Abstract
Background
The increasing prevalence of type 2 diabetes poses both clinical and public health challenges. Cost-effective approaches to prevent progression of the disease in primary care are needed. Evidence suggests that intensive multifactorial interventions including medication and behaviour change can significantly reduce cardiovascular morbidity and mortality among patients with established type 2 diabetes, and that patient education in self-management can improve short-term outcomes. However, existing studies cannot isolate the effects of behavioural interventions promoting self-care from other aspects of intensive primary care management. The ADDITION-Plus trial was designed to address these issues among recently diagnosed patients in primary care over one year.
Methods/Design
ADDITION-Plus is an explanatory randomised controlled trial of a facilitator-led, theory-based behaviour change intervention tailored to individuals with recently diagnosed type 2 diabetes. 34 practices in the East Anglia region participated. 478 patients with diabetes were individually randomised to receive (i) intensive treatment alone (n = 239), or (ii) intensive treatment plus the facilitator-led individual behaviour change intervention (n = 239). Facilitators taught patients key skills to facilitate change and maintenance of key behaviours (physical activity, dietary change, medication adherence and smoking), including goal setting, action planning, self-monitoring and building habits. The intervention was delivered over one year at the participant's surgery and included a one-hour introductory meeting followed by six 30-minute meetings and four brief telephone calls. Primary endpoints are physical activity energy expenditure (assessed by individually calibrated heart rate monitoring and movement sensing), change in objectively measured dietary intake (plasma vitamin C), medication adherence (plasma drug levels), and smoking status (plasma cotinine levels) at one year. We will undertake an intention-to-treat analysis of the effect of the intervention on these measures, an assessment of cost-effectiveness, and analyse predictors of behaviour change in the cohort.
Discussion
The ADDITION-Plus trial will establish the medium-term effectiveness and cost-effectiveness of adding an externally facilitated intervention tailored to support change in multiple behaviours among intensively-treated individuals with recently diagnosed type 2 diabetes in primary care. Results will inform policy recommendations concerning the management of patients early in the course of diabetes. Findings will also improve understanding of the factors influencing change in multiple behaviours, and their association with health outcomes.
Trial registration
ISRCTN: ISRCTN99175498
Background
Diabetes presents a global challenge to health and well being. The total number of people with the condition is projected to rise from 285 million in 2010 to 438 million in 2030 [1]. More than 2.5 million people in the UK are estimated to have diabetes, and the condition is the leading cause of kidney failure, blindness in adults, and amputations in the UK [2]. The condition can affect mental health and well-being, and is a major risk factor for heart disease and stroke, reducing life expectancy on average by 10 years [3].
The clinical care of people with diabetes currently consumes around 10% of the NHS budget, a total exceeding £9 billion [2]. People with diabetes (or their carers) are responsible for the day-to-day management of their condition, which includes eating a healthy diet, being physically active, taking medication as prescribed and not smoking. There is scope for improvement in the prevalence of health-promoting behaviours among people at risk of and with diabetes [4-6]. Developing effective approaches to prevent progression of the disease by intensification of primary care management, including prescribing and organisation of services, and by directly supporting individual patients in self care, is important in tackling the growing public health burden of type 2 diabetes [7,8].
Evidence to support behavioural interventions to reduce cardiovascular risk
There is good evidence that the onset of diabetes can be delayed, and cardiovascular risk factors reduced, by intensive interventions targeting multiple behaviours among large groups of individuals with impaired glucose tolerance followed up over several years [9]. The lifestyle interventions in these programmes were individually tailored and delivered by case managers or nutritionists with extended training. They drew on a range of theoretical perspectives and used techniques such as goal setting and self monitoring. In these studies the risk of diabetes was directly associated with change in lifestyle: the more behavioural targets that the participants achieved at follow-up, the lower their incidence of diabetes [10,11].
It has been recognised for some time that well organised care, including regular recall and review of patients, is associated with better outcomes [12]. More recently, there is also evidence from primary care that regular patient follow-up supported by prompting of doctors, feedback on goal attainment, and continuing medical education and guidelines can improve cardiovascular risk factors in patients followed 8 years from diagnosis compared with routine care [13]. Later in the disease trajectory there is evidence from secondary care that intensive multifactorial intervention including prescription of medication and behavioural advice compared with routine care, can significantly reduce cardiovascular mortality in patients with type 2 diabetes and microalbuminuria after 8 years. The results showed a 50% reduction in cardiovascular and microvascular events in the intervention group compared with routine care. However, the intervention had only minor effects on carbohydrate and fat intake, and no significant differences were observed for self-reported smoking and exercise habits between groups [14]. The Look AHEAD (Action for Health in Diabetes) trial is a multi-centre, clinic-based randomised trial comparing the effects of an intensive lifestyle intervention with diabetes support and education (control group) on cardiovascular risk factors and major cardiovascular disease among 5,145 overweight or obese individuals with clinically-detected type 2 diabetes [15]. Four-year results from the trial are encouraging, with participants in the intensive lifestyle arm achieving greater weight loss and greater improvements in fitness, glycated haemoglobin, systolic blood pressure, and HDL-cholesterol than those in the control group. The intervention was very intensive and participants were followed up annually. Overall, the evidence to date suggests that the greatest impact on cardiovascular risk is from intensified prescribing rather than patient education or behavioural interventions [7,8]. Despite this there is widespread introduction of healthy lifestyle facilitators, or health trainers, throughout England [16].
Limitations to the evidence to support diabetes management in primary care
While evidence from earlier studies is encouraging, it is not clear which approaches or combinations can cost-effectively support behavioural change in people with newly or recently diagnosed type 2 diabetes in primary care. Most of the studies that demonstrate benefits from interventions targeting multiple behavioural risk factors have been conducted in research clinics or specialist settings. In particular it is unclear whether direct intervention by specialist lifestyle trainers to facilitate behaviour change can add significantly to intensive management by core multidisciplinary primary care teams. In the primary care of type 2 diabetes there are now an increasing number of trials of case management, and self-management of diabetes that include a focus on key risk behaviours; lack of regular physical activity, unhealthy dietary intake and smoking. Reviews of randomised studies [17,18] support the effectiveness of patient self-management training in improving the management of type 2 diabetes in the short term. However, the contents of many interventions have not been clearly specified and measurement of quality of delivery or receipt is largely lacking. It is thus difficult to unravel why the effectiveness of behavioural interventions is so variable. With longer follow-up (>6 months), some of the behavioural interventions that used regular contact with patients for reinforcement were effective in improving glycaemic control. Educational interventions using a patient-centred approach to goal setting and problem-solving were more promising than didactic interventions in improving glycaemic control, weight, and lipid profiles [17-20]. Interventions based primarily on providing information about how to behave to avoid negative health outcomes are less effective than those using well-specified behaviour change techniques, building on participants' own motivations within a psychological framework [21,22].
Most of these studies omit the behaviour of medication taking. Yet intensive use of multiple medications to control cardiovascular risk factors has been a central tenet of effective interventions to improve cardiovascular outcomes in type 2 diabetes over the last ten years [23,24]. Patients can find taking medication regularly difficult, particularly for multiple drugs in asymptomatic conditions [25,26], emphasising the need for interventions to support medication taking as prescribed alongside other health-related behaviours.
