Abstract
Both bacteriopyropheophorbide-a and ring-B reduced pyropheophorbide-a on reacting with NBS (N-bromosuccinamide) undergo electrophilic bromination to provide 10-bromo analogs. The electronic nature of the substituents present at position-3 did not make any difference in regioselective outcome of the brominated products. These relatively stable brominated chlorins and bacteriochlorins provide an easy way of introducing a wide variety of functionalities, which could be extremely useful in developing improved agents for biomedical applications and supramolecular chemistry.
In recent years, chlorins and bacteriochlorins in which one or two pyrrole rings diagonal to each other are reduced have shown enormous interest in applications to material science, engineering, biology and medicine.1,2 Continued efforts are also underway to synthesize a large number of supramolecular structures to understand the plant and bacterial photosynthetic reaction centers.3,4 Due to long-wavelength absorption characteristics of both chlorins and bacteriochlorin, these chromophores have also generated immense interest in developing improved agents for tumor-imaging and phototherapy (PDT).5
For quite sometime, one of the main objectives of our laboratory has been to develop improved PDT and/or imaging agents with enhanced tumor-specificity.6,7
For developing such agents, the presence of multiple functionalities in the molecule could be advantageous as they provide additional opportunities to change the overall lipophilicity and target-specificity by adding desired tumor-targeting moieties to the molecule.8 For developing such agents, the presence of multiple functionalities in the molecule could be advantageous as they provide additional opportunities to change the overall lipophilicity and target-specificity by adding desired tumor-targeting moieties to the molecule.8 Further, presence of such a characteristic in the molecule could also help in developing a single agent for tumor-imaging and therapy using a “See & Treat Approach”.9–11
In porphyrin chemistry halogenation reactions have been used extensively to functionalize porphyrin and chlorin systems.12–14 Porphyrins can undergo fluorination, chlorination, bromination and iodination, although the last reaction is considerably less favored because of steric and electronic factors. When both the meso- and β-halogenation of unsubstituted positions at the porphyrin periphery are possible, meso- chlorination is usually observed, whereas β-substitution is favored for both bromination and iodination.1 The site of halogenation is significantly determined by the size and reactivity of the halogen. Since bromine is of an intermediate size among the halogens, meso-bromination is also observed.
In the chlorin system, e.g. methyl mesopyropheophorbide-a the halogens can be selectively introduced at position-20.15,16 For example, mesopyropheophorbide-a on reacting with either N-bromosuccinamide or pyridinium tribromide undergoes electrophilic bromination to give only the corresponding 20-bromo analogs due to high electron density at the meso-position next to the reduced ring. The other adjacent meso-position (position 15) is not available for any substitution because of its involvement in forming a fused isocyclic ring system (ring E). Substitution at the pyrrole periphery is also ruled out because all positions are occupied with alkyl or propionic ester functionalities. In recent years there has been considerable interest in using chlorophyll-a or bacteriochlorophyll-a based analogs to understand the photosynthetic reaction centers.17,18 For developing supramolecular structures, a variety of halogenated porphyrins, chlorins and bacteriochlorins are being used as substrates.19
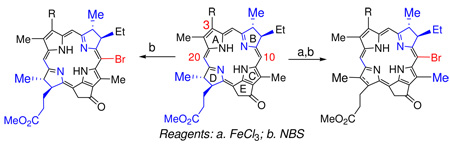
We have recently shown that bacteriopyropheophorbide-a on treating with appropriate oxidizing agents can be transformed into the corresponding ring-B or ring-D reduced chlorins in excellent yields.20 In this report, we present a remarkable regioselective bromination of the ring-B reduced chlorins and bacteriochlorins derived from bacteriochlorophyll-a. Although the syntheses and photophysical properties of the brominated porphyrins and synthetic bacteriochlorins have been investigated,21 comparatively little is known about the reactivity of naturally occurring chlorins and bacteriochlorins towards halogenation and functionalization.
