Abstract
LL-37, derived from human cathelicidin, stimulates immune responses in neutrophils. Although FPR2 and P2X7 were proposed as LL-37 receptors, we have shown that among 21 neutrophil receptors only CXCR2 was down-regulated by LL-37. LL-37 functions similarly to CXCR2 specific chemokines CXCL1 and CXCL7 in terms of receptor down-regulation and intracellular calcium mobilization on freshly-isolated neutrophils. Neutrophils pretreated with CXCL8, a chemokine that binds both CXCR1/2, completely blocked the calcium mobilization in response to LL-37, while LL-37 also partially inhibited 125I-CXCL8 binding to neutrophils. SB225002, a selective CXCR2 antagonist, blocked LL-37-induced calcium mobilization and migration of neutrophils. LL-37 stimulates calcium mobilization in CXCR2-transfected HEK293 cells, CXCR2+ THP-1 cells and monocytes, but not in CXCR1-transfected HEK293 cells. WKYMVm peptide (ligand for FPR2) does not block LL-37-stimulated calcium flux in either THP-1 (FPR2−) or monocytes (FPR2high), further confirming the specificity of LL-37 for CXCR2 and not FPR2. Among all ligands tested (ATP, BzATP, WKYMVm, CXCL1, and LL-37), only LL-37 stimulated migration of monocytes (CXCR2+ and FPR2+) and migration was inhibited by the CXCR2 inhibitor SB225002. Moreover, CXCR2 but not CXCR1 was internalized in LL-37-treated neutrophils. Thus, our data provide evidence that LL-37 may act as a functional ligand for CXCR2 on human neutrophils.
Keywords: LL-37, CXCR2, receptor, neutrophils
INTRODUCTION
Human CAP-18 (hCAP18), the only human cathelicidin identified in neutrophils [1] was later shown to be expressed in epithelial cells [2] and monocytes [3]. Cathelicidins are a family of antimicrobial peptides that are characterized by a conserved N-terminal cathelin domain and a variable C-terminal antimicrobial domain. This C-terminal domain is cleaved from the precursor by proteinase-3, releasing the ~4.5 kD active α-helical peptide LL-37 [4]. hCAP18 has been shown to inhibit LPS and other bacterial components (lipoteichoic acid and noncapped lipoarabinomannan) that induce a variety of cellular responses, including release of TNF-α, nitric oxide, and tissue factor [5, 6]. Other studies have revealed that LL-37 itself is a multifunctional modulator of innate immune responses. For example, LL-37 promotes wound neovascularization [7], re-epithelialization of healing skin [8], migration of human peripheral monocytes, neutrophils, CD4+ T cells, and mast cells [3, 9, 10], and stimulates the expression of over 40 genes by macrophages, including chemokines, such as CXCL8 (IL-8), and their receptors, such as CXCR2 [6]. These data suggest it is important to identify the specific receptors on cell surfaces responsible for LL-37 function. Initially, LL-37 was proposed to act through FPRL1 (newly named FPR2) to cause migration of monocytes with a general FPR2-like activity for neutrophil and T-lymphocyte migration [9], but subsequent studies challenged FPR2 as the receptor on epithelial cells, keratinocytes, and neutrophils [11–13]. Later, the ATP receptor P2X7 was proposed as the LL-37 receptor on monocytes [14]; however, it was unclear if LL-37 activated P2X7 through a direct or indirect mechanism. In our studies on the induction of neutrophil secondary necrosis on annexin V positive neutrophils [15], we found that among 21 cell surface receptors analyzed including FPR2 and P2X7, only CXCR2 was down-regulated when freshly isolated (annexin V negative) neutrophils were treated with LL-37. Thus, we hypothesized that LL-37 had differential effects on neutrophils depending on their apoptotic status.
In order to prove the utilization of GPCR (G protein-coupled receptor) as ligand specific receptors, it is critical to demonstrate three important properties. First, ligand treatment must cause a rapid down-regulation of the receptor in a dose dependent manner. Second, interaction with ligand should activate a rapid calcium flux that should be inhibited with GPCR specific inhibitors. Third, the binding of a radiolabeled, bona fide ligand should be inhibited by unlabeled putative ligand. If ligand induced down regulation of the receptor and induction of calcium flux does not occur within minutes, it is possible that the effect is indirect, a problem frequently encountered in assays that are performed on the hour time scale. Given the large number of GPCRs on any cell, the ligand-GPCRs interaction should not widely activate other GPCRs. In the study presented here, we demonstrate that interaction of LL-37 with CXCR2 in neutrophils meets all of three of the above criteria. Thus, our data provide evidence that LL-37 may act as a function ligand CXCR2 on human neutrophils.
RESULTS
LL-37 down-regulates surface CXCR2 expression of human neutrophils
Previously we demonstrated that out of 21 cell surface markers analyzed, including CXCR1, CXCR2, CXCR3, CCR1, CCR2, CCR3, CCR5, CCR7, TLR4, CD14, FPR2, CD88, CD125, CD32, CD64, CD15, CD66, CD11b, CD18, P2X7 and CD29, only CXCR2 was specifically down-regulated from the surface of LL-37 treated neutrophils [15]. As a first step in exploring the mechanism of CXCR2 down-regulation, we examine here the dose response and kinetics compared to CXCR1. As shown in Fig. 1A and Supporting information Fig. 1A, LL-37 (5 µM) treatment for 3h reduced neutrophil surface expression of CXCR2, but not CXCR1 by 50%. Although a further reduction was observed at 10 µM, our previous study indicates that general cytotoxicity is observed at this dosage. Since 5 µM is within the relevant range for LL-37 during infections and caused a 50% reduction in surface CXCR2 expression at 3h, the time course study was performed at this dosage. It can be seen that CXCR2 is down regulated by 50% at 3h compared to untreated controls (p<0.001), while CXCR1 was not affected by LL-37 treatment during the time course of treatment (Fig. 1B–C and Supporting infromation Fig. 1B). We also investigated the surface expression of FPR2, which like CXCR1, remained unchanged over the course of treatment with LL-37 (data not shown). These data show that LL-37 down-regulates CXCR2 in a dose and time dependent manner.
Figure 1. LL-37 down-regulates surface CXCR2 expression of human neutrophils.
5×106/mL neutrophils were incubated with LL-37 at four doses (1 µM, 2.5 µM, 5 µM, and 10 µM) for 3h or at 5 µM dose for four time points (0.5h, 1h, 2h, and 3h). The cells were washed and stained with anti-CXCR1 and anti-CXCR2 specific antibodies, analyzed by flow cytomety (GMFI, geometric mean of fluorescence intensity). (A) Dose response in 3 h stimulation. Time course of CXCR2 (B) and CXCR1 (C) expression in response to LL-37 (5 µM). Data show mean ± SE from four independent experiments. *p<0.05, **p<0.01, ***p<0.001 in comparison with CXCR2 expression of untreated control (unpaired Student’s t-test).
Down-regulation pattern of CXCR1/2 expression by LL-37 differs from fMLF, WKYMVm, or ATP treatment but is similar to CXCL1
While the above data suggest that LL-37 acts on CXCR2, it was necessary to investigate other mechanisms that may link it to the previously reported FPR2 and P2X7 receptors. Effectors may down-regulate neutrophil receptors by direct or indirect mechanisms in a process termed “inside-out” signaling. For example, TNFα and LPS stimulate neutrophils via their specific receptors, but in addition, cause down-regulation of CXCR2 and CXCR1 [16]. To investigate this possibility, neutrophils were treated with fMLF at three doses (50 nM, 100 nM, 200 nM) or with FPR2 receptor agonist WKYMVm (1 µM, 10 µM, 100 µM), and the expression of CXCR1 and CXCR2 were measured after 3h of treatment (Fig. 2A and 2B). fMLF and WKYMVm stimulation caused down-regulation of both CXCR2 and CXCR1 expression with similar kinetics. Similarly, WKYMVM treatment causes down-regulation of CXCR1 and CXCR2 (data not shown). Thus, we have shown that while LL-37 specifically down-regulates CXCR2 only, fMLF or the FPR2 agonist WKYMVm down-regulate both CXCR1 and CXCR2, most likely due to an indirect mechanism since they act via FPR2.
Figure 2. The patterns of surface CXCR1/2 expression of neutrophils treated with fMLF, WKYMVm, ATP, CXCL1, CXCL7, and CXCL8.