A major limitation of studies to date is the inability to isolate the effects of behavioural interventions from other aspects of intensified management including prescribing and organisation of services. This applies to both the effect of the intervention on behaviour, and the subsequent effect of behaviour change on clinical outcomes such as cardiovascular risk. The measurement of the behaviours themselves is a challenge, with the majority of trials relying on self-report, which is imprecise and susceptible to recall bias. Few behavioural interventions specify a hypothesised mechanism of action of behaviour change techniques, and only a minority measure behaviour and its hypothesised determinants along a specified causal pathway. This has limited the ability to identify active ingredients of interventions aimed at facilitating behaviour change and maintenance [27] and to replicate effective interventions in specific clinical settings.
No studies of behavioural management of type-2 diabetes in primary care early in the trajectory of the disease have demonstrated effects on cardiovascular disease-related events or mortality; economic analyses are absent or limited; and few studies examine health-care utilization or patient-centred outcomes such as quality of life or functional status. Performance, selection, attrition, and detection biases are common in the studies reviewed, and external generalisability is often limited.
Large gaps thus remain in the evidence concerning effectiveness and cost-effectiveness of interventions to improve multiple cardiovascular risk factors among patients with type 2 diabetes in primary care by behavioural means [20,28]. It is unclear whether interventions, based on theory and evidence from psychology [29,30] and designed to facilitate and maintain changes in behaviour (physical activity, diet, medication adherence and smoking), could be cost-effective among people detected early in the trajectory of the disease. It is also unclear how such interventions are best delivered within a health service. The existing evidence points to the importance of clinical and social context of both the patient and practitioner in enabling or obstructing health-related behaviour changes, and of a coherent approach by primary and secondary care [20]. The ADDITION-Plus trial was established to address these gaps.
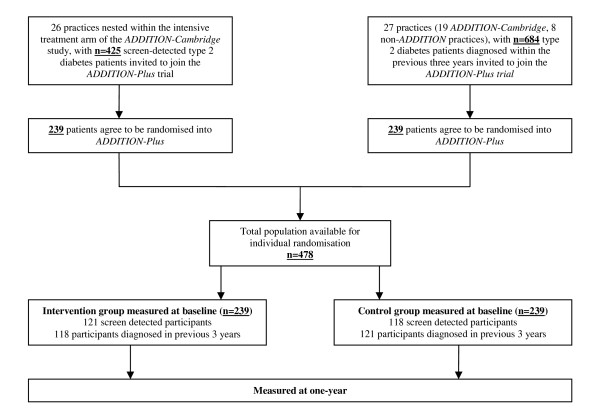
The ADDITION-Plus trial was originally established within the intensive treatment group in the Cambridge centre of the ADDITION study (Figure 1). In brief, the Anglo-Danish-Dutch Study of Intensive Treatment of people with newly diagnosed diabetes in primary care (ADDITION) study was set-up in 2001 [31]. It is a primary care-based study consisting of a screening phase followed by a pragmatic open-label cluster randomised controlled trial comparing the effect on cardiovascular risk of intensive multi-factorial therapy and theory-based diabetes education delivered by practice nurses (intensive treatment) with routine care in patients with screen-detected diabetes.
Figure 1.
Design and participant flows in the ADDITION-Plus trial (Cambridge, UK).
The aim of ADDITION-Plus was to assess whether a behaviour change intervention delivered by trained and quality-assured lifestyle facilitators, external to the primary care team, was a cost-effective addition to intensive treatment and could achieve and maintain changes in important health related behaviours (physical activity, dietary change, medication adherence and smoking cessation) when offered to people with recently diagnosed diabetes already receiving intensive general practice based care. Follow-up was at one year post-randomisation. The trial recruited screen-detected type 2 diabetes patients from within the ADDITION-Cambridge trial [32], and included patients with type 2 diabetes clinically diagnosed within the previous three years. As such, the ADDITION-Plus trial allows estimation of the additional contribution of a facilitator-led intervention to good practice care in the optimisation of healthy behaviours and well-being, and to the reduction in cardiovascular risk factors in individuals with recently diagnosed diabetes.
ADDITION-Plus objectives
To quantify the effectiveness and cost-effectiveness of adding a facilitator-led theory-based behaviour change intervention to intensive treatment of screen- and clinically- detected type 2 diabetes patients, and to analyse the psychological, socio-demographic and clinical predictors of behaviour change in the cohort. The following research questions are posed:
Trial analysis
1. Can a facilitator-led intervention, based on theory and evidence from psychology, to increase and maintain health-promoting behaviours (physical activity, dietary change, taking medication and smoking cessation) achieve clinically important and measurable change in these behaviours when offered to people with screen-detected diabetes and to those recently diagnosed with diabetes?
Primary outcomes include objectively measured physical activity, fruit and vegetable intake, medication adherence, and smoking status.
Secondary outcomes include (i) a composite measure of behaviour derived from the four primary outcomes; (ii) self-reported behaviours; (iii) modelled cardiovascular risk (UKPDS risk engine, v3[33]); (iv) levels of individual clinical risk factors; (v) functional status, health utility and quality of life; and (vi) costs and cost-effectiveness.
Cohort analysis
2. What and how changes were achieved in objectively measured health behaviours and health outcomes across the trial cohort?
2.1 What are the psychological, socio-demographic and clinical predictors of self-reported and objectively measured behaviour change?
2.2 What are the clinical consequences of behaviour change?
Methods and Design
Design and setting
The ADDITION-Plus study is nested within the intensive treatment arm of the ADDITION-Cambridge trial with additional practices (n = 8) added to increase the recruitment of recently diagnosed patients; see Figure 1 for details of study design and patient flows. Participants were recruited from 34 general practices in urban, suburban and rural Cambridgeshire, East Hertfordshire, West Suffolk and North Essex areas of England. 239/425 eligible screen-detected patients from ADDITION-Cambridge study, and 239/684 patients clinically diagnosed within the previous 3 years were individually randomised to receive intensive treatment alone or in conjunction with a facilitator-led behaviour change intervention.
Ethical approval was obtained from the Eastern Multi-Centre Research Ethics Committee (reference number: 02/5/54). Participants gave written informed consent to take part in the study. ISRCTN 99175498.
Participants
Eligibility criteria (initially assessed by general practice staff) included: age (40-69yrs) with type 2 diabetes following (i) screening in the ADDITION programme or (ii) clinical diagnosis during the three previous years in participating GP surgeries. Exclusion criteria included women who were pregnant or lactating or anybody who had a psychotic illness or an illness with a likely prognosis of less than one year.
Individual randomisation of participants to one of two carefully characterised interventions was independent of intervention facilitators, study coordination and measurement teams, and central using a partial minimisation procedure that dynamically adjusted the randomisation probabilities to balance stratifiers (age, sex and general practice, and within screen-detected and clinically-diagnosed subgroups: smoking, self-reported medication adherence [34] and BMI). The stratifiers were individually weighted in order that those with more categories (such as general practice) could achieve an even distribution between the two arms. A random element was included in the minimisation to allow a sound basis for inference.
