 2B, and 3B and 4B (after permutation of the smaller and larger coordinates for 2B and 4B placing them to the opposite side of the spectrum relative to the diagonal), fall well along two straight lines and belong to two exchangeable protons H1B and H2B with T~5.2 and 3.7 MHz. There are no other cross-features in the spectrum that can be correlated with cross-peaks 5B and 6B. The characteristics of the slopes and intercepts for the linear regressions shown in Figure 5 are presented in Table S3, together with two possible sets of (a, T) that satisfy Eqn. (1). Table 5 shows the preferred sets of the hyperfine couplings.
Figure 7.
Cross-peaks 1B-6B from 1H HYSCORE spectrum in Figure 6 plotted in the να2 vs. νβ2 coordinate system (see text for detailed explanations). The straight lines show the linear fit of plotted data points. The thick curved line is defined by (να +νβ)=2νH with proton Zeeman frequency 14.73 MHz. The dashed line corresponds to the diagonal line in the spectra.
Table 5.
Characteristics of hyperfine tensors of the protons H1B-H4B (MHz) derived from experimental HYSCORE spectra of the QB SQ.a,b
| Proton | a, T | A⊥=a–T | A||=a+2T |
|---|---|---|---|
| H1B (ridges 1B,2B) | −0.83±0.08, 5.2±0.16 | −6.0 | 9.6 |
| H2B (ridges 3B,4B) | −0.88±0.14, 3.71±0.26 | −4.6 | 6.5 |
| H3B (ridges 5B) | 1.09±0.35, 1.87±0.6 | −0.8 | 4.9 |
| H4B (ridges 6B) | 4.27±0.25, 1.92±0.35 | 2.4 | 8.1 |
| H4B (ridges 6B)c | 4.8±0.12, 1.59±0.25 | 3.2 | 8.0 |
The subscript B indicates assignments from experimental HYSCORE spectra of the QB semiquinone;
errors for a and T correspond to 95% prediction intervals for the linear regression results shown in Table S3;
sample in 2H2O.
Sum combination peaks in 1D 4-pulse ESEEM
Additional information about the exchangeable protons, supporting the HYSCORE data, was obtained from the 1D four-pulse ESEEM spectra (Figure 8). These spectra contain lines in the region of the double proton Zeeman frequency (2νH, at 29.4 MHz), which are sum-combination harmonics (να+νβ) of two basic frequencies να and νβ. These harmonics are not created in HYSCORE experiments. This approach is particularly useful for the resolution of protons with different anisotropic couplings.32
Figure 8.
Stacked presentations of sets of four-pulse ESEEM spectra of the QA and QB SQs in Rba. sphaeroides reaction centers prepared in 1H2O and 2H2O. The spectra show the modulus of the Fourier transform along the time (T) axis for different times between first and second pulses, τ. The initial time τ is 100 ns in the farthest trace, and was increased by 12 ns in successive traces. Microwave frequency and magnetic field were 9.711 GHz and 346.2 mT (QA in 1H2O), 9.705 GHz and 346.0 mT (QA in 2H2O). 9.708 GHz and 346.1 mT (QB in 1H2O), and 9.664 GHz and 344.5 mT (QB in 2H2O).
The four-pulse ESEEM spectrum of the QA SQ in 1H2O buffer contains two well resolved lines in the region of the proton 2νH, as shown in Figure 8. The most intense line appears exactly at the 2νH frequency and represents the contribution of weakly coupled protons from the protein environment with (να+νβ) ≅ 2νH. The spectrum also reveals a broad, asymmetrical peak of lower intensity shifted from 2νH to higher frequencies, with a shoulder on the high-frequency side. This peak does not possess a well-defined maximum and is consistent with two overlapping peaks with shifts of 0.8–1.1 MHz. This feature is greatly diminished in the spectrum of the sample prepared in 2H2O, indicating that it contains a major contribution from exchangeable protons.
The four-pulse spectrum of the QB SQ shows a single, narrow peak shifted by ~0.9 MHz from 2νH. In addition, there is a distinct, shoulder-like feature located near the 2νH peak. The flat maximum indicates a shift of ~0.25–0.50 MHz. Both features disappear in 2H2O.
