SUMMARY
Parkinson’s disease (PD) is characterized by a dramatic loss of dopamine that underlies complex structural and functional changes in striatal projection neurons. A key alteration that has been reported in various rodent models and PD patients is a significant reduction in striatal dendritic spine density. Our recent findings indicate that striatal spine loss is also a prominent feature of parkinsonism in MPTP-treated monkeys. In these animals, striatal spine plasticity is tightly linked with the degree of striatal dopamine denervation. It affects predominantly the sensorimotor striatal territory (i.e. the post-commissural putamen) and targets both direct and indirect striatofugal neurons. However, electron microscopic 3D reconstruction studies demonstrate that the remaining spines in the dopamine-denervated striatum of parkinsonian monkeys undergo major morphological and ultrastructural changes characteristic of increased synaptic efficacy. Although both corticostriatal and thalamostriatal glutamatergic afferents display such plastic changes, the ultrastructural features of pre- and post-synaptic elements at these synapses are consistent with a higher strength of corticostriatal synapses over thalamic inputs in both normal and pathological conditions. Thus, striatal projection neurons and their glutamatergic afferents are endowed with a high degree of structural and functional plasticity. In parkinsonism, the striatal dopamine denervation induces major spine loss on medium spiny neurons and generates a significant remodeling of corticostriatal and thalamostriatal glutamatergic synapses, consistent with increased synaptic transmission. Future studies are needed to further characterize the mechanisms underlying striatal spine plasticity, and determine if it represents a pathological feature or compensatory process of PD.
Keywords: Striatum, Dopamine, MPTP, Primate, Ultrastructure, Vesicular glutamate transporter, Corticostriatal, Thalamostriatal, Nigrostriatal, Glutamate
1. Introduction
The basal ganglia are a group of interconnected subcortical structures involved in the control of motor, cognitive and limbic functions. The striatum is the main entrance of information to the basal ganglia. It receives topographically organized glutamatergic excitatory inputs from the cerebral cortex and the thalamus. Once processed at the striatal level, the information is channeled via GABAergic projections to the external pallidum (GPe) and/or to the output structures of the basal ganglia, the internal pallidum (GPi) and the substantia nigra pars reticulata (SNr) which, in turn, project to the thalamus and brainstem [1,2]. The flow of information through the basal ganglia is organized into a “direct” (striato-GPi) pathway and “indirect” pathways that involve the GPe and the subthalamic nucleus (STN). The sources of the striatofugal direct and indirect pathways are all GABAergic neurons, that can be segregated into two populations by their peptide content, and by the preferential expression of dopamine receptors. Neurons of the direct pathway contain substance P/dynorphin and express preferentially dopamine D1-receptors, whereas neurons of the indirect pathway contain enkephalin and express preferentially D2-receptors [3]. In Parkinson’s disease (PD), the dopaminergic projection from the substantia nigra pars compacta (SNc) to the striatum degenerates. The resulting lack of striatal dopamine increases the activity of ‘indirect’ striatofugal neurons and decreases the striatal output along the ‘direct’ route. Together, these changes are thought to increase the GABAergic basal ganglia outflow to the thalamus [4]. Many aspects of this model have been challenged over the past decades [5,6]. One of the most substantial shortcomings of this model is the simplistic view by which dopamine mediates its functional effects through the basal ganglia network. The role of striatal dopamine is, indeed, much more complex than mere excitation or inhibition of striatal projection neurons [7]. Dopamine also plays a critical role in mediating long term synaptic plasticity of striatal glutamatergic afferents so that degeneration of the nigrostriatal system in parkinsonism not only directly affects the level of medium spiny neurons (MSN) activation, but also triggers substantial secondary changes of the synaptic morphology and function in the striatum [8–11].
Findings from our laboratory and others have demonstrated that the nigrostriatal dopaminergic system plays a key role in regulating morphological and functional spine plasticity in the striatum. In this review, we will summarize findings obtained in various animal models and PD patients indicating that spine loss in the striatum is a key morphological event of parkinsonism that, most likely, contributes to functional changes in corticostriatal transmission in parkinsonian condition. We will also highlight morphological differences and regulatory changes induced by dopamine depletion on glutamatergic transmission at axo-spinous synapses established by cortical versus thalamic inputs to the striatum. Finally, we will briefly discuss recent findings showing that the remaining spines in the striatum of MPTP-treated monkeys undergo complex ultrastructural changes consistent with increased synaptic activity, thereby raising doubt as to whether striatal spine loss and ultrastructural remodeling of axo-spinous glutamatergic synapses represent pathological or compensatory features of PD pathophysiology.
