Abstract
Cancer stem cells (CSCs) or tumor progenitor cells are involved in tumor progression and metastasis1. MicroRNAs (miRNAs) regulate both normal stem cells and CSCs2–5 and miRNA dysregulation has been implicated in tumorigenesis6. CSCs in many tumors, including cancers of the breast7, pancreas8, head and neck9, colon10,11, small intestine12, liver13, stomach14, bladder15, and ovary16 have been identified using adhesion molecule CD44, either individually or in combination with other marker(s). Prostate cancer (PCa) stem/progenitor cells with enhanced clonogenic17 and tumor-initiating and metastatic18,19 capacities are also enriched in the CD44+ cell population, but whether miRNAs regulate the CD44+ PCa cells and PCa metastasis remains unclear. Here we show, through expression analysis, that miR-34a, a p53 target20–24, was under-expressed in CD44+ PCa cells purified from xenograft and primary tumors. Enforced expression of miR-34a in bulk PCa cells inhibited clonogenic expansion and tumor development. miR-34a re-expression in CD44+ PCa cells blocked whereas miR-34a antagomirs in CD44− PCa cells promoted tumor regeneration and metastasis. Systemically delivered miR-34a inhibited PCa metastasis and extended animal survival. Of significance, CD44 was identified and validated as a direct and functional target of miR-34a and CD44 knockdown phenocopied miR-34a over-expression in inhibiting PCa regeneration and metastasis. Our study reveals miR-34a as a critical negative regulator of CD44+ PCa cells and establishes a strong rationale for developing miR-34a as a novel therapeutic against prostate CSCs.
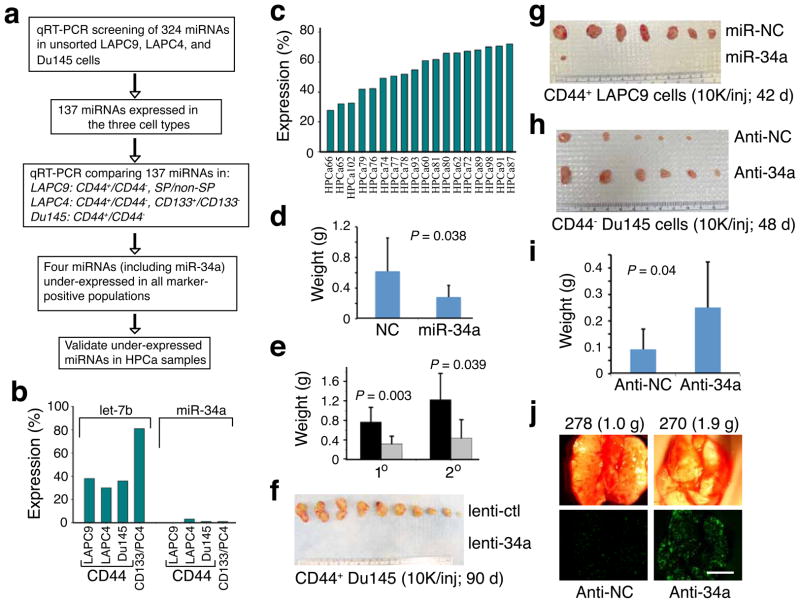
We used quantitative reverse transcription – polymerase chain reaction (qRT-PCR) to compare the miRNA expression25,26 of CD44+ and CD44− PCa cells. The CD44+ PCa cell population harbors tumor-initiating and metastatic cells18,19 and is enriched in self-renewal gene NANOG27. CD44+ PCa cells were purified from three xenograft models18,19,27,28: LAPC9, LAPC4, and Du145. For comparison, we also purified LAPC4 CD133+ and LAPC9 side population (SP) cells. The CD133+ PCa cells are clonogenic in vitro17 and LAPC9 SP is also enriched in tumor-initiating cells28. We first used unsorted cells to measure the levels of 324 sequence-validated human miRNAs and found that 137 miRNAs were expressed at reliably detectable levels (Fig. 1a). We then compared expression levels of these 137 miRNAs in marker-positive versus marker-negative PCa cell populations and found that miR-34a (1p36.22) was prominently under-expressed in all CD44+ populations (Fig. 1a), representing <3% of the levels in the corresponding CD44− cells (Fig. 1b). The other two miR-34 family members, miR-34b and miR-34c (11q23.1), did not show consistent differences between CD44+ and CD44− PCa cells (not shown). Under-expression of miR-34a in CD44+ PCa cells was more pronounced than that of let-7b, a tumor suppressive miRNA6 and an important regulator of both normal and cancer stem cells3,4. miR-34a was also under-expressed in LAPC4 CD133+ (Fig. 1b) and LAPC9 SP (not shown) cells. To validate miR-34a under-expression in CD44+ PCa cells and to determine the clinical relevance, we purified CD44+ and CD44− PCa cells from 18 primary tumors27,29 (HPCa; Supplementary Table 1) and compared the miR-34a levels. CD44+ HPCa cells expressed miR-34a at levels ~25–70% of those in CD44− cells from the same tumors (Fig. 1c). Altogether, these results suggest that miR-34a is under-expressed in the CD44+ PCa cells in both xenograft and primary tumors.
