Abstract
Every plant species examined to date harbors endophytic fungi within its asymptomatic aerial tissues, such that endophytes represent a ubiquitous, yet cryptic, component of terrestrial plant communities. Fungal endophytes associated with leaves of woody angiosperms are especially diverse; yet, fundamental aspects of their interactions with hosts are unknown. In contrast to the relatively species-poor endophytes that are vertically transmitted and act as defensive mutualists of some temperate grasses, the diverse, horizontally transmitted endophytes of woody angiosperms are thought to contribute little to host defense. Here, we document high diversity, spatial structure, and host affinity among foliar endophytes associated with a tropical tree (Theobroma cacao, Malvaceae) across lowland Panama. We then show that inoculation of endophyte-free leaves with endophytes isolated frequently from naturally infected, asymptomatic hosts significantly decreases both leaf necrosis and leaf mortality when T. cacao seedlings are challenged with a major pathogen (Phytophthora sp.). In contrast to reports of fungal inoculation inducing systemic defense, we found that protection was primarily localized to endophyte-infected tissues. Further, endophyte-mediated protection was greater in mature leaves, which bear less intrinsic defense against fungal pathogens than do young leaves. In vitro studies suggest that host affinity is mediated by leaf chemistry, and that protection may be mediated by direct interactions of endophytes with foliar pathogens. Together, these data demonstrate the capacity of diverse, horizontally transmitted endophytes of woody angiosperms to play an important but previously unappreciated role in host defense.
Symbiotic associations between fungi and photosynthetic organisms are both ancient and ubiquitous (1, 2). Through interactions spanning mutualism to antagonism, fungi associated with living plants shape both the diversity and species composition of terrestrial communities (3–5). Yet, ecological interactions with hosts have been catalogued for only an extreme minority (≪5%) of the 1.5 million species of fungi thought to exist (6), with most research focusing on plant pathogens and mycorrhizal fungi in temperate and agricultural systems (e.g., refs. 7 and 8). In particular, interactions of plants with fungal endophytes, fungi that colonize and grow asymptomatically within healthy aerial tissues of all plants sampled to date (e.g., mosses, liverworts, ferns, conifers, and angiosperms; refs. 9 and 10), are poorly known.
Among the best-studied endophytes are intercellular symbionts in the ascomycotan family Clavicipitaceae, which grow within above-ground tissues of many cool-season grasses in the temperate zone. Asexual clavicipitaceous endophytes are transmitted vertically from maternal plants to offspring and grow systemically throughout leaves and stems, with single genotypes typically infecting individual plants (11). Apparent host benefits include improved tolerance to heavy metals, increased drought resistance, reduced herbivory, systemic resistance against pathogens, and generally enhanced growth (11–14). Consistent with much theory regarding species interactions (15–18), these vertically transmitted and relatively nondiverse endophytes thus form mutualistic associations with their hosts, and are often cited as evidence for the general importance of microbial symbioses in defensive mutualisms (e.g., ref. 19, but see ref. 20).
In contrast, endophytes associated with healthy leaves of woody angiosperms are poorly known, despite their presence in aerial tissues of all trees and shrubs sampled thus far (9, 10, 21). Unlike the clavicipitaceous endophytes of grasses, endophytes associated with foliage of woody plants appear to be transmitted horizontally (22–24). Leaves accumulate numerous infections shortly after emergence by means of epiphytic germination of fungal propagules, followed by cuticular penetration or entry through stomates (23–25). Endophytes associated with woody angiosperms may be highly diverse within individual host plants, especially in tropical forests: tropical endophytes represent no fewer than five classes of Ascomycota, with ≈3–20 species often coexisting as highly localized infections within individual leaves (25, 26). Many endophytes of woody plants appear to be closely related to pathogens (14) and evidence for defensive mutualism with regard to herbivores or abiotic stress is rare (27). Therefore, it is generally thought that endophytes associated with leaves of woody angiosperms are unlikely to play protective or mutualistic roles with regard to the host plants they inhabit (28).
Here, we present evidence that fungal endophytes associated with a woody angiosperm reduce leaf damage and loss due to a major pathogen. We first use extensive field surveys in lowland Panama to characterize the diversity, spatial structure, and host affinity of natural endophyte infections in the economically important rainforest tree, Theobroma cacao (Malvaceae). We then demonstrate that inoculation of leaf tissues by an assemblage of endophytes frequently isolated from naturally infected, asymptomatic hosts significantly reduces damage by an important foliar pathogen (Phytophthora sp.). Coupled with the ecological context afforded by field surveys and in vitro experiments, the antipathogen protection documented here demonstrates the capacity of diverse, horizontally transmitted, and ubiquitous endophytes to play a previously unappreciated but important role in host plant defense.
Materials and Methods
Study Taxa and Sites. Fungal endophytes comprise a diverse group of primarily ascomycetous fungi that inhabit living plant tissues without inducing symptoms of disease (9). In lowland forest in Panama, endophytes have been recovered from every mature leaf sampled to date among woody species representing several major lineages of angiosperms (i.e., basal angiosperms, basal Eudicots, Eurosids I, Eurosids II, basal Asterids, and Asterids I; n = 9 leaves per species for 28 species in 14 orders; ref. 25). In these hosts, endophytes frequently occur in densities approaching one endophyte isolate per each 2 mm2 of mature leaf tissue (25, 26).
Theobroma cacao (Malvaceae, Malvales) is a small tree native to forests of north-central South America (29). Now cultivated pantropically for cocoa production, T. cacao (cacao) is well suited to conservation-based agriculture, provided that natural enemies can be kept in check (e.g., Phytophthora spp., Oomycota; refs. 30 and 31).