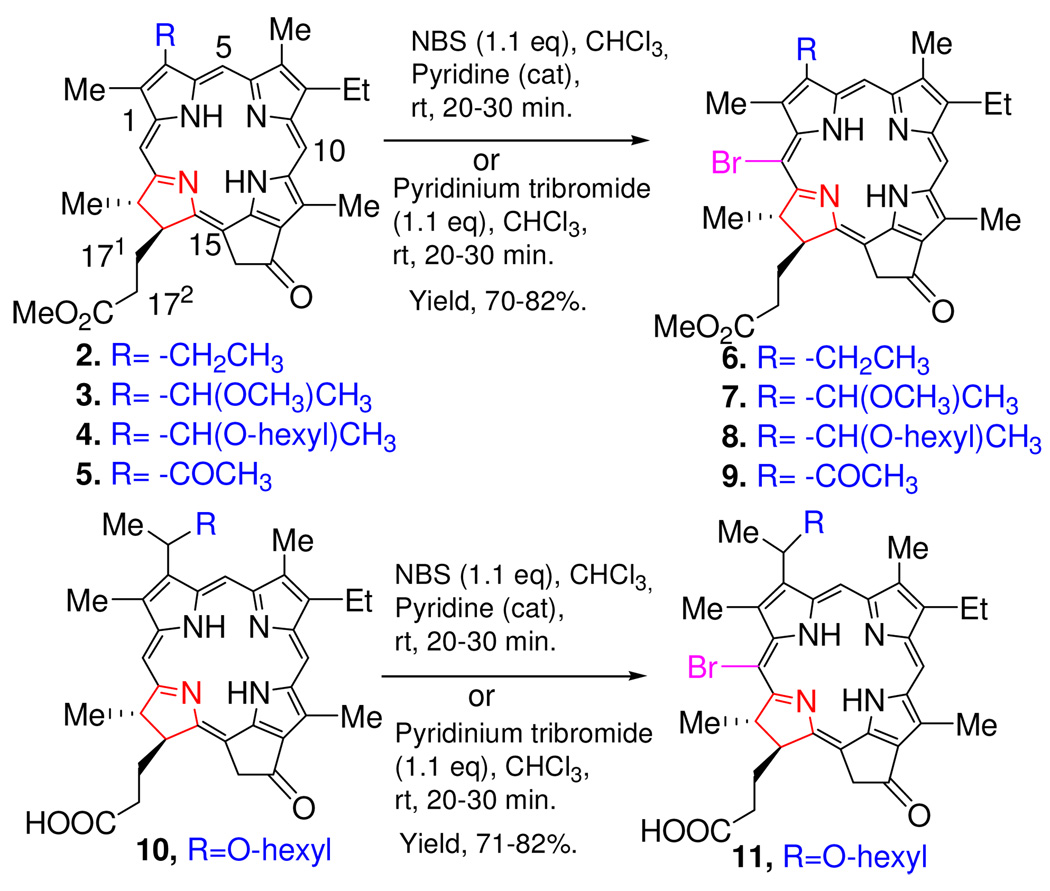
To establish the reaction conditions for bromination in chlorin and bacteriochlorin systems, we started with methyl pyropheophorbide a and, to avoid vinylic bromination or multiple bromination,22 the methyl pyropheophorbide-a was metallated with Zn(OAc)2. This was followed by reduction with Pd/C in THF and demetallation to give the 3-devinyl-3-ethyl pyropheophorbide-a (mesopyropheophorbide-a) 2. It was then treated with NBS in Chloroform in presence of a catalytic amount of pyridine, to obtain the corresponding bromo analog 6. We also employed other brominating agents; only pyridinium tribromide gave a similar yield and selectivity when compared with the NBS reaction products. The 2D-NMR analysis of compound 6 indicated that the bromine was attached at position-20 of the macrocyclic ring, as reported in the literature. To investigate the effect of different functionalities at position-3, a series of compounds containing an alkyl ether- or acetyl group 3, 4 and 5, respectively, were synthesized which, upon bromination (NBS/CHCl3/pyridine or pyridinium tribromide/CHCl3), only gave the corresponding 20-brominated compounds 7, 8 and 9, respectively (Scheme 1). These results suggest that the presence of electron donating or withdrawing (acetyl) groups at position-3 does not make any significant difference to the selectivity of halogenation. As expected, the presence of a free carboxylic acid at the 172-position (compound 10), instead of a methyl ester, did not alter the position of the bromine in product 11.