The shifts observed in the four-pulse ESEEM are well described32 by Equation 2
| [2] |
which yields the anisotropic component T for the exchangeable protons producing shifted lines (Table 6). The T values determined from the shifts of the sum-combination lines are consistent with the values obtained from the HYSCORE analysis for QA and QB site semiquinones. Particularly, T ~4.6–5.4 MHz was found for the QA-site. In the QB SQ spectrum, the line with the larger shift corresponds to T ~5 MHz and relates to H1B. The broad feature near the 2νH line corresponds to protons with T <3.7 MHz and can be accounted for by contributions of the exchangeable protons H2B and H3B, which possess significantly lower anisotropic hyperfine coupling than H1B. This contrasts with the QA SQ where both detected exchangeable protons, H1A and H2A, exhibit similar hyperfine tensors.
Table 6.
Anisotropic hyperfine couplings for exchangeable protons in the QA- and QB-sites estimated from the shift of the sum combination harmonics
| Site | Shift Δ, MHz | T, MHz |
|---|---|---|
| QA | 0.8–1.1 | 4.6–5.4 |
| QB | 0.9–1.0 0.2–0.5 |
4.9–5.1 2.3–3.6 |
Discussion
Exchangeable protons
The principal values of the hyperfine tensors determined in our HYSCORE studies for the exchangeable protons around the QA SQ are fully consistent with previously published orientation-selected Q-band ENDOR data,12 but were obtained with considerably greater ease of sample preparation, much shorter data acquisition time, and simpler analysis. We found the presence of two protons H1A and H2A, with similar anisotropic hyperfine couplings T~4.6–5.4 MHz, involved in the O4–His (M219) and O1–Ala (M260) hydrogen bonds.
Besides the cross-features assigned to these two protons, 1H/2H exchange did not result in any other significant loss of spectral intensity in the QA SQ spectrum, e.g., in areas remote from the diagonal peak where distant nuclei mostly contribute. This is in agreement with the current vision of the QA site where only two hydrogen bonds, one to each carbonyl, are responsible for coupling the SQ to the protein.
The HYSCORE spectra of the QB SQ have resolved features assigned to exchangeable protons with distinct anisotropic couplings T~5.2 MHz and ~3.7 MHz. These values exceed the T~3 MHz observed for in-plane hydrogen bonded protons in alcoholic solutions.33–35 Hydrogen bonding to the quinone carbonyl groups occurs via proton donation to one or both lone pairs on the sp2-hybridized carbonyl oxygen.36 Besides these two protons discussed here for the QB SQ, we found a marked loss of spectral intensity after 1H/2H exchange for a peak accompanying the antidiagonal, with a splitting of ~2.5 MHz (peak 5B). This indicates the presence of an additional exchangeable proton with T~1.9 MHz estimated from contour lineshape analysis.
As expected from the simulated spectra, the powder X-band HYSCORE spectra did not resolve all the cross-ridges of the QA SQ protons, but the the linear analysis clearly identified them through the correlations between features (Figure 5). Similarly, the four-pulse ESEEM spectra did not fully separate all the shifted proton sum-combination peaks, due to line broadening, although our model simulations of the four-pulse spectra with ENDOR derived tensors did resolve peaks from two QA protons. One likely factor contributing to broadening of the lines and influencing the resolution is the “strain” of the hyperfine parameters, i.e., the static distribution of hyperfine parameters. Analysis of the lineshapes in the “single-crystal-like” Q-band ENDOR spectra from the protons of hydrogen bonds in the QA-site of RCs from Rba. sphaeroides12 shows that the influence of this factor on the hyperfine coupling value would not exceed ±5%. For couplings of ~5 MHz, this could give broadening up to 0.5 MHz for basic frequencies and the spread in the position of line maximum for combination harmonics up to 0.3 MHz. In powder spectra such broadening does influence the resolution of the individual lines from protons H1A and H2A in the QA site.