To assess the endophyte community associated with T. cacao, we surveyed mature individuals in five lowland sites with mixed forest cover across the Isthmus of Panama [Instituto Nacional de Agricultura, Herrera, Panama (INA), Nombre de Dios, Colón, Panama (ND), near Almirante, Bocas del Toro, Panama (BT), Parque Nacional Soberanía, Panama (PNS), and Barro Colorado Island (BCI)]. Distances between sites range from 20 (BCI and PNS) to 325 km (ND and BT). All experimental assays were carried out at BCI (32).
Diversity, Spatial Structure, and Host Affinity of Endophytes Associated with T. cacao. From each of three individuals per site, we collected three young leaves (5–15 days old), three mature leaves (15–30 days old; toughened cuticles and fully expanded), and three old leaves (>60 days old). Focal leaves were chosen randomly from foliage lacking damage by herbivores or pathogens. Within 24 h, 16 segments (each 2 mm2) were cut from the middle lamina of each leaf and surface-sterilized through sequential immersion in 0.5% sodium hypochlorite (2 min) and 70% ethanol (2 min), which effectively renders epiphytic propagules inviable (25, 33, 34). Leaf pieces were plated on 2% malt extract agar (MEA), which encourages growth by diverse endophytic fungi (35). Sealed plates were incubated at room temperature for 21 days. Emergent fungi were isolated into pure culture and grouped to morphotaxa by using vegetative features that conservatively reconstruct species boundaries as defined by molecular markers (25, 36). Species boundaries were confirmed by analysis of nuclear ribosomal DNA sequence divergence and in vitro interactions among representative isolates (E.A.H., E.I.R., L.C.M., and Z.M., unpublished results).
Numerous studies have shown that ecological factors such as spatial structure and host affinity are important components in the evolution of species interactions (e.g., refs. 15–18). To assess spatial structure of endophytes associated with T. cacao within and among sites, we determined similarity using the abundance-based Morisita-Horn index (MH), and Jaccard's index (JI), which reports similarity based on presence/absence data only (37). Both MH and JI range from 0 (no congruence between samples) to 1 (full congruence) and were calculated using estimates (http://viceroy.eeb.uconn.edu/estimateS). To examine host affinity, we conducted a second survey in which we concurrently assessed endophyte infections in T. cacao and two co-occurring, but distantly related, host species for which aspects of the endophyte community have been characterized [Heisteria concinna (Olacaceae, Santalales) and Ouratea lucens (Ochnaceae, Malpighiales); refs. 33 and 38]. Endophytes were isolated from T. cacao at BCI, PNS, and ND, and H. concinna and O. lucens at BCI (n = 3 mature leaves per tree, and 3 trees per species per site). We used MH to assess similarity in endophyte species composition and isolation frequency among these host species at BCI, and among individuals of T. cacao within and between sites. In all analyses, only morphotaxa isolated from more than one leaf were considered.
Host Affinity: Empirical Tests. Experimental data are important for distinguishing true host affinity from spatial artifacts such as localized dispersal within host crowns. We used two in vitro experiments to investigate whether growth of endophytes is sensitive to leaf chemistry, which forms an important component of plant antifungal defense (39, 40) and differs markedly among species of tropical trees (41). First, we assessed growth of endophyte isolates representing morphotaxa frequently collected from T. cacao (n = 29) on media containing leaf extracts from T. cacao, H. concinna, or O. lucens. Extracts contained 10% wt/vol suspensions of healthy, mature leaves from at least three individuals of the focal species and were incorporated into water agar before autoclaving (final concentration = 10% vol/vol; ref. 24). For each replicated trial, cylindrical plugs of hyphae and agar (diameter = 5 mm) were cultivated concurrently on media of each type. Colony diameters after 60 h at 22°C were compared by ANOVA.
We then assessed whether growth rates of endophytes in vitro correspond to their relative abundance in planta. In a separate experiment, 13 endophyte taxa that occurred with different frequencies in all focal host taxa (T. cacao, H. concinna, and O. lucens) were cultivated on media containing leaf extracts of each host species. Colony diameters after 60 h at 22°C were analyzed by using a sign test, followed by a nonparametric Wilcoxon rank-sums test, to compare growth on extracts from hosts from which endophyte taxa were most- and least-frequently isolated.
Antipathogen Assays. To generate endophyte-free seedlings of T. cacao, we collected seeds from healthy, mature fruit borne on 10 randomly chosen trees at BT. Before planting in sterile soil, seeds were washed in 0.5% sodium hypochlorite (3 min) to limit viability of surface-borne fungi, and were randomized with respect to maternal tree. Seedlings were grown in a clean growing house without wetting of leaf surfaces (24, 25). After 100 days, leaves from 10% of seedlings were sampled intensively (n = 32 segments per leaf) to confirm that leaves were endophyte-free. Because leaf age is often implicated in susceptibility to fungal infection (see ref. 39), we established that young and mature leaves of T. cacao can be infected with equal success by endophytic fungi (24). We then randomly chose a subset of young and mature leaves to receive endophyte inoculum, such that each seedling (n = 70) bore endophyte-treated (E+) and endophyte-free (E–) leaves.
The inoculum contained propagules of seven endophyte species representing three common endophyte genera (Colletotrichum, Xylaria, and Fusarium/Nectria). Six inoculum species were isolated as endophytes from T. cacao in our field studies, including the morphotype most frequently isolated from naturally infected, asymptomatic leaves of cacao (Colletotrichum sp. M1). A seventh endophyte species (Xylaria sp.) was isolated frequently from healthy leaves of H. concinna. All isolates demonstrated antipathogen activity in in vitro assays on 2% MEA (see Discussion). To apply the inoculum, we misted individual leaves with 1.5-ml aliquots of a mixed suspension of propagules (6.4 × 106 propagules per ml in sterile water and 0.5% gelatin) under humid conditions. We confirmed infection of E+ leaves by inoculum taxa 14 and 29 days after inoculation (i.e., 4 days before and 10 days after infection by Phytophthora sp.; see below).