5×106/mL neutrophils were incubated with fMLF (A), FPR2 agonist WKYMVm (B), ATP (C), LL-37 (D), CXCL1 (E), CXCL7 (F), or CXCL8 (G) for 3h. The cells were washed and stained with anti-CXCR1-APC and anti-CXCR2-FITC (A–C and E–F) or anti-P2X7-FITC (D), and analyzed by flow cytomy (GMFI, geometric mean of fluorescence intensity). Data show mean ± SE from four independent experiments. * p<0.05 in comparison with control (D), All except (D), *p<0.05, **p<0.01, ***p<0.001 in comparison with CXCR2 expression of untreated control. #p<0.05, ##p<0.01, ###p<0.001 in comparison with CXCR1 expression of untreated control (unpaired Student’s t-test).
When neutrophils were treated with three doses of ATP (30 µM, 300 µM, 3000 µM) there was no down-regulation of either CXCR1 or CXCR2 (Fig. 2C). These results further confirm that LL-37 down-regulation of CXCR2 is a specific phenomena. Notably, our previous study [15] demonstrated that LL-37 induces ATP release from neutrophils. To extend this study, we demonstrate here that LL-37 treated neutrophils actually up-regulate the expression of P2X7 (Fig. 2D), perhaps explaining the fortuitous connection of LL-37 to P2X7 in previous studies.
Since CXCL1, CXCL7, and CXCL8 all directly interact with CXCR2, it was important to compare their effects on CXCR2 levels to LL-37. As shown in Fig. 2E, CXCL1 down-regulated CXCR2 but not CXCR1 in a limited dose-dependent manner (respectively, 1 nM, 5 nM, 10 nM), similar to LL-37 (Fig. 2D). Although similar results were obtained for CXCL7 (Fig. 2F), CXCL7 was less discriminatory towards CXCR2 compared to LL-37. Furthermore, both CXCL1 and CXCL7 are shown to down-regulate CXCR1 at higher doses (50 nM) in concordance with another report [17], whereas LL-37 does not down-regulate CXCR1 even at a dose of 10 µM. CXCL8 significantly down-regulated both CXCR1 and CXCR2 (Fig. 2G), in agreement with its reported activity towards both receptors. These results are similar to previous studies on CXCL1, CXCL7, and CXCL8 treatment of neutrophils [17–19]. We conclude that CXCR2 down-regulation by LL-37 closely resembles the activity profile of CXCL1, a predominantly CXCR2 specific chemokine.
We also explored the role of various ligands on the down-regulation of GPCRs on monocytes which express high CXCR2, low CXCR1, and high FPR2. LL-37, CXCL8, and CXCL1, but not ATP or BzATP, all down-regulate CXCR2, similar to their effect on neutrophils (Supporting information Fig. 2). Unexpectedly, WKYMVm down regulated FPR2 and CXCR2. This suggests that cross-talk between FPR2 and CXCR2 can occur in these cells, a result that may explain why some investigators suggested that LL-37 utilizes FPR2. The ability of GPCRs to cross-talk can be explained by the formation of heterodimers [20, 21].
Effect of LL-37 on calcium mobilization of neutrophils
Measurement of [Ca2+]i is a sensitive indicator of neutrophil activation as shown by various studies on fMLF, C5a, and ATP treatment of neutrophils [22]. More importantly, measurements of [Ca2+]i are the earliest (seconds to minutes) and among the most reliable indicators of GPCR activation. Since previous studies proposed both the ATP receptor P2X7 and WKYMVm receptor FPR2 as receptors for LL-37 on monocytes [9, 14], it was important to compare the calcium mobilization patterns for LL-37, ATP, fMLF, and WKYMVm, as well as CXCL1, CXCL7, and CXCL8 stimulated neutrophils. A dose response of [Ca2+]i for neutrophils treated with LL-37 (1 µM, 2.5 µM, 5 µM, 10 µM) exhibited a rapid rise followed by a slightly declining plateau region (Fig. 3A). When the [Ca2+]i patterns were compared to neutrophils treated with CXCL1, CXCL7 and CXCL8 (Fig. 3A), it can be seen that the LL-37 pattern is similar to CXCL1 and CXCL7, but dissimilar to CXCL8. The [Ca2+]i pattern of neutrophils treated with LL-37 shown here are similar to a previous study on neutrophils [23], and also to studies on endothelial cells [7] and monocytes [9] in which FPR2 was proposed as the receptor for LL-37. Thus, while our response curve agrees with previous studies, our comparative data provide new inferences, suggesting that LL-37 acts through the CXCR2 receptor shared with CXCL1 and CXCL7.
Figure 3. Effect of LL-37 on calcium mobilization of neutrophils.
A–B. Neutrophils loaded with the calcium indicator indo-1 AM were suspended in PBS with calcium and magnesium, then treated with LL-37 (red line 1 µM, blue line 2.5 µM, green line 5 µM, and orange line 10 µM), CXCL1 (10 nM), CXCL7 (10 nM), CXCL8 (10 nM), ATP (5 µM), fMLF (100 nM) or WKYMVm (5 µM). C. Neutrophils loaded with the calcium indicator indo-1 AM were suspended in PBS without calcium and magnesium, then treated with LL37 (5 µM), WKYMVm (5 µM), or ATP (5 µM). The ratio of 405:485 nm was detected as intracellular calcium mobilization. The data shown are representative of four independent experiments. Arrows indicate time point at which reagents were applied to the cells.
We next examined [Ca2+]i patterns of ATP and fMLF stimulated neutrophils. Neutrophils were first stimulated with 5 µM ATP, then after the return to baseline, with 100 nM fMLF (Fig. 3A). Both ATP and fMLF [Ca2+]i patterns exhibit a rapid rise and subsequent decline to baseline within 200s, in complete contrast to the LL-37 [Ca2+]i pattern in which LL-37 has sustained calcium mobilization over the 600s recording time. Notably, the decline to baseline for ATP or fMLF stimulated neutrophils also differ from CXCL8 (10 nM), which returns to base in less than 100s. Therefore, neither ATP nor fMLF stimulation of neutrophils mimics that of LL-37 or CXCL8 as measured by [Ca2+]i. In addition, we performed a control experiment with WKYMVm to demonstrate that the fMLF response was not due to its stimulation of other GPCRs (Fig. 3B). Since this measurement detects one of the earliest events in receptor activation, we conclude that LL-37 does not directly utilize the P2X7 or FPR2 receptors on neutrophils.
Given that the source of the Ca2+ signal is important, we also tested the response of neutrophils to LL-37, WKYMVm and ATP in the absence of extracellular Ca2+ (Fig. 3C). This study reveals that only the WKYMVm peptide is capable of generating a calcium flux response in the absence of extracellular Ca2+. These results suggest that LL-37 and ATP act via the plasma membrane Ca2+ channel, while WKYMVm acts (partially) through the ER Ca2+ channel. This is a further proof that LL-37 does not act through the FPR2 receptor.
Cross-desensitization of calcium mobilization of neutrophils by LL-37 and various ligands
Rapid successive exposure of chemokine receptors to their ligands is known to desensitize their signaling capacity. We have used heterologous desensitization of [Ca2+]i in neutrophils following ligand exposure to investigate whether ligands are signaling through the same types of chemokine receptors. The results of these cross-desensitization studies are shown in Fig. 4. Pre-exposure of neutrophils to CXCL8 (10 nM) completely blocked the response to LL-37 (5 µM), CXCL7 (10 nM), and CXCL1 (10 nM), but not to fMLF (100 nM). Notably, CXCL8 failed to cross-sensitize fMLF stimulation, a result in agreement with a previous report [16]. Similar to CXCL8, LL-37, CXCL7 and CXCL1 do not cross-sensitize fMLF stimulation (data not shown). These results demonstrate that LL-37, CXCL7, and CXCL1 share a common receptor with CXCL8. Again, there is no evidence that LL-37 acts through the FPR2 in neutrophils.
Figure 4. Cross-desensitization of neutrophils by CXCL8 and LL-37.
Neutrophils loaded with indo-1 AM were exposed to CXCL8 (10 nM). Cells were rechallenged 160 s later with a second stimulation by (A) LL-37 (5 µM), (B) CXCL7 (10 nM), (C) CXCL1 (10 nM), or (D) fMLF (100 nM). The ratio of 405:485 nm was detected as intracellular calcium mobilization. The data shown are representative of four independent experiments. Arrows indicate time point at which reagents were applied to the cells.
Since P2X7 has been suggested as a receptor for LL-37, it was also necessary to determine if ATP could cross-desensitize LL-37 stimulated calcium mobilization. As shown in Supporting information Fig. 3A–B, ATP was unable to block calcium mobilization stimulated by LL-37 in neutrophils, and vice versa. We also tested the ability of WKYMVm to cross-desensitize LL-37 stimulated calcium mobilization (Supporting information Fig. 3C–D), but in this case, WKYMVm was able to block LL-37 stimulated calcium mobilization, but not vice versa. This raises the question, does WKYMVm also block other ligands for CXCR2? Indeed, this was the case for CXCL1 (Supporting information Fig. 3E–F). These results may explain why, in some experiments, it may be concluded that LL-37 and WKYMVm share the same receptor.
