Abstract
Ceramide and sphingosine-1-phosphate are related sphingolipid metabolites that can be generated through a de novo biosynthetic route or derived from the recycling of membrane sphingomyelin. Both these lipids regulate cellular responses to stress, with generally opposing effects. Sphingosine-1-phosphate functions as a growth and survival factor, acting as a ligand for a family of G protein-coupled receptors, whereas ceramide activates intrinsic and extrinsic apoptotic pathways through receptor-independent mechanisms. A growing body of evidence has implicated ceramide, sphingosine-1-phosphate and the genes involved in their synthesis, catabolism and signaling in various aspects of oncogenesis, cancer progression and drug- and radiation resistance. This may be explained in part by the finding that both lipids impinge upon the PI3K/AKT pathway, which represses apoptosis and autophagy. In addition, sphingolipids influence cell cycle progression, telomerase function, cell migration and stem cell biology. Considering the central role of ceramide in mediating physiological as well as pharmacologically stimulated apoptosis, ceramide can be considered a tumor-suppressor lipid. In contrast, sphingosine-1-phosphate can be considered a tumor-promoting lipid, and the enzyme responsible for its synthesis functions as an oncogene. Not surprisingly, genetic mutations that result in reduced ceramide generation, increased sphingosine-1-phosphate synthesis or which reduce steady state ceramide levels and increase sphingosine-1-phosphate levels have been identified as mechanisms of tumor progression and drug resistance in cancer cells. Pharmacological tools for modulating sphingolipid pathways are being developed and represent novel therapeutic strategies for the treatment of cancer.
Introduction
Over the past twenty years, lipid metabolites have become recognized for their participation in membrane functions and signaling events that control a wide array of cellular activities. Two major sphingolipid metabolites, ceramide and sphingosine-1-phosphate (S1P), are involved in signaling pathways that regulate cell proliferation, apoptosis, motility, differentiation, stress responses, protein synthesis, carbohydrate metabolism, innate and adaptive immunity and angiogenesis.1 Ceramide and S1P exert opposing effects on cell survival, ceramide being pro-apoptotic and S1P generally promoting cell survival. In relation to their influence over cell fate, these two lipids and the proteins that mediate their signaling functions and metabolism have been implicated in various aspects of tumor biology and chemo- and radio-resistance. This review will summarize the main steps of sphingolipid metabolism and our current understanding of the interactions between ceramide, S1P and the cellular pathways that control cell fate. Recent in vitro and in vivo evidence supporting the roles these lipids play in oncogenesis, tumor progression and cancer therapeutics will be discussed. Readers interested in other aspects of sphingolipid metabolism and signaling are referred to several excellent recent review articles.2–7
Sphingolipid Metabolism
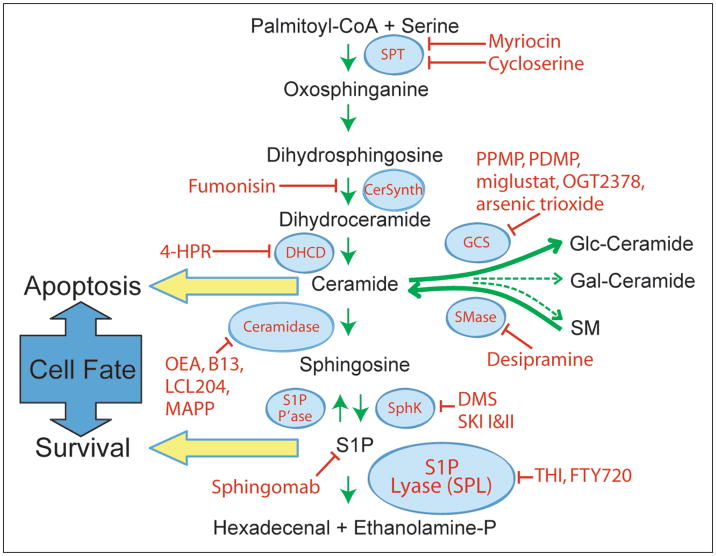
Ceramides are a class of hydrophobic molecules that contain a fatty acid moiety linked to sphingosine or a related long chain base.8 Ceramides are the basic constituents of higher order sphingolipids and also serve as bioactive intermediates that promote cell death. Ceramide is generated via a de novo biosynthetic pathway that is initiated by a condensation reaction between serine and palmitoyl CoA catalyzed by the essential enzyme serine palmitoyltransferase (SPT), resulting in the formation of 3-oxo-sphinganine (Fig. 1). The NADPH-dependent enzyme 3-oxo-sphinganine reductase converts 3-oxo-sphinganine to dihydrosphingosine. The latter is converted into dihydroceramide via an acylation reaction catalyzed by dihydroceramide synthase, encoded by the CerS genes, formerly known as LASS genes. Oxidation of dihydroceramide by dihydroceramide desaturase generates ceramide. Ceramide may be converted to sphingomyelin by sphingomyelin synthase, which catalyzes the transfer of phosphocholine from phosphatidylcholine to ceramide.9 Alternatively, ceramide may be modified via the addition of carbohydrate groups at the carbon 1 position by glucosylceramide synthase or galactosyl ceramide synthase and subsequent biochemical steps to generate glycosphingolipids. Ceramide can also be generated through a recycling pathway known as the “sphingomyelin cycle”.10 This process involves the hydrolysis of plasma membrane sphingomyelin, yielding ceramide and phosphorylcholine. Sphingomyelinases are specific phospholipases that carry out this reaction.11 Three different categories of sphingomyelinases have been characterized, based on their distinct pH optima. Similar hydrolytic reactions may generate ceramide from other higher order sphingolipids.12 Ceramide generated through de novo biosynthesis or sphingolipid recycling can be deacylated by ceramidases, giving rise to the long chain base sphingosine. Sphingosine is a bioactive lipid which inhibits cell cycle progression and/or promotes apoptosis, depending upon the cell type.13 Sphingosine is phosphorylated through the actions of sphingosine kinases (SK), encoded by SK1 and SK2, producing the mitogenic signaling lipid, S1P.14 S1P can be recycled back to sphingosine by lipid phosphatases.15,16 Alternatively, it can be irreversibly degraded by the enzyme S1P lyase (SPL), producing ethanolamine phosphate and hexadecenal.17
Figure 1.
Sphingolipid metabolism. The de novo pathway for generation and degradation of sphingolipids is depicted along with the enzymes that catalyze each step and their inhibitors. SPT, serine-palmitoyl transferase; DHCD, dihydroceramide desaturase; SphK, sphigosine kinase; S1P P’ase, S1P phosphatase; GCS, glucosyl ceramide synthase; PPMP, 1-phenyl-2-palmitoylamino- 3-morpholino-1-propanol; PDMP, l-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol; DMS, dimethyl sphingosine; 4-HPR, N-(4-hydroxyphenyl) retinamide; OEA, oleylethanolamide; MAPP, 2-(N-myristoylamino)-1-phenyl-1-propanol.
Ceramide Generated via Different Biochemical Routes Can Induce Apoptosis
Neutral sphingomyelinase (N-SMase) is found in the plasma membrane, cytoplasm and the cell nucleus.18,19 N-SMase has been implicated in cell death mediated by many stimuli including serum starvation,20 hypoxia,21 ethanol,22 nitric oxide (NO),23 reactive oxygen species (ROS) and chemotherapeutic agents such as daunorubicin24 and etoposide.25 In glioma cells treated with etoposide, activation of N-SMase was shown to occur downstream of p53-mediated ROS formation.26 N-SMase is activated by arachidonate,27 IL-1β and TNFαR1,28 iPLA2β,29 radiation30,31 and other cellular stimuli but is inhibited by glutathione, which is cytoprotective.32 These studies establish N-SMase as a central responder to cytokine- and stress-induced cell death.33
Acidic sphingomyelinase (A-SMase) is a lysosomal enzyme. An inherited deficiency of A-SMase activity is responsible for the lipid storage disorder Niemann–Pick disease (types A and B).34 It was shown as early as 1996 that lymphoblasts from Niemann–Pick patients are resistant to apoptosis induced by ionizing radiation.35,36 This observation served as a prelude to subsequent knockout mouse experiments revealing the significant role that A-SMase plays in mediating radiation-induced apoptosis through the generation of intracellular ceramide. A-SMase is upregulated by signaling events linked to induction of apoptosis including TNFα receptor ligation,37 UV-irradiation,38 and ischemia.39 A-SMase can also be secreted to the extracellular space.40 In a small study of patients receiving spatially fractionated high dose radiation, secretory SMase and ceramide increased in serum in response to radiation treatment and elevation of these markers correlated with clinical response.41 Although there have been no reports of A-SMase mutations in human cancers, an A-SMase-like protein was found to be elevated in bladder cancer.42
Alkaline sphingomyelinase activity is localized in the epithelial cells of the small intestines.43 The enzyme hydrolyses sphingomyelin in both intestinal lumen and the mucosal membrane in a bile salt-dependent manner.44 There is currently no direct evidence demonstrating that alkaline sphingomyelinase promotes cell death. However, the enzyme was recently found to be downregulated in colon cancer and aberrant forms of the enzyme with reduced activity have been associated with liver tumors, raising the possibility that ceramide generated in the enteric system by this enzyme could have an influence on gastrointestinal tumor biology.45
The identification of the sphingomyelin cycle represented the first causal connection between ceramide and cell fate. Later studies demonstrated that ceramide generated through the de novo pathway can also promote apoptosis. A recent study by Bose and colleagues showed that the anticancer drug daunorubicin induces apoptosis in P388 and U937 leukemia cells by increasing ceramide levels.46 The elevation in ceramide and apoptosis could be abrogated by addition of fumonisin B1, an inhibitor of ceramide synthase, indicating that de novo ceramide synthesis and not sphingomyelin hydrolysis, was required for daunorubicin-mediated cell death. Another study demonstrated that treatment of HL60 leukemia cells with H2O2 increased the intracellular levels of ceramide and sphinganine.47 Neither the levels of sphingomyelin nor sphingomyelinase activity were affected by H2O2 treatment, which indirectly suggested that H2O2 treatment may be activating the de novo pathway. Numerous additional examples of de novo ceramide synthesis contributing to apoptosis have been reported.48–52
Ceramide as a Mediator of Cell Death by Chemopreventive Agents
In addition to its role in promoting radiation- and chemotherapy-induced apoptosis, ceramide has been implicated in mediating the effects of chemopreventive agents including curcumin, resveratrol and nonsteroidal anti-inflammatory drugs. A number of reports have ascribed an anticarcinogenic effect and pro-apoptotic functions to curcumin, a polyphenol found in the spice turmeric. Moussavi et al53 recently demonstrated that curcumin induces ceramide generation and apoptosis in colon cancer cells. As apoptosis could be partially inhibited by myriocin, an inhibitor of SPT, it was concluded that ceramide generation via the de novo pathway was responsible.53 Resveratrol is a natural antioxidant found in grapes and wine that may potentially act as an anti-cancer agent.54 Resveratrol affects key targets involved in the regulation of cell cycle progression and apoptosis. For example, DU145 cells resistant to radiation became sensitive upon pretreatment with resveratrol. The sensitization effect of resveratrol was due to the induction of de novo synthesis of ceramide, since myriosin blocked the cell death caused by resveratrol/radiation.55 Nonsteroidal anti-inflammatory drugs (NSAIDs) comprise an important class of chemopreventive agents currently in use to protect patients prone to colon cancer. Studies indicate that NSAIDs stimulate ceramide production, which may contribute to their anticancer effects.56
Ceramide Influences Both the Intrinsic and Extrinsic Apoptotic Pathways
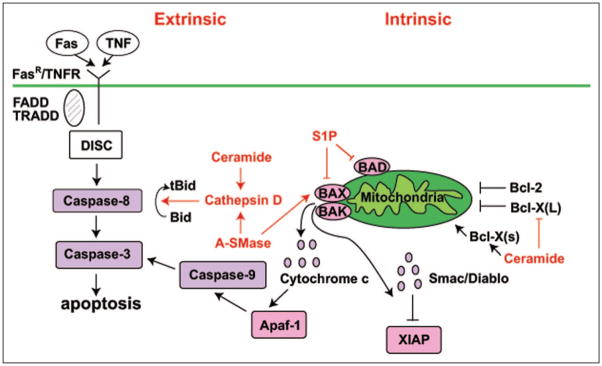
Apoptosis can be elicited in cells through two major routes, an extrinsic route initiated through activation of cell surface receptors such as TNFαR1 and Fas and an intrinsic route that is activated by permeabilization of the outer mitochondrial membrane via pro-apoptotic members of the Bcl-2 protein family (Fig. 2). Both pathways converge on caspases, which are responsible for executing subsequent steps in the apoptotic program.