Eighteen days after endophyte inoculations, we applied a strain of Phytophthora sp., isolated previously from symptomatic T. cacao in Panama, to a subset of young and mature E+ and E– leaves. To infect leaves, we placed agar plugs previously immersed in suspended zoospores (9.5 × 105 zoospores per ml) on leaf midveins, which were pricked with a sterile needle to facilitate infection. Infection sites were treated with an additional 10 μl of zoospore suspension 12 h after original applications. Because some seedlings lacked sufficient leaves to allow all treatments within the same plants, treatments were partitioned randomly among available leaves of each age class (n = 81 leaves of each age class treated with the pathogen). After treatment, plants were maintained in a screened growing house with a solid, light-admitting roof, where they were protected from contamination by other pathogens and endophytes.
After 72 h, necrotic lesions consistent with infection by Phytophthora sp. appeared on 97% of leaves treated with the pathogen. After 15 days, Phytophthora sp. was successfully reisolated from randomly chosen leaves showing symptoms of infection (n = 5 leaves). We then assessed pathogen damage by determining leaf mortality (proportion of leaves that were abscised or consumed entirely by necrosis) and the area of damage on surviving leaves (proportion of leaf area showing necrosis, measured using a leaf-area meter). A paired t test was used to compare rates of mortality for E–P+ and E+P+ leaves. Due to differential leaf mortality and treatment allocation, unpaired analyses were used to compare damage on surviving leaves.
Results
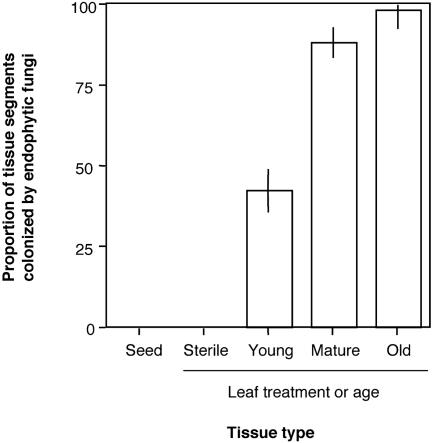
Endophyte Abundance and Richness in T. cacao. Naturally infected leaves of T. cacao were characterized by numerous and dense endophyte infections. Endophytes were present in sampled tissue from 82.2%, 95.6%, and 100% of young, mature, and old leaves, respectively (n = 135 leaves). Density of endophyte infections in mature and old leaves approached 100% of leaf segments infected and exceeded that in young leaves by a factor of two. In contrast, mature leaves of seedlings grown under protected conditions contained endophytic fungi in <1% of leaf segments (Fig. 1). Consistent with previous studies (24, 25), these data provide strong evidence that endophytes associated with T. cacao are transmitted horizontally.
Fig. 1.
Fungal endophytes associated with leaves of T. cacao are horizontally transmitted and accumulate over leaf lifetimes. Cultivable endophytes were not found in surface-sterilized seeds (data from ref. 24) and occurred in <1% of tissue segments of mature leaves of seedlings (100 days old) raised under sterile conditions. Under field conditions, proportions of leaf segments (each 2mm2) containing endophytes increased with leaf age (mean ± SE; data from BT, ND, PNS, and BCI; n = 3 leaves per age class per site).
From 126 naturally infected leaves of T. cacao, we recovered 1,172 endophyte isolates representing 344 morphotaxa. The most commonly isolated morphotaxa (n = 20) comprised 60% of isolates; all other morphotaxa were rarely encountered. Leaf samples comprising 32 mm2 per leaf (representing <5% of total leaf area; ref. 25) contained up to 13 distinct taxa of endophytic fungi. Fungal taxa used in subsequent seedling inoculations (Colletotrichum sp., Fusarium/Nectria spp., and Xylaria sp.) co-occurred frequently in asymptomatic tissues. Colletotrichum sp. M1 was the most frequently encountered endophyte, occurring in 71.9 ± 6.8% of mature and old leaves per tree (range: 33–100%).
Species richness of endophytes increased significantly with leaf age, ranging from 4.48 ± 0.46 to 6.23 ± 0.45 and 8.69 ± 0.31 morphotaxa per leaf for young, mature, and old leaves, respectively (χ2 = 37.35, P < 0.0001). Richness of endophytes recovered from individual trees (n = 9 leaves per tree) ranged from 38.3 ± 3.8 to 47.5 ± 4.9 morphotaxa per tree. Despite abiotic and biotic differences among sites (see below), richness of endophytes associated with focal trees did not differ significantly with regard to site (F4,10 = 2.34, P = 0.1256).
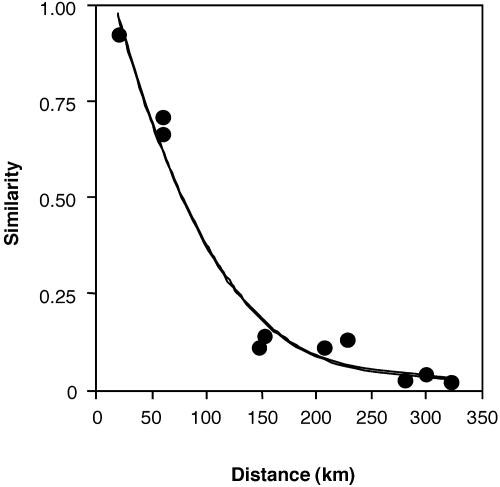
Spatial Structure of Endophytic Fungi. Similarity in endophyte assemblages decreased as a curvilinear function of distance between survey sites (R2 = 0.993, F3, 6 = 287.06, P < 0.0001; Fig. 2). Highest similarities occurred between T. cacao in intact forest (BCI) and in an abandoned plot under secondary forest at PNS (the closest site to BCI; MH = 0.928, JI = 0.458). These sites also share the most complex overstory and are similar in terms of rainfall regime. Relatively high values also were found for comparisons between BCI and a recently abandoned plot of T. cacao cultivated under scattered shade trees at ND, the second-closest site to BCI (MH = 0.714, JI = 0.387). These sites are ≈60 km from one another and differ in plant diversity, species composition, annual rainfall, and duration of the dry season. In contrast, comparisons between ND and an active plantation (BT), which are separated by ≈325 km but are similar in terms of general land use, rainfall, and plant diversity, approached zero (MH = 0.023, JI = 0.023).