Competition of LL-37 with 125I-labeled CXCL8 for binding to neutrophils
To further demonstrate that LL-37 shares a binding site with CXCL8, we performed competition experiments where increasing dosages of cold LL-37, CXCL1, or CXCL8 were used as competitors for the 1.25 nM 125I-labeled CXCL8 incubated with neutrophils. CXCL8 was chosen as the reference ligand because it binds to both CXCR1/2, can be easily iodinated and its binding to receptor has been studied extensively. According to the results shown in Fig. 5, cold LL-37, like cold CXCL1, in a dose-dependent manner was able to partly displace 125I-CXCL8 from its specific binding sites with the maximum competition of around 50%, while cold CXCL8 in a dose-dependent manner completely displaced 125I-CXCL8. Complete inhibition could not be observed due to the toxic activity of LL-37 on neutrophils beyond 10 µM, because > 10 µM LL-37 can cause cell membrane permeability allowing 125I-CXCL8 to enter cells non-specifically, in the case of the binding studies. Since LL-37 is known to bind to the phospholipids of cell membranes [24–26], the reverse inhibition experiment, namely displacement of labeled LL-37 from the neutrophil cell surface by unlabeled CXCL1 could not be performed (there is a vast excess of cell surface phospholipid over CXCR2). Based on this criterion alone, we cannot definitively state that CXCR2 acts as the only receptor for LL-37.
Figure 5. Competition of unlabelled LL-37, CXCL1, and CXCL8 with 125I-CXCL8 for binding to neutrophils.
A. Specific binding of 125I-CXCL8 to receptors on human neutrophils. Neutrophils were incubated with increasing concentrations of 125I-CXCL8. Unspecific binding was determined in the presence of an excess (400-fold molar at 40 nM CXCL8). Specific binding was calculated by subtraction of unspecific binding from total binding. B. Competition of unlabeled LL-37, CXCL1, or CXCL8 with 125I-CXCL8 for binding to neutrophils. Neutrophils were incubated with 1.25 nM 125I-CXCL8 in the presence or in the absence of increasing concentrations of unlabeled LL-37, CXCL1 or CXCL8. Data were corrected for unspecific binding, determined in the presence of a 400-fold molar excess of the unlabeled CXCL8. Values were expressed as percentage of specific binding obtained with the labeled 125I-CXCL8 from the triplicate experiments. Data show mean ± SE of triplicate experiments.
SB225002, a selective non-peptide CXCR2 antagonist, blocks LL-37-induced neutrophil calcium mobilization and migration
SB225002 is a selective non-peptide antagonist of CXCR2 that exhibits a dose dependent inhibition of [Ca2+]i and migration of neutrophils treated with CXCL1 or CXCL8 [27]. First, to further demonstrate the specificity of LL-37 to the CXCR2 receptor, we performed [Ca2+]i measurements for LL-37, CXCL7, CXCL1, CXCL8, ATP, WKYMVm, and fMLF treated neutrophils after blocking CXCR2 with SB225002. At a concentration of 10 µM, SB225002 caused more than a 90% reduction of [Ca2+]i for 5 µM LL-37 (Fig. 6A), and a complete inhibition of calcium mobilization for 10 nM CXCL7 (Fig. 6B) and 10 nM CXCL1 (Fig. 6C), but not for fMLF (Fig. 6E), ATP (Fig. 6F) or WKYMVm (Fig. 6G). CXCL8 (10 nM) stimulation was inhibited by about 50% (Fig. 6D), as expected for this chemokine that utilizes both CXCR1 and CXCR2.
Figure 6. SB225002 blocks LL-37-induced neutrophil calcium mobilization and migration.
A–G. Neutrophils were loaded with indo-1 AM and exposed to SB225002 (10 µM), a selective CXCR2 antagonist. Cells were rechallenged 180 s later with a second stimulation by A. LL-37 (5 µM), B. CXCL7 (10 nM), C. CXCL1 (10 nM), D. CXCL8 (10 nM), E. fMLF (100 nM), F. ATP (5 µM), or G. WKYMVm (5 µM). The ratio of 405:485 nm was detected as intracellular calcium mobilization. The data shown are representative of four independent experiments with similar results. Arrows indicate time point at which reagents were applied to the cells. H. SB225002 blocks LL-37-induced neutrophil migration. Chemotaxis was measured in 24-well transwell system as described in the Materials and methods. Data show mean ± SE from three independent experiments performed in quadruplicate. *p<0.05, in comparison with untreated control; # p<0.05 in comparison with 5 µM LL-37 treatment (unpaired Student’s t-test).
Second, we examined the ability of SB225002 to inhibit LL-37-induced neutrophil migration. We found that LL-37 induced neutrophil migration (over background) at a concentration of 5 µM (p<0.05), but not at 1 µM (Fig. 6H, open bars); however, pretreatment of neutrophils with 10 µM SB225002 for 20 min completely inhibited the cell migration response of neutrophils to 5 µM LL-37 (p<0.05) and did not interfere with cell migration of SB225002 treated controls (Fig. 6H, black bars).
Effects of LL-37 on HEK293/CXCR2 cells, monocytes, and THP-1 cells
In the case of CXCR2 or CXCR1 transfected HEK293 cells [28, 29], CXCL8, LL-37, and CXCL1 gave weak but similar patterns of calcium mobilization for CXCR2 transfected HEK293 cells, but only CXCL8 gave a response for CXCR1 transfected HEK293 cells (Fig. 7A and 7B). We also found that, in both CXCR1 and CXCR2 transfected HEK293 cells treated with LL-37, but not with CXCL1 results in the generation of secondary necrotic cells as demonstrated by the shift to smaller cell volumes (SSC vs FSC, Supporting information Fig. 4). We also determined the amount of secondary necrosis induced by LL-37 on fresh neutrophils, monocytes and THP-1 cells (Supporting information Fig. 4). The ability of LL-37 to cause cell secondary necrosis may complicate the interpretation of calcium mobilization experiments since when apoptotic cells are present, extra-cellular calcium will leak into necrotic cells [15, 30]. In the data reported here, we excluded apoptotic cells from the assay by gating on living cells.
Figure 7. Effect of LL-37 on calcium mobilization of HEK293/CXCR2/1 cells, THP-1 cells, and monocytes, and migration of THP-1 cells and monocytes.
A–B. HEK293/CXCR1/2 cells loaded with the calcium indicator indo-1 AM were suspended in PBS with calcium and magnesium, then treated with CXCL8, LL-37, or CXCL1. C–D. THP-1 cells (C) or monocytes (D) were loaded with indo-1 AM and exposed to the first stimulus, then rechallenged with a second stimulus. The ratio of 405:485 nm was detected as intracellular calcium mobilization. The data shown are representative of three independent experiments. Arrows indicate the time point at which reagents were applied to the cells. E–F. THP-1 (E) and monocytes (F) show difference in response to LL-37 in chemotaxis. Chemotaxis was measured in 24-well transwell system as described in the Materials and methods. Ctrl (control), CXCL1 (50 nM), LL-37 (5 µM), WK (WKYMVm 5 µM), ATP (50 µM), BzATP (50 µM). Data show mean ± SE from three independent experiments performed in quadruplicate. ***p<0.001, in comparison with untreated control; ##p<0.01 in comparison with 5µM LL-37 treatment (unpaired Student’s t-test).
Since the calcium mobilization with transfected cells is subject to the criticism that these cells do not naturally express these receptors and may not fully respond to ligands (eg, see the low calcium response for HEK293 transfected cells compared to neutrophils), we performed further calcium mobilization studies on a cell line that naturally expresses CXCR2 but not CXCR1 or FPR2, namely THP-1 cells. As shown in Fig. 7C, LL-37, but not WKYMVm gives a robust calcium signal, and pretreatment with WKYMVm does not block the LL-37 induced signal. Note: these results are in contrast to the ability of WKYMVm in neutrophils, which express FPR2 and both CXCR1 and CXCR2. ATP but not BzATP was able to partially block LL-37 induced calcium mobilization in THP-1 cells. Conversely, LL-37 was able to partially block ATP induced calcium mobilization. These results may explain why some investigators have suggested that LL-37 and ATP utilize the same receptor. However, the results of cross-desensitization with LL-37 and ATP are clearer when testing neutrophils or monocytes, both of which occur naturally in the body. In the case of monocytes, neither ATP nor WKYMVm cross-block LL-37 stimulated calcium mobilization and vice versa (Fig. 7D). Notably, THP-1 cells vs neutrophils and monocytes respond differently to LL-37 in that the Ca2+ response returns to baseline for THP-1 cells, but not for neutrophils and monocytes (Fig 3A and 7D vs 7C). This difference may be due to the differential formation of GPCR heterodimers in THP-1 cells vs neutrophils and monocytes. We also examined the ability of these ligands to induce migration of THP-1 and monocytes. LL-37, but no other ligands tested, was able to stimulate migration in monocytes, but not THP-1 cells, and this function was blocked by the CXCR2 inhibitor SB225002 (Fig. 7E–F). The relative levels of CXCR2, FPR2, and CXCR1 for neutrophils, THP-1 cells, monocytes, and HEK293/CXCR2 cells are shown in Supporting information Fig. 5. The additional experiments with THP-1 cells and monocytes further indicates that LL-37 may utilize CXCR2 and not FPR2 or P2X7.