Figure 2.
Sphingolipid effects on intrinsic and extrinsic apoptotic pathways. The two pathways to apoptosis are shown. The extrinsic pathway begins by ligand binding to cell surface receptors such as FasR or TNFR, followed by recruitment of Death Domain containing protein adaptors (e.g., TRADD) resulting in the formation of Death-Inducing Signaling Complex (DISC). This complex then activates the caspase cascade, culminating in cell death. The intrinsic or mitochondrial pathway begins when pro-apoptotic members of the Bcl-2 family cause mitochondrial release of cytochrome c, which binds to and activates Apaf-1 (apoptotic protease-activating factor), resulting in subsequent activation of the caspase cascade. Smac/DIABLO (Second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI) is also released, inhibiting XIAP. XIAP (X-linked Inhibitor of Apoptosis Protein) functions as an inhibitor of caspases 3, 7 and 9. Shown are also the influence of ceramide, A-SMase and S1P on different components of the pathway.
Numerous studies have shown that ceramide promotes apoptosis through the mitochondrial pathway, in part due to its effects on Bcl-2 family proteins.57–62 The Bcl-2 family can be subdivided into anti-apoptotic members such as Bcl-2 and Bcl-x(L) and pro-apoptotic family members such as Bcl-x(s), Bax, Bak, Bok, Bad, Bik, Bid, Puma, Bim, Bmf and Noxa. The posttranscriptional processing of the Bcl-x gene determines whether anti-apoptotic Bcl-x(L) or pro-apoptotic Bcl-x(s) will be expressed.63 The Bcl-x splice variant, Bcl-x(s), is produced by activation of an upstream 5′ splice site within Bcl-x exon 2. Importantly, ceramide was shown to regulate the 5′ splice site selection within the Bcl-x exon 2.64 Treatment of A549 lung adenocarcinoma cells with cell-permeable ceramide and/or agents that induce the synthesis of de novo ceramide downregulated Bcl-x(L) mRNA and protein levels and concomitantly increased Bcl-x(s) mRNA and protein.64 This effect correlated with increased sensitivity of A549 cells to daunorubicin. Furthermore, A549 cells resistant to chemotherapeutic agents and cell-permeable ceramides demonstrated increased Bcl-x(L) levels. Others have reported that UV light-induced Bax activation and ensuing cytochrome C release and apoptosis, require the actions of A-SMase.38 Thus, in HeLa cells treated with siRNA against A-SMase or in A-SMase−/− cells from NPD patients, UV light induction of Bax conformation change was drastically reduced. Further, restoration of A-SMase or addition of exogenous ceramide to A-SMase-deficient cells restored the UV pro-apoptotic response. These findings suggest that ceramide activates the intrinsic apoptotic pathway through its effects on Bcl-2 family proteins.38,64
Apoptosis can also be activated through the extrinsic, or death receptor pathway (Fig. 2). TNF receptor 1 and other members of the TNF family initiate this process when activated by ligand. Once activated, these receptors interact with an adaptor protein called FADD, leading to assembly of a protein complex that activates caspase-8, which in turn cleaves and activates the Bcl-2 family member, Bid. Bid then translocates to the mitochondrial outer membrane, initiating the intrinsic apoptotic pathway. Interestingly, Bid can also be cleaved by the lysosomal aspartate protease, capthepsin D. It was recently established that activation of cathepsin D by TNFα requires A-SMase activity.65,66 Further, ceramide was shown to bind directly to cathepsin D, causing autocatalytic proteolysis of the pre-pro-cathepsin D to form the enzymatically active isoforms of the enzyme, thereby implicating ceramide in regulation of Bid processing.
Lastly, some of ceramide’s effects may be due to its biophysical properties and influence on membrane organization. Endogenous ceramide aggregates into membrane microdomains, known as lipid rafts.67,68 These ceramide-enriched domains serve to cluster activated receptor molecules such as the Fas receptor67 and may thereby facilitate receptor-mediated (extrinsic pathway) apoptotic signaling events. Whether S1P exerts additional or opposing effects on membrane organization remains to be determined.
Sphingosine-1-Phosphate as a Counterbalance to Ceramide
Sphingosine 1-phosphate (S1P) is the final common product of sphingolipid catabolism, produced via phosphorylation of sphingosine by SK. S1P signaling inhibits apoptotic pathways, stimulates the proliferation, differentiation and migration of various cell types and promotes the development and maturation of nascent blood vessels, including tumor vessels.22,69–72 These effects are due to the ability of S1P to promote homotypic and heterotypic cell-cell interactions. S1P signaling is also required for lymphocyte egress from peripheral lymphoid organs and inhibition of this pathway using the pharmacological agent FTY720 is being tested as an immunomodulatory intervention in solid organ transplantation and autoimmune disease.73
A majority of S1P’s effects appear to be mediated through its ability to ligate and activate cell surface S1P1–5 receptors.74–76 These proteins represent a family of G protein-coupled receptors (GPCRs), first identified as endothelial differentiation genes. Due to its derivation from ceramide, its stimulatory effect on cell growth and proliferation and its ability to block apoptotic pathways, S1P has been considered a counterbalance to ceramide in the regulation of cell fate.76 Further, the anti-apoptotic and pro-angiogenic effects observed in response to activation of S1P receptors have led to the hypothesis that these signaling pathways may contribute to tumorigenesis and tumor progression. This notion is supported by the observation that SK functions as an oncogene in cell models of cancer and that blocking antibodies against S1P receptors inhibit xenograft growth in nude mice.77,78
Inhibitory Effects of S1P on Apoptotic Pathways
In contrast to the effects of ceramide, S1P signaling has been shown to block apoptotic pathways, primarily by inhibiting changes in mitochondrial membrane potential and preventing cytochrome c release from mitochondria.79 S1P stimulation of cells leads to downregulation of Bax expression and protection against Fas-induced apoptosis.80 In Jurkat T lymphoblasts, caspase-3 and -7 activation resulting from Fas ligation could be prevented by S1P addition or PKC activation via phorbol esters.81 S1P was also shown to inactivate Bad and prevent Bax translocation to mitochondria in response to Fas ligation.82 Interestingly, cathepsin B is responsible for proteolysis of SK1 during the onset of apoptosis.83 In light of the effects of ceramide on cathepsin D described above, it is intriguing to note the prominent role that lysosomes play in mediating effects of sphingolipids on cell fate decisions.
Interestingly, S1P may have different effects on cell fate depending upon its subcellular location. Spiegel and colleagues showed that the enzyme SK2 functions as a BH3-domain protein that counteracts the effects of SK1 and induces apoptosis.84 Experiments targeting SK1 expression to the ER, where SK2 normally resides, showed that SK1 can also promote apoptosis, indicating that S1P production at this site could have effects on local structures and also influences the biochemical conversion of sphingosine into ceramide, thereby perturbing the balance of sphingolipids that regulate cell fate.85,86
Sphingolipids Regulate Key Signaling Pathways at Control Cell Fate
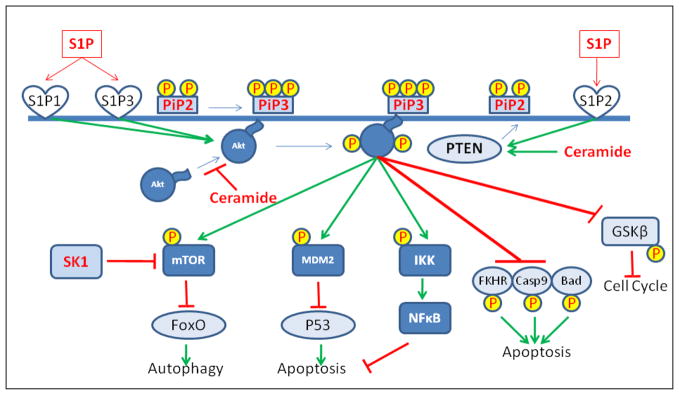
Another mechanism by which sphingolipids influence cell fate involves their ability to modulate key signaling pathways that control cell metabolism and survival in response to stress. One such pathway is the PI3 kinase/AKT pathway, which has emerged as a central signaling node responsible for adjusting the metabolic and biosynthetic operations of the cell in response to changing nutrient and environmental (stress) conditions (Fig. 3).87,88
Figure 3.
Effect of sphingolipids on the PI3K/AKT pathway. Upon phosphorylation of PIP2 to PIP3 in response to growth stimulatory signals, Akt and PDK1 (not shown) are recruited to the plasma membrane by virtue of their pleckstrin homology domains. Once there, PDK phosphorylates and activates Akt, which in turn either activates or inhibits a large number of substrates by phosphorylating them, thus exerting influence over various cell functions. The schematic focuses on the role of Akt in inhibition of apoptosis. The Akt substrates that have an inhibitory role on apoptosis are depicted in dark blue rectangles and those that exert a positive influence (which phosphorylation by Akt abrogates) are shown in light blue ovals. The figure also illustrates how different sphingolipids might influence the pathway both negatively (Ceramide) or positively (S1P).
Activation of the AKT pathway facilitates cell cycle progression, inhibits apoptotic responses, suppresses autophagy (see below) and enhances nutrient uptake, metabolism, ribosome biogenesis and protein synthesis.88 The cumulative effects of PI3K/AKT signaling lead to increased cell growth (cell mass) and cell survival. This critical function is coordinated through a system of cell surface receptors, typified by the insulin receptor which, when stimulated by ligand, leads to activation of the class I phosphoinositol-3 kinase (PI3K). Once activated, PI3K phosphorylates phosphatidylinositol-4,5-bisphosphate (PIP2), generating phosphatidylinositol-3,4,5-trisphosphate (PIP3). Generation of PIP3 at the plasma membrane leads to membrane recruitment of the serine/threonine protein kinase AKT, along with PDK1, each by virtue of their lipophilic pleckstrin homology domains (PHD). PDK1 then phosphorylates AKT on Thre308 of its activation loop. A second AKT residue (Ser473) is phosphorylated by mTORC2. Activated AKT subsequently phosphorylates downstream targets involved in protein synthesis, cell cycle progression and DNA repair, growth, survival and metabolism, angiogenesis, carbohydrate uptake, migration and proteins involved in feedback regulation of the AKT pathway itself. Antagonists to AKT signaling include the lipid phosphatase PTEN, which catalyzes the conversion of PIP3 to PIP2 and PHLPP, which dephosphorylates AKT at Ser 473. Importantly, activating mutations in the PI3K/AKT pathway have been identified in many types of human cancers.89–91 Considering the broad effects of PI3K/AKT signaling on cell growth and survival, it is not surprising that oncogenic mutations in the AKT pathway have been implicated in carcinogenesis, cytotoxic therapy, epithelial-mesenchymal transition and tumor progression within an inhospitable tumor environment.