Fig. 2.
Endophytic fungi associated with T. cacao demonstrate spatial structure. Similarity of endophyte assemblages, defined by the abundance-based MH for endophyte taxa occurring in more than one leaf, decreased as a function of distance between hosts. Points represent MH obtained in 10 pairwise comparisons of endophyte assemblages associated with T. cacao in each of five sites (n = 9 leaves per tree, 3 trees per site; P < 0.0001; JI data are congruent and are not shown).
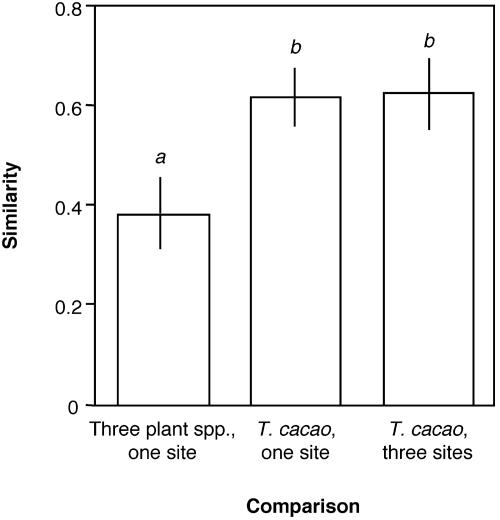
Host Affinity of Endophytes. Concurrent surveys of T. cacao, H. concinna, and O. lucens at BCI indicated that endophytes were present in 100% of mature leaves and leaf segments of all focal hosts. Among endophyte taxa recovered from more than one leaf, 65.5% were encountered in only one host species. Ten endophyte taxa were found concurrently in two or three host species; however, their relative abundances differed with regard to host. For example, Colletotrichum sp. M1 occurred in 88.9% of cacao leaves, but was recovered from only 33.3% of leaves of O. lucens, and was never recovered from H. concinna. When endophyte assemblages were compared on the basis of species composition and isolation frequencies (MH), endophytes associated with conspecific hosts within individual sites, or among sites separated by 20–60 km, were more similar to one another than were endophyte assemblages associated with three host species at one site (F2,24 = 3.85, P = 0.0356; Fig. 3). These data are consistent with previous findings of nonrandom associations of endophytes with H. concinna and O. lucens over a small sampling area (33, 38), as well as data from other guilds of fungi (42, 43).
Fig. 3.
Field surveys indicate host affinity among tropical endophytes. Endophyte assemblages associated with three host species at one site are less similar to one another than are endophytes of conspecific hosts at that site, and conspecific hosts at three sites separated by 20–60 km (P = 0.0356). MH for endophytes associated with T. cacao, Ouratea lucens, and Heisteria concinna at BCI (mean based on nine pairwise comparisons between individuals of each host species chosen randomly and without replacement) was significantly less than similarity among three individuals of T. cacao within each of three sites (BCI, PNS, and ND; n = 9 randomized pairwise comparisons), and among three sites (n = 9 randomized pairwise comparisons). Differing superscripts denote significant differences based on a posteriori Tukey-Kramer HSD tests (α = 0.05).
In in vitro trials, 86% of cacao endophytes differed significantly in growth rate when cultivated on media containing extracts from leaves of T. cacao vs. H. concinna or O. lucens (F2, 70 = 15.71, P < 0.0001). In a second experiment, growth rates for 77% of tested morphotaxa were greatest when endophytes were cultivated on media containing extracts of the host species from which they were most frequently isolated in field surveys (sign test, P = 0.0348). For those taxa, growth on extracts of their most frequent host exceeded growth on extracts of the host in which they occurred most rarely by >20% (χ2 = 13.23, P = 0.0013).
Efficacy of Inoculation. Both E– and E+ leaves were successfully produced on individual seedlings of T. cacao. Six endophyte taxa originally isolated from asymptomatic T. cacao were successfully reisolated from E+ leaves, whereas one endophyte species originally isolated from H. concinna (Xylaria sp.) was never recovered from E+ tissues.
After inoculation, endophyte infection densities in E+ leaves rapidly approached values comparable to those observed in field surveys. Fourteen days after inoculation (i.e., 4 days before infection with Phytophthora), inoculum endophytes were recovered from 85.7% of E+ leaves, and 37.5 ± 0.1% of E+ leaf segments in infected leaves (n = 7 leaves). In contrast, only one of 192 leaf segments assessed for E– leaves (0.5%) contained an inoculum endophyte (n = 6 leaves). Twenty nine days after inoculation (i.e., 10 days after infection with Phytophthora), endophytes were recovered from all E+ leaves (n = 9 leaves). At that time, endophytes were present in 72.4 ± 0.1% of E+ leaf segments, indicating proliferation of inoculum taxa in leaf tissues (percent of leaf segments infected at 14 vs. 29 days, F1, 14 = 7.07, P = 0.0187). The most common morphotaxon in our field surveys of T. cacao (Colletotrichum sp. M1) also was the most prevalent endophyte in E+ tissues, occurring in 100% of E+ leaves sampled 29 days after inoculation. Nonetheless, 89% of E+ leaves contained two to four endophyte species, indicating simultaneous infection by multiple taxa (mean = 3.78 ± 0.22 species per leaf). In contrast, only four of 384 segments examined for E– leaves (1%) contained inoculum endophytes, and simultaneous infection by multiple inoculum species was never observed (n = 6 leaves).