2. Striatal spines are the targets of both cortical and thalamic glutamatergic inputs to the striatum
Both the cerebral cortex and thalamus provide massive and highly topographic glutamatergic inputs to the striatum [10,12]. The recent cloning of the vesicular glutamate transporters 1 and 2 (vGluT1, vGluT2) has provided us with important tools to study the anatomy and synaptic connectivity of these two glutamatergic systems in the rat and monkey striatum because vGluT1 is expressed exclusively in corticostriatal terminals, while vGluT2 is confined to thalamostriatal terminals [9,10,12,13]. Using these specific markers, one can differentiate the two main populations of glutamatergic axon terminals in the mammalian striatum and study their synaptic relationships with striatal neurons in normal and parkinsonian conditions. Taking advantage of these tools, we have demonstrated that spines are the predominant targets of both cortical and thalamic afferents in the primate striatum, with the exception of thalamic inputs arising from the centre median and parafascicular nuclei (CM/Pf), which terminate predominantly on dendritic shafts [9,10,13].
Based on these and other findings, we proposed the existence of dual thalamostriatal systems. One of these is the thalamostriatal projection from the caudal intralaminar nuclei (CM/Pf) that innervates massively the striatum and provides a modest input to the cerebral cortex. This system terminates predominantly in the striatal matrix compartment, targets preferentially dendritic shafts of striatal projection neurons and interneurons, and, according to previous electrophysiologic studies, most likely transmits information related to attention [10,13]. The second system consists of other thalamostriatal projections that originate from collaterals of thalamocortical neurons in major relay nuclei, the rostral intralaminar and associative thalamic nuclei. The neurons giving rise to this system project preferentially to specific cortical areas, and provide modest, but highly topographic, striatal innervation that terminates almost exclusively on the dendritic spines of projection neurons [13]. The functional roles of these projections remain poorly understood, but it has been proposed that they may be part of subcortical loops that mediate connections between the basal ganglia and specific regions of the superior colliculus, acting independently or cooperatively with basal ganglia-thalamocortical loops to influence action selection [14]. Thalamostriatal neurons in VA/VL and the rostral intralaminar nuclei may also receive direct ascending inputs from the deep cerebellar nuclei and mediate the communication between cerebellar outflow and basal ganglia circuits, a pathway that could play a role in regulating motor and non-motor functions of the basal ganglia [15], and may be involved in some forms of dystonia [16].
3. Striatal spine loss versus dopamine denervation in parkinsonism
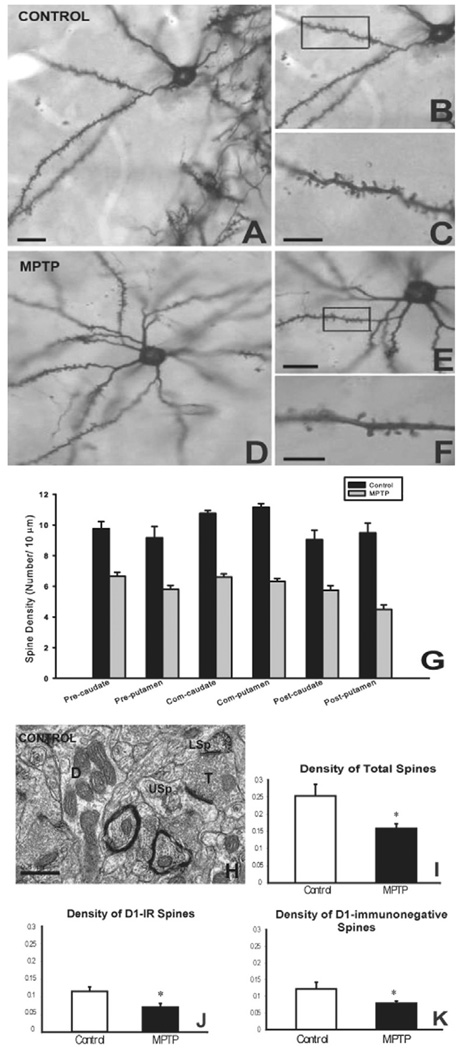