Assignment of hyperfine couplings through QM/MM calculations
To help assign the observed hyperfine couplings to specific interactions within the QA and QB sites we have obtained calculated values using QM/MM density functional theory-based electronic structure calculations. The excellent ability of DFT-based calculations to accurately calculate hyperfine couplings for semiquinone free radicals has been demonstrated some time ago.37 A recent report has shown how such calculations can be extended to model semiquinone protein binding sites using QM/MM calculations.20 The carbonyl and hydrogen bonding distances calculated for SQ in the QA and QB sites are given in Table 7, while the calculated hyperfine couplings for the exchangeable protons are given in Table 8. For the QA site, T (T=T33/2) values calculated for the His-M219 Nδ1H (5.3 MHz) and Ala-M260 peptide N1H (4.6 MHz) are in very good agreement with the respective T values of 5.2 MHz and 4.6 MHz from Q-band ENDOR,12 and with our HYSCORE results, 5.4 and 5.1 MHz. We can therefore confidently assign the experimental T values to these proton interactions within the QA site. In addition the calculated 14N isotropic hyperfine couplings for the His-M219 Nδ (2.6 MHz) and the Ala-M260 peptide N (1.5 MHz) are in good agreement with the experimental values of 2.5 MHz and 1.9 MHz estimated from the 14N and 15N HYSCORE spectra of this work (Figure S2). A previous two- and three-pulse ESEEM study14,15 reported hyperfine couplings of 1.8 and 1.1 MHz, which were not assigned to particular nitrogens due to uncertainty in the correlation of the single-quantum and double-quantum transitions from the same nitrogens in 1D ESEEM spectra.
Table 7.
| Bond | QA site | QB site |
|---|---|---|
| C4-O4 | 1.29 | 1.28 |
| C1-O1 | 1.27 | 1.28 |
| O4 - HNδ His-M219/L190 | 1.62 | 1.58 |
| O1 – HN Ala-M260/Gly-L225 | 1.84 | 2.00 |
| O1 - HO Ser-L223 | 1.81 | |
| O1 - HN Ile-L224 | 2.30 |
QA data taken from 20.
All distances in ångstroms.
Table 8.
Calculated 1H and 14N hyperfine couplings (MHz) for the QA and QB site hydrogen bonding interactions with SQ
| QA sitea | QB site | ||||
|---|---|---|---|---|---|
| Position | Anisotropic T33,T22,T11 | Isotropic a | Position | Anisotropic T33,T22,T11 | Isotropic a |
| 1HN His-M219 | 10.4 −5.3 −5.0 |
0.6 | 1HN His-L190 | 10.2 −5.3 −4.9 |
−0.4 |
| 1HN Ala-M260 | 9.3 −4.7 −4.6 |
−1.8 | 1HN Gly-L225 | 6.6 −3.7 −2.9 |
−0.6 |
| 1HN Ile-L224 | 3.9 −2.1 −1.8 |
−0.1 | |||
| 1HO Ser-L223 | 7.6 −4.0 −3.7 |
−0.8 | |||
| 14Nδ His-M219 | 0.6 −0.3 −0.3 |
2.6 | 14Nδ His-L190 | 0.4 −0.2 −0.2 |
1.5 |
| 14NH Ala-M260 | 0.6 −0.3 −0.3 |
1.5 | 14NH Gly-L225 | 0.3 −0.2 −0.1 |
0.6 |
| 14NH Ile-L224 | 0.2 −0.1 −0.1 |
0.1 | |||
QA data taken from 20.
For the QB site, the largest 1H tensor component in the calculations gives a value of 5.1 MHz for the coupling between the His-L190 NδH group and the O4 of the SQ (see Table 8). The experimentally observed T value of 5.2 MHz is therefore assigned to this interaction. Furthermore, the calculated 14N isotropic hyperfine coupling for the QB site Nδ His-L190 is 1.5 MHz, in agreement with the reported experimental value of 1.5 MHz.21 For the O1 side in the QB site there are three 1H hyperfine couplings calculated; two strong hydrogen bonding interactions, with the OH of Ser-L223 and the peptide NH of Gly-L225, and a weaker dipolar coupling with the peptide NH of Ile-L224. The calculated T value for the Ile-L224 peptide NH (1.9 MHz) is assigned to the same, experimentally observed T value of 1.9 MHz. The peptide NH of Gly-L225 is calculated to have a 14N isotropic value of 0.5 MHz whereas the corresponding value for Ile-L224 is 0.1 MHz. The experimentally observed 14N isotropic coupling of 0.5 MHz21 is therefore assigned to the NH Gly L-225 interaction. The much smaller value calculated for Ile-L224 would not be distinguishable from the matrix contributions on the diagonal.