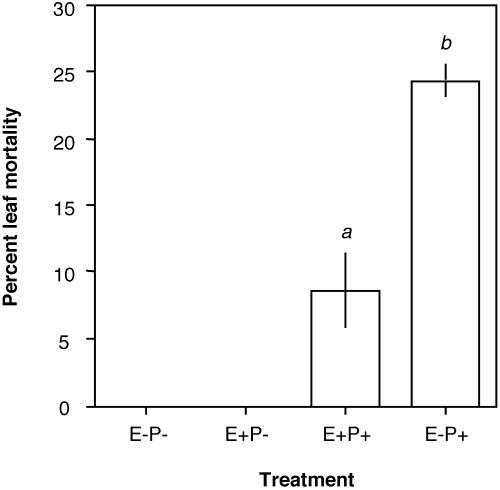
Antipathogen Assays. Neither leaf mortality nor necrosis was observed among control leaves (E–P–), nor among leaves that received endophytes alone (E+P–), for a period up to 100 days after the termination of data collection. In the presence of the pathogen, leaves lacking endophytes experienced leaf death and abscission 2.8 times more frequently than did leaves inoculated with endophytes (paired t1 = –10.00, P = 0.0317; Fig. 4). On pathogen-infected leaves that did survive, necrotic lesions were significantly larger on leaves without endophytes (E–P+: 12.01 ± 1.36% of leaf area, mean ± SE) than on leaves with endophytes (E+P+: 6.20 ± 1.28% of leaf area, F1,135 = 13.66, P = 0.0030, using logit-transformed data for surviving leaves). The apparently localized protection by endophytes bore implications for entire host plants: when both leaf loss and leaf damage on retained leaves are considered, surface area available for photosynthesis decreased by 32.3% for E–P+ treatments relative to controls, but only by 14.1% for E+P+ treatments relative to controls.
Fig. 4.
Endophyte colonization reduces leaf mortality due to a foliar pathogen in seedlings of T. cacao. No mortality occurred among control leaves lacking endophytes and the pathogen (E–P–), nor among leaves inoculated only with endophytes (E+P–). In the presence of the pathogen, mortality occurred 2.8 times more frequently among leaves lacking endophytes (E–P+) than among leaves with endophytes (E+P+) (P = 0.0317).
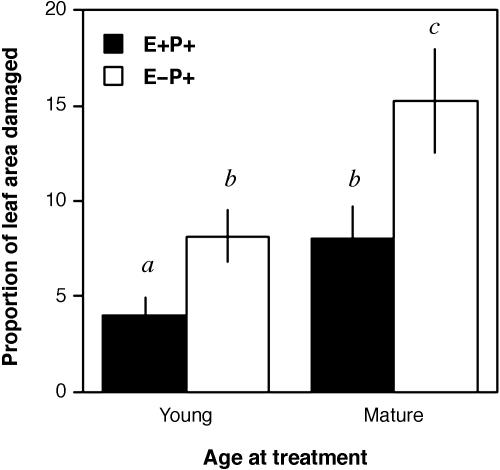
Endophyte Protection and Leaf Age. Mortality among E–P+ leaves did not differ as a function of leaf age (data not shown). Among surviving leaves, leaf area lost to necrosis was nearly twofold greater for mature E–P+ leaves (15.23 ± 2.76% of leaf area lost) than for young E–P+ leaves (8.13 ± 1.37% of leaf area lost; Fig. 5). Inoculation of both young and mature leaves with endophytes significantly reduced the area of pathogen-induced necroses relative to E–P+ leaves (P = 0.0130, P = 0.0241; linear contrasts from ANOVA using logit-transformed data; Fig. 5). Young leaves treated with the pathogen only (E–P+) lost ≈3.5% more leaf area than did young leaves with endophytes (E+P+). In contrast, mature E–P+ leaves lost ≈7.1% more leaf area than did mature E+P+ leaves (i.e.,≈3.9 cm2 of leaf area; ref. 25). Analysis by a random-effects/mixed-model nested ANOVA [leaf age as a random factor (P < 0.0001) with treatment nested within leaf age (P = 0.0105)] indicates that although both young and mature leaves benefited from endophyte colonization, endophyte-mediated protection was significantly greater for mature leaves.
Fig. 5.
Fungal endophytes reduce leaf area lost to a foliar pathogen for young and mature leaves of T. cacao, and the scale of beneficial effects by endophytes is specific to leaf age. For both young and mature leaves, inoculation with endophytes was associated with a decrease in mean proportion of leaf area damaged by Phytophthora (P = 0.0130, P = 0.0241). For young foliage, E–P+ leaves lost ≈3.5% more leaf area than did E+P+ leaves. Mature leaves lacking endophytes lost ≈7.1% more leaf area than did mature leaves with endophytes.
Discussion
In seedlings of T. cacao, inoculation by endophytes that commonly co-occur in naturally infected, asymptomatic tissues of conspecific hosts significantly reduced leaf necrosis and mortality due to a major foliar pathogen. Coupled with the ecological context afforded by field surveys, these data provide, to our knowledge, a first demonstration of an ecologically important, but cryptic, interaction between these diverse symbionts and their host plants. The present study bears upon investigations of plant–pathogen interactions, in which potential effects of endophytic fungi are generally not considered. Further, these data corroborate previous suggestions that endophytes hold potential as biological control agents for use in sustainable cultivation of cacao and other crops (44).
In lowland Panama, >10,000 fungal propagules are deposited as air spora on leaf surfaces each day (24, 25, 45). Coupled with previous studies (22–25), our data indicate that leaves at emergence, like seedlings at germination, lack cultivable endophytes. Given surface-wetting of leaves by dew, fog, or rainfall, a subset of these diverse fungi consistently enters foliage and persists as endophytes without causing detectable damage. Species composition and relative abundance of endophytes differ as a function of distance between individuals, indicating that T. cacao forms associations with diverse components of the tropical mycoflora in geographically distinct sites. These results are consistent with previous reports of associations between introduced plants and local fungi (22), suggesting a remarkable lability in the formation of endophyte symbioses.