Scheme 1.
Bromination of pyropheophorbide (ring D reduced) system.
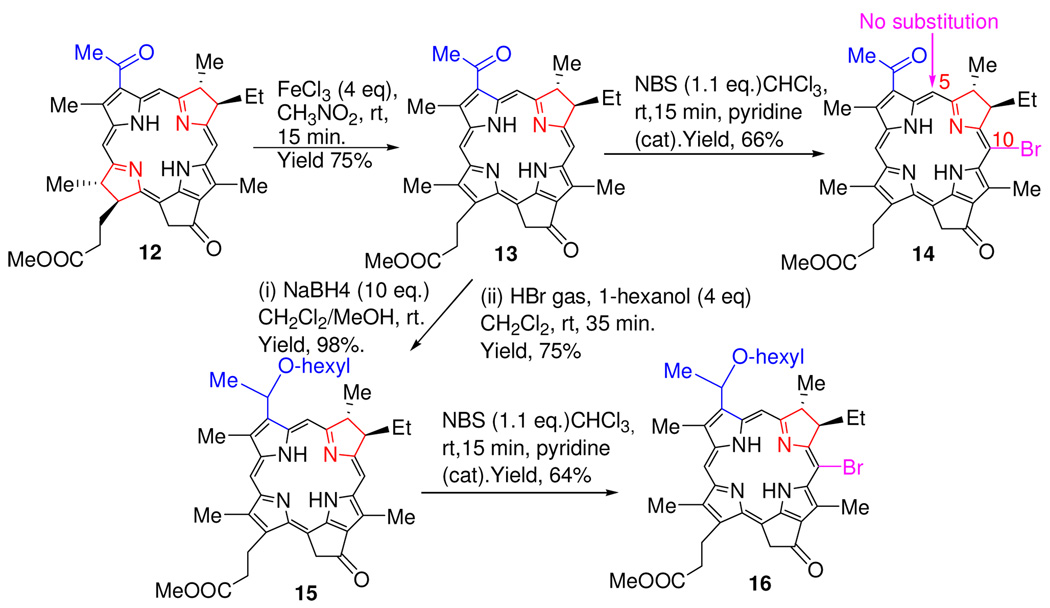
We next turned our attention to ring-B reduced chlorins 13 and 15 containing either an acetyl- or hexyloxyethylgroup at position-3, obtained by following the methodology recently developed in our laboratory.20,23 Both chlorins on reacting with NBS or pyridinium bromide gave the corresponding 10-bromo analogs 14 and 16, respectively. To our surprise, no substitution was observed at position-5 adjacent to reduced ring-B.
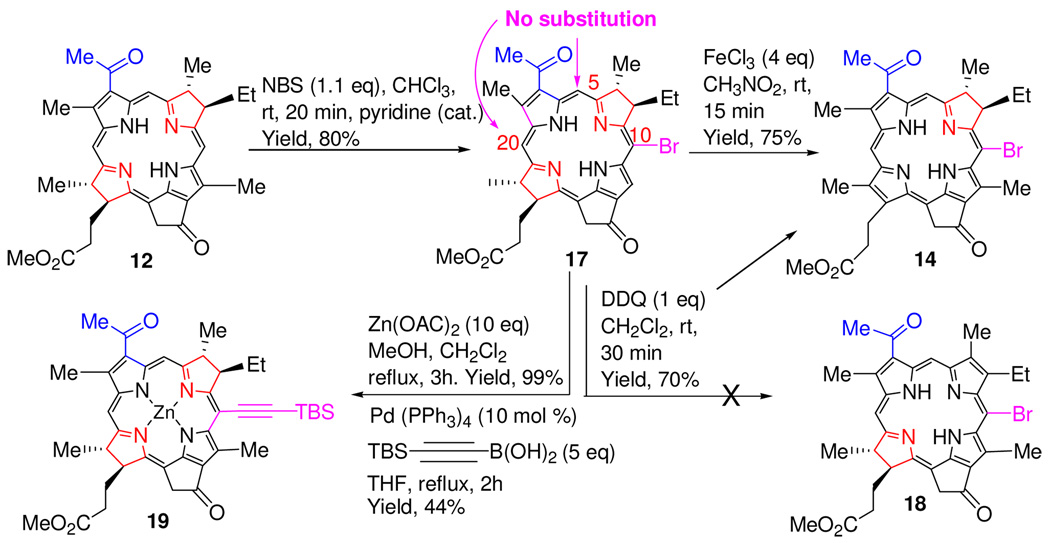
The selectivity of bromination reaction was then investigated in bacteriochlorin 12 containing both the ring-B and ring-D reduced pyrrole rings. We were expecting a mixture of 5, 10 and 20- brominated analogs, instead, only the 10-bromo-bacteriopyropheophorbide-a 17 was isolated as a sole product. Interestingly, oxidation of 17 on reacting with DDQ did not yield the expected ring-D reduced chlorin 18 rather gave the unexpected ring-B reduced chlorin 14 (Scheme 2).