Dopaminergic transmission regulates spine morphogenesis on striatal medium spiny neurons (MSNs) [10,17]. Changes in basal ganglia function in diseases that are associated with abnormal dopaminergic transmission, such as PD or drug addiction, may partly be the consequence of altered spine morphology and related changes in synaptic function and plasticity [17,18]. For instance, in rodent models of PD, loss of striatal dopamine is associated with a reduction of spine density, and a decrease in the number of putative glutamatergic synapses [19,20]. In Golgi-impregnated striatal tissue of brains of patients who died with PD, a similar 20–30% reduction in striatal spine density and a reduction of the size of the dendritic trees of MSNs has been demonstrated [21,22]. Recent findings from our laboratory have expanded these observations in the MPTP-treated nonhuman primate model of Parkinson’s disease showing that the sensorimotor striatum (i.e., the postcommissural putamen) was the most strongly affected striatal region, displaying as much as 50% spine loss in cases with severe dopamine denervation [11], while the ventral striatum was less affected (Fig. 1A–G), a pattern reminiscent of recent findings from postmortem human parkinsonian brains [22]. Another observation that came out of our studies was that striatal spine loss in parkinsonism is highly correlated with the degree of striatal dopamine denervation. For instance, the post-commissural putamen, the most severely dopamine-depleted striatal region in MPTP-treated monkeys, displays a significantly higher degree of spine loss than medial regions of the caudate nucleus, anterior putamen, and the nucleus accumbens in monkeys with partial striatal dopamine denervation [11]. There is controversy regarding the specificity of striatal spine loss towards direct or indirect striatofugal neurons. On one hand, a study using transgenic BAC D1 and D2 mice and 6-hydroxydopamine (6-OHDA)-treated rats, suggests that the striatal spine loss is exclusively confined to indirect D2-containing striatofugal neurons [23]. On the other hand, our recent electron microscopic data, based on the quantification of D1-positive spines in the striatum of MPTP-treated monkeys, indicate that both direct and indirect striatofugal neurons display significant spine loss in this animal model (Fig. 1H–K) [11]. The use of different animal models may underlie the differences in spine loss specificity between the dopamine-denervated rodents and our study in chronically treated monkeys with MPTP.
Fig. 1. Loss of striatal spines in MPTP-treated monkeys.
(A–F) Examples of Golgi-impregnated medium spiny neurons in the striatum of a control (A–C) and a MPTP-treated (D–F) monkey to illustrate the dramatic reduction in dendritic spines in the MPTP-treated animal compared with control. The boxed areas in B and E are shown at higher magnification in C and F, respectively. (G) Quantitative measurements of the density of dendritic spines in various striatal regions of control versus MPTP-treated monkeys. Note the significant reduction in spine density throughout the whole striatum. Abbreviations: Pre: Pre-commissural; Com: Commissural; Post: Post-commissural. (H–K) Significant reduction in the density of total striatal spines (I), or in D1-immunoreactive (D1-IR, J) and D1-immunonegative (K) spines in the striatum of MPTP-treated monkeys. The density values along the Y axis are number of spines/µm2 of striatal tissue. (H) depicts an example of labeled (LSp) and unlabeled (USp) spines in D1-immunostained striatal tissue of a control monkey. An unlabeled dendrite (D) and terminal (T) are also depicted in the neuropil. Scale bars: A: 25 µm (valid for D); C and F: 5 µm; E: 5 µm (valid for B); H: 0.5 µm. (See Villalba et al., 2009 [11] for more details).