Field surveys indicated that although some endophyte taxa were present in all surveyed host species, most occurred in only one (i.e., T. cacao, O. lucens, or H. concinna), and similarity of endophyte communities was low when assessed for these hosts at one study site. Host-specific leaf chemistry favored the growth of some endophytes over others in in vitro trials, and highest growth rates were observed when endophytes were cultivated on extracts of the host species in which they were most frequently encountered. By mediating the growth of particular endophyte species, leaf chemistry may influence the outcomes of competitive interactions among endophytes. To explore this hypothesis, we assessed in vitro interactions of nine endophyte taxa isolated from a single leaf of T. cacao (E.A.H., E.I.R., L.C.M., and Z.M., unpublished results). Trials were conducted on 2% MEA and on water agar containing extracts of mature cacao leaves (24). We found that the proportion of interactions with negative outcomes (competition, inhibition, or antagonism) differed markedly with regard to substrate. Further, isolates that were successful inhibitors or competitors on a given medium were not more likely to succeed in interactions on the other nutrient source. Finally, morphotaxa that were isolated frequently from naturally infected leaves of T. cacao consistently succeeded in interactions relative to more rarely encountered species when tested on cacao-leaf medium. Together, these observations indicate that host-specific leaf chemistry may mediate the outcomes of interactions among endophytes in planta, thereby influencing endophyte species composition and apparent host affinity.
In contrast to previous studies documenting systemic resistance after application of hypo- or avirulent fungal strains (e.g., refs. 11 and 46–53; see also ref. 54), the antipathogen defense documented here was localized to endophyte-infected tissues. By introducing a large inoculum volume of Phytophthora sp. through cuticular wounds, we provided advantageous conditions for infection and proliferation of the pathogen. Although 97% of leaves treated with the pathogen manifested symptoms of infection, subsequent proliferation of Phytophthora was significantly restricted in E+ leaves. In contrast, inoculum endophytes successfully proliferated in E+ tissues. Based on our observations of localized, postinfection protection in E+ leaves and interactions among endophytes in vitro, we suggest that interspecific interactions also may play an important role in mediating host defense.
To explore this hypothesis, we assessed in vitro interactions between 50 morphospecies of endophytic fungi isolated from T. cacao and three major cacao pathogens (Phytophthora sp., Moniliophthora roreri, and Crinipellis perniciosa; L.C.M. E.I.R., Z.M., and E.A.H., unpublished results). Whereas a large proportion of endophytes (40%) antagonized at least one of these species in pairwise trials on MEA, a subset had no effect, and a subset were themselves antagonized. Endophytes that effectively antagonized particular pathogen species were not more likely to antagonize the other pathogens examined here. Finally, repeated trials on media containing leaf extracts of T. cacao differed qualitatively and quantitatively relative to outcomes on MEA. Together, these observations suggest that direct interactions between endophytes and pathogens are complex, diverse, and sensitive to host-specific leaf chemistry. We suggest that the apparent plasticity and diversity of interspecific fungal interactions may contribute to effective antipathogen defense in woody plants. Given the ever changing and diverse pathogen assemblages in tropical forests, endophyte-mediated defense is likely to be enhanced when endophytes are highly diverse within and among leaves, plants, and host species.
The observation that beneficial effects of endophytes differ with leaf age underscores the potential ecological and evolutionary importance of endophyte-mediated protection. A consistent pattern among diverse tropical trees is that developing leaves bear high concentrations of chemicals with antifungal activity, which diminish at or immediately after leaf maturity (39, 55). Given that fungal pathogens represent a major selective force structuring plant communities (56, 57), long-term persistence of mature leaves without antipathogen defenses comparable to those of young leaves represents an apparent paradox. In T. cacao, antifungal anthocyanidins are present at high concentrations in young leaves, but are absent from fully expanded and toughened (mature) leaves (58). Consistent with other species of woody angiosperms in this lowland forest (25), young leaves of T. cacao initially lack endophytes, but multiple localized endophyte infections accumulate rapidly as leaves mature. When confronted with a pathogen, mature leaves of T. cacao appear to receive a relatively greater benefit from endophyte infection. The matching temporal pattern of increasing endophyte infection and reduction in intrinsic chemical defense corroborates the hypothesis that endophytes play an important role in host defense. In turn, endophytes appear to benefit by drawing apoplastic nutrients from host leaves, and from rapid growth and subsequent sporulation from leaves after senescence (see ref. 59).
Mutualistic interactions between hosts and vertically inherited symbionts such as endophytes associated with temperate grasses are easily reconciled with existing theory of species interactions. Indeed, many tenets of mutualism theory are based on these and similar cases (15–18, 46). However, horizontal inheritance of diverse, mutualistic symbionts coincides poorly with expectations based on current theory (see also ref. 60). It appears that tropical plants have the potential to develop differentially specific endophytic symbioses with components of the extremely diverse tropical mycota. Our results show that associations between a woody angiosperm and diverse, horizontally transmitted foliar endophytes can enhance and/or supplement host defense. How or whether communities of fungi within leaves are regulated intrinsically by competition, mutual antagonism, or other means (e.g., host chemistry); the relative importance of primacy of infection (53), induction of host defenses (61), and the abundance, diversity, or species composition of endophytes; the potential for endophytes to confer hidden costs on their hosts; and the degree to which endophytes may act as horizontally acquired immune systems represent only a few of many questions yet to be explored. Similarly, whether diverse, horizontally transmitted mutualists such as the mycorrhizal fungi (62, 63) and rhizobia (64) associated with terrestrial plants, zooxanthellae of corals (17), pollinating wasps associated with figs (65, 66), and fungal endophytes of woody angiosperms can be reconciled with existing theory of mutualism, or will redefine mutualism theory, provides a strong impetus for further study.