Intensive treatment (comparison arm)
The following features were added to routine multi-disciplinary primary care of diabetes to achieve intensive treatment in both trial groups:
1. Academic detailing for general practitioners and practice nurses: A practice-based academic detailing session for primary care teams was conducted by a local diabetologist or GP opinion leader to describe the treatment algorithms and targets, patient materials, and to present the evidence underpinning intensive treatment.
2. Treatment algorithms: These were based on trial data demonstrating the benefits of intensive treatment of several cardiovascular risk factors in people with diabetes [35-38], and recommended medication within licensed indications (Table 1). GPs were advised to consider prescribing an angiotensin converting enzyme (ACE) inhibitor to patients with a blood pressure≥120/80 and either a previous cardiovascular event or at least one other cardiovascular risk factor [39]. The remainder of the intervention was based on the stepwise target-led regimen from the Steno-2 study [23] aimed at optimising hyperglycaemia, hypertension, dyslipidaemia and microalbuminuria. GPs were advised to consider prescribing 75 mg of aspirin daily to patients without specific contraindications. Although targets for treatment were specified and classes of drugs recommended, where there was a choice between individual drugs the decision was made by the GPs and patients. The intensive treatment protocol was revised after publication of the Heart Protection Study [40] to include a recommendation to prescribe a statin to all patients with a cholesterol level of≥3.5 mmol/l.
Table 1.
Measures used at baseline and one year in the ADDITION-Plus trial (Cambridge, UK)
| Measures | Baseline | One year | ||
|---|---|---|---|---|
| C | I | C | I | |
| Objectively measured health behaviours | ||||
| Physical activity (ActiHeart)[47] | X | X | ||
| Dietary intake (plasma vitamin C concentration) | X | X | X | X |
| Medication adherence (plasma drug concentrations) | X | X | ||
| Smoking status (plasma cotinine concentration) | X | X | ||
| Self-reported health behaviours | ||||
| Physical activity: EPAQ-2 [52], IPAQ [53] | X | X | X | X |
| Dietary intake: EPIC food frequency questionnaire [54] | X | X | X | X |
| Medication adherence: | ||||
| All drugs during the last month [34] | X | X | X | X |
| Hypoglycaemic drugs during the last month [34] | X | X | ||
| Smoking status and alcohol consumption (questionnaire) | X | X | X | X |
| CVD risk | ||||
| Modelled ten-year CVD risk: UKPDS risk engine, v3 [33] | X | X | X | X |
| Self-reported history of angina, heart attack and stroke | X | X | X | X |
| Biochemical measures | ||||
| HbA1c, total cholesterol, HDL and LDL cholesterol, triglyceride, urea and electrolytes, creatinine, albumin, bilirubin, alanine amino transferanse (ALT), alkaline phosphatase, aspartate aminotransference (AST), thyroid stimulating hormone (TSH), urine albumin/creatinine ratio | X | X | X | X |
| Clinical measures | ||||
| Waist circumference, height, weight, blood pressure, pulse, body fat impedance and ECG | X | X | X | X |
| Cardiorespiratory fitness measured using a sub-maximal VO2 test[47] | X | X | ||
| Rose angina questionnaire [55] | X | X | ||
| Neuropathy questionnaire (adapted from the Michigan screening instrument) 1 | X | X | X | X |
| Health utility, functional status, quality of life, well-being, treatment satisfaction and anxiety | ||||
| Diabetes well-being (WBQ-28) [57] | X | X | ||
| SF-36 [74] | X | X | ||
| Diabetes-related quality of life (ADDQOL) [57] | X | X | ||
| Diabetes Treatment satisfaction (DTS) [57] | X | X | ||
| EuroQol EQ-5D [59] | X | X | X | X |
| Consultation and relational empathy measure (CARE) [61] | X | X | ||
| Spielberger Short form State anxiety inventory [60] | X | X | X | X |
| Assessment of diabetes services | X | X | ||
| Frequency of and views about self-monitoring of blood glucose (DiGEM questionnaire [62]) | X | X | ||
| Beliefs about behaviour change, illness perceptions and habit | ||||
| Beliefs about behaviour change: intention, perceived behavioural control, behavioural beliefs (physical activity, eating a low-fat diet; taking medication and smoking cessation) [29] | X | X | X | X |
| Illness perceptions (consequences and treatment control sub-scales IPQ-R) [65] | X | X | X | X |
| Diabetes knowledge1 | X | X | ||
| Habit [67]) in relation to physical activity and dietary change | X | X | ||
| Intervention evaluation | ||||
| Facilitator assessment | X | |||
| Skills in last 12 months in relation to lifestyle change and medication taking1 | X | |||
| Costs | ||||
| Self-reported current medication/vitamins | X | X | X | X |
| Personal patient costs1 | X | X | ||
| Health service and medication use in the previous three months (adapted from the Aberdeen Health Service Research Unit questionnaire) 1 | X | X | ||
C = control group; I = intervention group; 1 Questionnaire developed for the study
3. Treatment targets: HbA1c <7% (with a recommendation to initiate treatment at a threshold of 6.5%), blood pressure≤135/85 mmHg, total cholesterol <5 mmol/l or < 4.5 mmol/l for people with a history of ischaemic heart disease.
4. Audit and feedback: Interactive practice-based audit and feedback sessions were organised around 6 and 14 months after the academic detailing session and annually thereafter. They consisted of discussion of overall achievement of treatment targets and optimisation of the management of individual patients.
5. Provision of glucometers for patients: Blood glucometers and training in their use were provided to the primary care team. However, the decision to offer a glucometer to a patient was left to practitioners.
6. Theory-based diabetes education: Practice nurses were provided with theory-based education materials to discuss with, and give to patients in order to provide a shared framework for the causes, consequences and treatment of diabetes ('Getting Started with Diabetes'). The materials were developed by a multidisciplinary team and drew on Leventhal's Common-Sense Model [41]. They cross-referred to 'Diabetes for Beginners-Type 2' a Diabetes UK publication [42] that was included in the patient information pack. The materials stressed the importance of a healthy lifestyle for the control of diabetes and associated health problems and provided targets for behaviour change. Specifically, participants with a BMI > 28 kg/m2 were encouraged to lose 5-10% of their body weight, and all participants to increase physical activity gradually (recommendations to reach the equivalent of 35 minutes of brisk walking per day for 7 days per week), decrease the consumption of fatty foods and sugar, increase the consumption of fruit, vegetables, and whole grains, avoid excessive alcohol intake, take their medication regularly, self-monitor their blood glucose level (if applicable) and attend annual health checks. Participants who smoked were encouraged to stop.
7. Funding to support more frequent contact between patients and practitioners: Practices received funding equivalent to three 10-minute consultations with a GP and three 15-minute nurse appointments per patient per year.