4. Functional plasticity of corticostriatal glutamatergic transmission in parkinsonism
The loss of spines and possible reduction of glutamatergic synapses in the striatum of dopamine-depleted animals would suggest a reduction of glutamatergic transmission in the dopamine-denervated striatum of parkinsonians. However, this assumption is at odds with our recent ultrastructural data [9], the increased cellular expression of AMPA-receptor subunits reported in striatal neurons [24], and with most electrophysiologic studies of corticostriatal transmission in animal models of PD, which are strongly in favor of the hypothesis that parkinsonism is, in fact, a state of increased striatal glutamatergic transmission [8]. For instance, brain slice recording studies have indicated that dopamine denervation augments neuronal excitability in the striatum due to increased corticostriatal transmission [8], although these findings have recently been challenged based on results collected in reserpinized mice [23]. In vivo, the discharge activity of striatal MSNs is enhanced in 6-OHDA-treated rats [25,26], and potentially also in MPTP-treated monkeys [27]. Furthermore, a decrease in the threshold current required to obtain cortical-evoked responses has been reported in dopamine-depleted rats [28], consistent with the possibility that the glutamatergic corticostriatal transmission is overactive in parkinsonian condition. Recent in vivo data from anesthetized 6-OHDA-lesioned rats have indicated that the responsiveness of striatal output neurons may be increased, at least in the D2-receptor containing indirect striatopallidal neurons [26].
5. Reorganization of the synaptic connectivity of corticostriatal glutamatergic synapses in parkinsonism
Various rodent studies have reported morphological changes in the striatum of dopamine-depleted rats that are in line with the electrophysiological data discussed above, suggesting possible increased synaptic efficacy of striatal glutamatergic transmission in parkinsonism. Changes that have been noticed include an increased density of perforated asymmetric synapses [20,29], considered as an index of increased synaptic strength in other brain regions [30], an increase in the volume of presynaptic terminals forming asymmetric synapses, and an increased volume of dendritic spines [29,31].
Recent data from our laboratory have expanded these observations and provided strong evidence for ultrastructural changes in the morphology and increased vGluT1 expression at corticostriatal synapses in MPTP-treated parkinsonian monkeys [9]. First, we demonstrated that the overall pattern of synaptic connectivity of vGluT1- (i.e. corticostriatal) and vGluT2- (i.e. thalamostriatal) positive terminals in striatum of normal monkeys is strikingly different. While almost all vGluT1 terminals form axo-spinous synapses, the vGluT2 boutons are evenly divided between axospinous and axo-dendritic synapses in both the caudate nucleus and putamen [9]. This pattern remains the same in the striatum of MPTP-treated monkeys suggesting that the severe loss of dendritic spines described above does not lead to a significant shift in the synaptic connectivity of cortical and thalamic terminals between spines and dendrites in parkinsonism. However, a striking finding of this study was an apparent relative increase in the prevalence of vGluT1- (66.5% of total striatal spine population in control vs 51.9% in MPTP-treated), but not vGluT2- (21.5% in control vs 21.7% in MPTP-treated) positive terminals in the striatum of MPTP-treated monkeys [9], a finding consistent with a recent report from postmortem human brain describing an increased level of vGluT1 protein in the putamen of parkinsonian patients [32]. At first glance, these observations are paradoxical in light of findings showing a loss of spines in the striatum of animal models and Parkinson’s disease patients (see above).
However, there are different possibilities that could reconcile these data. In the next two sections, we will discuss various data from other brain regions that highlight the importance of ultrastructural plastic remodeling of glutamatergic synapses as key substrates for functional changes in glutamatergic transmission in normal and pathological conditions. Finally, we will present recent findings gathered in MPTP-treated monkeys indicating that both the pre- and postsynaptic elements of corticostriatal and thalamostriatal axo-spinous synapses undergo striking ultrastructural changes consistent with increased synaptic efficacy in MPTP-treated monkeys.