Ceramide acts as a negative regulator of the PI3K/AKT pathway.58,92–94 In vascular smooth muscle cells, ceramide was shown to block PDGF-induced AKT activation in a PKCζ-dependent manner.92 Ceramide disrupts interactions between 14-3-3 scaffolding proteins and PKCζ, thereby recruiting the latter to lipid rafts. Additional studies in 3T3 L1 pre-adipocytes have shown that ceramide blocks AKT translocation from cytosol to plasma membrane via its PHD.95 In addition to its effect on AKT transloction, ceramide also appears to block AKT signaling through dephosphorylation of the active AKT protein by PP2A, a direct target of ceramide.95–97
These findings indicate several independent effects of ceramide on AKT signaling. They are consistent with the frequent observations correlating ceramide accumulation in skeletal muscle and liver with insulin resistance and decreased insulin-dependent glycogen synthesis and glucose uptake, which are AKT-dependent activities. Similar effects of ceramide have been observed in parasite-infected macrophages, neuronal cells, myotubes and intestinal epithelial cells.58,98–100 Whereas most reports have described the effects of ceramide to be independent of phosphatidylinositol levels (i.e., downstream of PI3K), one study in PC12 pheochromocytoma cells found that ceramide recruited PTEN to lipid rafts and that ceramide-dependent cell death was dependent on PTEN.101 Interestingly, another lipid mediator, ceramide-1-phosphate, was found to activate AKT, likely through indirect effects on sphingomyelinase resulting in reduced ceramide generation.102
Whereas the effects of ceramide are inhibitory to AKT signaling, S1P activates this pathway. In endothelial cells and cardiac myocytes, S1P activation of AKT led to eNOS activation, hypertrophy, stress fiber formation and resistance to ischemia/reperfusion injury.103,104 S1P receptors are required for each of these effects, as demonstrated by studies employing chemical inhibitors, siRNA and murine knockout models. In endothelial cells, the effect of S1P on AKT appears to require S1P receptor-mediated activation of AMPK, followed by activation of the small GTPase, Rac.105 VEGF treatment of endothelial cells was found to stimulate expression of S1P3, which stabilized Apkt3 mRNA and increased Akt3 protein expression.106 These results suggest that S1P may exert short-term, long-term and Akt isoform-specific effects on the PI3K/AKT system in a variety of cell types. Transactivation of the VEGF receptor Flk-1/KDR by ligation of S1P receptors resulted in AKT activation and eNOS phosphorylation.107 Further, overexpression of SK1 was found to induce AKT signaling, leading to enhancement of endothelial cell-cell interactions.108 In a CHO cell expression system, S1P activated AKT signaling in a PLD- and S1P3-dependent manner, leading to membrane ruffling.109 S1P also appears to activate PI3Kb in endothelial cells.110 Interestingly, AKT phosphorylation of S1P1 is necessary for S1P-induced Rac activation and cell migration, indicating that S1P and AKT signaling are mutually activating systems. In mouse embryonic fibroblasts (MEFs) and other cell types, S1P activation of S1P3 led to phosphorylation of the PDGFR and phosphorylation of AKT on Ser473. Both S1P3 and PDGFR were required for AKT activation by S1P, indicating crosstalk between S1P and PDGF receptors.111 In some ovarian cell lines, S1P mediates its effects on PI3K-dependent AKT activation through the stress-activated kinases MEK and p38.112 Finally, there is evidence suggesting that S1P activation of S1P2 inhibits cell migration and enhances vascular permeability through Rho-dependent activation of PTEN, leading to inhibition of AKT signaling through reduction of PIP3.113
Sphingolipids and Autophagy
In addition to regulating apoptosis and protein translation, the PI3K/AKT signaling pathway plays a central role in regulating autophagy, another important mechanism of cell death upon which sphingolipids exert their influence. Autophagy is the evolutionarily conserved lysosomal degradation of a cell’s own macromolecules and organelles. Autophagy is important in energy homeostasis, providing a means by which cells and organisms recycle their own parts when nutritional intake is low and energy stores are depleted. However, autophagy also plays a role in innate immunity, stress responses, neurodegeneration, aging and cancer. The latter has been shown by the finding that some tumor suppressors induce autophagy.114
During autophagy, a doubled-membraned vesicle called an autophagosome sequesters the target molecules or organelles followed by the fusion of the outer membrane with lysosomes and culminating in the degradation of the sequestered material via lysosomal enzymes.115 This process is mediated by the actions of a protein complex involving beclin-1, which serves as a platform for recruitment of autophagy activators and inhibitors. For example, the anti-apoptosis protein Bcl-2 inhibits autophagy by interacting with beclin-1. In response to growth deprivation, Jnk1 phosphorylates Bcl-2 on T69, S70 and S87, disrupting its interaction with beclin in favor of Bcl-2/Bad interactions (Patrice Codogno, personal communication).
Under favorable growth conditions the AKT pathway inhibits the onset of autophagy via activation of mTOR kinase.116 Once a cell senses the lack of nutrients or growth factors, signaling through the AKT pathway ceases and the block on autophagy is lifted. Autophagy can be induced in malignant glioma cells by inhibition of PI3K (by LY294002), AKT (by UCN-01) or mTOR (by rapamycin).117 In contrast, overexpression of constitutively active AKT affords the cells more resistance to autophagic cell death induced by rapamycin treatment.
C2-ceramide was shown to induce autophagy by upregulating beclin-1 in MCF-7 cells.118 In the same study it was shown that tamoxifen induced autophagy and concurrent beclin-1 upregulation. Both of these endpoints were inhibited by the SPT inhibitor myriocin, indicating a role for the de novo ceramide synthesis in mediating tamoxifen-induced autophagy. Ceramide has also been implicated in mediating autophagy in human umbilical vein endothelial cells exposed to glycated collagen I.119 Daido and colleagues showed that C2-ceramide induced autophagic cell death in U373-MG and T98G glioblastoma cells.120 The cell death was linked to an increase in the transcription of BNIP3 gene, a Bcl-2 family member that induces nonapoptotic cell death.
Whereas ceramide and S1P usually exert opposing effects on cell fate, both lipids function as positive regulators of autophagy. It has been show that overexpression of SK1 induces autophagy and that SK1 is activated when cells are starved, leading to increased autophagy.121 However, the autophagy induced by S1P seems to be independent of its effects on AKT. Rather, it is exerted by inhibition of mTOR and, in contrast to the effect of ceramide, the increase in beclin-1 is nominal. Furthermore, the autophagy induced by SK1 upregulation during starvation seems to be cytoprotective, as siRNA against SK1 inhibited autophagy and increased cell death during starvation.122 It remains unclear to what extent sphingolipid metabolism affects autophagy in physiological circumstances and in cancer, where autophagy may initially promote cell survival in the harsh environment of a tumor but may eventually be a mechanism for targeted cancer cell death and induction of apoptotic cell death by chemotherapy and radiation.
In summary, sphingolipid metabolism wields a double-edged sword with respect to cell fate. In response to stressful conditions or cytotoxic therapy, ceramide generated from either de novo biosynthetic or recycling metabolic pathways triggers activation of signals affecting intrinsic and extrinsic apoptotic pathways and cell cycle control, while simultaneously inhibiting signaling events that promote cell growth and survival mediated by the AKT pathway. In contrast, S1P generally reverses the effects of ceramide on these pathways through the stimulation of receptor-mediated signaling events and activation of the AKT pathway. The two effectors meet, however, in the autophagy response, where both lipids serve as activators of the pathway.
Other Signaling Pathways Influenced by Sphingolipids
In addition to effects on the AKT pathway, ceramide been shown to induce members of the stress-activated protein kinases such as JNK,123 kinase suppressor of Ras (KSR)124 and the atypical protein kinase C (PKC) isoform, PKC ζ.92 Each of these proteins influences cell survival and apoptosis. S1P opposes the actions of ceramide, suppressing JNK activation mediated by ceramide and activating ERK. Both these effects of S1P can contribute to cell survival,125 whereas in other cases, S1P blocks ceramide-induced apoptosis through effects that appear to be independent of ERK.126 S1P can also stimulate production and secretion of EGF, PDGF and VEGF.127 The increase in levels of these pro-growth and -angiogenesis factors in turn results in transactivation of their cognate receptors, leading to downstream signals that regulate cell proliferation, migration and vascular remodeling. Hsieh and colleagues showed that S1P induced both transcription and translation of EGFR via activation of p42/p44 MAPK, AP-1, PI3K/AKT and NF-κB-signaling pathways in rat primary cultured vascular smooth muscle cells.128 High levels of the EGFR have been detected in various human cancers, including glioblastomas and ovarian cervical and kidney tumors.129–131 S1P treatment enhanced proliferation of these cells in a process that could be inhibited either by treatment with EGFR kinase inhibitor AG1478 or EGFR shRNA.
COX-2 is a critical enzyme required for synthesis of prostaglandins and the target of the anti-inflammatory and chemopreventive agent celecoxib. In HT29 colon cancer cells, downregulation of SK1 expression by siRNA significantly inhibited TNFα- or IL-1β-induced COX-2 expression and PGE2 production. These findings suggest that S1P signaling may promote carcinogenic inflammatory effects through upregulation of COX-2 and generation of prostaglandin metabolites.
Ceramide Regulates Cell Cycle Progression
In some cells, ceramide has been shown to prevent cell proliferation through inhibition of cell cycle progression. Treatment of MOLT4 leukemia cells with ceramide or its metabolite sphingosine results in dephosphorylation of the retinoblastoma gene product, leading to cell cycle arrest.132 Ceramide has also been shown to regulate cyclin dependent kinase 2 (CDK2) activity.22 Ceramide can cause G2 cell cycle arrest via induction of p21 expression, even in the absence of DNA damage.133 Interestingly, the negative regulator of p53, MDM2 was found to abrogate ceramide-induced growth arrest and apoptosis.133 In RD rhabdomyosarcoma cells, overexpression of MDM2 prevented ceramide-induced G2 arrest, p21 induction and apoptosis, whereas siRNA against MDM2 sensitized the cells to ceramide-induced G2 arrest. Accumulation of p21 as well as reduction in cyclin D1 and CDK7 were responsible for ceramide-induced cell cycle arrest in Be7402 hepatocarcinoma cells.134
Ceramide and Telomerases
Telomerase is a ribonucleoprotein composed of a reverse transcriptase (hTERT) and an RNA partner (hTR). The complex is responsible for addition of TTAGGG tandem repeat sequences to the ends of chromosomes.135–138 Telomerase is overexpressed in approximately 90% of human cancers. Its expression, usually absent in somatic cells, allows malignant cells to maintain telomere length, thereby insuring infinite replicative capacity. Increased telomerase activity has been suggested to offer protection against apoptosis. Both exogenous short-chain ceramide and stimulation of endogenous ceramide production were found to inhibit telomerase expression.139 Ceramide affected the promoter activity of hTERT, decreasing the half-life of its trans-activator, cMyc, via increased ubiquitination and rapid proteolysis. More recent findings suggest that ceramide modulates the promoter activity of hTERT via a pathway that involves epigenetic effects on the hTERT promoter, culminating in transcriptional repression.140
Ceramide and S1P in Cancer Stem Cells
Cancer cells are usually a heterogeneous population, of which only a small fraction referred to as “cancer stem cells” is capable of continuous self-renewal, as demonstrated by colony formation in soft agar or development into tumors following transplantation into a host organism. A recent study found mammary cancer stem cells to be more resistant than their nonstem cell counterparts to serum deprivation and ceramide-induced apoptosis.141 This characteristic of the cancer stem cells was attributed to the unique overexpression of sphingomyelin synthase 1 and Bcl-2 in the stem cells, two properties that would serve to lower ceramide levels.
Effects of S1P on Migration and Metastasis
S1P activation of its receptors influences endothelial cell migration by promoting changes in cytoskeletal organization that are needed for the formation of lamellipodia and attachment to the basement membrane.142 These effects may be important targets for the prevention of pathological angiogenesis, including tumor angiogenesis, which is an important contributor to the progression of cancer.
S1P has also been shown to influence epithelial cancer cell migration, which could contribute to metastatic potential. S1P was found to stimulate chemotaxis and invasion of ovarian cancer cell line (OVCAR3) in a receptor-dependent fashion that involved activation of ERK, AKT and p38.143 A link between S1P signaling, cell migration and tumor metastasis was uncovered when it was observed that overexpression of the KAI1 gene downregulates SK1. The KAI1 gene was originally isolated as a prostate-specific tumor metastasis suppressor gene that inhibited metastases without affecting primary tumor formation. When KAI1 was overexpressed using an adenovirus vector in pancreatic carcinoma cells (PANC I) both SK-1 activity and migration of PANC I cells was significantly reduced.144
Cancer Cells Exhibit Molecular and Genetic Changes in Sphingolipid Metabolism
Considering the central role of ceramide in mediating physiological as well as pharmacologically stimulated apoptosis, ceramide could be considered a tumor-suppressor lipid. If ceramide-mediated apoptosis functions as a form of cancer surveillance, one would predict that the tumor cells might evolve mechanisms to avoid ceramide accumulation either by inhibiting the production of ceramide or by enhancing its degradation. Recent research has provided evidence that both these types of molecular changes occur in cancer cells.
Ceramide levels in fresh human specimens of primary and metastatic colon cancer were found to be half that of normal colon mucosa from the same patient.145 The difference seemed to stem from higher ceramidase activity in the tumor tissue. Importantly, ceramidase inhibition increased the ceramide content of tumor cells and caused activation of the apoptotic cascade, whereas this maneuver had no effect on normal cells.