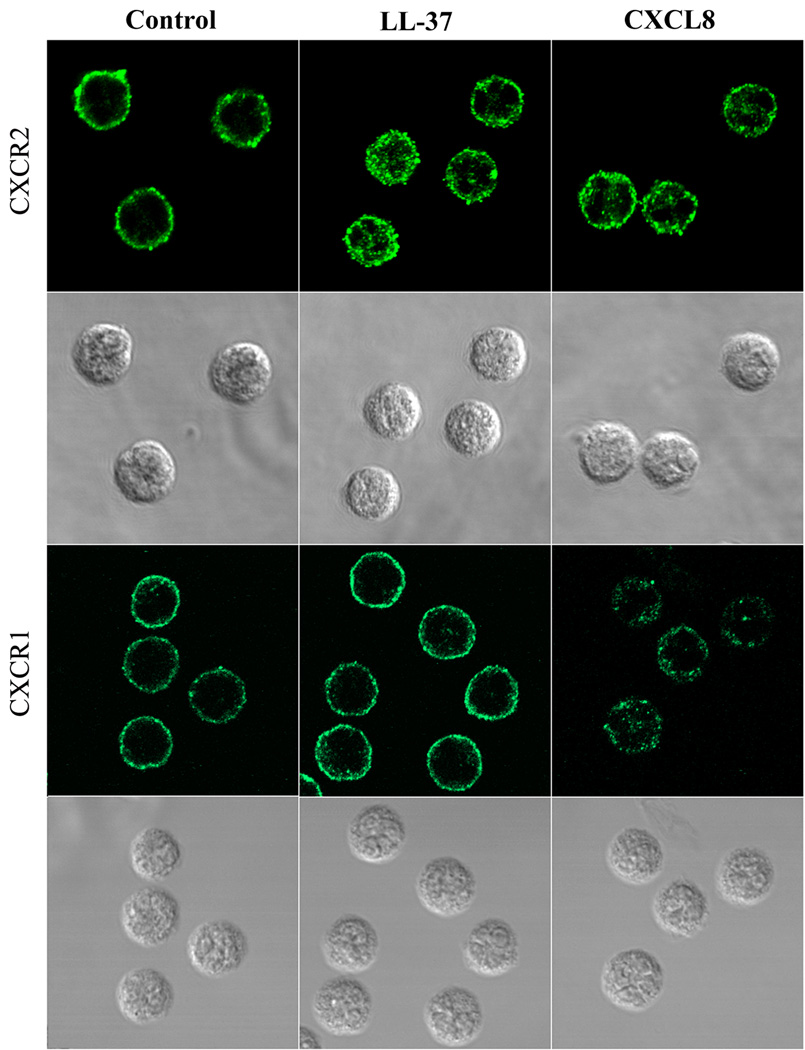
LL-37 induces CXCR2 internalization of neutrophils
Previous studies have shown that treatment of neutrophils with CXCL7 and CXCL8 reduced the cell surface levels of CXCR2 due to CXCR2 internalization [31–33]. We used confocal analysis to visualize the process of receptor internalization in neutrophils after treatment with 5 µM LL-37. As shown in Fig. 8, when untreated neutrophils were stained with antibodies to CXCR2 or CXCR1, membrane expression patterns were found; however, after treatment with 5 µM LL-37 (1 h, 37°C), translocation of CXCR2 but not CXCR1 to the cytoplasmic granules was observed. In contrast, CXCL8 treatment was able to cause translocation of both CXCR1 and CXCR2 to the cytoplasm, in agreement with its ability to bind both receptors. Similar to LL-37, treatment with CXCL7 (50 nM) or CXCL1 (50 nM) caused only CXCR2 receptor translocation to the cytoplasm (data not shown). These results provide visual evidence for CXCR2 internalization after LL-37 treatment and suggest that the mechanism of CXCR2 down-regulation by LL-37 is due to receptor internalization. We also demonstrated that LL-37 treatment of neutrophils did not block anti-CXCR2 antibodiy binding (Supporting Informatioin Fig. 6A). We further analyzed the protein levels of CXCR2 in neutrophils by western blot after treatement with LL-37 (Supporting Information Fig. 6B). The results demonstrate no changes in total amounts, suggesting that CXCR2 was not degraded after internalization.
Figure 8. Confocal analysis of CXCR2 internalization in the LL-37 treated neutrophils.
Neutrophils were incubated in the presence or absence of LL-37 (5 µM) or CXCL8 (50 nM) for 1 h. The cells were incubated with mouse anti-CXCR2 or anti-CXCR1 mAb, followed by staining with Alexa 488-conjugated rabbit anti-mouse IgG. Results are representative of three independent experiments.
DISCUSSION
Antimicrobial peptides such as LL-37 are potent effector molecules of the innate immune system and contribute to host defense and regulation of inflammation. To explain these activities, FPR2 [9], and later, the ATP receptor P2X7 [14] were proposed as LL-37 receptors on monocytes; however, these studies were later challenged for a variety of reasons [11–13]. Later, we found that LL-37 in less than 10 µM dose had a toxic effect (causing secondary necrosis) on aged or annexin V positive neutrophils [15], but had no toxic effect on freshly isolated neutrophils (annexin V negative). Thus, we found it is important to specify the apoptotic state of the analyzed neutrophils prior to dissecting the role of LL-37 on these cells. Furthermore, we considered a functional assay as primary proof that LL-37 affected neutrophils, since neutrophils themselves produce LL-37 and are cells that naturally migrate in response to natural signals. In this respect, others had shown that LL-37 induces neutrophil migration [9], and we show that this function was blocked by the CXCR2 inhibitor SB225002. Since receptor down-regulation is a hall-mark of receptor activation, especially for GPCRs, we found that out of 21 candidates only CXCR2 was down-regulated. This is especially interesting, since neutrophils utilize CXCR1/2 as major activation receptors for the structurally related chemokines CXCL8, CXCL1, and CXCL7, but they differ in their relative affinities towards CXCR1/2. Thus, CXCL8 will interact with both CXCR1/2 while CXCL1 and CXCL7 are rather CXCR2 specific [34]. The down-regulation result immediately suggested that LL-37 behaved more like CXCL1 and CXCL7 than CXCL8, since CXCR2 but not CXCR1 was down-regulated by LL-37.
As predicted, the functional profiles for LL-37 more closely resembled CXCL1 and CXCL7 than CXCL8, or for that matter, FPR2 and P2X7 for calcium mobilization. The calcium mobilization experiments are of special importance because they measure GPCR activation on the second time scale. On this time scale, there is little opportunity for cross-activation of one receptor to another. More importantly, the calcium mobilization was blocked by a CXCR2 specific inhibitor whose specificity was further demonstrated by only a partial blockade with CXCL8, which, as mentioned above, can interact with both CXCR1/2. In cross-stimulation experiments, CXCL8 was able to block calcium mobilization for LL-37, CXCL1 and CXCL7, but not for fMLF. Cross stimulation experiments are also an important part of our proof, in that they show that calcium mobilization is ligand specific across receptors (in this case CXCR1/2 vs FPR2). Thus, it is unlikely that LL-37 really interacts with FPR2 or P2X7 on neutrophils, which in turn activates CXCR1/2.