6. Ultrastructural features of spines and synaptic transmission
Although the functional significance of changes in spine morphology in the striatum remains poorly understood, there is ample evidence from the hippocampus and cerebral cortex indicating that the regulation of spine morphogenesis is a critical component of the strength and plasticity of glutamatergic transmission [33,34]. In general, dendritic spines consist of a bulbous head attached to the dendrite by a narrow stalk or neck, although morphologic variants exist even within dendrites of individual neurons. In the normal brain, all spines receive excitatory glutamatergic inputs, and the size of these axo-spinous synapses is related to the volume of the spine head (for instance, large spines have large glutamatergic synaptic areas). Various features of spine morphology, including the volume of the head, the length and volume of the neck, the volume of the spine apparatus and the area of the postsynaptic density (PSD) are known to be highly plastic. Morphological changes of these parameters in cortical or hippocampal neurons have been associated with increased synaptic efficacy, learning and normal memory formation [34]. However, changes in the number and morphological features of dendritic spines are also the hallmark pathology of brain diseases such as PD, Fragile X syndrome, schizophrenia and drug addiction [34–36]. There is strong evidence that morphological spine plasticity can directly affect the physiological responses of their parent neurons to glutamatergic synaptic inputs. For instance, because spine volume is related to the size of the active zones, the extent of the PSD, and the number of postsynaptic glutamate receptors [34], large spine heads may mediate stronger excitatory transmission than small spines. This is, indeed, the case in hippocampal pyramidal neurons where large ‘mushroom’ spines express a larger number of AMPA receptors than thin spines and filopodia, indicating that the distribution of functional AMPA receptors is tightly correlated with spine geometry [37]. The fate of calcium that enters spine heads through AMPA and NMDA receptor channels is governed by the shape (length and diameter) of the spine neck [33,34]. The size of spine necks is critical to regulate the diffusion of calcium from the spine head to the parent dendrite. Thin spine necks restrict more efficiently calcium into the spine head, whereas large spine necks allow for a more pronounced diffusion of calcium into the parent dendrites.
Another key element responsible for changes in synaptic function and plasticity in dendritic spines is the spine apparatus, a specialized part of the endoplasmic reticulum network, found in most spines in the telencephalon, including the striatum [38]. Although its mechanistic role remains elusive, recent data have implicated the spine apparatus in the regulation of spine calcium kinetics and in the post-translational modification and transport of locally synthesized proteins [39,40], two important phenomena that could play major roles in regulating spine plasticity and morphogenesis.
7. 3D reconstruction of striatal spines in parkinsonism: structural evidence for increased efficacy at glutamatergic synapses
In light of findings discussed in the previous section showing clear evidence for correlation between the ultrastructural features of dendritic spines and function of glutamatergic synapses in the CNS, we undertook a detailed comparative analysis of the ultrastructural features of dendritic spines that receive cortical or thalamic inputs in normal and parkinsonian monkeys using 3D reconstruction method of serial ultrathin sections at the electron microscopic level.
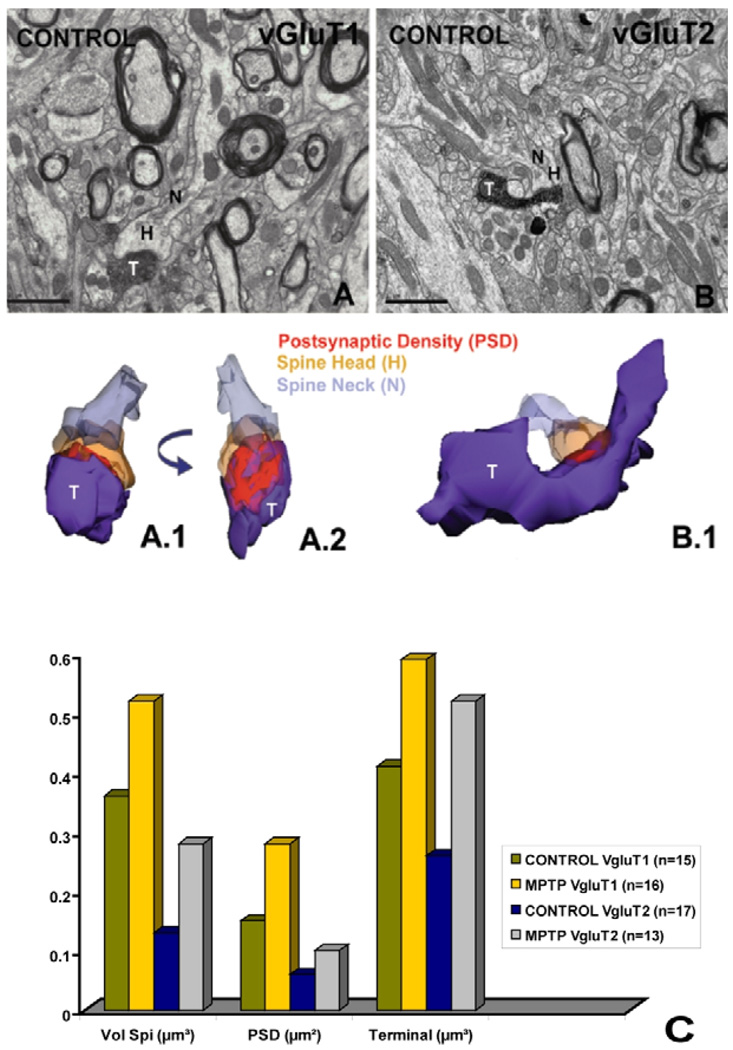