Consistent with this notion, acid ceramidase was found to be over-expressed in 70% of head and neck squamous cell tumors compared with normal tissues.146 Forced expression of acid ceramidase in tumor-derived cells increased resistance to Fas-induced apoptosis, whereas its down-regulation using specific siRNA sensitized the cells to Fas. Inhibition of acid ceramidase using the small molecule LCL204 sensitized head and neck squamous cancer cell lines to Fas-induced apoptosis both in vitro and in a xenograft model. The increased expression of acid ceramidase has also been observed in prostate cancer cells.146 Treatment of DU145 prostate cancer cells with LCL204 resulted in increased ceramide and decreased sphingosine levels along with rapid destabilization of lysosomes and the release of lysosomal proteases into the cytosol, culminating in the activation of the intrinsic apoptotic pathway.147
Another mechanism that cancer cells utlize to increase drug resistance is the enhancement of ceramide transport via the actions of a protein called CERT (ceramide transporter). CERT is involved in ATP-dependent ER-to-Golgi trafficking of ceramide molecules. CERT expression was recently shown to be elevated in paclitaxel-resistant cancer cell lines and in ovarian cancer cells that survived paclitaxel treatment. Reduction of CERT expression using siRNA increased cellular sensitivity to paclitaxel. CERT may attenuate the effects of ceramide via its removal from ER, where it has been postulated to potentiate the drug-induced ER stress response.148
Given the role of S1P as a mitogen and inhibitor of apoptotic pathways, genetic changes resulting in S1P accumulation in cancer cells might also be selected for during carcinogenesis and cancer progression. In fact, overexpression of SK1 has been observed in a variety of solid tumors including lung, colon and other cancers.149–152 SK1 upregulation was also observed in a transgenic mouse model of erythroleukemia caused by overexpression of the transcription factor Spi-1.153 In this model, transcriptional upregulation of the SK1 gene was a recurrent event in the development of malignant erythroblasts, where SK1 expression and activity conferred a proliferative advantage, increased clonogenicity, resistance to apoptosis and in vivo tumorigenic potential. Conversely, Kohno et al154 reported that an SK1−/− genotype reduces adenoma size and attenuates epithelial cell proliferation in the ApcMin/+ mouse model of colon cancer, implicating SK1 as a mediator of adenoma progression. Interestingly, this effect was independent of S1P receptor expression and therefore extracellular S1P signaling, since polyp incidence or size was unaltered in ApcMin/+ in which S1P receptor genes were disrupted.
Observations of SK1 upregulation in human cancer specimens and the tumor suppressive effects of mutations preventing S1P accumulation in mouse models of intestinal tumorigenesis suggest that conversion of sphingosine to S1P may promote tumor progression, whereas S1P catabolism might inhibit it.149,154 Consistent with this hypothesis is the recent finding that SPL and S1P phosphatase, two enzymes responsible for degradation of S1P, are downregulated in colon cancer and in mouse models of intestinal tumorigenesis.155 Downregulation of SPL and reduced SPL enzyme activity were consistent hallmarks of early adenomas in the ApcMin/+ mouse model and were associated with increased S1P levels in polyp compared to surrounding tissue. Sphingosine derived from dietary sphingolipids has been shown to have protective effects against colon cancer in rodent models.156–158 Thus, while loss of SPL or phosphatase has not yet been shown to directly contribute to tumorigenesis, it seems plausible that neoplastic intestinal tissue may be under selection pressure to facilitate conversion of dietary sphingosine to S1P and to prevent S1P degradation, thereby converting a growth-inhibitory pathway into a growth activating and angiogenic stimulus.
The inhibitory effects of S1P on apoptotic pathways may be responsible for mechanisms of cancer cell drug resistance. In HL60 leukemia cells, drug sensitivity correlated with low SK activity and the ability to generate ceramide.159 In contrast, drug-resistant derivatives of the parent cell line exhibited greater SK activity, failure to accumulate ceramide and inability to activate the mitochondrial apoptotic pathway in response to drug treatment. Inhibition of SK in these cells restored drug sensitivity. The opposing actions of ceramide and S1P are illustrated by a study in which apoptosis-resistant human melanoma cell lines M221- and Mel-2a were found to have higher SK activity, lower ceramide levels and higher S1P-to-ceramide ratios than the corresponding drug-sensitive cell lines A-375 and M186.160 Overexpression of functional (but not mutant) SK1 increased resistance of A-375 melanoma cells to short-chain ceramide, Fas antibody and doxorubicin-induced apoptosis. In contrast, siRNA-mediated downregulation of SK1 expression rendered ceramide-resistant Mel-2a cells sensitive to ceramide- and Fas-induced cell death. Similar effects of S1P were observed in response to imatinib, a small molecule inhibitor of Bcr-Abl tyrosine kinase.161 Forced SK1 downregulation and reduction of S1P levels resulted in imatinib-resistant cells becoming markedly more sensitive to the drug, whereas overexpression of SK1 prevented the apoptotic response to imatinib.162
Targeting Sphingolipids for Cancer Therapy
The crucial role that sphingolipids play in regulating cell fate in combination with the genetic alteration of sphingolipid metabolic pathways observed in cancer specimens and in association with cellular drug resistance have prompted efforts to harness sphingolipid signaling for antitumor therapy. Approaches have included the development of: (1) synthetic ceramide analogs, (2) small molecule inhibitors of enzymes that catalyze ceramide catabolism or its conversion to other molecular species, (3) inhibitors of SK and (4) S1P receptor antagonists. Additional approaches that have been proposed include reactivation of genes such as SPL, S1P phosphatase and SMase that have been silenced in cancer tissue, targeting dihydroceramide desaturase (see below) and activation of SMase enzymes using small molecules. Pharmacological modulation of sphingolipid targets singly, as adjuvant therapy in combination with conventional cytotoxic approaches and in combinations that serve to enhance ceramide generation while preventing auto-regulatory effects caused by conversion of ceramide to S1P are all being considered.
Numerous ceramide analogs with unique physicochemical and biological properties have been developed, including cell-permeable, short chain ceramides, analogs with additional double bonds in the long chain base, analogs containing disulfide linkages, conjugates of ceramides and dihydroceramides with pyridinium salts and a variety of alkyl- acyl and urea-analogs.163 Some of these molecules have the advantage of improved solubility, efficient cellular uptake and the ability to be targeted to different cellular compartments such as mitochondria and lysosomes for enhanced efficacy. Some have been shown to promote mitochondrial apoptosis, deplete cellular glutathione and cause cytotoxicity in malignant cells. However, it is important to keep in mind that ceramide analogs have the potential to interact with numerous cellular and biochemical targets in the sphingolipid metabolic pathway. Thus, the toxicity profiles and efficacy of these novel drugs will need to be tested in vivo before conclusions about their utility in the clinic can be drawn.
As discussed above, analysis of the gene expression patterns associated with the acquisition of cytotoxic drug resistance phenotypes suggest that preventing ceramide accumulation through enhanced degradation or glycosylation is an important mechanism of drug resistance. In contrast to the use of ceramide analogs, which may exert effects that are not specific to cancer cells, targeting genes that are overexpressed in cancer may afford some selectivity. Interestingly, the pharmacological inhibition of glucosylceramide synthase by miglustat and similar agents has been used for many years in “substrate depletion” approaches to treat sphingolipidosis storage diseases. These agents act by preventing formation of the first intermediate in the synthesis of many higher order sphingolipids, glucosylceramide.164 While these drugs have not been tested as anticancer agents in humans, targeting glucosylceramide synthase has shown some efficacy in cancer models. For example, the imino sugar OGT2378, an inhibitor of glucosylceramide synthase, inhibits melanoma tumor growth in vitro and in a syngeneic orthotopic mouse model.165 In the case of melanoma, the positive effects of the drug may be related to prevention of ganglioside formation, rather than ceramide accumulation, as the former contributes to tumorigenicity in melanoma.166 Dbaibo and colleagues reported that arsenic trioxide, an agent used in the treatment of promyelocytic leukemia, induces apoptosis via a combination of glucosylceramide synthase inhibition and induction of de novo ceramide synthesis.167 As these and other specific small molecule inhibitors of ceramide-metabolizing enzymes are tested in a range of tumor types, the utility of enhancing endogenous ceramide accumulation to reactivate apoptotic pathways in drug-resistant cancer cells should become clear.
Inhibition of S1P synthesis by blocking SK activity has proven useful in enhancing cancer cell responses to cytotoxic therapy in vitro and in animal models.152,159,168–170 French and colleagues developed a panel of nonlipid inhibitors of SK1 that prevented proliferation and induced apoptosis in various tumor cell lines, including some that displayed multidrug resistant phenotypes.151 A recent study171 provided evidence suggesting that apoptosis induced by the SK inhibitor dimethylsphingosine occurred in a Bcl-2-independent fashion in human prostatic adenocarcinoma cell lines. Since Bcl-2 upregulation is a common event in cancer, Bcl-2 independence could be advantageous in treating drug-resistant cancers. A preclinical study demonstrating the efficacy of inhibiting S1P signaling using blocking antibodies to prevent tumorigenesis/tumor progression was also recently reported.78 In this hallmark study, a specific monoclonal antibody recognizing S1P was administered to mice harboring human cancer xenografts. The intervention reduced and in some cases completely eliminated, tumor formation and accompanying tumor angiogenesis. These studies suggest that blocking tumor angiogenesis through antibody-mediated inhibition of S1P signaling, similar to or in combination with VEGF-directed antibodies, may be developed as a strategy for treating advanced malignant disease. In a different approach to blocking S1P signaling, the immunomodulatory drug FTY720 promoted apoptosis in drug resistant multiple myeloma cells by inducing mitochondrial membrane potential changes, Bax cleavage and caspase activation.172 FTY720 acts by downregulating S1P receptors and interfering with S1P signaling. This study suggests that additional small molecule inhibitors of S1P signaling may be useful targets to enhance cancer cell apoptosis and inhibit tumor angiogenesis. Due to the immunomodulatory effects of FTY720, development of S1P receptor-specific agonists and antagonists is an area of active research. As these agents become available, testing for anticancer efficacy could be included in biological assays.
Another promising chemotherapeutic agent is retinimide or fenretinide (4-HPR). The drug is a synthetic retinoid N-(4-hydroxyphenyl) that is cytotoxic to a variety of cancers including neuroblastoma. Interestingly, the drug does not appear to be dependent on retinoid receptors, for which it has a very low binding affinity.173,174 Recently, fenretinide was found to increase ceramide generation via the de novo pathway, through activation of both SPT and ceramide synthase.175 Importantly, fenretinide does not increase ceramide levels in nonmalignant cells. Furthermore, it induces apoptosis in a p53- and caspase-independent manner, making the drug useful even in cancer cells harboring mutations in these molecular pathways.176 Fenretinide was also found to be cytotoxic to endothelial cells, in which it induced ceramide accumulation and caspase-dependent apoptosis. The ceramide generation could be suppressed by ceramide synthesis inhibitors fumonisin B1, myriocin and L-cycloserine but not by the A-SMase inhibitor desipramine. These studies suggest that fenretinide’s antitumor mechanism of action may involve an anti-angiogenic component.177
Interestingly, one report has provided evidence that fenretinide functions as an inhibitor of ceramide desaturase, suggesting that dihydroceramide or dihydrosphingosine rather than ceramide may be the cytotoxic intermediate.178 This possibility is consistent with the finding that γ-tocopherol induces apoptosis in prostate cancer cells by causing accumulation of dihydrosphingosine and dihydroceramide.179 If these studies hold true, dihydroceramide desaturase may become another important anticancer drug target. Additional studies have explored the role of the dihydrosphingosine analog safingol, which is also toxic to human prostate cancer cell lines and other malignant cell lines.176,180 Safingol acts through inhibition of PKC and has been shown to promote a caspase-independent form of apoptosis. However, a wide range of toxicities are associated with safingol, its N-methyl metabolites and potentially its conversion to dihydroceramide-like molecules on murine liver, kidney and blood.
Sphingomyelinases are activated by cytotoxic agents, resulting in the generation of ceramide through the recycling pathway and the promotion of mitochondrial apoptosis. Overexpression of A-SMase and other forms of sphingomyelinase have been shown to sensitize glioma cells and other cancer cell types to chemotherapy.181 Activation of sphingomyelinases by small molecules, thus, represents another potential avenue by which to enhance cancer cell death. Aside from conventional chemotherapeutic agents and radiation, sphingomyelinase activation by reactive oxygen species, ursolic acid and the histone deacetylase inhibitor LAQ824 have been reported.182–184 Interestingly, however, treatment of normal and cancer cells with a thiourea derivative of sphingomyelin resulted in accumulation of ceramide and induction of apoptotic cell death, despite inhibition of acid and neutral sphingomyelinase activity by the agent.185 These results appear to be due to the drug’s ability to inhibit formation of sphingomyelin from ceramide, illustrating the fact that sphingolipid analogs may have multiple targets and may induce unexpected effects on cell fate.