Figure 1. Underexpression and tumor-inhibitory effects of miR-34a.
a. Experimental scheme. b,c. Lower miR-34a levels in CD44+ xenograft (b; PC4, LAPC4) or primary tumor (HPCa; c) cells. Results are expressed as the mean % of marker-positive over marker-negative cells. d,e. miR-34a inhibited LAPC9 (d) and HPCa58 (e; black, lenti-ctl; grey, lenti-34a; n = 10 and n = 7 for 1° and 2° experiments) tumor growth (mean ± S.D). f. CD44+ Du145 cells infected with lenti-ctl or lenti-34a were injected (10,000 cells each) s.c in NOD-SCID mice. Tumor incidence was 10/10 and mean weight was 0.6 g for lenti-ctl group whereas the incidence for lenti-34a group was 0/10. g. Purified CD44+ LAPC9 cells were transfected with miR-NC or miR-34a and s.c injected. Tumor incidence was 7/8 and mean weight was 0.5 g for miR-NC group whereas incidence was 1/8 and tumor weight was 0.03 g for miR-34a group (P = 0.016, incidence). h. Purified CD44− Du145 cells were transfected with anti-NC or anti-34a and s.c injected. Tumor incidence was 5/8 and mean weight was 0.05 g for anti-NC group whereas incidence was 6/8 and tumor weight was 0.2 g for anti-34a group (P = 0.038, weight). i. Bulk LAPC9 cells were transfected with anti-NC or anti-34a oligos and implanted (100,000 cells) in the DP. Mice were terminated at d 46. Tumor incidences were 5/8 and 7/8 for anti-NC and anti-34a groups, respectively. j. Representative microphotographs (animal number and tumor weight indicated on top; scale bar, 100 μm) showing increased lung metastasis by anti-34a (also see Supplementary Fig. 7c–d).
miR-34a is regulated by p53 and induces apoptosis, cell-cycle arrest, or senescence when introduced into cancer cells20–24,30. miR-34a levels in ten prostate (cancer) cell types correlated with the p53 status (Supplementary Fig. 1) and transfection of synthetic miR-34a oligonucleotides (oligos), but not the negative control (NC) miRNA oligos, induced cell-cycle arrest, apoptosis or senescence in p53-mutant PCa cells (Supplementary Data and Supplementary Figs. 2 and 3). To determine whether miR-34a possesses tumor-inhibitory effects, we manipulated miR-34a levels (Supplementary Fig. 4) in a variety of PCa cell types and then implanted the cells subcutaneously (s.c) or orthotopically in the dorsal prostate (DP) in NOD-SCID mice (Fig. 1d,e; Supplementary Fig. 5). miR-34a transfected LAPC9 (Fig. 1d; Supplementary Fig. 5a) and HPCa58 (Fig. 1e) cells produced significantly smaller tumors than the same cells transfected with miR-NC oligos. LAPC9 cells are androgen-dependent whereas HPCa58 cells were from an early-generation xenograft tumor (Supplementary Methods). miR-34a also inhibited the secondary transplantation of HPCa58 cells (Fig. 1e). Similar tumor-inhibitory effects of miR-34a were observed with androgen-dependent LAPC4 (Supplementary Fig. 5b) and androgen-independent Du145 (Supplementary Fig. 5d) and PPC-1 (Supplementary Fig. 5g) cells. We also infected PCa cells with lentiviral or retroviral vectors encoding pre-miR-34a (Supplementary Fig. 1d) prior to implantation. The viral vector-mediated miR-34a overexpression also inhibited tumor regeneration of LAPC4 (Supplementary Fig. 5c), Du145 (Supplementary Fig. 5e,f), and LAPC9 (not shown) cells. Histological and immunohistochemical (IHC) examination of tumor sections (Supplementary Fig. 6) revealed increased necrotic areas and reduced Ki-67+ cells in miR-34a transfected tumors, which also showed increased expression of HP-1γ, a protein associated with cell-cycle arrest and senescence.