The data were gathered using vGluT1- and vGluT2-immunostained sections from the postcommisural putamen of two control and two MPTP-treated monkeys. A summary of the results and examples of reconstructions are shown in Fig. 2. The total number of spines that have been reconstructed from each group is indicated in parenthesis in figure 2. Thus, measurements gathered from 15 spines contacted by vGluT1 terminals and 13 spines receiving vGluT2-positive inputs in normal monkeys, indicate that the volume of vGluT1-receiving spines (green column) is almost twice as large as that of vGluT2-receiving elements (blue column). The reconstructions also highlighted the relative differences in the areas of the PSDs associated with the two sets of terminals. The PSDs at vGluT1 synapses are more extensive and complex than the contacts established by vGluT2 boutons (Fig. 2). These findings provide the first evidence that the two major glutamatergic afferents to the striatum target preferentially spines with strikingly different morphological features, suggesting potential differences in synaptic strength between these two inputs. We also found that the volume of spine heads and their afferent pre-synaptic terminals as well as the PSD areas of cortical and thalamic glutamatergic synapses are much larger in the striatum of MPTP-treated monkeys than in controls (Fig. 2), suggesting that thalamic and cortical axospinous synapses undergo significant ultrastructural changes in chronic parkinsonism that favor increased strength of individual synapses, as discussed above in the section related to the physiology of glutamatergic transmission in parkinsonism. In addition to the parkinsonism-related changes in the size of the PSD, our studies also indicate that the volume and extent of the spine apparatus (SA) increase substantially in spines that receive inputs from vGluT1-immunoreactive cortical terminals in MPTP-treated monkeys relative to controls. This increase is not merely due to the larger size of spine heads, because the ratio of the spine apparatus volume over the volume of spine heads is much higher in MPTP-treated animals than in controls. In normal monkeys, the spine apparatus is mainly confined to the lower part of the head and the neck of the spine, while in MPTP-treated animals, the spine apparatus are fragmented, display a more complex organization and extend significantly in the head of the dendritic spine up to the PSD. In other brain regions, such changes have been interpreted as evidence for increased protein synthesis and changes in the regulation of the calcium concentration in spines [41,42].
Fig. 2. 3D reconstruction of dendritic spines in the monkey striatum.
(A, B) Electron micrographs showing a vGluT1- (A) and a vGluT2- (B) immunoreactive axon terminal (T) forming asymmetric synapses with the heads (H) of dendritic spines in the striatum of a control monkey. The neck of the spine (N) is also labeled in these micrographs. (A.1–B.1) 3D reconstruction of the two labeled terminals and their postsynaptic targets shown in A and B. In A.2 the axo-spinous complex has been rotated to better illustrate the entire extent of the synaptic junction. (C) Quantitative measurements of the volumes of spines and terminals (in µm3) and the surface areas of postsynaptic densities (PSD; in µm2) at axo-spinous synapses formed by vGLuT1- or vGluT2-immunoreactive boutons. The total number of reconstructed terminals in each group is indicated in parentheses. Scale bars: 1 µm.
8. Conclusions
Together, these observations demonstrate two main points related to glutamatergic transmission in the striatum: First, they highlight important ultrastructural differences between corticostriatal and thalamostriatal glutamatergic synapses, suggesting differential strength of these two major synaptic inputs to striatal projection neurons. Second they provide further evidence that striatal projection neurons are endowed with a significant degree of structural plasticity which, most likely, underlies functional changes in corticostriatal glutamatergic transmission in parkinsonism. Future studies are needed to better understand the mechanisms and functional significance of striatal spine remodeling in Parkinson’s disease. Although it might appear as an additional pathological feature induced by striatal dopamine depletion in parkinsonism, the possibility that these changes are initiated as compensatory mechanisms in response to abnormal regulation of glutamatergic and dopaminergic systems must be considered.