Another area of proof is provided by competition of radioligand binding to a given receptor. The binding of 125I-CXCL8 to neutrophils via the CXCR1/2 is well documented, including its inhibition of binding by unlabled CXCL8, CXCL1, and CXCL7. In general, competition experiments are better in demonstrating ligand-receptor specificity than direct binding studies, because they are more specific and sensitive. In competition experiments, LL-37 behaved like CXCL1, in a dose-dependent manner by partly displacing 125I-CXCL8 from its specific binding sites on neutrophils with a maximum competition of about 50%. However, we were not able to continue the inhibition beyond this point because we reached the toxic threshold of LL-37 for neutrophils (10 µM). Furthermore, we were unable to perform the reverse inhibition study (inhibition of labeled LL-37 by cold CXCL1) because LL-37 binds to the phospholipids of the plasma membrane [26] and there is a vast excess of phospholipid over CXCR2 in the membrane. Thus, based on this criterion alone, we cannot definitely state that LL-37 utilizes CXCR2 as its sole receptor.
Finally, the specific CXCR2 antagonist, SB225002, was able to completely block the migration of LL-37 stimulated neutrophils. While many stimuli can cause neutrophils to migrate, only CXCL1 and CXCL7 stimulated migration was completely inhibited by SB225002, and in the case of CXCL8, partially inhibited since it also utilizes CXCR1 [27]. In this assay, LL-37 behaves more like CXCL1 and CXCL7 than CXCL8, confirming its CXCR2 specificity in a functional way. It is also customary to transfect receptor negative cells with the receptor of interest and then demonstrate functionality. When we attempted to do this with CXCR2 transfected HEK293 cells, we found that these cells migrate poorly in response to CXCR2 ligands because they are epithelial cells. To support the neutrophil results, we also demonstrate that LL-37 cause monocyte migration, and monocytes are a natural cell with migratory function. This function was blocked by the CXCR2 specific antagonist SB225002, demonstrating that LL-37 may utilize CXCR2 on either neutrophils or monocytes.
The importance of CXCR1 and CXCR2 in neutrophils is well documented. They share 77% identical amino acid identity, and their genes are colocalized on chromosome 2q35 [35, 36]. While both receptors are present in similar numbers on neutrophils, CXCR2 expression appears to prevail on other leukocytes [37]. CXCR2 has a high affinity for CXCL8 and all other CXC chemokines (e.g. CXCL1,CXCL2, CXCL3, CXCL7, CXCL6, and CXCL5) that attract neutrophils, while CXCR1 has high affinity for CXCL8 and CXCL6 [33, 34]. CXCR1/2 are also found on monocytes, basophils [38], eosinophils, and T lymphocytes [39–41]. Expression of CXCR2 was reported in cultured melanocytes and fibroblasts [39], epithelial cells and smooth muscle cells [42], endothelial cells [43], mast cells [44], articular chondrocytes [45], cerebellar granule neurons [46], and carcinoma cell lines [47–49]. Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer and delayed wound healing was observed in CXCR2 knockout mice [49, 50]. Anti-CXCR2 antibodies, but not anti-CXCR1 antibodies, were found to block CXCL8-induced endothelial cell migration [51] and stress fiber assembly [52]. Furthermore, the signaling pathways regulated by CXCR2 are essential for cell proliferation, migration, and survival, which are necessary events in angiogenesis [53]. Thus, CXCR2 meets the criteria as a widely expressed receptor on leukocytes, tissue cells, and carcinoma cells, all of which would be expected to react to LL-37. In addition, CXCR1 and CXCR2 may form heterodimers [21], allowing cross-talk between these two receptors. Since the main sources of LL-37 are neutrophils and epithelial cells, one can expect that LL-37 has major effects throughout the body.
In the report proposing FPR2 as the LL-37 receptor on monocytes [9], the experimental evidence showed that LL-37 induces in vitro migration of human peripheral blood monocytes, but not immature dendritic cells, which were differentiated from monocytes. The authors reasoned that of 9 human chemotactic receptors, namely FPR1, FPR2, platelet activating factor receptor (PAFR), C5aR, CXCR4, CCR1, CCR2, CCR5, and CCR8 that have been demonstrated to be expressed by freshly isolated peripheral blood monocytes at mRNA, protein, and functional levels [38, 54, 55] only 7 (FPR1, PAFR, C5aR, CXCR4, CCR1, CCR5, and CCR8) are functionally expressed by monocyte-derived immature dendritic cells [55, 56]. Thus, they focused on CCR2 and FPR2 which are not expressed by monocyte-derived immature dendritic cells. In fact, they did not examine CXCR1/2 which are found on monocytes [39]. DCs also express detectable levels of mRNA for CXCR1/2, but it is interesting to note that although DCs express the transcripts for CXCR1/2, they do not functionally respond to these chemokines [57, 58]. In view of this, the missing piece of information regarding CXCR1/2 may have led these authors to not test these two key chemokine receptors. One more arguable result in their paper is that they used pertussis toxin (PTX), a reagent known to selectively block Gi protein–coupled signaling, as a specific probe for FPR2. However, it is well known that fMLF, LTB4, CXCL8, and CXCL6 all bind to Gi-coupled cell-surface receptors [33, 59–61], although they activate different intracellular signal cascades [62] and stimulate different cellular functions [63, 64]. Indeed, PTX is able to block the activities of CXCL8, fMLF, LTB4, CXCL6, and MNCF (macrophage-derived neutrophil chemotactic factor) in the stimulation of neutrophil migration [33, 63, 65] and FPR- and CXCR-induced oxidase activity of neutrophils [66]. These reports indicate that chemokines and other neutrophil attractants trigger directional cell movement by binding to GPCRs, and PTX, as a general GPCRs blocker, is not a specific blocker for FPR2, especially for neutrophils and monocytes. Furthermore, a report on neutrophils, in which the capacity of LL-37 to act as a potent inhibitor of spontaneous neutrophil apoptosis was demonstrated to involve signaling via G-protein-coupled receptors other than FPR2, also challenges the idea that FPR2 as the receptor of LL-37 [13].
Regarding the report that LL-37 utilized the P2X7 receptor to induce IL-1β processing and release from monocytes [14], the specific P2X7 receptor blockers (KN04, KN62, and oxATP) used in the study did not completely block the release of IL-1β, and it has been proposed that ATP itself is involved in the IL-1β secretory pathway [67]. Our data regarding CXCR2 expression and calcium mobilization by neutrophils treated with either LL-37 or ATP showed that LL-37 induces P2X7 up-regulation on neutrophils, and ATP release [15], similar to other studies on monocytes [14]. Although the requirement of ATP for IL-1β release in vivo has been questioned because of the low concentration of extracellular ATP in physiological tissues [14], LL-37 stimulated ATP release and the increased sensitivity of P2X7 up-regulation shown in our studies suggest that ATP may indeed be a physiological regulator of IL-1β release.
In summary, we provide evidence that LL-37 may act as a functional ligand of the CXCR2 receptor on human neutrophils. Given the expression of CXCR2 in a multitude of cells with diverse functions and the expression of LL-37 in both neutrophils and epithelial cells, this finding provides a new opportunity for further research of human LL-37 function in physiologically relevant cells.
MATERIALS AND METHODS
Reagents
Recombinant human CXCL8, CXCL7, CXCL1, ATP, BzATP, fMLF, WKYMVm (FPR2 agonist), saponin, and anti-purinergic receptor P2X7-FITC polyclonal antibody were purchased from Sigma-Aldrich (St. Louis, MO 63103, USA). Anti-CXCR1-APC (clone 5A12), anti-CXCR2-FITC (Clone 6C6), anti-CD16-PerCP-Cy5.5 (Clone 3G8), anti-CD14-FITC (M5E2), isotype controls, and 24-well Transwell System (3-µm pore size) were from BD Bioscences (Chicago, IL 60673, USA), anti-CXCR1 (Clone 501) and anti-CXCR2 (Clone 19) monoclonal antibody were from Cell Sciences Inc (Sharon, MA 02067, USA), CFSE (carboxy fluorescein diacetate succimidyl ester) and Indo-1-AM were from Molecular Probes (Eugene, OR 97402, USA), FBS and human serum were from Irvine scientific (Santa Ana, CA 92705, USA), anti-FPR2-PE (Clone 904405) was from R&D Systems Inc (Minneapolis, MN 55413, USA), Alexa Fluor 488 goat anti-mouse IgG (H+L) was from Invitrogen (Chicago, IL 60693, USA), and Hard Set mounting medium with DAPI was from Vector Laboratories Inc (Burlingame, CA 94010, USA). LL-37 was synthesized by N-(9-fluorenyl) methoxycarbonyl chemistry at the Nucleic Acid/Protein Service unit at the City of Hope Medical Center. Peptides were purified by reverse-phase high-performance liquid chromatography to at least 98% purity and were LPS-free as analyzed by Limulus Amebocyte Lysate from Cambrex Bio Science Walkersville Inc (Walkersville, MD 21793, USA). LL-37 was dissolved in endotoxin-free water from B. Braun Medical Inc (Philadelphia, PA 19178, USA) and stored at −20°C until further use. The concentration of the peptides in solution was determined by amino acid analysis. All reagents were tested to ensure that they were free of endotoxin and reconstituted in endotoxin-free water.