Scheme 2.
Regioselective Bromination of Ring-B Reduced Chlorins.
To exemplify the application of these brominated derivatives, we performed the Pd-cross coupling reaction (Suzuki coupling)24 on 10-bromobacteriochlorin 17 by following the reaction sequences shown in Scheme 3 and the desired Zn(II) 10-silyl acetylene analog 19 was isolated in good yield, which could be converted into a series of novel supramolecular structures and these studies are currently in progress. The regioselective electrophilic substitution of a particular meso- position adjacent to the reduced ring of the chlorins and bacteriochlorins can be explained on the basis of its higher electron density.
Scheme 3.
Synthesis of 10-Bromo Bacteriopyropheophorbide-a, 10-Bromo- Ring-B reduced Chlorin and the Corresponding 10-Silyl Analog.
Density functional calculations were carried out to clarify the difference in reactivities for bromination at 10 and 20-position. Tables 1 & 2 show the electronic charges of the starting materials and the Hartree energies of the brominated products obtained by DFT with the B3LYP/6-31G(d) basis set.25 For compounds 2–5 and 10 shown in Scheme 1, the calculated electron densities at position-20 were higher than those at position-10 (Table 1).
Table 1.
Calculated Electron Densities at Positions- 5-, 10-, 20- of Certain Chlorins and Bacteriochlorinsa
| Position-20 | Position-10 | Position-5 | |
|---|---|---|---|
| Ring D reduced | |||
| 2 | −0.316 | −0.226 | −0.248 |
| 3 | −0.318 | −0.229 | −0.244 |
| 4 | −0.317 | −0.227 | −0.241 |
| 5 | −0.303 | −0.233 | −0.236 |
| 10 | −0.318 | −0.227 | −0.240 |
| Ring B reduced | |||
| 13 | −0.223 | −0.313 | −0.316 |
| 15 | −0.242 | −0.311 | −0.316 |
| Rings B & D reduced | |||
| 12 | −0.288 | −0.298 | −0.302 |
Most negative values are presented by bold numbers.
Table 2.
Calculated Energies (in Hartreea) of Certain Chlorins and Bacteriochlorins at Positions -5, 10- and 20b
| Position-20 | Position-10 | Position-5 | |
|---|---|---|---|
| Ring D reduced | |||
| 6 | −4334.3282435 | −4334.325446 | −4334.3174495 |
| 7 | −4409.5330262 | −4409.5308662 | −4409.5151262 |
| 8 | −4645.4141285 | −4645.4120693 | −4645.3961645 |
| 9 | −4408.3363894 | −4408.3355856 | −4408.3290654 |
| 11 | −4606.1070822 | −4606.1049602 | −4606.0890733 |
| Ring B reduced | |||
| 14 | −4408.3244631 | −4408.3393993 | −4408.3328343 |
| 16 | −4645.4012942 | −4645.4144882 | −4645.4006457 |
| Rings B & D reduced | |||
| 17 | −4409.5395198 | −4409.5458701 | −4409.5387054 |
1 hartree = 629.51 kcal mol−1.
Most stable energy values are presented by bold numbers.
In contrast, the ring B reduced compounds 13, 15 and the ring- B and D reduced bacteriochlorin 12 exhibit higher electron density at position-5. However, no bromination at the 5-position was observed even though higher electron density at position-5. However, no bromination at the 5-position was observed even though the calculated electron density was the highest. Perhaps, interference from adjacent substituents at 31-position such as carboxymethyl or hexyloxy group prevents bromination at 5-position. This could be the reason for the higher stability of the 10-brominated isomer.