Acknowledgements
The authors thank Jean-Francois Pare and Susan Jenkins for technical assistance. This work was supported by a grant from the National Institutes of Health to YS (R01 NS037948) and the NIH base grant (RR-00165) of the Yerkes National Primate Research Center.
Footnotes
Conflict of interests
The authors certify that there is no conflict of interest related to the content of this publication.
References
- 1.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The corticobasal ganglia-thalamo-cortical loop. Brain Res Rev. 1996;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 2.Smith Y, Shink E, Bevan MD, Bolam JP. Synaptology of the direct and indirect striatofugal pathways. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 3.Gerfen CR, Wilson CJ. The basal ganglia. In: Björklund A, Hökfelt T, Swanson L, editors. Handbook of Chemical Neuroanatomy, Integrated Systems of the CNS, Part III. Amsterdam: Elsevier; 1996. pp. 369–466. [Google Scholar]
- 4.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 5.Chesselet MF, Delfs JM. Basal ganglia and movement disorders: an update. Trends Neurosci. 1996;19:417–422. doi: 10.1016/0166-2236(96)10052-7. [DOI] [PubMed] [Google Scholar]
- 6.Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Lanciego JL, Artieda J, Gonzalo N, et al. Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci. 2000;23:S8–S19. doi: 10.1016/s1471-1931(00)00028-8. [DOI] [PubMed] [Google Scholar]
- 7.Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- 8.Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Raju DV, Ahern TH, Shah DJ, Wright TM, Standaert DG, Hall RA, et al. Differential synaptic plasticity of the corticostriatal and thalamostriatal systems in an MPTP-treated monkey model of parkinsonism. Eur J Neurosci. 2008;27:1647–1658. doi: 10.1111/j.1460-9568.2008.06136.x. [DOI] [PubMed] [Google Scholar]
- 10.Smith Y, Raju D, Nanda B, Pare JF, Galvan A, Wichmann T. The thalamostriatal systems: anatomical and functional organization in normal and parkinsonian states. Brain Res Bull. 2009;78:60–68. doi: 10.1016/j.brainresbull.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villalba RM, Lee H, Smith Y. Dopaminergic denervation and spine loss in the striatum of MPTP-treated monkeys. Exp Neurol. 2009;215:220–227. doi: 10.1016/j.expneurol.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Raju DV, Shah DJ, Wright TM, Hall RA, Smith Y. Differential synaptology of vGluT2-containing thalamostriatal afferents between the patch and matrix compartments in rats. J Comp Neurol. 2006;499:231–243. doi: 10.1002/cne.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McHaffie JG, Stanford TR, Stein BE, Coizet V, Redgrave P. Subcortical loops through the basal ganglia. Trends Neurosci. 2005;28:401–407. doi: 10.1016/j.tins.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- 16.Neychev VK, Fan X, Mitev VI, Hess EJ, Jinnah HA. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131:2499–2509. doi: 10.1093/brain/awn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deutch AY, Colbran RJ, Winder DJ. Striatal plasticity and medium spiny neuron dendritic remodeling in parkinsonism. Parkinsonism Relat Disord. 2007;13 Suppl 3:S251–S258. doi: 10.1016/S1353-8020(08)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingham CA, Hood SH, Arbuthnott GW. Spine density on neostriatal neurones changes with 6-hydroxydopamine lesions and with age. Brain Res. 1989;503:334–338. doi: 10.1016/0006-8993(89)91686-7. [DOI] [PubMed] [Google Scholar]
- 20.Ingham CA, Hood SH, Taggart P, Arbuthnott GW. Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. J Neurosci. 1998;18:4732–4743. doi: 10.1523/JNEUROSCI.18-12-04732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephens B, Mueller AJ, Shering AF, Hood SH, Taggart P, Arbuthnott GW, et al. Evidence of a breakdown of corticostriatal connections in Parkinson’s disease. Neuroscience. 2005;132:741–754. doi: 10.1016/j.neuroscience.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Zaja-Milatovic S, Milatovic D, Schantz AM, Zhang J, Montine KS, Samii A, et al. Dendritic degeneration in neostriatal medium spiny neurons in Parkinson’s disease. Neurology. 2005;64:545–547. doi: 10.1212/01.WNL.0000150591.33787.A4. [DOI] [PubMed] [Google Scholar]