Cell preparation
This study was approved by the Institutional Human Subject’s Review Board (City of Hope National Medical Center). Neutrophils were isolated from citrated blood by dextran sedimentation of erythrocytes, followed by centrifugation over Ficoll-Paque Plus (GE healthcare biosciences, Pittsburgh, PA 15264) density gradient. Cell purities were determined by forward light scatter/side light scatter gating of cells stained with PerCP-Cy5.5-conjugated anti-CD16 mAb using a flow cytometer (FACSCaliber, BD Biosciences, San Jose, CA 95131, USA). Neutrophils were defined as CD16 positive cell and the purity of neutrophils were more than 95%. Neutrophils were suspended at 5×106 cells/mL in RPMI 1640 medium supplement with 1% FBS (FBS contained < 5 pg/100 mL LPS).
Monocytes were collected and separated using Monocyte Isolation Kit II (Miltenyi Biotec Inc., Auburn, CA 95602, USA) from peripheral blood mononuclear cells. The purified monocytes were stained with anti-CD14-FITC and checked by using flow cytometry FACSCalibur. HEK293-CXCR2 and HEK293-CXCR1 cell lines were previously described [28], THP-1 cells were purchased from ATCC (Manassas, VA 20108, USA).
Determination of receptor modulation by flow cytometry analysis
Neutrophils at 5×106 cells/mL were treated with 5 µM LL-37 for 0.5h, 1h, 2h, 3h, or LL-37 (1 µM, 2.5 µM, 5 µM, 10 µM), fMLF (50 nM, 100 nM, 200 nM), ATP (30 µM, 300 µM, 3000 µM), CXCL8 (1 nM, 5 nM, 10 nM, 50 nM), CXCL7 (1 nM, 5 nM, 10 nM, 50 nM), and CXCL1 (1 nM, 5 nM, 10 nM, 50 nM) for 3h, washed with PBS, blocked with 10% human serum in PBS, stained with anti-CXCR1-APC, anti-CXCR2-FITC, and anti-CD16-PerCP-Cy5.5 or anti-P2X7-FITC and anti-CD16-PerCP-Cy5.5, and analyzed on a FACSCalibur using FLOWJO software. Expression of neutrophil surface receptors were shown as geometric mean of fluorescence intensity (GMFI).
Calcium mobilization assay
Neutrophils, monocytes, THP-1 cells all at 107 /mL were loaded with 5 µM Indo-1-AM (Molecular Probes, Eugene, OR) for 7 min at 37 °C and kept at room temperature for an additional 10 min. HEK293/CXCR2 cells or HEK293/CXCR1 cells were loaded with 5 µM Indo-AM in 5 µM pluronic F-127/PBS for 30 min at RT. Cells were washed with a 5-fold excess of cold PBS and sedimented at 200 × g for 10 min at 4 °C. Flow cytometric measurements of intracellular Ca2+ was performed using a MoFlo MLS (Cytomation Inc, Fort Collins, CO 80525). Samples were excited at 351 nm and emission fluorescence was recorded at both 405/20 and 485/20 nm for Indo-1 AM. FACS analyses were performed on suspensions of 2 × l06 cells/mL in KRP (Krebs Ringer phosphate buffer: PBS containing 1.5 mM CaCl2 and 1.5 mM MgSO4). In some cases CaCl2 and MgSO4 were omitted. Base-line fluorescence was determined and cells were then stimulated with LL-37 (5 µM), CXCL8 (10 nM), CXCL7 (10 nM), CXCL1 (10 nM), fMLF (100 nM), ATP (5 µM), WKYMVm (5 µM), BzATP (50 µM), and SB225002 (10 µM) were added to suspended cells and monitored as described [68]. The ratio of 405:485 nm was detected as intracellular calcium mobilization ([Ca2+]i).
Iodination of CXCL8 and competition assay
CXCL8 was iodinated by the iodogen method to a specific activity of 0.216 µCi/pmole. Neutrophils were suspended at 1 × 107 cells/mL in assay buffer (D-PBS supplemented with 0.2% BSA). The binding curve was established by measuring CPM vs increasing amounts of 125I-CXCL8. For inhibition studies, triplicate samples of 2 × 106 cells were incubated on ice for 2h at various concentrations in the presence or in the absence of unlabeled competitors (LL-37, CXCL1, or CXCL8) with 1.25 nM iodinated CXCL8 in a total volume of 200 µL. The bulk of unspecifically bound material was removed by three washing steps after adding 600 µL of assay buffer to each tube, followed by centrifugation at 2000 rpm for 3 min at 4°C. Finally, the cells were suspended in 100 µL of assay buffer and layered on top of 400 µL of D-PBS, containing 10% sucrose. Cell-bound radioactivity was determined in a gamma counter. Nonspecific binding was determined by incubation parallel samples in the presence of a 400-fold molar excess of unlabeled CXCL8. Values were expressed as percentage of specific binding obtained with the labeled 125I-CXCL8 [69].
Cell migration assay
Neutrophils, monocytes, THP-1 cells were labeled with 5 µM CFSE for 5 min at room temperature in PBS. After two washes, neutrophils, monocytes, or THP-1 cells were suspended respectively in migration medium (RPMI 1640 without phenol red, supplemented with 100 U/ml penicillin, 100 mg/ml streptomycin, 1 mM L-glutamine, and 1% FBS) with a cell concentration of 5 × 106/ mL for neutrophils, and 2 × 106/mL for monocytes, and THP-1 cells.
Migration of neutrophils, monocytes, or THP-1 cells was quantified using the 24-well Transwell System (3-µm pore size). Briefly, the lower compartment of the chamber was loaded with 1% FBS RPMI 1640 medium containing LL-37 (1 µM or 5 µM), CXCL8 (10 nM), CXCL1 (50 nM), WKYMVm (5 µM), BzATP (50 µM), ATP (50 µM) or not, while the upper compartment of the chamber was loaded with CFSE (5 μM) labeled neutrophils, monocytes, or THP-1 cells in quadruplicate without or with preincubation 20 min of SB225002 (10 µM). After incubated at 37°C for 1 h for neutrophils, 90 min for monocytes, or 2 h for THP-1 cells, the inserts were removed by gently scraping the bottom surfaces of the filters several times to dislodge the adherent cells. Cell numbers in the lower wells were measured at 495 nm on the Victor V plate reader (PerkinElmer, Woodbridge, Canada) according to standard curve.
Confocal analyses of receptor down-modulation
Neutrophils at 5×106 /mL were incubated in the absence, or in the presence of LL-37 (5 µM), CXCL8 (50 nM), CXCL7 (50 nM), or CXCL1 (50 nM) for 60 min at 37°C. The cells were washed in PBS, fixed with 2% paraformaldehyde for 15 min. Permeabilized cells were obtained by incubating the cells with 0.01% saponin for 30 min, followed by blocking with 10% human serum and 0.01% saponin (Sigma) in PBS for 30 min. After washing the cells in washing buffer (0.01% saponin in PBS), the cells were stained with monoclonal anti-human CXCR1 or CXCR2 for 30 min at 4°C. Following additional washing, the cells were stained with Alexa Fluor 488-conjugated goat anti-mouse IgG (H+L) for 30 min. After washing, stained cells were mounted with Hard Set mounting medium with DAPI and analyzed using a Carl Zeiss confocal laser scanning microscope (Carl Zeiss Inc, Thornwood, NY). Zeiss inverted LSM 510 META-2-Photon Microscope was equipped with an argon laser (458, 488, 514 nm), HeNe 1 Laser 543 nm, HeNe 2 Laser 633 nm, tunable IR Ti-Sapphire Pulse Laser 700–950 nm for DAPI, and transmitted light mode. A 63 × NA/1.2 C-apochromat water-immersion lens was used for all imaging.
Statistical analysis
Assay results are expressed as means ± SE and unpaired Student’s t-tests were used for comparisons. All p values are two-sided. Data were analyzed with SPSS software (release 10.0, SPSS, Chicago, IL) and GraphPad Prism software (version 5.0, GraphPad Software, San Diego, CA).
Supplementary Material
Acknowledgement
We thank Tung Nguyen, Dr. Christine Chen, and Ian Zhang in our laboratory for their support, Lucy Brown, Claudio Spalla, and Alexander Spalla in the analytical cytometry core for intracellular calcium mobilization analysis, Mariko Lee in electron microscopy core for confocal microscopy, Bruce Kaplan in synthetic and biopolymer chemistry core for peptide synthesis. This work was partially supported by NIH grant CA84202.