The T values calculated for protons of Ser-L223 (3.8 MHz) and Gly-L225 (3.3 MHz) are both likely to contribute to the experimentally derived T=3.7 MHz value. The HYSCORE spectra calculated with the hyperfine coupling values for protons of Ser-L223, Gly-L225 and His-L190 from Table 8 showed that the cross-peaks from Ser-L223 and Gly-L225 protons are indistinguishable in the sum spectrum (Figure S7, A–F). The addition of the His-L190 proton gives the total spectrum, reproducing the shape of the overlapped cross-features (corresponding to the features assigned to 2B and 3B in the experimental spectra in Figures 6 and S5) and its location relative to the cross-ridge (equivalent to 1B in Figure 6 and S5) from the His proton (Figure S7, G–L). Thus, these calculations qualitatively support the suggested contribution of the Ser-L223 and Gly-L225 protons to the feature with T=3.7 MHz in the experimental spectra. Further work using orientation-selected Q-band ENDOR or pulsed EPR at lower microwave frequencies is needed to confirm this result experimentally.
Nonexchangeable protons
The hyperfine couplings of nonexchangeable protons in the QA and QB semiquinones have been extensively studied by multifrequency (X-, Q-, and W-band) ENDOR in frozen solutions and X-band ENDOR in single-crystals.11,38,39 These studies reported the couplings for the protons of a rotating methyl group (C5′) and one proton from a methylene group of the isoprenoid chain of QA. All experiments with QA SQ have consistently shown that the maximum and minimum principal values of the hyperfine tensor are about 6.8 and 3.0 MHz for the methyl protons and 8.8 and 5.0 MHz for the methylene proton (Table S4). This suggests that the lines from methyl and methylene protons will partially overlap in powder X-band HYSCORE spectra.
ENDOR studies of the QB SQ have reported the coupling for methyl protons only, with larger isotropic coupling than for the QA SQ. Q- and W-band studies indicated maximum and minimum principal values of the hyperfine tensor of 7.8 and 3.9 MHz.11,39
The HYSCORE spectra of the QA and QB semiquinones after deuterium exchange show only one pair of cross-peaks, i.e., 4A and 6B, respectively, along the antidiagonal and well-separated from the diagonal peak and remaining shoulders. Analysis of the contour line-shape of these peaks in the samples prepared in 1H2O and 2H2O gives similar results for both SQs, and with only very minor contributions from exchangeable protons in this vicinity. The formal axial tensors are: (7.2, 2.4, 2.4) MHz with a=4.0 MHz and T=1.6 MHz for the QA SQ, and (8.0, 3.2, 3.2) MHz with a=4.8 MHz and T=1.6 MHz for the QB SQ. These values are in reasonable agreement with those derived from ENDOR, although the isotropic contributions are somewhat smaller and the anisotropic components larger (T~1.1–1.2 MHz for CH3 and CH2 protons). The HYSCORE analysis consequently yields a larger difference between the maximum and minimum principal values of the hyperfine tensors. The difference likely arises from the overlap of the methyl and methylene proton lines, which increases the apparent length of the HYSCORE cross-peak and leads to a larger anisotropic component in the formal analysis.
Proton resonances with smaller splittings have also been observed in ENDOR spectra, but have not been assigned. They should presumably belong to the second proton of the CH2 group, other protons of the isoprenoid chain, the methoxy groups, and protons from the protein matrix. These protons contribute to the shoulders of the diagonal line in HYSCORE spectra.