8. Dietary counselling: GPs were recommended to refer all newly diagnosed patients to a dietician.
Intervention group: Intensive treatment plus facilitator led behaviour change intervention
Participants in this group received intensive treatment (described above) plus a facilitator-led, individually tailored behaviour change intervention, based on psychological theory and evidence. The intervention was delivered by trained lifestyle facilitators, who were not part of the practice team. The facilitators used detailed protocols for each contact with the participant and received on-going supervision and feedback from a Clinical Psychologist, informed by assessment of tape-recorded consultations, enabling tight quality assurance of intervention delivery. The intervention was designed to build on the diabetes education delivered by practice nurses and intensive treatment by the practice team. The behaviours targeted in the intervention were physical activity, dietary intake, medication adherence, and smoking cessation. Hypothesised mediators of behaviour change targeted in the intervention included illness perceptions based on Leventhal's Common-Sense Model [41] and beliefs about behaviour change based on the Theory of Planned Behaviour (TPB) [29]. The intervention targeted instrumental (e.g. health) and affective beliefs about changing specific behaviours, (lack of) encouragement by important others (subjective norm), and perceived barriers and facilitators of behaviour change (perceived behavioural control). The behaviour change techniques used by the facilitators could be mapped onto the TPB (e.g., giving information, strengthening motivation), Operant Theory (identifying cues for action and reinforcement of behaviour change or effort), Carver and Scheier's Control Theory (e.g., goal setting, action planning, self-monitoring) and Relapse Prevention Theory (e.g., preparing for and dealing with setbacks) [29,43-45]. Facilitators taught patients this range of self-regulatory skills to achieve behaviour change and maintenance over time, which was supported by a manual describing the skills. Each patient initially selected to work on the behaviour(s) they most wanted to change and felt most confident about changing. The facilitator later discussed with them other domains in which change was possible and encouraged patients to consider setting additional goals. Interested patients were offered pedometers and/or glucometers. The intervention was delivered over one year at the participants' surgeries. It included a one-hour introductory meeting followed by six 30-minute meetings and four brief phone calls. Facilitators visited participant's homes or workplaces if it was not possible to meet in the surgery.
Measurement
Table 1 shows the measures taken at each stage in the ADDITION-Plus study. Baseline measurements were carried out on all eligible patients including the completion of questionnaires, physiological and anthropometric measures, and venesection. Similar measurements were conducted one year after recruitment. Measurements were undertaken at outpatient clinical research facilities by trained staff following standard operational procedures and unaware of participants' study group allocation. Double data entry of all anthropometric and questionnaire measures was undertaken by experienced, independent agencies, blind to study group (Wyman Dillon Research and Data Management, Bristol, UK and Document Technologies and Imaging Solutions Ltd, Chalgrove, Oxford, UK).
Objectively measured health behaviours
Physical activity was assessed at one-year using a combined heart rate and movement sensor (Actiheart, CamNtech, Cambridge, UK), which was worn continuously for at least 4 days [46]. A graded treadmill walk test was used for individual calibration of heart rate [47], a procedure which also provides an estimate of cardio-respiratory fitness by extrapolation of the HR-VO2 relationship to age-predicted maximal heart rate [48]. Heart rate data collected during the free-living period were pre-processed [49] and activity intensity (J/min/kg) was estimated using a branched equation framework [47,50]. Resulting time-series data were summarised into physical activity energy expenditure (PAEE, in kJ/kg/day) and time spent in sedentary and moderate-to-vigorous intensity physical activity (SPA and MVPA, min/day), whilst minimising diurnal information bias caused by non-wear periods (segments of non-physiological data). Participants without individual calibration data but who provided data during free-living were processed using an age, sex, beta blocker, and sleeping heart rate adjusted group calibration equation for the translation of heart rate into activity intensity.
Dietary intake was assessed at baseline and one-year by plasma vitamin C levels. Vitamin C is not endogenously produced and therefore provides a robust measurement of consumption. Medication adherence was assessed at one-year by plasma drug levels. Concentrations of metformin, simvastatin and atorvastatin in plasma samples were measured by liquid-chromatography mass-spectrometry (LC-MS/MS) after protein precipitation extraction. The LC-MS/MS methods for simvastatin and atorvastatin were established at Quotient Bioresearch Ltd. (Fordham) for the ADDITION-Plus study. The metformin method was validated at Quotient Bioresearch Ltd. (Fordham) for a previous study [51]. The calibration ranges for each of the analytes were as follows: metformin 5.00-5000 ng/mL, simvastatin 0.100-50.0 ng/mL and atorvastatin 0.0600 to 30.0 ng/mL. Objective measurement of smoking was assessed by analysis of cotinine levels in plasma samples using an Immulite® Nicotine metabolite solid phase competitive chemiluminescent immunoassay (Siemens).
Self-reported health behaviours
Physical activity was assessed using the validated EPAQ2 [52] and IPAQ [53] questionnaires. Dietary intake was evaluated using a validated food frequency questionnaire [54]. Medication adherence was assessed by the Medication Adherence Report Schedule (MARS) questionnaire [34]. Smoking status and alcohol consumption were assessed by questionnaire.
Modelled CVD risk
The UKPDS model [33] uses information on sex, ethnicity, smoking status, presence or absence of atrial fibrillation, systolic blood pressure, HbA1c, total cholesterol, and HDL-cholesterol to predict the 10-year risk of primary CVD. Predicted events include myocardial infarction, sudden cardiac death, other incident ischaemic heart disease, stroke, and peripheral vascular disease death.
Biochemical measures
HbA1c was analysed in venous samples at the time of diagnostic testing by ion-exchange high-performance liquid chromatography on a Tosoh machines (Tosoh Bioscience, Redditch, UK). Serum total cholesterol, HDL-cholesterol and triglycerides was measured by means of enzymatic techniques (Dade Behring Dimension analyser, Newark, USA). Plasma creatinine was analysed with kinetic colorimetric methods, and urine albumin by rate nephelemetry (Dade Behring Nephelometer II, Newark, USA). Plasma levels of urea and electrolytes, bilirubin, alanine aminotansferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, and thyroid stimulating hormone (TSH) and urine levels of creatinine were assayed by means of the Dade Behring Dimension analyser. Metaphosphoric acid was added to plasma samples for vitamin C analysis before being stored at -80C°. Plasma vitamin C level was measured with a Fluoroskan Ascent FL fluorometer. The albumin-to-creatinine ratio (ACR) was measured on a random spot urine specimen.
Clinical measures
Blood pressure was calculated as the mean of three measurements performed after at least 10 minutes rest, while participants were seated with the cuff on the predominant arm at the level of the heart, using an automatic sphygmomanometer (Omron M4, UK). ECG was recorded by a 12 lead machine. Body height and weight were measured in light indoor clothing and without shoes using a fixed rigid stadiometer and a scale (SECA, UK) respectively. Waist circumference was estimated as the average of two measurements taken with a tape measure halfway between the lowest point of the rib cage and the anterior superior iliac crests when standing. Body fat percentage was measured by bio-electrical impedance (TANITA, Tokyo, Japan). Angina was assessed using the Rose angina questionnaire [55]. Neuropathy was evaluated using an adapted version of the Michigan Neuropathy Screening Instrument [56].