S1P Signaling to Protect Normal Tissues from Therapy-Related Cytotoxicity
Many cancer interventions exhibit a high therapeutic index, causing unacceptable toxicity to normal tissues. Identification of ways to protect normal cells from iatrogenic apoptosis in response to radiation and chemotherapy would improve overall outcomes for cancer patients. Interestingly, S1P administration to mice has been shown to suppress male germ cell apoptosis and ovarian failure/infertility in response to radiation and other cancer therapies.60,186 Similar observations suggest that S1P pathways are involved in regulating cardiac cell fate in response to oxidative stress.187 While still untested, these findings suggest the possibility that S1P may be able to protect against radiation and anthracycline-induced cardiotoxicity as well. These promising findings suggest that targeted protection of normal tissues may be yet another method for improving the therapeutic index of commonly employed chemotherapeutic drugs and radiation.
Conclusion
Sphingolipids are an important class of membrane lipids that give rise to signaling metabolites such as ceramide and S1P, which exert control over cell fate and senescence. rough effects on mitochondrial membrane potential, Bcl-2 family proteins, cell survival and stress-response signaling pathways, telomerase and other critical cellular machinery, these two molecules modulate cell fate in response to environmental stress, chemotherapy, radiation and genotoxic stress associated with continual DNA replication. Not surprisingly, genes involved in metabolism and signaling of S1P and ceramide have been implicated in tumorigenesis, tumor progression and patterns of drug resistance. The expression of these genes and their protein products may become useful indicators of tumor progression and modulation of these pathways through pharmacological means holds promise as novel approaches for the treatment of cancer.
Acknowledgments
This work was supported by National Insitutes of Health Grants CA77528 and CA129438 (JDS).
We apologize to the many colleagues whose work could not be cited directly in this review due to space limitations. We thank Tom McElderry for expert administrative assistance.
References
- 1.Morales A, Lee H, Goñi F, et al. Sphingolipids and cell death. Apoptosis. 2007;12:923–39. doi: 10.1007/s10495-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 2.Futerman A, Riezman H. The ins and outs of sphingolipid synthesis. Trends Cell Biol. 2005;15:312–8. doi: 10.1016/j.tcb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Kihara A, Mitsutake S, Mizutani Y, et al. Metabolism and biological functions of two phosphorylated sphingolipids, sphingosine 1-phosphate and ceramide 1-phosphate. Prog Lipid Res. 2007;46:126–44. doi: 10.1016/j.plipres.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Prinetti A, Chigorno V, Mauri L, et al. Modulation of cell functions by glycosphingolipid metabolic remodeling in the plasma membrane. J Neurochem. 2007;103(Suppl 1):113–25. doi: 10.1111/j.1471-4159.2007.04714.x. [DOI] [PubMed] [Google Scholar]
- 5.Zeidan Y, Hannun Y. Translational aspects of sphingolipid metabolism. Trends Mol Med. 2007;13:327–36. doi: 10.1016/j.molmed.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2007 doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murph M, GBM Targeting the lipids LPA and S1P and their signalling pathways to inhibit tumour progression. Expert Rev Mol Med. 2007;9:1–18. doi: 10.1017/S1462399407000476. [DOI] [PubMed] [Google Scholar]
- 8.Kolesnick RN, Goni FM, Alonso A. Compartmentalization of ceramide signaling: physical foundations and biological effects. J Cell Physiol. 2000;184(3):285–300. doi: 10.1002/1097-4652(200009)184:3<285::AID-JCP2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Itoh M, Kitano T, Watanabe M, et al. Possible role of ceramide as an indicator of chemoresistance: decrease of the ceramide content via activation of glucosylceramide synthase and sphingomyelin synthase in chemoresistant leukemia. Clin Cancer Res. 2003;9(1):415–23. [PubMed] [Google Scholar]
- 10.Okazaki T, Bell R, Hannun Y. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. J Biol Chem. 1989;264(32):19076–80. [PubMed] [Google Scholar]
- 11.Liu B, Obeid L, Hannun Y. Sphingomyelinases in cell regulation. Semin Cell Dev Biol. 1998;8(3):311–22. doi: 10.1006/scdb.1997.0153. [DOI] [PubMed] [Google Scholar]
- 12.Valaperta R, Chigorno V, Basso L, et al. Plasma membrane production of ceramide from ganglioside GM3 in human fibroblasts. FASEB J. 2006;20:1227–9. doi: 10.1096/fj.05-5077fje. [DOI] [PubMed] [Google Scholar]
- 13.Nikolova-Karakashian M, Merril AH., Jr Ceramidases. Methods Enzymol. 2000;311(Part A):194–207. doi: 10.1016/s0076-6879(00)11081-x. [DOI] [PubMed] [Google Scholar]
- 14.Olivera A, Spiegel S. Sphingosine kinase: a mediator of vital cellular functions. Prostaglandins Other Lipid Mediat. 2001;64(1–4):123–34. doi: 10.1016/s0090-6980(01)00108-3. [DOI] [PubMed] [Google Scholar]
- 15.Le Stunff H, Peterson C, Liu H, et al. Sphingosine-1-phosphate and lipid phosphohydrolases. Biochim Biophys Acta. 2002;1582(1–3):8–17. doi: 10.1016/s1388-1981(02)00132-4. [DOI] [PubMed] [Google Scholar]
- 16.Mandala SM, Thornton R, Tu Z, et al. Sphingoid base 1-phosphate phosphatase: a key regulator of sphingolipid metabolism and stress response. Proc Natl Acad Sci USA. 1998;95(1):150–5. doi: 10.1073/pnas.95.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandhuvula P, Saba J. Sphingosine-1-phosphate lyase in immunity and cancer: silencing the siren. Trends Mol Med. 2007;13(5):210–7. doi: 10.1016/j.molmed.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe M, Kitano T, Kondo T, et al. Increase of nuclear ceramide through caspase-3-dependent regulation of the “sphingomyelin cycle” in Fas-induced apoptosis. Cancer Res. 2004;64(3):1000–7. doi: 10.1158/0008-5472.can-03-1383. [DOI] [PubMed] [Google Scholar]
- 19.Clarke C, Snook C, Tani M, et al. The extended family of neutral sphingomyelinases. Biochemistry. 2006;45:11247–56. doi: 10.1021/bi061307z. [DOI] [PubMed] [Google Scholar]
- 20.Jayadev S, Liu B, Bielawska A, et al. Role for ceramide in cell cycle arrest. J Biol Chem. 1995;270(5):2047–52. doi: 10.1074/jbc.270.5.2047. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimura S, Banno Y, Nakashima S, et al. Ceramide formation leads to caspase-3 activation during hypoxic PC12 cell death. Inhibitory effects of Bcl-2 on ceramide formation and caspase-3 activation. J Biol Chem. 1998;273(12):6921–7. doi: 10.1074/jbc.273.12.6921. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Wada R, Yamashita T, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106(8):951–61. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeda Y, Tashima M, Takahashi A, et al. Ceramide generation in nitric oxide-induced apoptosis. Activation of magnesium-dependent neutral sphingomyelinase via caspase-3. J Biol Chem. 1999;274(15):10654–60. doi: 10.1074/jbc.274.15.10654. [DOI] [PubMed] [Google Scholar]
- 24.Mansat V, Laurent G, Levade T, et al. The protein kinase C activators phorbol esters and phosphatidylserine inhibit neutral sphingomyelinase activation, ceramide generation and apoptosis triggered by daunorubicin. Cancer Res. 1997;57(23):5300–4. [PubMed] [Google Scholar]
- 25.Sawada M, Nakashima S, Banno Y, et al. Ordering of ceramide formation, caspase activation and Bax/Bcl-2 expression during etoposide-induced apoptosis in C6 glioma cells. Cell Death Differ. 2000;7(9):761–72. doi: 10.1038/sj.cdd.4400711. [DOI] [PubMed] [Google Scholar]
- 26.Hara S, Nakashima S, Kiyono T, et al. p53-Independent ceramide formation in human glioma cells during gamma-radiation-induced apoptosis. Cell Death Differ. 2004;11:853–61. doi: 10.1038/sj.cdd.4401428. [DOI] [PubMed] [Google Scholar]
- 27.Jayadev S, Linardic C, Hannun Y. Identification of arachidonic acid as a mediator of sphingomyelin hydrolysis in response to tumor necrosis factor a. J Biol Chem. 1994;269(8):5757–63. [PubMed] [Google Scholar]
- 28.Schütze S, Machleidt T, Krönke M. The role of diacylglycerol and ceramide in tumor necrosis factor and interleukin-1 signal transduction. J Leukoc Biol. 1994;56:533–41. doi: 10.1002/jlb.56.5.533. [DOI] [PubMed] [Google Scholar]
- 29.Lei X, Zhang S, Bohrer A, et al. The group VIA calcium-independent phospholipase A2 participates in ER stress-induced INS-1 insulinoma cell apoptosis by promoting ceramide generation via hydrolysis of sphingomyelins by neutral sphingomyelinase. Biochemistry. 2007;46:10170–85. doi: 10.1021/bi700017z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruno A, Laurent G, Averbeck D, et al. Lack of ceramide generation in TF-1 human myeloid leukemic cells resistant to ionizing radiation. Cell Death Differ. 1998;5:172–82. doi: 10.1038/sj.cdd.4400330. [DOI] [PubMed] [Google Scholar]
- 31.Maddens S, Charruyer A, Plo I, et al. Kit signaling inhibits the sphingomyelin-ceramide pathway through PLC gamma 1: implication in stem cell factor radioprotective effect. Blood. 2002;100:1294–301. [PubMed] [Google Scholar]
- 32.Liu B, Hannun Y. Inhibition of the neutral magnesium-dependent sphingomyelinase by glutathione. J Biol Chem. 1997;272:16281–7. doi: 10.1074/jbc.272.26.16281. [DOI] [PubMed] [Google Scholar]
- 33.Marchesini N, Hannun Y. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochem Cell Biol. 2004;82:27–44. doi: 10.1139/o03-091. [DOI] [PubMed] [Google Scholar]
- 34.Schneider PB, Kennedy EP. Sphingomyelinase in normal human spleens and in spleens from subjects with Niemann-Pick disease. J Lipid Res. 1967;8(3):202–9. [PubMed] [Google Scholar]
- 35.Santana P, Pena L, Haimovitz-Friedman A, et al. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189–99. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 36.Zundel W, Giaccia A. Inhibition of the anti-apoptotic PI(3)K/Akt/Bad pathway by stress. Genes Dev. 1998;12(13):1941–6. doi: 10.1101/gad.12.13.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schutze S, Potthoff K, Machleidt T, et al. TNF activates NF-kB by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell. 1992;71:765–76. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 38.Kashkar H, Wiegmann K, Yazdanpanah B, et al. Acid sphingomyelinase is indispensable for UV light-induced Bax conformational change at the mitochondrial membrane. J Biol Chem. 2005;280(21):20804–13. doi: 10.1074/jbc.M410869200. [DOI] [PubMed] [Google Scholar]
- 39.Yu ZF, Nikolova-Karakashian M, Zhou D, et al. Pivotal role for acidic sphingomyelinase in cerebral ischemia-induced ceramide and cytokine production and neuronal apoptosis. J Mol Neurosci. 2000;15(2):85–97. doi: 10.1385/JMN:15:2:85. [DOI] [PubMed] [Google Scholar]
- 40.Schissel S, Keesler G, Schuchman E, et al. The cellular trafficking and zinc dependence of secretory and lysosomal sphingomyelinase, two products of the acid sphingomyelinase gene. J Biol Chem. 1998;273:18250–9. doi: 10.1074/jbc.273.29.18250. [DOI] [PubMed] [Google Scholar]