The Hartree energies in Table 2 suggest that 20-brominated ring-D reduced compound, 6–9 and 11 are the most stable isomers. Thus, the bromination favors the 20-position rather than 5- or 10-positions. On the other hand with B-ring reduced chlorins (13 and 15) and bacteriochlorin (12), the bromination takes place at 10-position which has the lowest Hartree energy among these isomers.
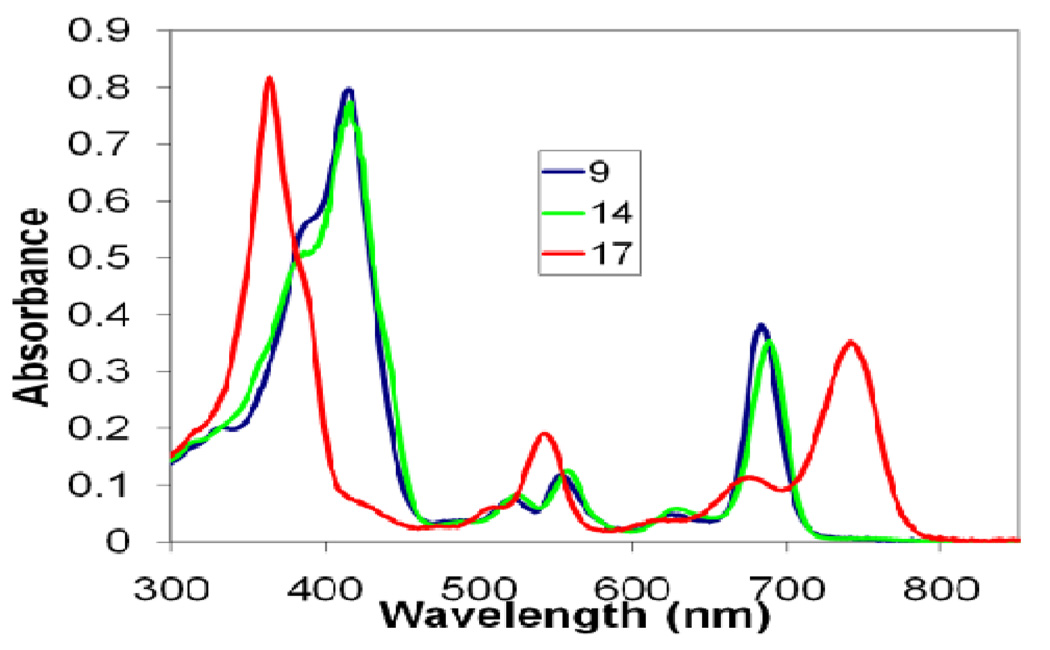
The electronic absorption spectra of key products; ring-Dreduced 2—bromo analog 9, its isomer ring-B reduced-20-bromo analog 14 and the methyl 20-bromo-bacteriopyropheophorbide-a 17 are shown in Figure 1. As can be seen, the electronic absorption spectra of both chlorins 9 and 14 were almost identical. The Soret band exhibited strong absorption at 416 nm and the long wavelength absorption was observed at 686 & 691 nms respectively. As expected the bacteriochloin 17 showed longer wavelength absorption appeared at 365 nm and the Soret band was observed at 746 nm. Thus compared to chlorin 9 and 14 a blue shift on 55 nm was observed. The spectra were measured at equimolar concentrations in dichloromethane.
Figure 1.
Electronic absorption spectra of methyl 3-acetyl-20-bromo-3-devinyl pyropheophorbide-a 9, 3-acetyl-10-bromo-ring-B reduced chlorin 14 and the methyl 3-acetyl-10-bromo-bacterio pyropheophorbide-a 17 in dichloromethane.
In summary, we report herein a facile approach for introducing bromo- functionality at position-10 or 20- of certain chlorins and bacteriochlorins. The regioselective approach provides an opportunity to develop a variety of novel supramolecular structures for biomedical applications and to understand more about the photosynthetic reaction center, especially the reasons for the selection of ring-D reduced chlorins (chlorophyll-a) over the corresponding ring-B reduced isomer by the nature.