Acknowledgments
We thank R. H. Robichaux, J. L. Bronstein, E. G. Leigh, Jr., J. Dalling, J. F. Bishoff, A. Read, D. R. Maddison, S. Van Bael, two anonymous referees, and especially L. A. McDade for critical review; P. K. Hebbar, R. Lumsden, and G. S. Gilbert for training and advice; and the Smithsonian Tropical Research Institute and the Republic of Panama for logistical support. This work was supported by grants from the American Cocoa Research Institute, World Cocoa Foundation, and the M&M/Mars Division of Masterfoods to the Smithsonian Tropical Research Institute Sustainable Cacao Research Group (all authors), the Andrew Mellon Foundation (E.A.H.), the Smithsonian Tropical Research Institute (E.A.H. and A.E.A.), a National Science Foundation Graduate Research Fellowship (to A.E.A.) and Doctoral Dissertation Improvement Grant DEB-9902346 (to L. A. McDade and A.E.A.), and Research Training Grant in Biological Diversification at the University of Arizona DIR-9113362, BIR-9602246 (to A.E.A.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: E+, endophyte-treated; E–, endophyte-free; BCI, Barro Colorado Island, Panama; BT, near Almirante, Bocas del Toro, Panama; ND, Nombre de Dios, Colón, Panama; PNS, Parque Nacional Soberanía, Panama; MH, Morisita-Horn index of similarity; JI, Jaccard's index of similarity.
References
- 1.Berbee, M. L. (2001) Physiol. Mol. Plant Pathol. 59, 165–187. [Google Scholar]
- 2.Alexopoulos, C. J., Mims, C. W. & Blackwell, M. (1996) Introductory Mycology (Wiley, New York).
- 3.Clay, K. & Holah J. (1999) Science 285, 1742–1744. [DOI] [PubMed] [Google Scholar]
- 4.Packer, A. & Clay, K. (2000) Nature 404, 278–228. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert, G. S. (2002) Annu. Rev. Phytopathol. 40, 13–43. [DOI] [PubMed] [Google Scholar]
- 6.Hawksworth, D. L. (1991) Mycol. Res. 95, 641–655. [Google Scholar]
- 7.Carlsson-Graener, U. & Thrall, P. H. (2002) Oikos 97, 97–110. [Google Scholar]
- 8.Douds, D. D. & Millner, P. (1999) Agric. Ecosyst. Environ. 74, 77–93. [Google Scholar]
- 9.Petrini, O. (1991) in Microbial Ecology of Leaves, eds. Andrews, J. H. & Hirano, S. S. (Springer, New York), pp. 179–197.
- 10.Stone, J. K., Bacon C. W. & White, J. F., Jr. (2000) in Microbial Endophytes, eds. Bacon, C. W. & White, J. F., Jr. (Dekker, New York), pp. 3–29.
- 11.Clay, K. & Schardl, C. (2002) Am. Nat. 160, S99–S127. [DOI] [PubMed] [Google Scholar]
- 12.Clay, K. (1988) Ecology 69, 10–16. [Google Scholar]
- 13.Malinowski, D. P. & Belesky, D. P. (1999) J. Plant Nutr. 22, 1335–1349. [Google Scholar]
- 14.Saikkonen, K., Faeth, S. H., Helander, M. & Sullivan, T. J. (1998) Annu. Rev. Ecol. Syst. 29, 319–343. [Google Scholar]
- 15.Bull, J. J. (1994) Evolution (Lawrence, Kans.) 48, 1423–1437. [Google Scholar]
- 16.Maynard Smith, J. & Szathmary, E. (1995) The Major Transitions in Evolution (Freeman, New York).
- 17.Herre, E. A., Knowlton, N., Mueller, U. G. & Rehner, S. A. (1999) Trends Ecol. Evol. 14, 49–53. [DOI] [PubMed] [Google Scholar]
- 18.Leigh, E. G., Jr. (1999) Tropical Forest Ecology (Oxford Univ. Press, New York).
- 19.Oliver, K. M., Russell, J. A., Moran, N. A. & Hunter, M. S. (2003) Proc. Natl. Acad. Sci. USA 100, 1803–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faeth, S. H. & Sullivan, T. J. (2003) Am. Nat. 161, 310–325. [DOI] [PubMed] [Google Scholar]
- 21.Arnold, A. E. (2001) in Tropical Ecosystems: Structure, Diversity, and Human Welfare, eds. Ganeshaiah, K. N., Uma Shaanker, R. & Bawa, K. S. (Oxford and IBH Publishing Co. Pvt. Ltd. New Delhi, India), pp. 739–743.
- 22.Bayman, P., Angulo-Sandoval, P., Baez-Ortiz, Z. & Lodge, D. J. (1998) Mycol. Res. 102, 944–948. [Google Scholar]
- 23.Lebrón, L., Lodge, D. J., Laureano, S. L. & Bayman, P. (2001) Phytopathology 91, S116. [Google Scholar]
- 24.Arnold, A. E. & Herre, E. A. (2003) Mycologia 95, 388–398. [PubMed] [Google Scholar]
- 25.Arnold, A. E. (2002) Ph.D. thesis (Univ. of Arizona).
- 26.Lodge, D. J., Fisher, P. J. & Sutton, B. C. (1996) Mycologia 88, 733–738. [Google Scholar]
- 27.Carroll, G. (1995) Can. J. Bot. 73, S1316–S1324. [Google Scholar]
- 28.Faeth, S. H. & Fagan, W. F. (2002) Int. Comp. Biol. 42, 360–368. [DOI] [PubMed] [Google Scholar]
- 29.Young, A. M. (1994) The Chocolate Tree: A Natural History of Cacao (Smithsonian Institution Press, Washington, DC).