- 41.Sathishkumar S, Boyanovsky B, Karakashian AA, et al. Elevated sphingomyelinase activity and ceramide concentration in serum of patients undergoing high dose spatially fractionated radiation treatment: implications for endothelial apoptosis. Cancer Biol Ther. 2005;4(9):979–86. doi: 10.4161/cbt.4.9.1915. [DOI] [PubMed] [Google Scholar]
- 42.Wright K, Messing E, Reeder J. Increased expression of the acid sphingomyelinase-like protein ASML3a in bladder tumors. J Urol. 2002;168:2645–9. doi: 10.1016/S0022-5347(05)64236-X. [DOI] [PubMed] [Google Scholar]
- 43.Nilsson A. The presence of spingomyelin- and ceramide-cleaving enzymes in the small intestinal tract. Biochim Biophys Acta. 1969;176(2):339–47. doi: 10.1016/0005-2760(69)90192-1. [DOI] [PubMed] [Google Scholar]
- 44.Duan RD. Alkaline sphingomyelinase: an old enzyme with novel implications. Biochim Biophys Acta. 2006;1761(3):281–91. doi: 10.1016/j.bbalip.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Duan RD. Anticancer compounds and sphingolipid metabolism in the colon. In Vivo. 2005;19(1):293–300. [PubMed] [Google Scholar]
- 46.Bose R, Verheij M, Haimovitz-Friedman A, et al. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995;82(3):405–14. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- 47.Son JH, Yoo HH, Kim DH. Activation of de novo synthetic pathway of ceramides is responsible for the initiation of hydrogen peroxide-induced apoptosis in HL-60 cells. J Toxicol Environ Health A. 2007;70(15–16):1310–8. doi: 10.1080/15287390701434364. [DOI] [PubMed] [Google Scholar]
- 48.Petrache I, Natarajan V, Zhen L, et al. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med. 2005;11:491–8. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turpin S, Lancaster G, Darby I, et al. Apoptosis in skeletal muscle myotubes is induced by ceramides and is positively related to insulin resistance. Am J Physiol Endocrinol Metab. 2006;291:E1341–350. doi: 10.1152/ajpendo.00095.2006. [DOI] [PubMed] [Google Scholar]
- 50.Itoh G, Tamura J, MS, et al. DNA fragmentation of human infarcted myocardial cells demonstrated by the nick end labeling method and DNA agarose gel electrophoresis. Am J Pathol. 1995;146:1325–31. [PMC free article] [PubMed] [Google Scholar]
- 51.Seumois G, Fillet M, Gillet L, et al. De novo C16- and C24-ceramide generation contributes to spontaneous neutrophil apoptosis. J Leukoc Biol. 2007;81:1477–86. doi: 10.1189/jlb.0806529. [DOI] [PubMed] [Google Scholar]
- 52.Itoh Y, Yano T, Sendo T, et al. Involvement of de novo ceramide synthesis in radiocontrast-induced renal tubular cell injury. Kidney Int. 2006;69:288–97. doi: 10.1038/sj.ki.5000057. [DOI] [PubMed] [Google Scholar]
- 53.Moussavi M, Assi K, Gomez-Munoz A, et al. Curcumin mediates ceramide generation via the de novo pathway in colon cancer cells. Carcinogenesis. 2006;27(8):1636–44. doi: 10.1093/carcin/bgi371. [DOI] [PubMed] [Google Scholar]
- 54.Signorelli P, Ghidoni R. Resveratrol as an anticancer nutrient: molecular basis, open questions and promises. J Nutr Biochem. 2005;16:449–66. doi: 10.1016/j.jnutbio.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 55.Scarlatti F, Sala G, Somenzi G, et al. Resveratrol induces growth inhibition and apoptosis in metastatic breast cancer cells via de novo ceramide signaling. FASEB J. 2003;17(15):2339–41. doi: 10.1096/fj.03-0292fje. [DOI] [PubMed] [Google Scholar]
- 56.Chan T, Morin P, Vogelstein B, et al. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci USA. 1998;95:681–6. doi: 10.1073/pnas.95.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruvolo PP, Clark W, Mumby M, et al. A functional role for the B56 alpha-subunit of protein phosphatase 2A in ceramide-mediated regulation of Bcl2 phosphorylation status and function. J Biol Chem. 2002;277(25):22847–52. doi: 10.1074/jbc.M201830200. [DOI] [PubMed] [Google Scholar]
- 58.Stoica BA, Movsesyan VA, Lea PMt, et al. Ceramide-induced neuronal apoptosis is associated with dephosphorylation of Akt, BAD, FKHR, GSK-3beta and induction of the mitochondrial-dependent intrinsic caspase pathway. Mol Cell Neurosci. 2003;22(3):365–82. doi: 10.1016/s1044-7431(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 59.von Haefen C, Wieder T, Gillissen B, et al. Ceramide induces mitochondrial activation and apoptosis via a Bax-dependent pathway in human carcinoma cells. Oncogene. 2002;21(25):4009–19. doi: 10.1038/sj.onc.1205497. [DOI] [PubMed] [Google Scholar]
- 60.Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22(37):5897–906. doi: 10.1038/sj.onc.1206702. [DOI] [PubMed] [Google Scholar]
- 61.Scorrano L, Oakes SA, Opferman JT, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300(5616):135–9. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 62.Kim HJ, Mun JY, Chun YJ, et al. Bax-dependent apoptosis induced by ceramide in HL-60 cells. FEBS Lett. 2001;505(2):264–8. doi: 10.1016/s0014-5793(01)02836-8. [DOI] [PubMed] [Google Scholar]
- 63.Boise L, González-García M, Postema C, et al. Bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:579–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 64.Chalfant C, Rathman K, Pinkerman R, et al. De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. J Biol Chem. 2002;277:12587–95. doi: 10.1074/jbc.M112010200. [DOI] [PubMed] [Google Scholar]
- 65.Heinrich M, Wickel M, Schneider-Brachert W, et al. Cathepsin D targeted by acid sphingomyelinase-derived ceramide. EMBO J. 1999;18(19):5252–63. doi: 10.1093/emboj/18.19.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heinrich M, Neumeyer J, Jakob M, et al. Cathepsin D links TNF-induced acid sphingomyelinase to Bid-mediated caspase-9 and -3 activation. Cell Death Differ. 2004;11(5):550–63. doi: 10.1038/sj.cdd.4401382. [DOI] [PubMed] [Google Scholar]
- 67.Grassme H, Jekle A, Riehle A, et al. CD95 signaling via ceramide-rich membrane rafts. J Biol Chem. 2001;276(23):20589–96. doi: 10.1074/jbc.M101207200. [DOI] [PubMed] [Google Scholar]
- 68.Kilkus J, Goswami R, Testai FD, et al. Ceramide in rafts (detergent-insoluble fraction) mediates cell death in neurotumor cell lines. J Neurosci Res. 2003;72(1):65–75. doi: 10.1002/jnr.10549. [DOI] [PubMed] [Google Scholar]
- 69.Spiegel S, Merrill AHJ. Sphingolipid metabolism and cell growth regulation. FASEB J. 1996;10:1388–97. doi: 10.1096/fasebj.10.12.8903509. [DOI] [PubMed] [Google Scholar]
- 70.Yatomi Y, Ohmori T, Rile G, et al. Sphingosine 1-phosphate as a major bioactive lysophospholipid that is released from platelets and interacts with endothelial cells. Blood. 2000;96(10):3431–8. [PubMed] [Google Scholar]
- 71.Radeff-Huang J, Seasholtz TM, Matteo RG, et al. G protein mediated signaling pathways in lysophospholipid induced cell proliferation and survival. J Cell Biochem. 2004;92(5):949–66. doi: 10.1002/jcb.20094. [DOI] [PubMed] [Google Scholar]
- 72.Hobson JP, Rosenfeldt HM, Barak LS, et al. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science. 2001;291(5509):1800–3. doi: 10.1126/science.1057559. [DOI] [PubMed] [Google Scholar]
- 73.Brinkmann V, Cyster J, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;4:1019–25. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 74.Spiegel S, Milstien S. Functions of a new family of sphingosine-1-phosphate receptors. Biochim Biophys Acta. 2000;1484(2–3):107–16. doi: 10.1016/s1388-1981(00)00010-x. [DOI] [PubMed] [Google Scholar]
- 75.Hla T, Lee MJ, Ancellin N, et al. Lysophospholipids-receptor revelations. Science. 2001;294(5548):1875–8. doi: 10.1126/science.1065323. [DOI] [PubMed] [Google Scholar]
- 76.Payne SG, Milstien S, Spiegel S. Sphingosine-1-phosphate: dual messenger functions. FEBS Lett. 2002;531(1):54–7. doi: 10.1016/s0014-5793(02)03480-4. [DOI] [PubMed] [Google Scholar]
- 77.Xia P, Gamble JR, Wang L, et al. An oncogenic role of sphingosine kinase. Curr Biol. 2000;10(23):1527–30. doi: 10.1016/s0960-9822(00)00834-4. [DOI] [PubMed] [Google Scholar]
- 78.Visentin B, Vekich J, Sibbald B, et al. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–38. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 79.Cuvillier O, Levade T. Sphingosine 1-phosphate antagonizes apoptosis of human leukemia cells by inhibiting release of cytochrome c and Smac/DIABLO from mitochondria. Blood. 2001;98:2828–36. doi: 10.1182/blood.v98.9.2828. [DOI] [PubMed] [Google Scholar]
- 80.Goetzl E, Kong Y, Mei B. Lysophosphatidic acid and sphingosine 1-phosphate protection of T-cells from apoptosis in association with suppression of Bax. J Immunol. 1999;162(4):2049–56. [PubMed] [Google Scholar]
- 81.Cuvillier O, Rosenthal DS, Smulson ME, et al. Sphingosine 1-phosphate inhibits activation of caspases that cleave poly(ADP-ribose) polymerase and lamins during Fas- and ceramide- mediated apoptosis in Jurkat T-lymphocytes. J Biol Chem. 1998;273(5):2910–6. doi: 10.1074/jbc.273.5.2910. [DOI] [PubMed] [Google Scholar]
- 82.Betito S, Cuvillier O. Regulation by sphingosine 1-phosphate of Bax and Bad activities during apoptosis in a MEK-dependent manner. Biochem Biophys Res Commun. 2006;340(4):1273–7. doi: 10.1016/j.bbrc.2005.12.138. [DOI] [PubMed] [Google Scholar]
- 83.Taha TA, Kitatani K, Bielawski J, et al. Tumor necrosis factor induces the loss of sphingosine kinase-1 by a cathepsin B-dependent mechanism. J Biol Chem. 2005;280(17):17196–202. doi: 10.1074/jbc.M413744200. [DOI] [PubMed] [Google Scholar]
- 84.Liu H, Toman RE, Goparaju S, et al. Sphingosine kinase type 2 is a putative BH3-Only protein that induces apoptosis. J Biol Chem. 2003;278:40330–6. doi: 10.1074/jbc.M304455200. [DOI] [PubMed] [Google Scholar]
- 85.Le Stunff H, Giussani P, Maceyka M, et al. Recycling of sphingosine is regulated by the concerted actions of sphingosine-1-phosphate phosphohydrolase 1 and sphingosine kinase 2. J Biol Chem. 2007;282:34372–80. doi: 10.1074/jbc.M703329200. [DOI] [PubMed] [Google Scholar]
- 86.Spiegel S, Milstien S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J Biol Chem. 2006 doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- 87.Woodgett JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17(2):150–7. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 88.Ruggero D, Sonenberg N. The Akt of translational control. Oncogene. 2005;24(50):7426–34. doi: 10.1038/sj.onc.1209098. [DOI] [PubMed] [Google Scholar]
- 89.Tohma Y, Gratas C, Biernat W, et al. PTEN (MMAC1) mutations are frequent in primary glioblastomas (de novo) but not in secondary glioblastomas. J Neuropathol Exp Neurol. 1998;57(7):684–9. doi: 10.1097/00005072-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 90.Carpten JD, Faber AL, Horn C, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448(7152):439–44. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 91.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 92.Fox TE, Houck KL, O’Neill SM, et al. Ceramide recruits and activates protein kinase C zeta (PKC zeta) within structured membrane microdomains. J Biol Chem. 2007;282(17):12450–7. doi: 10.1074/jbc.M700082200. [DOI] [PubMed] [Google Scholar]