From the QM/MM calculations (Table 9), the 1H CH3 values for QA are in good agreement with the experimentally determined values (see also20). For QB the calculated isotropic hyperfine coupling is predicted to be larger than that found for QA in agreement with the experimental determinations. However, the QB value calculated is significantly higher than that observed experimentally, suggesting that the model in its current form overestimates the spin density at C5 of the QB SQ. Since the 17O and 13C coupling values calculated for the carbonyls are in good agreement with experiment, the asymmetry along the C1-C4 quinone axis is well reproduced. However, asymmetry across the C2-C5 axis could be affected by the torsional angle of the C2 methoxy group, which we have shown to be absolutely required for QB activity.2,40 Proper understanding of the C5 spin anomaly will require further knowledge of the unpaired spin density distribution over the ring carbons. This can be obtained from studies of 13C ring-labeled QA and QB SQs, and such experiments are under consideration.
Table 9.
| QA sitea | QB site | ||||
|---|---|---|---|---|---|
| Position | Anisotropic T33,T22,T11 | Isotropic a | Position | Anisotropic T33,T22,T11 | Isotropic a |
| 13C1 | 20.1 −11.8 −8.3 |
−7.0 | 13C1 | 30.6 −16.6 −14.0 |
3.3 |
| 13C4 | 33.1 −17.6 −15.5 |
8.4 | 13C4 | 26.3 −14.4 −11.9 |
1.0 |
| 17O1 | −78.8 39.7 39.1 |
−19.9 | 17O1 | −66.9 −33.6 −33.3 |
−18.3 |
| 17O4 | −54.0 27.3 26.7 |
−16.0 | 17O4 | −62.6 −31.4 −31.2 |
−18.2 |
| 1H CH3(5′) | 2.4 −1.6 −0.8 |
4.3 | 1H CH3(5′) | 2.6 −1.7 −1.0 |
7.6 |
QA data taken from20;
Experimentally determined hyperfine couplings from 11:
13C couplings, Aobs=a+T33, QA site: 22.7 MHz (C1) and 35 MHz (C4); QB site: 27.7 MHz (C1) and 32.2 MHz (C4).
17O couplings, Aobs=a+T33, QA site: −94 MHz (O1) and −75 MHz (O4); QB site: −88 MHz (O1) and −82 MHz (O4).
Reported couplings for methyl group are summarized in Table S4.
Asymmetry in spin density distribution
Differences in the hydrogen bonding interactions with the quinone oxygens is expected to lead to asymmetry of the spin distribution in the radical anion. Comparison of the 1H, 13C and 17O couplings for SQ in the QA and QB sites with those of ubiquinone-10 anion radicals in alcoholic solution led to the conclusion that the QA quinone binding site is more asymmetric than the QB site.11 Based on the results and discussion above it can be inferred that the interactions with both SQs at the O4 atom are similar, where a strong hydrogen bond is formed with the Nδ atom of a histidine residue. The O1 interactions, however, are significantly different. For the QA site only one hydrogen bonding interaction is found with the peptide NH of Ala-M260, whereas in the QB site there are interactions with the peptide NH of Gly-L225 and the OH group of Ser-L223, and a weaker interaction with the peptide NH group of Ile-L224.
In the QA site, the stronger hydrogen bond to the O4 oxygen gives rise to an asymmetric spin density distribution for the ubisemiquinone. For the QB site, the strong hydrogen bonding interaction to the O4 atom is balanced by the double hydrogen bond interaction at the O1 atom in addition to a weaker interaction with the NH group of Ile-L224. This leads to a more symmetric spin density distribution for the QB SQ as previously indicated by the experimental 13C and 17O hyperfine couplings.11
Table 9 gives the calculated isotropic and anisotropic 13C and 17O hyperfine couplings for the QA and QB SQs. Here the asymmetry difference between the two sites is clearly demonstrated by the large differences in the calculated values of the 1 and 4 positions for the QA SQ, whereas similar values are calculated for the QB SQ. The calculated total tensors show good agreement with the experimentally determined values of reference provided in the notes to Table 9.11
The difference in methyl group hyperfine couplings is another indicator of the different spin density asymmetries exhibited by the QA and QB SQs. For QA, the stronger hydrogen bond at the O1 atom leads to a low spin density at C5 (see spin populations in Figure 9), which, in turn, leads to a lower hyperfine coupling for the methyl group protons. For the QB SQ, the more symmetric spin density distribution leads to a higher spin density value at C5, which gives rise to a larger methyl group hyperfine coupling. This is similar to the situation in protic solvents where similar hydrogen bond strengths to each oxygen are expected.