To evaluate whether the miR-34a-mediated tumor inhibition might be due to an effect on the CSC populations, we performed tumor experiments using purified CD44+ and CD44− PCa cells. Remarkably, when purified CD44+ Du145 cells were infected with lenti-34a, tumor development was completely blocked (Fig. 1f). Similarly, when CD44+ LAPC9 cells were transfected with miR-34a oligos (Fig. 1g) or infected with lenti-34a (Supplementary Fig. 5h), tumor incidence was virtually abolished. Conversely, introducing an antisense inhibitor of miR-34a (i.e., anti-34a or miR-34a antagomir) into purified CD44− Du145 cells promoted tumor growth (Fig. 1h; Supplementary Fig. 5i). Likewise, bulk or CD44− LAPC9 cells transfected with anti-34a oligos generated larger tumors than those with anti-NC oligos (Fig. 1i; Supplementary Fig. 7a,b). Importantly, we observed more lung metastasis in the anti-34a transfected group (Fig. 1j; Supplementary Fig. 7c,d). Collectively, these results suggest that miR-34a possesses tumor-inhibitory effects in both bulk and purified CD44+ PCa cells.
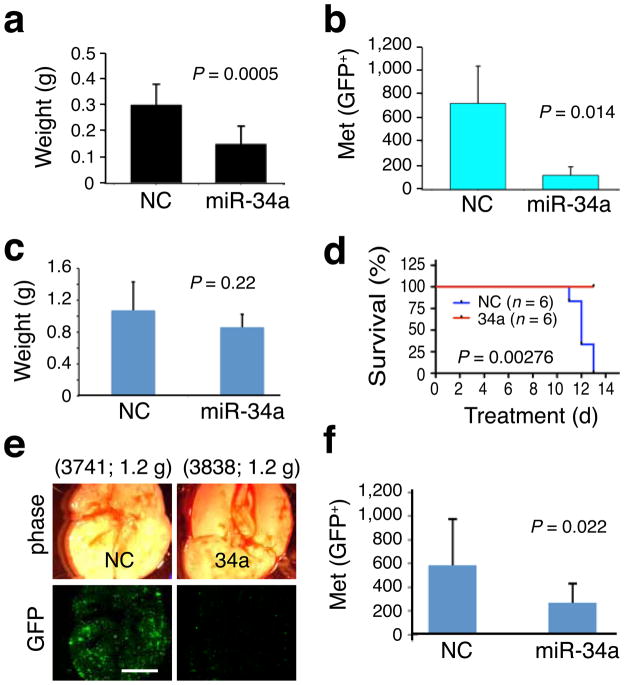
Subsequently, we performed 4 sets of therapeutic experiments (Fig. 2; see Online Methods) in NOD-SCID mice bearing pre-established PCa. We first observed that repeated intratumoral injections of miR-34a into PPC-1 tumors halted tumor growth (Supplementary Fig. 5g). We then established orthotopic PC3 tumors and, 3 weeks later, injected miR-34a or miR-NC oligos complexed with a lipid-based delivery agent26 into tail veins of mice every two days. Systemically delivered miR-34a reduced PC3 tumor burden by 50% (Fig. 2a). In two therapeutic experiments with orthotopic LAPC9 tumors, miR-34a reduced lung metastasis (Fig. 2b,e,f; Supplementary Fig. 8) without affecting tumor growth (Fig. 2c). miR-34a also promoted survival of tumor-bearing animals (Fig. 2d). These results indicate that miR-34a possesses therapeutic efficacy against pre-established prostate tumors.