- 93.Powell DJ, Hajduch E, Kular G, et al. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol Cell Biol. 2003;23(21):7794–808. doi: 10.1128/MCB.23.21.7794-7808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Salinas M, Lopez-Valdaliso R, Martin D, et al. Inhibition of PKB/Akt1 by C2-ceramide involves activation of ceramide-activated protein phosphatase in PC12 cells. Mol Cell Neurosci. 2000;15(2):156–69. doi: 10.1006/mcne.1999.0813. [DOI] [PubMed] [Google Scholar]
- 95.Stratford S, Hoehn K, Liu F, et al. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem. 2004;279:36608–15. doi: 10.1074/jbc.M406499200. [DOI] [PubMed] [Google Scholar]
- 96.Law B, Rossie S. The dimeric and catalytic subunit forms of protein phosphatase 2A from rat brain are stimulated by C2-ceramide. J Biol Chem. 1995;270(21):12808–13. doi: 10.1074/jbc.270.21.12808. [DOI] [PubMed] [Google Scholar]
- 97.Dobrowsky R, Kamibayashi C, Mumby M, et al. Ceramide activates a heterotrimeric protein phosphatase 2A. J Biol Chem. 1993;268(21):15523–30. [PubMed] [Google Scholar]
- 98.Dey R, Majumder N, Bhattacharjee S, et al. Leishmania donovani-induced ceramide as the key mediator of Akt dephosphorylation in murine macrophages: role of protein kinase Czeta and phosphatase. Infect Immun. 2007;75:2136–42. doi: 10.1128/IAI.01589-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schmitz-Peiffer C, Craig D, Biden T. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274:24202–10. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- 100.Guan L, Song K, Pysz M, et al. Protein kinase C-mediated down-regulation of cyclin D1 involves activation of the translational repressor 4E-BP1 via a phosphoinositide 3-kinase/Akt-independent, protein phosphatase 2A-dependent mechanism in intestinal epithelial cells. J Biol Chem. 2007;282:14213–25. doi: 10.1074/jbc.M610513200. [DOI] [PubMed] [Google Scholar]
- 101.Goswami R, Singh D, Phillips G, et al. Ceramide regulation of the tumor suppressor phosphatase PTEN in rafts isolated from neurotumor cell lines. J Neurosci Res. 2005;81:541–50. doi: 10.1002/jnr.20550. [DOI] [PubMed] [Google Scholar]
- 102.Gómez-Muñoz A, Kong J, Parhar K, et al. Ceramide-1-phosphate promotes cell survival through activation of the phosphatidylinositol 3-kinase/protein kinase B pathway. FEBS Lett. 2005;579:3744–50. doi: 10.1016/j.febslet.2005.05.067. [DOI] [PubMed] [Google Scholar]
- 103.Means C, Xiao C, Li Z, et al. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H2944–H2951. doi: 10.1152/ajpheart.01331.2006. [DOI] [PubMed] [Google Scholar]
- 104.Igarashi J, Bernier SG, Michel T. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J Biol Chem. 2001;276(15):12420–6. doi: 10.1074/jbc.M008375200. [DOI] [PubMed] [Google Scholar]
- 105.Levine Y, Li G, Michel T. Agonist-modulated regulation of AMP-activated protein kinase (AMPK) in endothelial cells. Evidence for an AMPK -> Rac1 -> Akt -> endothelial nitric-oxide synthase pathway. J Biol Chem. 2007;282:20351–64. doi: 10.1074/jbc.M702182200. [DOI] [PubMed] [Google Scholar]
- 106.Fieber C, Eldgridge J, Taha T, et al. Modulation of total Akt kinase by increased expression of a single isoform: requirement of the sphingosine-1-phosphate receptor Edg3/S1P3, for the VEGF-dependent expression of Akt3 in primary endothelial cells. Exp Cell Res. 2006;312:1164–73. doi: 10.1016/j.yexcr.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 107.Tanimoto T, Jin ZG, Berk BC. Transactivation of vascular endothelial growth factor (VEGF) receptor Flk-1/KDR is involved in sphingosine 1-phosphate-stimulated phosphorylation of Akt and endothelial nitric-oxide synthase (eNOS) J Biol Chem. 2002;277(45):42997–3001. doi: 10.1074/jbc.M204764200. [DOI] [PubMed] [Google Scholar]
- 108.Limaye V, Li X, Hahn C, et al. Sphingosine kinase-1 enhances endothelial cell survival through a PECAM-1-dependent activation of PI-3K/Akt and regulation of Bcl-2 family members. Blood. 2005;105:3169–77. doi: 10.1182/blood-2004-02-0452. [DOI] [PubMed] [Google Scholar]
- 109.Banno Y, Takuwa Y, Akao Y, et al. Involvement of phospholipase D in sphingosine 1-phosphate-induced activation of phosphatidylinositol 3-kinase and Akt in Chinese hamster ovary cells overexpressing EDG3. J Biol Chem. 2001;276:35622–8. doi: 10.1074/jbc.M105673200. [DOI] [PubMed] [Google Scholar]
- 110.Igarashi J, Michel T. Sphingosine 1-phosphate and isoform-specific activation of phosphoinositide 3-kinase beta. Evidence for divergence and convergence of receptor-regulated endothelial nitric-oxide synthase signaling pathways. J Biol Chem. 2001;276:36281–8. doi: 10.1074/jbc.M105628200. [DOI] [PubMed] [Google Scholar]
- 111.Baudhuin LM, Jiang Y, Zaslavsky A, et al. S1P3-mediated Akt activation and cross-talk with platelet-derived growth factor receptor (PDGFR) Faseb J. 2004;18(2):341–3. doi: 10.1096/fj.03-0302fje. [DOI] [PubMed] [Google Scholar]
- 112.Baudhuin L, Cristina K, Lu J, et al. Akt activation induced by lysophosphatidic acid and sphingosine-1-phosphate requires both mitogen-activated protein kinase kinase and p38 mitogen-activated protein kinase and is cell-line specific. Mol Pharmacol. 2002;62:660–71. doi: 10.1124/mol.62.3.660. [DOI] [PubMed] [Google Scholar]
- 113.Sanchez T, Skoura A, Wu M, et al. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol. 2007;27:1312–8. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 114.Liang C, Feng P, Ku B, et al. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat Cell Biol. 2006;8:688–99. doi: 10.1038/ncb1426. [DOI] [PubMed] [Google Scholar]
- 115.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21(22):2861–73. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 116.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 117.Takeuchi H, Kondo Y, Fujiwara K, et al. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65(8):3336–46. doi: 10.1158/0008-5472.CAN-04-3640. [DOI] [PubMed] [Google Scholar]
- 118.Scarlatti F, Bauvy C, Ventruti A, et al. Ceramide-mediated macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. J Biol Chem. 2004;279:18384–91. doi: 10.1074/jbc.M313561200. [DOI] [PubMed] [Google Scholar]
- 119.Patschan S, Chen J, Polotskaia A, et al. Lipid mediators of autophagy in stress-induced premature senescence of endothelial cells. Am J Physiol Heart Circ Physiol. 2008;294:H1119–H29. doi: 10.1152/ajpheart.00713.2007. [DOI] [PubMed] [Google Scholar]
- 120.Daido S, Kanzawa T, Yamamoto A, et al. Pivotal role of the cell death factor BNIP3 in ceramide-induced autophagic cell death in malignant glioma cells. Cancer Res. 2004;64(12):4286–93. doi: 10.1158/0008-5472.CAN-03-3084. [DOI] [PubMed] [Google Scholar]
- 121.Lavieu G, Scarlatti F, Sala G, et al. Regulation of autophagy by sphingosine kinase 1 and its role in cell survival during nutrient starvation. J Biol Chem. 2006;281:8518–27. doi: 10.1074/jbc.M506182200. [DOI] [PubMed] [Google Scholar]
- 122.Boya P, Gonzalez-Polo RA, Casares N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25(3):1025–40. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Verheij M, Bose R, Lin X, et al. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–9. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 124.Basu S, Bayoumy S, Zhang Y, et al. BAD enables ceramide to signal apoptosis via Ras and Raf-1. J Biol Chem. 1998;273(46):30419–26. doi: 10.1074/jbc.273.46.30419. [DOI] [PubMed] [Google Scholar]
- 125.Cuvillier O, Pirianov G, Kleuser B, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381(6585):800–3. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 126.Edsall LC, Cuvillier O, Twitty S, et al. Sphingosine kinase expression regulates apoptosis and caspase activation in PC12 cells. J Neurochem. 2001;76(5):1573–84. doi: 10.1046/j.1471-4159.2001.00164.x. [DOI] [PubMed] [Google Scholar]
- 127.Usui S, Sugimoto N, Takuwa N, et al. Blood lipid mediator sphingosine 1-phosphate potently stimulates platelet-derived growth factor-A and -B chain expression through S1P1-Gi-Ras-MAPK-dependent induction of Kruppel-like factor 5. J Biol Chem. 2004;279(13):12300–11. doi: 10.1074/jbc.M305025200. [DOI] [PubMed] [Google Scholar]
- 128.Hsieh HL, Sun CC, Wu CB, et al. Sphingosine 1-phosphate induces EGFR expression via Akt/NF-kappaB and ERK/AP-1 pathways in rat vascular smooth muscle cells. J Cell Biochem. 2007 doi: 10.1002/jcb.21563. [DOI] [PubMed] [Google Scholar]
- 129.Libermann TA, Nusbaum HR, Razon N, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313(5998):144–7. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- 130.Kohler M, Janz I, Wintzer HO, et al. The expression of EGF receptors, EGF-like factors and c-myc in ovarian and cervical carcinomas and their potential clinical significance. Anticancer Res. 1989;9(6):1537–47. [PubMed] [Google Scholar]
- 131.Yoshida K, Tosaka A, Takeuchi S, et al. Epidermal growth factor receptor content in human renal cell carcinomas. Cancer. 1994;73(7):1913–8. doi: 10.1002/1097-0142(19940401)73:7<1913::aid-cncr2820730723>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 132.Dbaibo G, Pushkareva M, Jayadev S, et al. Retinoblastoma gene product as a downstream target for a ceramide-dependent pathway of growth arrest. Proc Natl Acad Sci USA. 1995;92:1347–51. doi: 10.1073/pnas.92.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Phillips DC, Hunt JT, Moneypenny CG, et al. Ceramide-induced G2 arrest in rhabdomyosarcoma (RMS) cells requires p21Cip1/Waf1 induction and is prevented by MDM2 overexpression. Cell Death Differ. 2007;14(10):1780–91. doi: 10.1038/sj.cdd.4402198. [DOI] [PubMed] [Google Scholar]
- 134.Wang J, Lv XW, Shi JP, et al. Mechanisms involved in ceramide-induced cell cycle arrest in human hepatocarcinoma cells. World J Gastroenterol. 2007;13(7):1129–34. doi: 10.3748/wjg.v13.i7.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12(10):1133–8. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 136.Greider CW, Blackburn EH. Telomeres, telomerase and cancer. Sci Am. 1996;274(2):92–7. doi: 10.1038/scientificamerican0296-92. [DOI] [PubMed] [Google Scholar]
- 137.Blackburn EH, Greider CW, Henderson E, et al. Recognition and elongation of telomeres by telomerase. Genome. 1989;31(2):553–60. doi: 10.1139/g89-104. [DOI] [PubMed] [Google Scholar]
- 138.Weinrich SL, Pruzan R, Ma L, et al. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat Genet. 1997;17(4):498–502. doi: 10.1038/ng1297-498. [DOI] [PubMed] [Google Scholar]
- 139.Ogretmen B, Kraveka JM, Schady D, et al. Molecular mechanisms of ceramide-mediated telomerase inhibition in the A549 human lung adenocarcinoma cell line. J Biol Chem. 2001;276(35):32506–14. doi: 10.1074/jbc.M101350200. [DOI] [PubMed] [Google Scholar]
- 140.Wooten-Blanks LG, Song P, Senkal CE, et al. Mechanisms of ceramide-mediated repression of the human telomerase reverse transcriptase promoter via deacetylation of Sp3 by histone deacetylase 1. FASEB J. 2007;21(12):3386–97. doi: 10.1096/fj.07-8621com. [DOI] [PubMed] [Google Scholar]
- 141.Xu JX, Morii E, Liu Y, et al. High tolerance to apoptotic stimuli induced by serum depletion and ceramide in side-population cells: high expression of CD55 as a novel character for side-population. Exp Cell Res. 2007;313(9):1877–85. doi: 10.1016/j.yexcr.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 142.Ozaki H, Hla T, Lee MJ. Sphingosine-1-phosphate signaling in endothelial activation. J Atheroscler Thromb. 2003;10(3):125–31. doi: 10.5551/jat.10.125. [DOI] [PubMed] [Google Scholar]