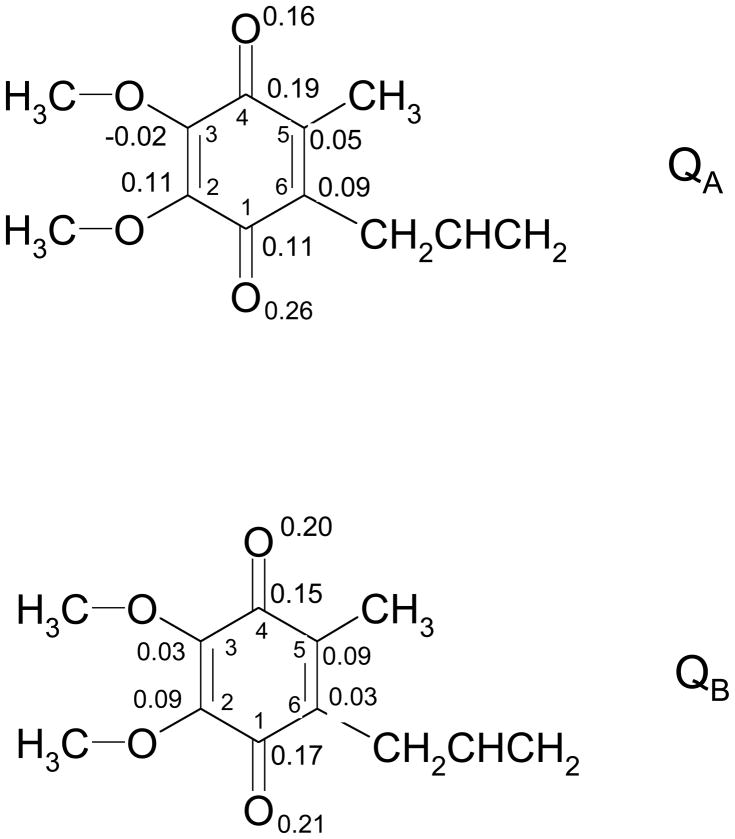
Figure 9.
Calculated Mulliken spin populations for the semiquinone in the QA and QB sites.
The difference in spin asymmetry for the QA and QB site semiquinones is well illustrated by the calculated Mulliken spin populations shown in Figure 9, where a much more symmetric spin population is shown for the QB SQ. This difference in spin density distribution for the two semiquinones may well contribute to their different functions in electron transfer. The asymmetry of the QA site in bacterial RCs polarizes the electron density of the QA SQ towards QB and it has been suggested that this might contribute to the electron transfer to QB.11 However, in the similar, Type II reaction center of oxygenic photosystem II, the H-bond strengths that are presumed to underlie the spin density asymmetry have been reported to be opposite to that in bacterial RCs, i.e., the interaction with the peptide nitrogen was strongest.41 Thus, the directional component of the electron distribution in QA may not be a design requirement of functional QA to QB electron transfer. However, the asymmetry will likely affect the redox potential regardless of its particular polarity. For the QB site the more symmetric spin density resembles the in vitro SQ in protic solvents favoring double reduction and formation of the quinol form. The extra hydrogen bonding interactions found in the QB site will also increase the electron affinity of QB compared with QA, which should lead to a higher redox potential for QB, as observed experimentally.2
Conclusion
We have found that a principal difference between the SQ in the QA and QB sites of purple bacterial reaction centers is an increased number of hydrogen bond interactions at the O1 atom for the QB SQ. This leads to a symmetric spin density distribution for the QB SQ and also an enhanced stabilization. The hydrogen bonding pattern in the binding site is able to control the reduction properties of the quinone. In other type II reaction centers, such as Photosystem II, the hydrogen bonding interactions with the QA semiquinone are also highly asymmetrical, suggesting that a similar control mechanism exists.41 The fine tuning of SQ properties through hydrogen bonding is likely to be a key mechanism for controlling quinone reduction in biological systems.