Figure 2. Therapeutic effects of miR-34a.
a. Tail vein-injected miR-34a inhibited orthotopic PC3 tumor growth (n = 9 each). b–d. Tail vein-injected miR-34a oligos inhibited metastasis (GFP+ foci in the endpoint lungs; mean ± S.D, n = 6/group) of orthotopic LAPC9-GFP tumors (b) without significantly affecting tumor growth (c) and extended animal survival (d; Kaplan-Meier analysis and Log-Rank test). e,f. The fourth set of therapeutic experiment in LAPC9 cells. Shown are representative lung images (e; animal number and tumor weight indicated on top; scale bar, 100 μm) and quantification of lung metastases (f; mean ± S.D, n = 10/group).
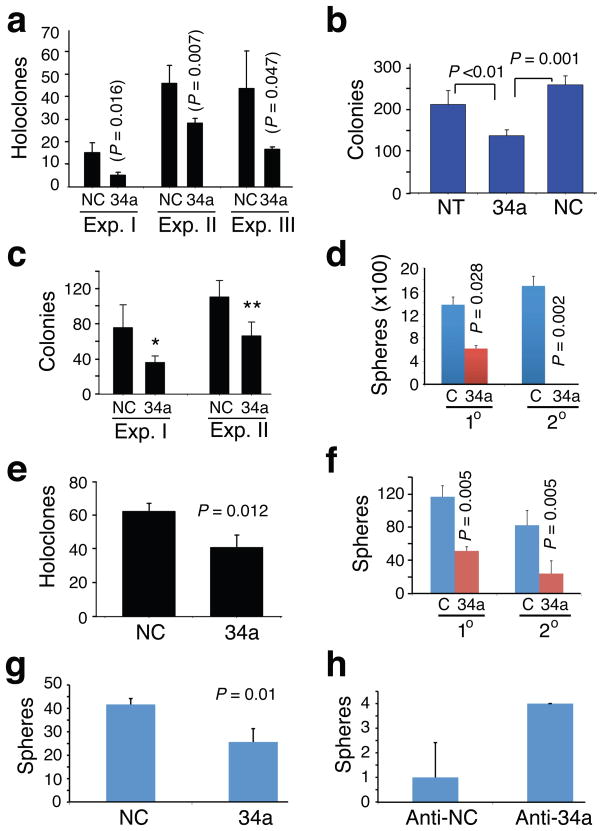
Since the CD44+ PCa cell population is enriched in CSCs, we performed holoclone, colonogenic, and sphere formation assays18,19,27,33,34 to determine whether miR-34a might regulate certain stem cell-associated properties. PCa cell holoclones contain self-renewing cancer cells34 and sphere-formation assays have been widely used to measure stem/progenitor cell activities1,35. We first established stringent assay conditions in which clones (i.e., holoclones formed in culture dish), colonies (formed in Matrigel or methylcellulose), and (floating) spheres were all of clonal origin (Supplementary Fig. 9). Under these conditions, miR-34a overexpression inhibited holoclone formation, clonogenic capacity, and sphere establishment in Du145 (Fig. 3a,b; Supplementary Fig. 2d,e), LAPC4 (Fig. 3c,d), and PPC-1 (Fig. 3e; Supplementary Fig. 3h,i) cells. Importantly, miR-34a inhibited sphere formation in primary HPCa cells (Fig. 3f; Supplementary Fig. 10) and abrogated secondary sphere establishment (Fig. 3d,f). Moreover, HPCa cells infected with lenti-34a formed tiny or differentiated spheres (Supplementary Fig. 10b). Of significance, miR-34a overexpression in purified CD44+ HPCa116 cells inhibited sphere formation (Fig. 3g) and, by contrast, anti-34a increased the inherently low sphere-forming capacity of CD44− HPCa116 cells by several fold (Fig. 3h). Taken together, these observations indicate that miR-34a negatively regulates stem cell properties of PCa cells.