Abbreviations
- hCAP18
Human CAP-18
- [Ca2+]i
intracellular calcium mobilization
- CFSE
carboxy fluorescein diacetate succimidyl ester
- GPCR
G protein-coupled receptor
Footnotes
Conflict of Interest: The authors declare no financial or commercial conflict of interest.
REFERENCES
- 1.Sorensen O, Arnljots K, Cowland JB, Bainton DF, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–2803. [PubMed] [Google Scholar]
- 2.Frohm Nilsson M, Sandstedt B, Sorensen O, Weber G, Borregaard N, Stahle-Backdahl M. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect Immun. 1999;67:2561–2566. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agerberth B, Charo J, Werr J, Olsson B, Idali F, Lindbom L, Kiessling R, Jornvall H, Wigzell H, Gudmundsson GH. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96:3086–3093. [PubMed] [Google Scholar]
- 4.Sorensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS, Hiemstra PS, Borregaard N. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–3959. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
- 5.Larrick JW, Hirata M, Balint RF, Lee J, Zhong J, Wright SC. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169:3883–3891. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- 7.Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, Issbrucker K, Unterberger P, Zaiou M, Lebherz C, Karl A, Raake P, Pfosser A, Boekstegers P, Welsch U, Hiemstra PS, Vogelmeier C, Gallo RL, Clauss M, Bals R. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heilborn JD, Nilsson MF, Kratz G, Weber G, Sorensen O, Borregaard N, Stahle-Backdahl M. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120:379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 9.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niyonsaba F, Iwabuchi K, Someya A, Hirata M, Matsuda H, Ogawa H, Nagaoka I. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology. 2002;106:20–26. doi: 10.1046/j.1365-2567.2002.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sorensen OE, Borregaard N, Rabe KF, Hiemstra PS. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol. 2003;171:6690–6696. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- 12.Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y, Yahata Y, Dai X, Tohyama M, Nagai H, Yang L, Higashiyama S, Yoshimura A, Sugai M, Hashimoto K. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol. 2005;175:4662–4668. doi: 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- 13.Barlow PG, Li Y, Wilkinson TS, Bowdish DM, Lau YE, Cosseau C, Haslett C, Simpson AJ, Hancock RE, Davidson DJ. The human cationic host defense peptide LL-37 mediates contrasting effects on apoptotic pathways in different primary cells of the innate immune system. J Leukoc Biol. 2006;80:509–520. doi: 10.1189/jlb.1005560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Z, Cherryholmes G, Shively JE. Neutrophil secondary necrosis is induced by LL-37 derived from cathelicidin. J Leukoc Biol. 2008;84:780–788. doi: 10.1189/jlb.0208086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khandaker MH, Mitchell G, Xu L, Andrews JD, Singh R, Leung H, Madrenas J, Ferguson SS, Feldman RD, Kelvin DJ. Metalloproteinases are involved in lipopolysaccharide- and tumor necrosis factor-alpha-mediated regulation of CXCR1 and CXCR2 chemokine receptor expression. Blood. 1999;93:2173–2185. [PubMed] [Google Scholar]
- 17.Ludwig A, Petersen F, Zahn S, Gotze O, Schroder JM, Flad HD, Brandt E. The CXC-chemokine neutrophil-activating peptide-2 induces two distinct optima of neutrophil chemotaxis by differential interaction with interleukin-8 receptors CXCR-1 and CXCR-2. Blood. 1997;90:4588–4597. [PubMed] [Google Scholar]
- 18.Ehlert JE, Ludwig A, Grimm TA, Lindner B, Flad HD, Brandt E. Down-regulation of neutrophil functions by the ELR(+) CXC chemokine platelet basic protein. Blood. 2000;96:2965–2972. [PubMed] [Google Scholar]
- 19.Chuntharapai A, Kim KJ. Regulation of the expression of IL-8 receptor A/B by IL-8: possible functions of each receptor. J Immunol. 1995;155:2587–2594. [PubMed] [Google Scholar]
- 20.Parenty G, Appelbe S, Milligan G. CXCR2 chemokine receptor antagonism enhances DOP opioid receptor function via allosteric regulation of the CXCR2-DOP receptor heterodimer. Biochem J. 2008;412:245–256. doi: 10.1042/BJ20071689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson S, Wilkinson G, Milligan G. The CXCR1 and CXCR2 receptors form constitutive homo- and heterodimers selectively and with equal apparent affinities. J Biol Chem. 2005;280:28663–28674. doi: 10.1074/jbc.M413475200. [DOI] [PubMed] [Google Scholar]
- 22.Hagenlocker BE, Walker BA, Ward PA. Superoxide responses of immune complex-stimulated rat alveolar macrophages. Intracellular calcium and priming. J Immunol. 1990;144:3898–3906. [PubMed] [Google Scholar]
- 23.Zheng Y, Niyonsaba F, Ushio H, Nagaoka I, Ikeda S, Okumura K, Ogawa H. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br J Dermatol. 2007;157:1124–1131. doi: 10.1111/j.1365-2133.2007.08196.x. [DOI] [PubMed] [Google Scholar]
- 24.Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem J. 1999;341(Pt 3):501–513. [PMC free article] [PubMed] [Google Scholar]
- 25.Henzler-Wildman KA, Martinez GV, Brown MF, Ramamoorthy A. Perturbation of the hydrophobic core of lipid bilayers by the human antimicrobial peptide LL-37. Biochemistry. 2004;43:8459–8469. doi: 10.1021/bi036284s. [DOI] [PubMed] [Google Scholar]
- 26.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 27.White JR, Lee JM, Young PR, Hertzberg RP, Jurewicz AJ, Chaikin MA, Widdowson K, Foley JJ, Martin LD, Griswold DE, Sarau HM. Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J Biol Chem. 1998;273:10095–10098. doi: 10.1074/jbc.273.17.10095. [DOI] [PubMed] [Google Scholar]
- 28.Zhao M, Wimmer A, Trieu K, Discipio RG, Schraufstatter IU. Arrestin regulates MAPK activation and prevents NADPH oxidase-dependent death of cells expressing CXCR2. J Biol Chem. 2004;279:49259–49267. doi: 10.1074/jbc.M405118200. [DOI] [PubMed] [Google Scholar]
- 29.Schraufstatter IU, Trieu K, Zhao M, Rose DM, Terkeltaub RA, Burger M. IL-8-mediated cell migration in endothelial cells depends on cathepsin B activity and transactivation of the epidermal growth factor receptor. J Immunol. 2003;171:6714–6722. doi: 10.4049/jimmunol.171.12.6714. [DOI] [PubMed] [Google Scholar]
- 30.Bjorstad A, Askarieh G, Brown KL, Christenson K, Forsman H, Onnheim K, Li HN, Teneberg S, Maier O, Hoekstra D, Dahlgren C, Davidson DJ, Bylund J. The host defense peptide LL-37 selectively permeabilizes apoptotic leukocytes. Antimicrob Agents Chemother. 2009;53:1027–1038. doi: 10.1128/AAC.01310-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose JJ, Foley JF, Murphy PM, Venkatesan S. On the mechanism and significance of ligand-induced internalization of human neutrophil chemokine receptors CXCR1 and CXCR2. J Biol Chem. 2004;279:24372–24386. doi: 10.1074/jbc.M401364200. [DOI] [PubMed] [Google Scholar]
- 32.Zaslaver A, Feniger-Barish R, Ben-Baruch A. Actin filaments are involved in the regulation of trafficking of two closely related chemokine receptors, CXCR1 and CXCR2. J Immunol. 2001;166:1272–1284. doi: 10.4049/jimmunol.166.2.1272. [DOI] [PubMed] [Google Scholar]
- 33.Feniger-Barish R, Belkin D, Zaslaver A, Gal S, Dori M, Ran M, Ben-Baruch A. GCP-2-induced internalization of IL-8 receptors: hierarchical relationships between GCP-2 and other ELR(+)-CXC chemokines and mechanisms regulating CXCR2 internalization and recycling. Blood. 2000;95:1551–1559. [PubMed] [Google Scholar]
- 34.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 35.Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278–1280. [PubMed] [Google Scholar]
- 36.Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253:1280–1283. [PubMed] [Google Scholar]