Supplementary Material
Acknowledgments
This investigation was supported by the NIH GM062954 Grant and DE-FG02-08ER15960 Grant from Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Sciences, US DOE (S.A.D.), NSF Grant MCB0818121 (C.A.W.) and NCRR/NIH Grant S10-RR15878 for pulsed EPR instrumentation.
Footnotes
Supporting Information Available: two-pulse ESEEM for QB SQs, 14N,15N HYSCORE spectra of the QA site SQ, additional 1H HYSCORE spectra for QA and QB SQs, complete ref.23, additional tables with results of HYSCORE analyses. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Okamura MY, Paddock ML, Graige MS, Feher G. Biochim Biophys Acta. 2000;1458:148–163. doi: 10.1016/s0005-2728(00)00065-7. [DOI] [PubMed] [Google Scholar]
- 2.Wraight CA. Frontiers in Biosciences. 2004;9:309–337. doi: 10.2741/1236. [DOI] [PubMed] [Google Scholar]
- 3.Wraight CA, Gunner MR. In: The Purple Phototrophic Bacteria. Hunter CN, Daldal F, Thurnauer MC, Beatty JT, editors. Springer Science + Business Media B.V; 2009. pp. 379–405. [Google Scholar]
- 4.Lancaster CRD. FEBS Lett. 1998;545:52–60. doi: 10.1016/s0014-5793(03)00393-4. [DOI] [PubMed] [Google Scholar]
- 5.Lancaster CRD, Ermler U, Michel H. In: Anoxygenic Photosynthetic Bacteria. Blankenship RE, Madigan MT, Bauer CE, editors. Kluwer Academic Publishers; Dordrecht: 1995. pp. 503–526. [Google Scholar]
- 6.Xu Q, Gunner MR. Biochemistry. 2002;41:2694–2701. doi: 10.1021/bi011834c. [DOI] [PubMed] [Google Scholar]
- 7.Stowell MH, McPhillips TM, Rees DC, Soltis SM, Abresch E, Feher G. Science. 1997;276:812–816. doi: 10.1126/science.276.5313.812. [DOI] [PubMed] [Google Scholar]
- 8.Axelrod HL, Abresch EC, Paddock ML, Okamura MY, Feher G. Proc Natl Acad Sci USA. 2000;97:1542–1549. doi: 10.1073/pnas.97.4.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koepke J, Krammer EM, Klingen AR, Sebban P, Ullmann GM, Fritzsch G. J Mol Biol. 2007;371:396–409. doi: 10.1016/j.jmb.2007.04.082. [DOI] [PubMed] [Google Scholar]
- 10.Paddock ML, Flores M, Isaacson R, Chang C, Abresch EC, Okamura MY. Biochemistry. 2007;46:8234–8243. doi: 10.1021/bi7005256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lubitz W, Feher G. Appl Magn Reson. 1999;17:1–48. [Google Scholar]
- 12.Flores M, Isaacson R, Abresch E, Calvo R, Lubitz W, Feher G. Biophys J. 2007;92:671–682. doi: 10.1529/biophysj.106.092460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flores M, Isaacson R, Abresch E, Calvo R, Lubitz W, Feher G. Biophys J. 2006;90:3356–3362. doi: 10.1529/biophysj.105.077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosch MK, Gast P, Hoff AJ, Spoyalov AP, Tsvetkov YuD. Chem Phys Lett. 1995;239:306–312. [Google Scholar]
- 15.Spoyalov AP, Hulsebosch RJ, Shochat S, Gast P, Hoff AJ. Chem Phys Lett. 1996;263:715–720. [Google Scholar]
- 16.Lendzian F, Rautter J, Käβ H, Gardiner A, Lubitz W. Ber Bunsenges Phys Chem. 1996;100:2036–2040. [Google Scholar]
- 17.Höfer P, Grupp A, Nebenführ H, Mehring MM. Chem Phys Lett. 1986;132:279–284. [Google Scholar]