Health utility, functional status, quality of life, well-being, treatment satisfaction and anxiety
The generic and disease-specific instruments used were diabetes well-being questionnaire (W-BQ12) [57], SF-36 [58], Audit of Diabetes-Dependent Quality of Life (ADDQoL) [57], diabetes treatment satisfaction (DTS) [57], and EuroQol (EQ-5D) [59], and the short form of the state scale of the Spiegelberger State-Trait Anxiety Inventory [60]. All patients completed the consultation and relational empathy (CARE) measure in relation to the GP and practice nurse; participants in the intervention group also completed this measure in relation to the lifestyle facilitator [61]. Participants were also asked to complete six items at one year asking about their views on the diabetes service they had received in the last 12 months, including frequency of contact, information and advice received, and satisfaction with the service. Participants were also asked about the frequency of and views about self-monitoring of blood glucose using the Diabetes Glycaemic Education and Monitoring (DiGem) study questionnaire [62].
Beliefs about behaviour change, illness perceptions, and habit
A questionnaire, developed according to TPB guidelines [29,63], assessed selected cognitions about becoming more physically active, eating a lower fat diet, taking medication and smoking: intention, perceived behavioural control, and behavioural beliefs. Each construct was assessed with two items, measured on a 5-point Likert-type scale ranging from 'strongly disagree' to 'strongly agree'. Examples of perceived control items are: 'I am confident that I could be more physically active/eat a lower fat diet in the next 12 months, if I wanted to'; for intention: 'I intend to be more physically active/eat a lower fat diet in the next 12 months'; and for behavioural beliefs: 'If I was more physically active/did eat a lower fat diet in the next 12 months, it is likely that I would lose weight'. We used the consequences and treatment control subscales (11 items) of the Illness Perception Questionnaire-Revised (IPQ-R) [64,65]. The consequences scale assessed seriousness of diabetes and the impact of diabetes on various aspects of life. An example is: 'My diabetes is a serious condition'. The treatment control scale assessed beliefs and feelings associated with the management of diabetes. An example is: 'My treatment can control my diabetes'. Both scales were measured on 5-point Likert-type scales ranging from 'strongly disagree' to 'strongly agree'. Participants were also asked to complete a nine-item closed-response questionnaire covering basic knowledge of diabetes and its' management [66]. At one year, participants were asked to write down the most important change that they had made in their physical activity and dietary intake, and 12 items were used to assess to what extent these changes had become a habit [67].
Intervention evaluation
Participants in the intervention group completed 10 items at one year about their views on the frequency of contacts with the lifestyle facilitators, clarity of advice given, educational materials, and overall intervention. They also completed a skills questionnaire assessing their confidence in relation to using nine skills (e.g., goal setting, action planning, self-monitoring) on a 10-point scale ranging from 'not at all confident' to 'very confident', and use of these skills in the past 11 months in order to increase physical activity, eat a lower-fat diet, try to stop smoking (if applicable) and try to take medicines regularly as prescribed (if applicable) on a binary scale (yes/no).
Costs
Health service use in the three months prior to follow-up was quantified using an adapted version of the Health Services Research Unit Aberdeen questionnaire that enquires about the use of services (consultations with healthcare professionals and hospitalisations) and medications [68].
Participant safety
Treatment algorithms were developed with advice from local diabetes specialists who also contributed to the initial and follow-up practice-based training sessions for primary care staff involved in diabetes care. The responsibility for prescribing and management decisions remained with the general practitioners. Classes of medication were only recommended within licensed indications. Before taking part in the treadmill test, participants completed a pre-test screening questionnaire incorporating the Rose angina questionnaire. If they answered positively to any of the questions, a short medical review was completed by a clinical member of the team. Blood pressure, current medications and ECG (if available) were also reviewed following strict protocols before a participant was allowed to begin the treadmill test.
Statistical analysis
The additional benefit of the facilitator-led behaviour change intervention on objective measures of physical activity level, medication adherence, and change from baseline in plasma vitamin C and smoking status will be assessed using an intention to treat analysis comparing the intervention and comparison groups, using linear regression for continuous outcomes and a comparison of proportions for binary outcomes. Continuous outcomes will be analysed with adjustment for the baseline value of the outcome, where this has been measured, in order to improve precision. Those participants missing the baseline value will remain included in the analysis by using the missing indicator method, which is valid for pre-randomisation measures in trials [69]. Due to the large number of stratifier categories, there will be no adjustment for stratifiers in order to ensure that estimation of intervention effects is stable. The modelled ten-year cardiovascular risk will be log transformed before analysis and computed for those with and without a history of previous CVD events. The primary intention to treat analysis will be supported by per protocol analyses of primary outcomes, where the per protocol population is defined to be those participants attending the introductory and initial three core intervention sessions. The impact of missing primary outcome data will be examined within sensitivity analyses using the multiple imputation method of Rubin [70]. This will involve fitting an imputation model from the observed data, from which missing data will be imputed under two opposing scenarios. In the first scenario it is assumed that the same full intervention-minus-comparison effect applies amongst participants with missing data as has been observed amongst their counterparts with observed data. In the second scenario it is assumed that the intervention-minus-comparison effect is zero for participants with missing data. Ten complete datasets will be imputed, as recommended, to incorporate the uncertainty in the residuals and parameter estimates of the imputation model. Subgroup analyses will be confined to the comparison of intervention effects on primary outcomes by the route of diagnosis (screen-detected versus already-diagnosed).
The perspective for cost analysis will be the health service and will include recent use of services (GP/hospital appointments & admissions) and prescribed medications in the previous 3 months. The costs of intensive treatment with and without the facilitator-led behaviour change intervention will be compared with unit change in health utility. In addition, costs at one-year and future costs derived from existing data [71] will be compared with risk of death and cardiovascular events, with appropriate sensitivity analysis.
Cohort analyses will be reported separately and will test causal pathways between behavioural determinants, behaviour change and consequences of behaviour change.
Sample size
The trial was designed within the main ADDITION-Cambridge trial. It was anticipated that 500 patients would participate in the ADDITION-Plus trial and that 200 individuals per group would be available with follow-up data. With these numbers, there is 80% power at the 5% level of significance level to detect the following relevant differences between arms in primary outcomes:
Physical activity:
A 0.017 kJ/kg/min difference in PAEE. This is based on a standard deviation of 0.058 at baseline in the ProActive trial dataset [72] with mean 0.078. A 0.017 absolute difference represents a 22% relative difference in mean energy expenditure between groups.
Diet:
A 5.5 umol/l difference in plasma vitamin C (control group mean 53, SD 19, EPIC study [73]). Adjustment for baseline level in the analysis allows a finer difference of 4.0 umol/l to be detected between arms (EPIC test-rest correlation of 0.67).
Medication adherence:
A 10% absolute difference in the proportion of low adherers to hypoglycaemic medications (control group 18%, Supported Adherence to Medication pilot study [51]).
Smoking:
A 9.5% absolute difference in the proportion smoking (control group 17.9%).