- 143.Park KS, Kim MK, Lee HY, et al. S1P stimulates chemotactic migration and invasion in OVCAR3 ovarian cancer cells. Biochem Biophys Res Commun. 2007;356(1):239–44. doi: 10.1016/j.bbrc.2007.02.112. [DOI] [PubMed] [Google Scholar]
- 144.Guo XZ, Zhang WW, Wang LS, et al. Adenovirus-mediated overexpression of KAI1 suppresses sphingosine kinase activation and metastasis of pancreatic carcinoma cells. Zhonghua Nei Ke Za Zhi. 2006;45(9):752–4. [PubMed] [Google Scholar]
- 145.Selzner M, Bielawska A, Morse MA, et al. Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Res. 2001;61(3):1233–40. [PubMed] [Google Scholar]
- 146.Elojeimy S, Liu X, McKillop JC, et al. Role of acid ceramidase in resistance to FasL: erapeutic approaches based on acid ceramidase inhibitors and FasL gene therapy. Mol Ther. 2007;15(7):1259–63. doi: 10.1038/sj.mt.6300167. [DOI] [PubMed] [Google Scholar]
- 147.Holman DH, Turner LS, El-Zawahry A, et al. Lysosomotropic acid ceramidase inhibitor induces apoptosis in prostate cancer cells. Cancer Chemother Pharmacol. 2008;61(2):231–42. doi: 10.1007/s00280-007-0465-0. [DOI] [PubMed] [Google Scholar]
- 148.Swanton C, Marani M, Pardo O, et al. Regulators of mitotic arrest and ceramide metabolism are determinants of sensitivity to paclitaxel and other chemotherapeutic drugs. Cancer Cell. 2007;11(6):498–512. doi: 10.1016/j.ccr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 149.Kawamori T, Osta W, Johnson KR, et al. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB J. 2006;20(2):386–8. doi: 10.1096/fj.05-4331fje. [DOI] [PubMed] [Google Scholar]
- 150.Johnson KR, Johnson KY, Crellin HG, et al. Immunohistochemical distribution of sphingosine kinase 1 in normal and tumor lung tissue. J Histochem Cytochem. 2005;53(9):1159–66. doi: 10.1369/jhc.4A6606.2005. [DOI] [PubMed] [Google Scholar]
- 151.French K, Schrecengost R, Lee B, et al. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–9. [PubMed] [Google Scholar]
- 152.French K, Upson J, Keller S, et al. Antitumor activity of sphingosine kinase inhibitors. J Pharmacol Exp Ther. 2006;318:596–603. doi: 10.1124/jpet.106.101345. [DOI] [PubMed] [Google Scholar]
- 153.Le Scolan E, Pchejetski D, Banno Y, et al. Overexpression of sphingosine kinase 1 is an oncogenic event in erythroleukemic progression. Blood. 2005;106(5):1808–16. doi: 10.1182/blood-2004-12-4832. [DOI] [PubMed] [Google Scholar]
- 154.Kohno M, Momoi M, Oo M, et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol. 2006;26:7211–23. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Oskouian B, Sooriyakumaran P, Borowsky A, et al. Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is downregulated in colon cancer. Proc Natl Acad Sci USA. 2006;103:17384–9. doi: 10.1073/pnas.0600050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Symolon H, Schmelz E, Dillehay D, et al. Dietary soy sphingolipids suppress tumorigenesis and gene expression in 1,2-dimethylhydrazine-treated CF1 mice and ApcMin/+ mice. J Nutr. 2004;134:1157–61. doi: 10.1093/jn/134.5.1157. [DOI] [PubMed] [Google Scholar]
- 157.Schmelz EM, Dillehay DL, Webb SK, et al. Sphingomyelin consumption suppresses aberrant colonic crypt foci and increases the proportion of adenomas versus adenocarcinomas in CF1 mice treated with 1,2-dimethylhydrazine: implications for dietary sphingolipids and colon carcinogenesis. Cancer Res. 1996;56(21):4936–41. [PubMed] [Google Scholar]
- 158.Schmelz EM, Roberts PC, Kustin EM, et al. Modulation of intracellular beta-catenin localization and intestinal tumorigenesis in vivo and in vitro by sphingolipids. Cancer Res. 2001;61(18):6723–9. [PubMed] [Google Scholar]
- 159.Bonhoure E, Pchejetski D, Aouali N, et al. Overcoming MDR-associated chemoresistance in HL-60 acute myeloid leukemia cells by targeting sphingosine kinase-1. Leukemia. 2006;20(1):95–102. doi: 10.1038/sj.leu.2404023. [DOI] [PubMed] [Google Scholar]
- 160.Bektas M, Jolly PS, Muller C, et al. Sphingosine kinase activity counteracts ceramide-mediated cell death in human melanoma cells: role of Bcl-2 expression. Oncogene. 2005;24(1):178–87. doi: 10.1038/sj.onc.1208019. [DOI] [PubMed] [Google Scholar]
- 161.Schindler T, Bornmann W, Pellicena P, et al. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289(5486):1938–42. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- 162.Baran Y, Salas A, Senkal CE, et al. Alterations of ceramide/sphingosine 1-phosphate rheostat involved in the regulation of resistance to imatinib-induced apoptosis in K562 human chronic myeloid leukemia cells. J Biol Chem. 2007;282(15):10922–34. doi: 10.1074/jbc.M610157200. [DOI] [PubMed] [Google Scholar]
- 163.Bittman R. In: Synthetic sphingolipids as bioactive molecules: roles in regulation of cell function. Wiley, editor. Wiley Encyclopedia; 2008. [Google Scholar]
- 164.McCormack P, Goa K. Miglustat. Drugs. 2003;63:2427–36. doi: 10.2165/00003495-200363220-00006. [DOI] [PubMed] [Google Scholar]
- 165.Weiss M, Hettmer S, Smith P, et al. Inhibition of melanoma tumor growth by a novel inhibitor of glucosylceramide synthase. Cancer Res. 2003;63(13):3654–8. [PubMed] [Google Scholar]
- 166.Guerrera M, Ladisch S. N-butyldeoxynojirimycin inhibits murine melanoma cell ganglioside metabolism and delays tumor onset. Cancer Lett. 2003;201(1):31–40. doi: 10.1016/s0304-3835(03)00459-2. [DOI] [PubMed] [Google Scholar]
- 167.Dbaibo GS, Kfoury Y, Darwiche N, et al. Arsenic trioxide induces accumulation of cytotoxic levels of ceramide in acute promyelocytic leukemia and adult T-cell leukemia/lymphoma cells through de novo ceramide synthesis and inhibition of glucosylceramide synthase activity. Haematologica. 2007;92(6):753–62. doi: 10.3324/haematol.10968. [DOI] [PubMed] [Google Scholar]
- 168.Billich A, Bornancin F, Mechtcheriakova D, et al. Basal and induced sphingosine kinase 1 activity in A549 carcinoma cells: function in cell survival and IL-1beta and TNF-alpha induced production of inflammatory mediators. Cell Signal. 2005;17:1203–17. doi: 10.1016/j.cellsig.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 169.Gamble J, Xia P, Hahn C, et al. Phenoxodiol, an experimental anticancer drug, shows potent antiangiogenic properties in addition to its antitumour effects. Int J Cancer. 2006;118:2412–20. doi: 10.1002/ijc.21682. [DOI] [PubMed] [Google Scholar]
- 170.Leroux M, Auzenne E, Evans R, et al. Sphingolipids and the sphingosine kinase inhibitor, SKI II, induce BCL-2-independent apoptosis in human prostatic adenocarcinoma cells. Prostate. 2007;67:1699–717. doi: 10.1002/pros.20645. [DOI] [PubMed] [Google Scholar]
- 171.Leroux ME, Auzenne E, Evans R, et al. Sphingolipids and the sphingosine kinase inhibitor, SKI II, induce BCL-2-independent apoptosis in human prostatic adenocarcinoma cells. Prostate. 2007;67(15):1699–717. doi: 10.1002/pros.20645. [DOI] [PubMed] [Google Scholar]
- 172.Yasui H, Hideshima T, Raje N, et al. FTY720 induces apoptosis in multiple myeloma cells and overcomes drug resistance. Cancer Res. 2005;65(16):7478–84. doi: 10.1158/0008-5472.CAN-05-0850. [DOI] [PubMed] [Google Scholar]
- 173.Sani BP, Shealy YF, Hill DL. N-(4-hydroxyphenyl)retinamide: interactions with retinoid-binding proteins/receptors. Carcinogenesis. 1995;16(10):2531–4. doi: 10.1093/carcin/16.10.2531. [DOI] [PubMed] [Google Scholar]
- 174.Sheikh MS, Shao ZM, Li XS, et al. N-(4-hydroxyphenyl)retinamide (4-HPR)-mediated biological actions involve retinoid receptor-independent pathways in human breast carcinoma. Carcinogenesis. 1995;16(10):2477–86. doi: 10.1093/carcin/16.10.2477. [DOI] [PubMed] [Google Scholar]
- 175.Wang H, Maurer BJ, Reynolds CP, et al. N-(4-hydroxyphenyl)retinamide elevates ceramide in neuroblastoma cell lines by coordinate activation of serine palmitoyltransferase and ceramide synthase. Cancer Res. 2001;61(13):5102–5. [PubMed] [Google Scholar]
- 176.Maurer BJ, Melton L, Billups C, et al. Synergistic cytotoxicity in solid tumor cell lines between N-(4-hydroxyphenyl)retinamide and modulators of ceramide metabolism. J Natl Cancer Inst. 2000;92(23):1897–909. doi: 10.1093/jnci/92.23.1897. [DOI] [PubMed] [Google Scholar]
- 177.Erdreich-Epstein A, Tran LB, Bowman NN, et al. Ceramide signaling in fenretinide-induced endothelial cell apoptosis. J Biol Chem. 2002;277(51):49531–7. doi: 10.1074/jbc.M209962200. [DOI] [PubMed] [Google Scholar]
- 178.Kraveka J, Li L, Szulc Z, et al. Involvement of dihydroceramide desaturase in cell cycle progression in human neuroblastoma cells. J Biol Chem. 2007;282:16718–28. doi: 10.1074/jbc.M700647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jiang Q, Wong J, Fyrst H, et al. gamma-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc Natl Acad Sci USA. 2004;101(51):17825–30. doi: 10.1073/pnas.0408340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Morales PR, Dillehay DL, Moody SJ, et al. Safingol toxicology after oral administration to TRAMP mice: demonstration of safingol uptake and metabolism by N-acylation and N-methylation. Drug Chem Toxicol. 2007;30(3):197–216. doi: 10.1080/01480540701375018. [DOI] [PubMed] [Google Scholar]
- 181.Grammatikos G, Teichgraber V, Carpinteiro A, et al. Overexpression of acid sphingomyelinase sensitizes glioma cells to chemotherapy. Antioxid Redox Signal. 2007;9(9):1449–56. doi: 10.1089/ars.2007.1673. [DOI] [PubMed] [Google Scholar]
- 182.Won JS, Singh I. Sphingolipid signaling and redox regulation. Free Radic Biol Med. 2006;40(11):1875–88. doi: 10.1016/j.freeradbiomed.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 183.Andersson D, Cheng Y, Duan RD. Ursolic acid inhibits the formation of aberrant crypt foci and affects colonic sphingomyelin hydrolyzing enzymes in azoxymethane-treated rats. J Cancer Res Clin Oncol. 2008;134(1):101–7. doi: 10.1007/s00432-007-0255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rosato RR, Maggio SC, Almenara JA, et al. The histone deacetylase inhibitor LAQ824 induces human leukemia cell death through a process involving XIAP down-regulation, oxidative injury and the acid sphingomyelinase-dependent generation of ceramide. Mol Pharmacol. 2006;69(1):216–25. doi: 10.1124/mol.105.017145. [DOI] [PubMed] [Google Scholar]
- 185.Darroch PI, Dagan A, Granot T, et al. A lipid analogue that inhibits sphingomyelin hydrolysis and synthesis, increases ceramide and leads to cell death. J Lipid Res. 2005;46(11):2315–24. doi: 10.1194/jlr.M500136-JLR200. [DOI] [PubMed] [Google Scholar]
- 186.Suomalainen L, Hakala JK, Pentikainen V, et al. Sphingosine-1-phosphate in inhibition of male germ cell apoptosis in the human testis. J Clin Endocrinol Metab. 2003;88(11):5572–9. doi: 10.1210/jc.2003-030776. [DOI] [PubMed] [Google Scholar]
- 187.Pchejetski D, Kunduzova O, Dayon A, et al. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circ Res. 2007;100(1):41–9. doi: 10.1161/01.RES.0000253900.66640.34. [DOI] [PubMed] [Google Scholar]