Figure 3. miR-34a inhibits clonal and clonogenic properties of PCa cells.
a. Holoclone assays in Du145 cells. Cells transfected with miR-NC (NC) or miR-34a (34a) oligos were used in three experiments (Exp. I, 100 cells/well scored on d 9; Exp. II, 100 cells/well scored on d 13; Exp. III, 500 cells/well scored on d 7). b. Clonogenic assays in Du145 cells. Cells (3,000/well) were plated in MG and colonies counted on day 13. NT, non-transfected. c. MG clonogenic assays in LAPC4 cells. Two experiments were performed (Exp. I, 1,250 cells/well scored on d 5, *P = 0.005; Exp. II, 25,000 cells/well scored on d 5, **P = 0.015). d. Sphere assays in LAPC4 cells. LAPC4 cells infected with lenti-ctl (C) or lenti-34a were plated (10,000 cells/well) for both 1° and 2° assays and spheres scored on d 15. e. Holoclone assays in PPC-1 cells. Cells transfected with miR-NC or miR-34a oligos were plated (500 cells/well) in triplicate and holoclones quantified on d 5. f. Sphere assays in HPCa101 (Gleason 9) cells. Purified HPCa101 cells infected with lenti-ctl (C) or lenti-34a were plated (20,000 cells/well) for both 1° and 2° and spheres scored 3 weeks later. g,h. Sphere assays in purified CD44+ HPCa116 (Gleason 7) cells transfected with NC or miR-34a oligos (g) or CD44− HPCa116 cells transfected with anti-NC or anti-34a oligos (h). Spheres were scored on d 15.
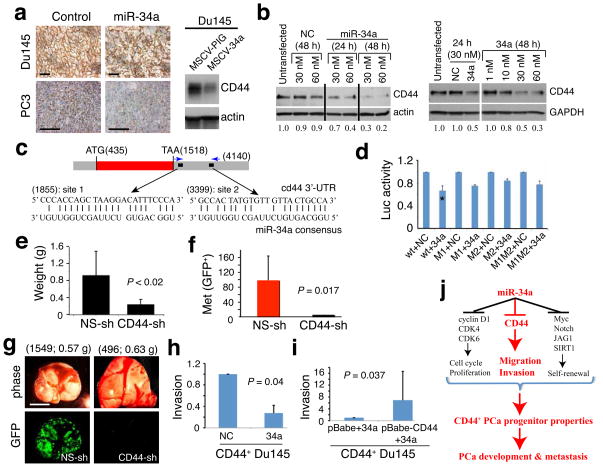
Cyclin D1, CDK4 and 6, E2F3, N-Myc, c-MET, and BCL-2 have been reported to be direct targets of miR-34a20–24,26,30–32. A survey of some of these molecules revealed that miR-34a affected the levels of cyclin D1, CDK4, CDK6 and c-MET in our PCa models (Supplementary Fig. 4d,e; Supplementary Fig. 6d,e). Interestingly, we consistently observed a strong inverse correlation between miR-34a levels and CD44 (Fig. 4a,b; Supplementary Fig. 1a,4e,11a–c; Supplementary Table 2). For example, CD44 protein and CD44+ PCa cells were reduced in miR-34a treated tumors (Fig. 4a). Transfected miR-34a downregulated CD44 in PCa cells (Fig. 4b; Supplementary Fig. 11a,b). In contrast, CD44 mRNA (Supplementary Fig. 4e) and protein (Supplementary Fig. 11c) were increased in anti-34a transfected tumors. Of interest, the target-prediction program rna22 (36) revealed 2 putative miR-34a binding sites in the 3’-UTR of CD44 mRNA (Fig. 4c). When we cloned the 3’-UTR fragment harboring both putative miR-34a binding sites downstream of a luciferase coding sequence (Supplementary Fig. 11d,e), co-transfection of the luciferase reporter and miR-34a oligos into three PCa cell types produced lower luciferase activity than cells co-transfected with the NC oligos but mutation of the seed sequence in either site, especially the distal site, partially abrogated the suppressive effect of miR-34a (Fig. 4d; Supplementary Fig. 11f,g). These results suggest that miR-34a regulates CD44 expression via two binding sites located in the 3’-UTR of the CD44 gene.
Figure 4. CD44 as a direct and functional target of miR-34a.
a. Representative CD44 IHC images in Du145 tumors from cells infected with MSCV-PIG (control) or MSCV-34a vectors (Western blot on the right) and PC3 tumors harvested from animals treated with miR-NC or miR-34a oligos. Scale bars, 10 μm. b. miR-34a downregulates CD44 in Du145 (left) and PPC-1 (right) cells. Relative levels of CD44 indicated at the bottom. c. Schematic of two putative miR-34a binding sites in the CD44 3’-UTR. d. Luciferase experiments in Du145 cells (*P <0.01). e. CD44 knockdown inhibits LAPC4 tumor regeneration (see Supplementary Fig. 12). f,g. CD44 knockdown inhibits PC3 cell metastasis evidenced by both quantification (f) and images (g; scale bar, 100 μm). h,i. Invasion assays. miR-34a oligos inhibit Matrigel invasion of CD44+ Du145 cells (h), which was partially overcome by overexpression of a human CD44 cDNA lacking the miR-34a binding sites at the 3’-UTR (i). Invasion was expressed as values relative to the corresponding controls. j. A schematic summary. The part highlighted in red refers to the novel findings made in the present study.