Discussion
The ADDITION-Plus trial is designed to establish the medium-term effectiveness and cost-effectiveness of a facilitator-led intervention tailored to support change in multiple behaviours among intensively-treated individuals with recently diagnosed diabetes in primary care. Key features of this trial include (i) a carefully characterised target group and intervention; (ii) an intervention based on theory and evidence from psychology to support change in key behaviours affecting CVD risk - physical activity, dietary intake, medication adherence and smoking; (iii) quality assured delivery enabled by training, ongoing supervision and protocols; (iv) objective measurements of behaviours as well as self-report of behaviours, functional status and well-being, and; (v) an examination of the psychological, socio-demographic and clinical predictors of behaviour change as well as the clinical consequences of behaviour change in the cohort.
Results will inform policy recommendations concerning the introduction of health trainer initiatives out-with the primary-secondary care team to assist in early management of patients with type 2 diabetes. They will inform both the education of patients and practitioners, the quality assurance of behaviour change programmes, and will improve understanding of the association between changes in health behaviours and health outcomes.
Competing interests
The authors declare that they have no competing interest.
Authors' contributions
SJG, NJW, SS and ALK are the principal investigators for the ADDITION-Plus trial. AT is the trial statistician, WH is intervention programme co-ordinator, KMW is the trial co-ordinator, JG and FW are trial assistants, SB is an intervention facilitator, IH is a Clinical Psychologist, TF helped with data management and analysis, and SB and KW are part of the Physical Activity Technical team. RKS, SJG, WH, and ALK drafted the manuscript. All authors read and approved the final manuscript. SJG is the paper guarantor.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Simon J Griffin, Email: simon.griffin@mrc-epid.cam.ac.uk.
Rebecca K Simmons, Email: rebecca.simmons@mrc-epid.cam.ac.uk.
Kate M Williams, Email: kmw36@medschl.cam.ac.uk.
A Toby Prevost, Email: toby.prevost@kcl.ac.uk.
Wendy Hardeman, Email: wh207@medschl.cam.ac.uk.
Julie Grant, Email: jg274@medschl.cam.ac.uk.
Fiona Whittle, Email: fcw30@medschl.cam.ac.uk.
Sue Boase, Email: sab72@medschl.cam.ac.uk.
Imogen Hobbis, Email: I.Hobbis@uea.ac.uk.
Soren Brage, Email: soren.brage@mrc-epid.cam.ac.uk.
Kate Westgate, Email: kate.westgate@mrc-epid.cam.ac.uk.
Tom Fanshawe, Email: t.fanshawe@lancaster.ac.uk.
Stephen Sutton, Email: srs34@medschl.cam.ac.uk.
Nicholas J Wareham, Email: nick.wareham@mrc-epid.cam.ac.uk.
Ann Louise Kinmonth, Email: alk25@medschl.cam.ac.uk.
Acknowledgements
We are grateful to all participants and the practices teams for taking part in the ADDITION-Plus trial - Arbury Road Surgery, Ashwell Surgery, Birchwood Surgery, Bottisham Medical Practice, Brookfields/Cherry Hinton, Buckden Surgery, Clarkson Surgery, Cornerstone Practice, Cornford House Surgery, Cottenham Surgery, Dr Eaton & Partners (Saffron Walden), George Clare Surgery, Great Staughton Surgery, Haddenham Surgery, Hilton House Surgery, Manea Surgery, Milton Surgery, New Roysia Surgery, Orchard House Surgery, Orton Medical Practice, Lensfield Road Surgery, Parkhall Road Surgery, Park Medical Centre, Petersfield Medical Practice, Riverside Practice, Rookery Medical Centre, Rosalind Franklin House, South Street Surgery, St Mary's Surgery, Thaxted Surgery, The Old Exchange, The Spinney Surgery, The Surgery (Over), and Woolpit Surgery.
The trial is supported by the Wellcome Trust, the Medical Research Council, Diabetes UK and National Health Service R&D support funding. ALK, KMW & SJG were members of the National Institute for Health Research (NIHR) School for Primary Care Research. ALK and SS are NIHR Senior Investigators. The General Practice and Primary Care Research Unit is supported by NIHR Research funds. SJG receives support from the Department of Health NIHR Programme Grant funding scheme [RP-PG-0606-1259]. The views expressed in this publication are those of the authors and not necessarily those of the Department of Health.
We thank the Cambridge University Hospitals NHS Foundation Trust Department of Clinical Biochemistry and the NIHR Cambridge Biomedical Research Centre, Core Biochemical Assay Laboratory for carrying out the biochemical assays.
The ADDITION- Plus team includes
Ruhul Amin, Judith Argles, Gisela Baker, Rebecca Bale, Roslyn Barling, Mark Betts, Sue Boase, Soren Brage, James Brimicombe, Ryan Butler, Adam Dickinson, Justin Basile Echouffo Tcheugui, Ulf Ekelund, Tom Fanshawe, Francis Finucane, Philippa Gash, Julie Grant, Simon Griffin, Wendy Hardeman, Imogen Hobbis, Ann Louise Kinmonth, Stefanie Mayle, Tom McGonigle, Joanna Mitchell, Nicola Popplewell, Andrew T. Prevost, Paul Roberts, Lincoln Sargeant, Rebecca Simmons, Matt Sims, Jenny Smith, Megan Smith, Stephen Sutton, Nicholas Wareham, Kate Westgate, Fiona Whittle, Kate Williams, the Field Epidemiology, Data Management, IT, Physical Activity Technical and Study Coordination teams of the Medical Research Council Epidemiology Unit. The General Practice and Primary Care Research Unit at the University of Cambridge and the Medical Research Council Epidemiology Unit in Cambridge jointly coordinated the baseline and one-year follow-up phases of the study.