Supplementary Material
Acknowledgment
The financial support from the NIH (CA55791, CA 127369), the Roswell Park Alliance Foundation, a Grant-in-Aid (No 20108010 to S.F.) and a Global COE program, “the Global Education and Research Center for Bio-Environmental Chemistry” from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and KOSEF/MEST through WCU projects (R31-2008-000-10010-0), and the shared resources of the Roswell Park Cancer Support Grant (P30CA15056 is gratefully acknowledged. The Mass spectrometry analyses were performed at the Biopolymer Facility, Roswell Park Cancer Institute, Buffalo and the Michigan State University, East Lansing, Michigan.
Footnotes
Supporting Information Available. Experimental procedures, 1H and 13C NMR spectra of new chlorins and bacteriochlorins.
References
- 1.Kadish KM, Smith KM, Guilard R, editors. Porphyrin Handbook, Vol. 6, Applications: Past, Present and Future. San Diego: Academic Press; 2000. [Google Scholar]
- 2.Kadish KM, Smith KM, Guilard R, editors. Handbook of Porphyrin Science, Vol. 4, Phototherapy, Radioimmunotherapy and Imaging. World Scientific; 2010. [Google Scholar]
- 3.(a) Wasielewski MJ. Acc. Chem. Res. 2009;42:1910–1921. doi: 10.1021/ar9001735. [DOI] [PubMed] [Google Scholar]; (b) Moore AL. Acc. Chem. Res. 2009;42:1890–1898. doi: 10.1021/ar900209b. [DOI] [PubMed] [Google Scholar]
- 4.(a) Wohri A, Katona G, Johansson LC, Fritz E, Malmerberg E, Anderson M, Vicent J, Eklund M, Commarata M, Wulff M, Davidson J, Groenhot G, Neutze R. Science. 2010;328:630–633. doi: 10.1126/science.1186159. [DOI] [PubMed] [Google Scholar]; (b) Warshel A. Proc. Natl. Acad. Sci. USA. 1980;77:3105–3109. doi: 10.1073/pnas.77.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Fleming GR, Martin JL, Breton J. Nature. 1988;333:190–192. [Google Scholar]
- 5.(a) Ethirajan M, Chen Y, Joshi P, Pandey RK. Chem. Soc. Rev. 2011;40:340–362. doi: 10.1039/b915149b. and references therein. [DOI] [PubMed] [Google Scholar]; (b) Pandey RK. Oncology Issues. 2008:22–23. [Google Scholar]
- 6.Chumakov DE, Khoroshutin AV, Anisimov AV, Kobrakov KI. Chem. Heterocycl. Comp. 2009;45:259–283. [Google Scholar]
- 7.Esdaile LJ, Senge MO, Arnold DP. Chem. Commun. 2006:4192–4194. doi: 10.1039/b608365j. [DOI] [PubMed] [Google Scholar]
- 8.Henderson BW, Bellnier DA, Greco WR, Sharma A, Pandey RK, Vaughan L, Weishaupt WR, Dougherty TJ. Cancer Res. 1997;57:4000–4007. [PubMed] [Google Scholar]
- 9.Pandey SK, Sajjad M, Chen Y, Zheng X, Yao R, Missert JR, Batt C, Nabi HA, Oseroff AR, Pandey RK. J. Med. Chem. 2009;52:445–455. doi: 10.1021/jm8012213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spernyak JA, White WH, Ethirajan M, Patel NJ, Goswami L, Chen Y, Turowski S, Missert JR, Batt C, Mazurchuk R, Pandey RK. Bioconjugate Chem. 2010;21:828–835. doi: 10.1021/bc9005317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Chen Y, Gryshuk A, Achilefu S, Ohulchanskyy T, Potter W, Zhong T, Morgan J, Chance B, Prasad PN, Henderson BW, Oseroff A, Pandey RK. Bioconjugate Chem. 2005;16:1264–1274. doi: 10.1021/bc050177o. [DOI] [PubMed] [Google Scholar]; (b) Pandey SK, Chen Y, Zwada RH, Oseroff A, Pandey RK. Proceedings of SPIE; 2006. pp. 813905–813910. [Google Scholar]