To determine whether CD44 represents a functionally important target of miR-34a in the context of regulating PCa development, we reduced CD44 expression using a lentiviral CD44-shRNA vector (Supplementary Fig. 1d) in LAPC4, PC3, and Du145 cells. CD44 knockdown in LAPC4 cells inhibited both orthotopic tumor regeneration (Fig. 4e) and lung metastasis (Supplementary Fig. 12). CD44 knockdown in PC3 cells dramatically inhibited metastasis (Fig. 4f,g; Supplementary Fig. 13) without affecting tumor regeneration (not shown). CD44 knockdown in Du145 cells inhibited tumor development in both s.c and orthotopic sites (Supplementary Fig. 14a,b) as well as metastasis (not shown). These results not only reveal a critical role of CD44 itself in determining the tumorigenic and metastatic capacity of PCa cells but also indicate that CD44 knockdown phenocopies the anti-PCa effects of miR-34a. Mechanistically, we observed that the CD44+ PCa cells demonstrated higher migratory (Supplementary Fig. 14c,d) and invasive (Supplementary Fig. 14e) capacities than CD44− cells, which were partially inhibited by miR-34a (Fig. 4h; Supplementary Fig. 14f,g). ‘Rescue’ experiments wherein CD44 was overexpressed using a cDNA that lacked the 3’-UTR containing the miR-34a binding sites abrogated miR-34a-mediated inhibition of invasion of CD44+ Du145 cells (Fig. 4i), reinforcing CD44 as a direct and functional target of miR-34a. In contrast, CD44 overexpression did not significantly relieve miR-34a inhibition of PCa cell proliferation (Supplementary Fig. 15).
We report herein underexpression of miR-34a in tumorigenic CD44+ PCa cells and demonstrate its potent anti-tumor and anti-metastasis effects. We establish miR-34a as a critical negative regulator of CD44+ PCa cells and CD44 itself as an important target of miR-34a. Our results suggest that reduced expression of miR-34a in CD44+ PCa cells contributes to PCa development and metastasis by allowing for the elevated expression of CD44 and manifestation of their migratory, invasive and metastatic properties (Fig. 4j). It is interesting that p53, which directly activates miR-34a, also negatively regulates CD44 through a non-canonical p53-binding site in the promoter37, suggesting the importance in controlling CD44 expression. Considering the widespread expression of CD44 in CSCs (7–16) and functional involvement of CD44 in mediating CSC migration and homing38 and in metastasis of many cancers including PCa, the newly identified miR-34a suppression of CD44 reveals a key role for miRNA-based gene regulation. The emerging role of miR-34a in regulating other CSC32,39 properties (Fig. 4j), coupled with the therapeutic effects of miR-34a on lung26 and prostate (this study) tumors, establishes a strong rationale for developing miR-34a as a novel therapeutic targeting tumorigenic PCa cells.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/naturemedicine/.
Note: Supplementary information is available on the Nature Medicine website.
Supplementary Material
Acknowledgments
We thank K. Claypool and P. Whitney for FACS, the Histology Core for help in IHC, K. Lin for statistical analysis, G. Calin for critically reading the manuscript, and other members of the Tang lab for support and helpful discussions. We also thank Dr. G. Hannon (CSHL, NY, USA) for providing MSCV-PIG vector. This work was supported in part by grants from US National Institutes of Health (R01-AG023374, R01-ES015888, R21-ES015893, R21-CA150009), Department of Defense (W81XWH-07-1-0616, W81XWH-08-1-0472), and Elsa Pardee Foundation (D.G.T) and by two Center Grants (CCSG-5 P30 CA016672-34 and ES07784). C. Liu and H. Li were supported in part by predoctoral fellowships from US Department of Defense.