References
- IDF. The Diabetes Atlas, Fourth Edition. IDF. Brussels; 2009. [Google Scholar]
- Diabetes UK. Diabetes UK Impact Report. Diabetes UK. London; 2008. [Google Scholar]
- Diabetes UK. Diabetes in the UK 2004: A report from Diabetes UK. Diabetes UK; 2004. [Google Scholar]
- Nothwehr F, Stump T. Health-promoting behaviors among adults with type 2 diabetes: findings from the Health and Retirement Study. Prev Med. 2000;30(5):407–414. doi: 10.1006/pmed.2000.0658. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Fisher EB, Anderson BJ, LaGreca A, Marrero D, Johnson SB, Rubin RR, Cox DJ. Behavioral science in diabetes. Contributions and opportunities. Diabetes care. 1999;22(5):832–843. doi: 10.2337/diacare.22.5.832. [DOI] [PubMed] [Google Scholar]
- Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004;363(9421):1589–1597. doi: 10.1016/S0140-6736(04)16202-8. [DOI] [PubMed] [Google Scholar]
- Shojania KG, Ranji SR, McDonald KM, Grimshaw JM, Sundaram V, Rushakoff RJ, Owens DK. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. Jama. 2006;296(4):427–440. doi: 10.1001/jama.296.4.427. [DOI] [PubMed] [Google Scholar]
- Sperl-Hillen JM, O'Connor PJ. Factors driving diabetes care improvement in a large medical group: ten years of progress. The American journal of managed care. 2005;11(5):S177–185. [PubMed] [Google Scholar]
- Lindstrom J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemio K, Hamalainen H, Harkonen P, Keinanen-Kiukaanniemi S, Laakso M. et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368(9548):1673–1679. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M. et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Griffin S. Diabetes care in general practice: meta-analysis of randomised control trials. BMJ (Clinical research ed. 1998;317(7155):390–396. doi: 10.1136/bmj.317.7155.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivarius NF, Beck-Nielsen H, Andreasen AH, Horder M, Pedersen PA. Randomised controlled trial of structured personal care of type 2 diabetes mellitus. BMJ (Clinical research ed. 2001;323(7319):970–975. doi: 10.1136/bmj.323.7319.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Archives of internal medicine. 2010;170(17):1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Psychological Society Health Psychology Team. Improving health: changing behaviour - NHS health trainer handbook. DOH; 2008. [Google Scholar]
- Norris SL, Nichols PJ, Caspersen CJ, Glasgow RE, Engelgau MM, Jack L, Isham G, Snyder SR, Carande-Kulis VG, Garfield S. et al. The effectiveness of disease and case management for people with diabetes. A systematic review. Am J Prev Med. 2002;22(4 Suppl):15–38. doi: 10.1016/S0749-3797(02)00423-3. [DOI] [PubMed] [Google Scholar]
- Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes care. 2001;24(3):561–587. doi: 10.2337/diacare.24.3.561. [DOI] [PubMed] [Google Scholar]
- Norris SL, Zhang X, Avenell A, Gregg E, Bowman B, Serdula M, Brown TJ, Schmid CH, Lau J. Long-term effectiveness of lifestyle and behavioral weight loss interventions in adults with type 2 diabetes: a meta-analysis. Am J Med. 2004;117(10):762–774. doi: 10.1016/j.amjmed.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Goldstein MG, Whitlock EP, DePue J. Multiple behavioral risk factor interventions in primary care. Summary of research evidence. Am J Prev Med. 2004;27(2):61–79. doi: 10.1016/j.amepre.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Padgett D, Mumford E, Hynes M, Carter R. Meta-analysis of the effects of educational and psychosocial interventions on management of diabetes mellitus. J Clin Epidemiol. 1988;41(10):1007–1030. doi: 10.1016/0895-4356(88)90040-6. [DOI] [PubMed] [Google Scholar]
- Griffin S, Kinmonth AL, Skinner C, Kelly J. Educational and psychosocial interventions for adults with diabetes. London: British Diabetic Association; 1999. [Google Scholar]
- Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- Kinmonth AL, Griffin S, Wareham NJ. Implications of the United Kingdom Prospective Diabetes Study for general practice care of type 2 diabetes. Br J Gen Pract. 1999;49(446):692–694. [PMC free article] [PubMed] [Google Scholar]
- Mallion JM, Dutrey-Dupagne C, Vaur L, Genes N, Renault M, Elkik F, Baguet P, Boutelant S. Benefits of electronic pillboxes in evaluating treatment compliance of patients with mild to moderate hypertension. J Hypertens. 1996;14(1):137–144. [PubMed] [Google Scholar]
- Donnan PT, Brennan GM, MacDonald TM, Morris AD. For the DARTS/MEMO Collaboration UoD. Population-based adherence to prescribed medication in type 2 diabetes: a cause for concern. Diabet Med. 2000;17(1):1. doi: 10.1046/j.1464-5491.2000.00251.x. [DOI] [Google Scholar]
- Rothman AJ, Baldwin A, Hertel A. In: The handbook of self-regulation. Vohs K, Baumeister R, editor. New York: Guildford Press; 2004. Self-regulation and behaviour change: Disentangling behavioral initiation and behavioral maintenance; pp. 130–148. [Google Scholar]
- Ellis SE, Speroff T, Dittus RS, Brown A, Pichert JW, Elasy TA. Diabetes patient education: a meta-analysis and meta-regression. Patient Educ Couns. 2004;52(1):97–105. doi: 10.1016/S0738-3991(03)00016-8. [DOI] [PubMed] [Google Scholar]
- Ajzen I. The Theory of Planned Behavior. Organizational Behavior and Human Decision Processes. 1991;50:179–211. doi: 10.1016/0749-5978(91)90020-T. [DOI] [Google Scholar]
- Gollwitzer PM, Brandstatter V. Implementation intentions and effective goal pursuit. Journal of Personality and Social Psychology. 1997;73:186–199. doi: 10.1037/0022-3514.73.1.186. [DOI] [PubMed] [Google Scholar]
- Lauritzen T, Griffin S, Borch-Johnsen K, Wareham NJ, Wolffenbuttel BH, Rutten G. The ADDITION study: proposed trial of the cost-effectiveness of an intensive multifactorial intervention on morbidity and mortality among people with Type 2 diabetes detected by screening. Int J Obes Relat Metab Disord. 2000;24(3):S6–11. doi: 10.1038/sj/ijo/0801420. [DOI] [PubMed] [Google Scholar]
- Echouffo-Tcheugui JB, Simmons RK, Williams KM, Barling RS, Prevost AT, Kinmonth AL, Wareham NJ, Griffin SJ. The ADDITION-Cambridge trial protocol: a cluster -- randomised controlled trial of screening for type 2 diabetes and intensive treatment for screen-detected patients. BMC Public Health. 2009;9:136. doi: 10.1186/1471-2458-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R, Stevens R, Holman R. American Diabetic Association: 2007. Chicago, USA; 2007. The Oxford Risk Engine: A Cardiovascular Risk Calculator for Individuals with or without Type 2 Diabetes; p. A170. Diabetes. [Google Scholar]
- Horne R, Weinman J. In: Adherence to treatment in medical conditions. Myers L, Midence K, editor. London Harwood Academic; 1998. Predicting treatment adherence: an overview of theoretical models; pp. 25–50. [Google Scholar]
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. Bmj. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. doi: 10.1016/S0140-6736(98)07019-6. [DOI] [PubMed] [Google Scholar]
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854–865. doi: 10.1016/S0140-6736(98)07037-8. [DOI] [PubMed] [Google Scholar]
- Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S) Diabetes Care. 1997;20(4):614–620. doi: 10.2337/diacare.20.4.614. [DOI] [PubMed] [Google Scholar]
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet. 2000;355(9200):253–259. doi: 10.1016/S0140-6736(99)12323-7. [DOI] [PubMed] [Google Scholar]
- Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361(9374):2005–2016. doi: 10.1016/S0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- Leventhal H, Brissette I, Leventhal EA. In: The Self-Regulation of Health and Illness Behaviour. Cameron LD, Leventhal H, editor. London: Routledge; 2003. The common-sense model of self-regulation of health and illness; pp. 42–65. [Google Scholar]
- Diabetes for beginners- Type2. http://www.diabetes.org.uk http://www.diabetes.org.uk.