- 12.(a) Liu C, Shen D-M, Chen Q-Y. J. Org. Chem. 2007;72:2732–2736. doi: 10.1021/jo0618728. [DOI] [PubMed] [Google Scholar]; (b) Liu C, Shen D-M, Chen Q-Y. Chem. Commuun. 2006:770–772. doi: 10.1039/b514767k. [DOI] [PubMed] [Google Scholar]
- 13.Sahoo AK, Mori S, Shinokubo H, Osuka A. Angew. Chem. 2006;118:8140–8143. doi: 10.1002/anie.200603580. [DOI] [PubMed] [Google Scholar]
- 14.Muzzi CM, Medforth CJ, Voss L, Cancilla M, Lebrilla C, Ma J-G, Shelnutt JA, Smith KM. Tetrahedron Lett. 1999;40:6159–6162. [Google Scholar]
- 15.(a) Woodward RB, Skaric V. J. Am. Chem. Soc. 1961;83:4676–4678. [Google Scholar]; (b) Smith KM, Goff DA, Simpson DP. J. Am. Chem. Soc. 1985;107:4946–4954. [Google Scholar]
- 16.Kelly RF, Lee SK, Wilson TM, Nakamura Y, Tiede DM, Osuka A, Hupp JT, Wasielewski M. J. Am. Chem. Soc. 2008;130:4277–4284. doi: 10.1021/ja075494f. [DOI] [PubMed] [Google Scholar]
- 17.Aratani N, Osuka A. Chap. 1. In: Kadish KM, Smith KM, Guilard R, editors. Handbook of Porphyrin Science. New Jersey: World Scientific; 2010. and references therein. [Google Scholar]
- 18.Balaban TS. In: Handbook of Porphyrin Science, Chapter 2. Kadish KM, Smith KM, Guilard R, editors. New Jersey: World. Scientific; 2010. and references therein. [Google Scholar]
- 19.Balaban TS, Tamiaki H, Holzwarth AR. In: Topics in Current Chemistry, Supramelocular Dye Chemistry. Wurthner F, editor. Vol. 258. Berlin: Springer-Verlag; 2005. pp. 1–38. [Google Scholar]
- 20.Liu C, Dobhal MP, Ethirajan M, Missert JR, Pandey RK, Balasubramaniam S, Sukumaran D, Zhang M, Kadish KM, Ohkubo K, Fukuzumi S. J. Am. Chem. Soc. 2008;130:14311–14323. doi: 10.1021/ja8050298. [DOI] [PubMed] [Google Scholar]
- 21.(a) Fan D, Taniguchi M, Lindsey JS. J. Org. Chem. 2007;72:5350–5357. doi: 10.1021/jo070785s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Pereira M, Monteiso CJP, Simoes AR, Pinto SMA, Abreu AR, Sa CFF, Silva EFF, Rocha LB, Dabrowsk JM, Arnaut LG. Tetrahedron. 2010;49:9345–9551. [Google Scholar]
- 22.(a) Han G-F, Wang J-J, Shim YK. J. Photosci. 2001;8:71–73. [Google Scholar]; (b) Bold B, Barkhuu B, Lee W-K, Shim YK. Bull. Korean Chem. Soc. 2008;29:237–240. [Google Scholar]; (c) Tamiaki H, Kotegawa Y, Mizutani K. Bioorg. Med. Chem. Lett. 2008;18:6037–6040. doi: 10.1016/j.bmcl.2008.10.031. [DOI] [PubMed] [Google Scholar]; (d) Sasaki S-I, Mizoguchi T, Tamiaki H. J. Org. Chem. 2007;72:4566–4569. doi: 10.1021/jo0703855. [DOI] [PubMed] [Google Scholar]
- 23.Fukuzumi S, Ohkubo K, Chen Y, Pandey RK, Zhan R, Shao J, Kadish M. J. Phys. Chem. A. 2002;106:5105–5113. [Google Scholar]
- 24.Sharman WM, Van Lier JE. J. Porphyrins Phthalocyanines. 2000;4:441–453. [Google Scholar]
- 25.Becke AD. J. Chem. Phys. 1993;98:5648–5652. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.