- 23.Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- 24.Betarbet R, Porter RH, Greenamyre JT. GluR1 glutamate receptor subunit is regulated differentially in the primate basal ganglia following nigrostriatal dopamine denervation. J Neurochem. 2000;74:1166–1174. doi: 10.1046/j.1471-4159.2000.741166.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen MT, Morales M, Woodward DJ, Hoffer BJ, Janak PH. In vivo extracellular recording of striatal neurons in the awake rat following unilateral 6-hydroxydopamine lesions. Exp Neurol. 2001;171:72–83. doi: 10.1006/exnr.2001.7730. [DOI] [PubMed] [Google Scholar]
- 26.Mallet N, Ballion B, Le Moine C, Gonon F. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J Neurosci. 2006;26:3875–3884. doi: 10.1523/JNEUROSCI.4439-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang L, DeLong MR, Papa SM. Inversion of dopamine responses in striatal medium spiny neurons and involuntary movements. J Neurosci. 2008;28:7537–7547. doi: 10.1523/JNEUROSCI.1176-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Florio T, Di Loreto S, Cerrito F, Scarnati E. Influence of prelimbic and sensorimotor cortices on striatal neurons in the rat: electrophysiological evidence for converging inputs and the effects of 6-OHDA-induced degeneration of the substantia nigra. Brain Res. 1993;619:180–188. doi: 10.1016/0006-8993(93)91610-5. [DOI] [PubMed] [Google Scholar]
- 29.Meshul CK, Emre N, Nakamura CM, Allen C, Donohue MK, Buckman JF. Timedependent changes in striatal glutamate synapses following a 6-hydroxydopamine lesion. Neuroscience. 1999;88:1–16. doi: 10.1016/s0306-4522(98)00189-4. [DOI] [PubMed] [Google Scholar]
- 30.Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Ann Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 31.Meshul CK, Cogen JP, Cheng HW, Moore C, Krentz L, McNeill TH. Alterations in rat striatal glutamate synapses following a lesion of the cortico- and/or nigrostriatal pathway. Exp Neurol. 2000;165:191–206. doi: 10.1006/exnr.2000.7467. [DOI] [PubMed] [Google Scholar]
- 32.Kashani A, Betancur C, Giros B, Hirsch E, El Mestikawy S. Altered expression of vesicular glutamate transporters vGluT1 and vGluT2 in Parkinson’s disease. Neurobiol Aging. 2007;28:568–578. doi: 10.1016/j.neurobiolaging.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arellano JI, Benavides-Piccione R, Defelipe J, Yuste R. Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front Neurosci. 2007;231:131–143. doi: 10.3389/neuro.01.1.1.010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segal M. Dendritic spines for neuroprotection: a hypothesis. Trends Neurosci. 1995;18:468–471. doi: 10.1016/0166-2236(95)92765-i. [DOI] [PubMed] [Google Scholar]
- 36.Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Rev. 2002;39:29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- 37.Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Lino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 39.Steward O, Schuman EM. Protein synthesis at synaptic sites on dendrites. Annu Rev Neurosci. 2001;24:299–325. doi: 10.1146/annurev.neuro.24.1.299. [DOI] [PubMed] [Google Scholar]
- 40.Pierce JP, van Leyen K, McCarthy JB. Translocation machinery for synthesis of integral membrane and secretory proteins in dendritic spines. Nat Neurosci. 2000;3:311–313. doi: 10.1038/73868. [DOI] [PubMed] [Google Scholar]
- 41.Burgoyne RD, Barron J, Geisow MJ. Cytochemical localisation of calcium binding sites in adrenal chromaffin cells and their relation to secretion. Cell Tissue Res. 1983;229:207–217. doi: 10.1007/BF00217893. [DOI] [PubMed] [Google Scholar]
- 42.Fifkova E, Markham JA, Delay RJ. Calcium in the spine apparatus of dendritic spines in the dentate molecular layer. Brain Res. 1983;266:163–168. doi: 10.1016/0006-8993(83)91322-7. [DOI] [PubMed] [Google Scholar]