- 18.Dikanov SA, Samoilova RI, Kolling DRJ, Holland JT, Crofts AR. J Biol Chem. 2004;279:15814–15823. doi: 10.1074/jbc.M313417200. [DOI] [PubMed] [Google Scholar]
- 19.Yap LL, Samoilova RI, Gennis RB, Dikanov SA. J Biol Chem. 2006;281:16879–16887. doi: 10.1074/jbc.M602544200. [DOI] [PubMed] [Google Scholar]
- 20.Lin TJ, O’Malley PJ. J Mol Struct: THEOCHEM. 2008;870:31–35. [Google Scholar]
- 21.Martin E, Samoilova RI, Narasimhulu KV, Wraight CA, Dikanov SA. J Am Chem Soc. 2010;132:11671–11677. doi: 10.1021/ja104134e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vreven T, Byun KS, Komaromi I, Dapprich S, Montgomery JA, Morokuma K, Frisch MJ. J Chem Theor Comput. 2006;2:815–826. doi: 10.1021/ct050289g. [DOI] [PubMed] [Google Scholar]
- 23.Frisch MJ, et al. Gaussian 03, Revision D.01. Gaussian, Inc; Pittsburgh PA: 2003. [Google Scholar]
- 24.Schweiger A, Jeschke G. Principles of pulse electron paramagnetic resonance. Chapters 10 and 11 Oxford University Press; Oxford: 2001. [Google Scholar]
- 25.Dikanov SA. In: New Advances in Analytical Chemistry. Atta-ur-Rahman, editor. Amsterdam: Gordon and Breach; 2000. pp. 523–568. [Google Scholar]
- 26.Van Doorslaer S, Vinck E. Phys Chem Chem Phys. 2007;9:4620–4638. doi: 10.1039/b701568b. [DOI] [PubMed] [Google Scholar]
- 27.Dikanov SA, Bowman MK. J Magn Reson Ser A. 1995;116:125–128. [Google Scholar]
- 28.Dikanov SA, Tyryshkin AM, Bowman MK. J Magn Reson. 2000;144:228–242. doi: 10.1006/jmre.2000.2055. [DOI] [PubMed] [Google Scholar]
- 29.Gemperle C, Aebli G, Schweiger A, Ernst RR. J Magn Reson. 1990;88:241–256. [Google Scholar]
- 30.Stoll S, Calle C, Mitrikas G, Schweiger A. J Magn Reson. 2005;177:93–101. doi: 10.1016/j.jmr.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Kolling DRJ, Samoilova RI, Shubin AA, Crofts AR, Dikanov SA. J Phys Chem A. 2009;113:653–667. doi: 10.1021/jp806789x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reijerse EJ, Dikanov SA. J Chem Phys. 1991;95:836–845. [Google Scholar]
- 33.O’Malley PJ, Babcock GT. J Am Chem Soc. 1986;108:3995–4001. [Google Scholar]
- 34.MacMillan F, Lendzian F, Lubitz W. Magn Reson Chem. 1995;33:581–593. [Google Scholar]
- 35.Flores M, Isaacson RA, Calvo R, Feher G, Lubitz W. Chem Phys. 2003;294:401–413. [Google Scholar]
- 36.O’Malley PJ. Chem Phys Lett. 1998;291:367–374. [Google Scholar]
- 37.O’Malley PJ. Chem Phys Lett. 1996;262:797–800. [Google Scholar]
- 38.Rohrer M, MacMillan F, Prisner TF, Gardiner AT, Möbius K, Lubitz W. J Phys Chem B. 1998;102:4648. [Google Scholar]
- 39.Schnegg A, Dubinskii AA, Fuchs MR, Grishin YuA, Kirilina EP, Lubitz W, Plato M, Savitsky A, Möbius K. Appl Magn Reson. 2007;31:59–98. [Google Scholar]
- 40.Wraight CA, Vakkasoglu AS, Poluektov Y, Mattis A, Takahashi E, Nihan D, Lipshutz BH. Biochim Biophys Acta. 2008;1777:631–636. doi: 10.1016/j.bbabio.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 41.Deligiannakis Y, Hanley J, Rutherford AW. J Am Chem Soc. 1999;121:7653–7664. [Google Scholar]
- 42.Humphrey W, Dalke A, Schulten K. J Mol Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.