Footnotes
AUTHOR CONTRIBUTIONS
C.L., K.K., B.L., X.C. and L.P designed and performed the experiments with help from C.J., T.D., H.L., S.H., J.W., and A.B. R. F. provided all HPCa samples. C.L. and D.G.T. prepared the manuscript. D.G.T., with help from D.B., designed the experiments and supervised the whole project. All authors discussed the results and commented on the manuscript.
COMPETING INTERESTS STATEMENT
K.K, J.F.W, A.G.B, and D.B are employees of Mirna Therapeutics, Inc., which develops miRNA-based therapeutics. Other authors declare no competing financial interests.
References
- 1.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 2.Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122:6–7. doi: 10.1016/j.cell.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 3.Melton C, Judson RL, Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature. 2010;463:621–626. doi: 10.1038/nature08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu F, et al. let-7 regulates self-renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 5.Shimono Y, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 7.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 9.Prince ME, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalerba P, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du L, et al. CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res. 2008;14:6751–6760. doi: 10.1158/1078-0432.CCR-08-1034. [DOI] [PubMed] [Google Scholar]
- 12.Zeilstra J, et al. Deletion of the WNT target and cancer stem cell marker CD44 in Apc(Min/+) mice attenuates intestinal tumorigenesis. Cancer Res. 2008;68:3655–3661. doi: 10.1158/0008-5472.CAN-07-2940. [DOI] [PubMed] [Google Scholar]
- 13.Yang ZF, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Takaishi S, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan KS, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci USA. 2009;106:14016–14021. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang S, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 18.Patrawala L, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 19.Patrawala L, Calhoun-Davis T, Schneider-Broussard R, Tang DG. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+α2β1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67:6796–6805. doi: 10.1158/0008-5472.CAN-07-0490. [DOI] [PubMed] [Google Scholar]
- 20.He L, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raver-Shapira N, et al. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell. 2007;26:731–743. doi: 10.1016/j.molcel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Chang TC, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bommer GT, et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol. 2007;17:1298–1307. doi: 10.1016/j.cub.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 24.Tarasov V, et al. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle. 2007;6:1586–1593. doi: 10.4161/cc.6.13.4436. [DOI] [PubMed] [Google Scholar]
- 25.Johnson CD, et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–7722. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 26.Wiggins JF, et al. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010;70:5923–5930. doi: 10.1158/0008-5472.CAN-10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeter C, et al. Functional evidence that the self-renewal gene NANOG regulates human tumor development. Stem Cells. 2009;27:993–1005. doi: 10.1002/stem.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patrawala L, et al. Side population (SP) is enriched in tumorigenic, stem-like cancer cells whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207–6219. doi: 10.1158/0008-5472.CAN-05-0592. [DOI] [PubMed] [Google Scholar]
- 29.Bhatia B, et al. Critical and distinct roles of p16 and telomerase in regulating the proliferative lifespan of normal human prostate epithelial progenitor cells. J Biol Chem. 2008;283:27957–27972. doi: 10.1074/jbc.M803467200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17:193–199. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 31.Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci USA. 2008;105:13421–3426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, et al. MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Res. 2009;69:7569–7576. doi: 10.1158/0008-5472.CAN-09-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li HW, et al. Methodologies in assaying prostate cancer stem cells. Methods Mol Biol. 2009;569:85–138. doi: 10.1007/978-1-59745-280-9_7. [DOI] [PubMed] [Google Scholar]
- 34.Li HW, Chen X, Calhoun-Davis T, Claypool K, Tang DG. PC3 Human prostate carcinoma cell holoclones contain self-renewing tumor-initiating cells. Cancer Res. 2008;68:1820–1825. doi: 10.1158/0008-5472.CAN-07-5878. [DOI] [PubMed] [Google Scholar]
- 35.Dontu G, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miranda KC, et al. A pattern-based method for the identification of microRNA-target sites and their corresponding RNA/RNA complexes. Cell. 2006;126:1203–1217. doi: 10.1016/j.cell.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 37.Godar S, et al. Growth-inhibitory and tumor-suppressive functions of p53 depend on its repression of CD44 expression. Cell. 2008;134:62–73. doi: 10.1016/j.cell.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 39.Ji Q, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009;4:e6816. doi: 10.1371/journal.pone.0006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.