- Carver CS, Scheier MF. In: Perspectives on behavioral self-regulation Advances in social cognition, Volume XII. Wyer RS, editor. London: Lawrence Erlbaum Associates; 1999. Themes and issues in the self-regulation of behavior. [Google Scholar]
- Kazdin AE. Behavior Modification in Applied Settings. Belmont, CA: Wadsworth; 2001. [Google Scholar]
- Marlatt GA, Gordon JR. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. New York: Guildford Press; 1985. [Google Scholar]
- Brage S, Brage N, Franks PW, Ekelund U, Wareham NJ. Reliability and validity of the combined heart rate and movement sensor Actiheart. Eur J Clin Nutr. 2005;59(4):561–570. doi: 10.1038/sj.ejcn.1602118. [DOI] [PubMed] [Google Scholar]
- Brage S, Ekelund U, Brage N, Hennings MA, Froberg K, Franks PW, Wareham NJ. Hierarchy of individual calibration levels for heart rate and accelerometry to measure physical activity. J Appl Physiol. 2007;103(2):682–692. doi: 10.1152/japplphysiol.00092.2006. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. Journal of the American College of Cardiology. 2001;37(1):153–156. doi: 10.1016/S0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- Stegle O, Fallert SV, MacKay DJ, Brage S. Gaussian process robust regression for noisy heart rate data. IEEE transactions on bio-medical engineering. 2008;55(9):2143–2151. doi: 10.1109/TBME.2008.923118. [DOI] [PubMed] [Google Scholar]
- Brage S, Brage N, Franks PW, Ekelund U, Wong MY, Andersen LB, Froberg K, Wareham NJ. Branched equation modeling of simultaneous accelerometry and heart rate monitoring improves estimate of directly measured physical activity energy expenditure. J Appl Physiol. 2004;96(1):343–351. doi: 10.1152/japplphysiol.00703.2003. [DOI] [PubMed] [Google Scholar]
- Farmer AJ, Prevost AT, Hardeman W, Craven A, Sutton S, Griffin SJ, Kinmonth AL. Protocol for SAMS (Support and Advice for Medication Study): a randomised controlled trial of an intervention to support patients with type 2 diabetes with adherence to medication. BMC family practice. 2008;9:20. doi: 10.1186/1471-2296-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareham NJ, Rennie KL. The assessment of physical activity in individuals and populations: why try to be more precise about how physical activity is assessed? Int J Obes Relat Metab Disord. 1998;22(2):S30–38. [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF. et al. International physical activity questionnaire: 12-country reliability and validity. Medicine and science in sports and exercise. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Bingham SA, Gill C, Welch A, Cassidy A, Runswick SA, Oakes S, Lubin R, Thurnham DI, Key TJ, Roe L. et al. Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers. International journal of epidemiology. 1997;26(1):S137–151. doi: 10.1093/ije/26.suppl_1.S137. [DOI] [PubMed] [Google Scholar]
- Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bulletin of the World Health Organization. 1962;27:645–658. [PMC free article] [PubMed] [Google Scholar]
- Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- Bradley C. Handbook of psychology and diabetes: a guide to psychological measurement in diabetes research and practice. Harwood Academic Press; 1994. [Google Scholar]
- Ware J, Snow KK, Kosinski M, Gandek B. Manual & Interpretation Guide. Boston, Massachusetts: Nimrod press; 1993. SF-36 Health Survey. [Google Scholar]
- Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. Bmj. 1998;316(7133):736–741. doi: 10.1136/bmj.316.7133.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI) Br J Clin Psychol. 1992;31(3):301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- Mercer SW, Maxwell M, Heaney D, Watt GC. The consultation and relational empathy (CARE) measure: development and preliminary validation and reliability of an empathy-based consultation process measure. Family practice. 2004;21(6):699–705. doi: 10.1093/fampra/cmh621. [DOI] [PubMed] [Google Scholar]
- Farmer A, Wade A, French DP, Goyder E, Kinmonth AL, Neil A. The DiGEM trial protocol--a randomised controlled trial to determine the effect on glycaemic control of different strategies of blood glucose self-monitoring in people with type 2 diabetes [ISRCTN47464659] BMC family practice. 2005;6:25. doi: 10.1186/1471-2296-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner M, Sparks P. In: Predicting health behaviour. Conner M, Norman P, editor. Open University Press; 2005. Theory of planned behaviour and health behaviour; pp. 171–222. [Google Scholar]
- Leventhal L, Benyamino Y, Brownlee S, Diefenbach M, Leventhal EL, Patrick-Miller L, Robitaille C. In: Perceptions of health and illness. Petrie KJ, Weinman J, editor. Amsterdam: Harwood Academic Publisher; 1997. Illness representations: theoretical foundations; pp. 19–45. [Google Scholar]
- Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D. The revised illness perception questionnaire (IPQ-R) Psychology & Health. 2002;17:1–16. [Google Scholar]
- Kinmonth AL, Woodcock A, Griffin S, Spiegal N, Campbell MJ. Randomised controlled trial of patient centred care of diabetes in general practice: impact on current wellbeing and future disease risk. The Diabetes Care From Diagnosis Research Team. BMJ (Clinical research ed. 1998;317(7167):1202–1208. doi: 10.1136/bmj.317.7167.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplanken B, Orbell S. Reflections on past behavior: A self-report index of habit strength. Journal of Applied Pscyhology. 2003;33:1313–1330. doi: 10.1111/j.1559-1816.2003.tb01951.x. [DOI] [Google Scholar]
- Knapp M, Beecham J. Reduced list costings: examination of an informed short cut in mental health research. Health economics. 1993;2(4):313–322. doi: 10.1002/hec.4730020404. [DOI] [PubMed] [Google Scholar]
- White IR, Thompson SG. Adjusting for partially missing baseline measurements in randomized trials. Stat Med. 2005;24(7):993–1007. doi: 10.1002/sim.1981. [DOI] [PubMed] [Google Scholar]
- Schafer JL. Multiple imputation: a primer. Statistical methods in medical research. 1999;8(1):3–15. doi: 10.1191/096228099671525676. [DOI] [PubMed] [Google Scholar]
- Currie CJ, Williams DR, Peters JR. Patterns of in and out-patient activity for diabetes: a district survey. Diabet Med. 1996;13(3):273–280. doi: 10.1002/(SICI)1096-9136(199603)13:3<273::AID-DIA57>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Williams K, Prevost AT, Griffin S, Hardeman W, Hollingworth W, Spiegelhalter D, Sutton S, Ekelund U, Wareham N, Kinmonth AL. The ProActive trial protocol - a randomised controlled trial of the efficacy of a family-based, domiciliary intervention programme to increase physical activity among individuals at high risk of diabetes [ISRCTN61323766. BMC Public Health. 2004;4(1):48. doi: 10.1186/1471-2458-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham SA, Welch AA, McTaggart A, Mulligan AA, Runswick SA, Luben R, Oakes S, Khaw KT, Wareham N, Day NE. Nutritional methods in the European Prospective Investigation of Cancer in Norfolk. Public Health Nutr. 2001;4(3):847–858. doi: 10.1079/PHN2000102. [DOI] [PubMed] [Google Scholar]
- Ware JE SK, Kosinski M, Gandek B. SF-36 Health Survey. Manual and Interpretation Guide. Boston, MA, New England Medical Centre, The Health Institute; 1993. [Google Scholar]