- 30.Rice, R. A. & Greenberg, R. (2000) Ambio 29, 167–173. [Google Scholar]
- 31.Krauss, U. & Soberanis, W. (2002) Biol. Control 24, 82–89. [Google Scholar]
- 32.Leigh, E. G., Jr., Rand, A. S. & Windsor, D. M. (1996) The Ecology of a Tropical Forest (Smithsonian Institution Press, Washington, DC).
- 33.Arnold, A. E., Maynard, Z., Gilbert, G. S., Coley, P. D. & Kursar, T. A. (2000) Ecol. Lett. 3, 267–274. [Google Scholar]
- 34.Schulz, B., Wanke, U., Draeger, S. & Aust, H. J. (1993) Mycol. Res. 97, 1447–1450. [Google Scholar]
- 35.Fröhlich, J. & Hyde, K. D. (1999) Biodivers. Conserv. 8, 977–1004. [Google Scholar]
- 36.Lacap, D. C., Hyde, K. D. & Liew, E. C. Y. (2003) Fungal Divers. 12, 53–66. [Google Scholar]
- 37.Magurran, A. E. (1988) Ecological Diversity and Its Measurement (Princeton Univ. Press, Princeton).
- 38.Arnold, A. E., Maynard, Z. & Gilbert, G. S. (2001) Mycol. Res. 105, 1502–1507. [Google Scholar]
- 39.Coley, P. D. & Barone, J. A. (1996) Annu. Rev. Ecol. Syst. 27, 305–335. [Google Scholar]
- 40.Coley, P. D. & Aide, T. M. (1989) J. Trop. Ecol. 5, 293–300. [Google Scholar]
- 41.Coley, P. D. (1983) Ecol. Monogr. 53, 209–233. [Google Scholar]
- 42.Lovelock, C. E., Andersen, K. & Morton, J. B. (2003) Oecologia 135, 268–279. [DOI] [PubMed] [Google Scholar]
- 43.Gilbert, G. S., Ferrer, A. & Carranza, J. (2002) Biodivers. Conserv. 11, 947–957. [Google Scholar]
- 44.Arnold, A. E. (1999) in Proceedings of the Research Methodology of Biocontrol of Plant Diseases Workshop, eds. Krauss, U. & Hebbar, P. (CATIE, San Jose, Costa Rica), pp. 44–54.
- 45.Gilbert, G. S. (2002) in Conservación de Bosques Tropicales, eds. Guariguata, M. & Kattan, G. (Libro Universitario Regional, Cartago, Costa Rica), pp. 435–463.
- 46.Carroll, G. C. (1986) in Microbiology of the Phyllosphere, eds. Fokkema N. J. & van den Huevel, J. (Cambridge Univ. Press, Cambridge, U.K.), pp. 205–222.
- 47.Clay, K., Cheplick, G. P. & Marks, S. M. (1989) Oecologia 80, 374–380. [DOI] [PubMed] [Google Scholar]
- 48.Gwinn, K. D. & Gavin, A. M. (1992) Plant Dis. 76, 911–914. [Google Scholar]
- 49.Freeman, S. & Rodriguez, R. J. (1993) Science 260, 75–78. [DOI] [PubMed] [Google Scholar]
- 50.Sneh, M. & Ichielevic-Auster, M. (1998) Phytoparasitica 26, 27–38. [Google Scholar]
- 51.Reuveni, M. & Reuveni, R. (2000) Eur. J. Plant Path. 106, 633–638. [Google Scholar]
- 52.Borowicz, V. A. (2001) Ecology 82, 3057–3068. [Google Scholar]
- 53.Redman, R. S., Dunigan, D. D. & Rodriguez, R. J. (2001) New Phytol. 151, 705–716. [DOI] [PubMed] [Google Scholar]
- 54.Boyle, C., Gotz, M., Dammann-Tugend, U. & Schulz, B. (2001) Symbiosis 31, 259–281. [Google Scholar]
- 55.Lee, D. W. & Collins, T. M. (2001) Int. J. Plant Sci. 162, 1141–1153. [Google Scholar]
- 56.Wills, C., Condit, R., Foster, R. B. & Hubbell, S. P. (1997) Proc. Natl. Acad. Sci. USA 94, 1252–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Givnish, T. J. (1999) J. Ecol. 87, 193–210. [Google Scholar]
- 58.Lee, D. W., Brammeier, S. & Smith, A. P. (1987) Biotropica 19, 40–49. [Google Scholar]
- 59.Clay, K. (2001) Am. Zool. 41, 810–824. [Google Scholar]
- 60.Wilkinson, D. M. & Sherratt, T. N. (2001) Oikos 92, 377–384. [Google Scholar]
- 61.Schulz, B., Rommert, A. K., Dammann, U., Aust, H. J. & Strack, D. (1999) Mycol. Res. 103, 1275–1283. [Google Scholar]
- 62.Husband, R., Herre, E. A. & Young, J. P. W. (2002) FEMS Microbiol. Ecol. 42, 131–136. [DOI] [PubMed] [Google Scholar]
- 63.Husband, R., Herre, E. A., Turner, S. L., Gallery, R. & Young, J. P. W. (2002) Mol. Ecol. 11, 2669–2678. [DOI] [PubMed] [Google Scholar]
- 64.Kiers, E. T., Rousseau, R. A., West, S. A. & Denison, R. F. (2003) Nature 425, 78–81. [DOI] [PubMed] [Google Scholar]
- 65.Herre, E. A. (1999) in Levels of Selection, ed. Keller, L. (Princeton Univ. Press, Princeton), pp. 209–237.
- 66.Molbo, D., Machado, C. A., Sevenster, J. G., Keller, L. & Herre, E. A. (2003) Proc. Natl. Acad. Sci. USA 100, 5867–5872. [DOI] [PMC free article] [PubMed] [Google Scholar]