- 37.Chuntharapai A, Lee J, Hebert CA, Kim KJ. Monoclonal antibodies detect different distribution patterns of IL-8 receptor A and IL-8 receptor B on human peripheral blood leukocytes. J Immunol. 1994;153:5682–5688. [PubMed] [Google Scholar]
- 38.Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 39.Moser B, Barella L, Mattei S, Schumacher C, Boulay F, Colombo MP, Baggiolini M. Expression of transcripts for two interleukin 8 receptors in human phagocytes, lymphocytes and melanoma cells. Biochem J. 1993;294(Pt 1):285–292. doi: 10.1042/bj2940285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu L, Kelvin DJ, Ye GQ, Taub DD, Ben-Baruch A, Oppenheim JJ, Wang JM. Modulation of IL-8 receptor expression on purified human T lymphocytes is associated with changed chemotactic responses to IL-8. J Leukoc Biol. 1995;57:335–342. doi: 10.1002/jlb.57.2.335. [DOI] [PubMed] [Google Scholar]
- 41.Qin S, LaRosa G, Campbell JJ, Smith-Heath H, Kassam N, Shi X, Zeng L, Buthcher EC, Mackay CR. Expression of monocyte chemoattractant protein-1 and interleukin-8 receptors on subsets of T cells: correlation with transendothelial chemotactic potential. Eur J Immunol. 1996;26:640–647. doi: 10.1002/eji.1830260320. [DOI] [PubMed] [Google Scholar]
- 42.Nanney LB, Mueller SG, Bueno R, Peiper SC, Richmond A. Distributions of melanoma growth stimulatory activity of growth-regulated gene and the interleukin-8 receptor B in human wound repair. Am J Pathol. 1995;147:1248–1260. [PMC free article] [PubMed] [Google Scholar]
- 43.Schraufstatter IU, Chung J, Burger M. IL-8 activates endothelial cell CXCR1 and CXCR2 through Rho and Rac signaling pathways. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1094–L1103. doi: 10.1152/ajplung.2001.280.6.L1094. [DOI] [PubMed] [Google Scholar]
- 44.Lippert U, Artuc M, Grutzkau A, Moller A, Kenderessy-Szabo A, Schadendorf D, Norgauer J, Hartmann K, Schweitzer-Stenner R, Zuberbier T, Henz BM, Kruger-Krasagakes S. Expression and functional activity of the IL-8 receptor type CXCR1 and CXCR2 on human mast cells. J Immunol. 1998;161:2600–2608. [PubMed] [Google Scholar]
- 45.Merz D, Liu R, Johnson K, Terkeltaub R. IL-8/CXCL8 and growth-related oncogene alpha/CXCL1 induce chondrocyte hypertrophic differentiation. J Immunol. 2003;171:4406–4415. doi: 10.4049/jimmunol.171.8.4406. [DOI] [PubMed] [Google Scholar]
- 46.Limatola C, Ciotti MT, Mercanti D, Santoni A, Eusebi F. Signaling pathways activated by chemokine receptor CXCR2 and AMPA-type glutamate receptors and involvement in granule cells survival. J Neuroimmunol. 2002;123:9–17. doi: 10.1016/s0165-5728(01)00472-6. [DOI] [PubMed] [Google Scholar]
- 47.Metzner B, Hofmann C, Heinemann C, Zimpfer U, Schraufstatter I, Schopf E, Norgauer J. Overexpression of CXC-chemokines and CXC-chemokine receptor type II constitute an autocrine growth mechanism in the epidermoid carcinoma cells KB and A431. Oncol Rep. 1999;6:1405–1410. doi: 10.3892/or.6.6.1405. [DOI] [PubMed] [Google Scholar]
- 48.Li A, Varney ML, Singh RK. Expression of interleukin 8 and its receptors in human colon carcinoma cells with different metastatic potentials. Clin Cancer Res. 2001;7:3298–3304. [PubMed] [Google Scholar]
- 49.Keane MP, Belperio JA, Xue YY, Burdick MD, Strieter RM. Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer. J Immunol. 2004;172:2853–2860. doi: 10.4049/jimmunol.172.5.2853. [DOI] [PubMed] [Google Scholar]
- 50.Devalaraja RM, Nanney LB, Du J, Qian Q, Yu Y, Devalaraja MN, Richmond A. Delayed wound healing in CXCR2 knockout mice. J Invest Dermatol. 2000;115:234–244. doi: 10.1046/j.1523-1747.2000.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY, Buechi L, Walz A, Richmond A, Strieter RM. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol. 2000;165:5269–5277. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- 52.Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, Otterson MF, Ota DM, Lugering N, Domschke W, Binion DG. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508–8515. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- 53.Shyamala V, Khoja H. Interleukin-8 receptors R1 and R2 activate mitogen-activated protein kinases and induce c-fos, independent of Ras and Raf-1 in Chinese hamster ovary cells. Biochemistry. 1998;37:15918–15924. doi: 10.1021/bi9811415. [DOI] [PubMed] [Google Scholar]
- 54.Zlotnik A, Morales J, Hedrick JA. Recent advances in chemokines and chemokine receptors. Crit Rev Immunol. 1999;19:1–47. [PubMed] [Google Scholar]
- 55.Sozzani S, Allavena P, Vecchi A, Mantovani A. The role of chemokines in the regulation of dendritic cell trafficking. J Leukoc Biol. 1999;66:1–9. doi: 10.1002/jlb.66.1.1. [DOI] [PubMed] [Google Scholar]
- 56.Yang D, Chen Q, Stoll S, Chen X, Howard OM, Oppenheim JJ. Differential regulation of responsiveness to fMLP and C5a upon dendritic cell maturation: correlation with receptor expression. J Immunol. 2000;165:2694–2702. doi: 10.4049/jimmunol.165.5.2694. [DOI] [PubMed] [Google Scholar]
- 57.Sozzani S, Luini W, Borsatti A, Polentarutti N, Zhou D, Piemonti L, D'Amico G, Power CA, Wells TN, Gobbi M, Allavena P, Mantovani A. Receptor expression and responsiveness of human dendritic cells to a defined set of CC and CXC chemokines. J Immunol. 1997;159:1993–2000. [PubMed] [Google Scholar]
- 58.Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, Qin S, Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 59.Damaj BB, McColl SR, Mahana W, Crouch MF, Naccache PH. Physical association of Gi2alpha with interleukin-8 receptors. J Biol Chem. 1996;271:12783–12789. doi: 10.1074/jbc.271.22.12783. [DOI] [PubMed] [Google Scholar]
- 60.Wenzel-Seifert K, Hurt CM, Seifert R. High constitutive activity of the human formyl peptide receptor. J Biol Chem. 1998;273:24181–24189. doi: 10.1074/jbc.273.37.24181. [DOI] [PubMed] [Google Scholar]
- 61.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 62.Huang R, Lian JP, Robinson D, Badwey JA. Neutrophils stimulated with a variety of chemoattractants exhibit rapid activation of p21-activated kinases (Paks): separate signals are required for activation and inactivation of paks. Mol Cell Biol. 1998;18:7130–7138. doi: 10.1128/mcb.18.12.7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berger M, Budhu S, Lu E, Li Y, Loike D, Silverstein SC, Loike JD. Different G(i)-coupled chemoattractant receptors signal qualitatively different functions in human neutrophils. J Leukoc Biol. 2002;71:798–806. [PubMed] [Google Scholar]
- 64.Loike JD, Cao L, Budhu S, Marcantonio EE, El Khoury J, Hoffman S, Yednock TA, Silverstein SC. Differential regulation of beta1 integrins by chemoattractants regulates neutrophil migration through fibrin. J Cell Biol. 1999;144:1047–1056. doi: 10.1083/jcb.144.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moreno AN, Pereira-da-Silva G, Oliver C, Jamur MC, Panunto-Castelo A, Roque-Barreira MC. The macrophage-derived lectin, MNCF, activates neutrophil migration through a pertussis toxin-sensitive pathway. J Histochem Cytochem. 2005;53:715–723. doi: 10.1369/jhc.4A6562.2005. [DOI] [PubMed] [Google Scholar]
- 66.Fu H, Bylund J, Karlsson A, Pellme S, Dahlgren C. The mechanism for activation of the neutrophil NADPH-oxidase by the peptides formyl-Met-Leu-Phe and Trp-Lys-Tyr-Met-Val-Met differs from that for interleukin-8. Immunology. 2004;112:201–210. doi: 10.1111/j.1365-2567.2004.01884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meshulam T, Herscovitz H, Casavant D, Bernardo J, Roman R, Haugland RP, Strohmeier GS, Diamond RD, Simons ER. Flow cytometric kinetic measurements of neutrophil phospholipase A activation. J Biol Chem. 1992;267:21465–21470. [PubMed] [Google Scholar]
- 69.Petersen F, Flad HD, Brandt E. Neutrophil-activating peptides NAP-2 and IL-8 bind to the same sites on neutrophils but interact in different ways. Discrepancies in binding affinities, receptor densities, and biologic effects. J Immunol. 1994;152:2467–2478. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.