Abstract
A putative mannitol operon of the phosphoenolpyruvate phosphotransferase (PTS) type was cloned from Vibrio cholerae O395 and its activity studied in Escherichia coli. The 3.9 kb operon comprising of three genes is organized as mtlADR. Based on the sequence analysis, these were identified as genes encoding a putative mannitol-specific enzyme IICBA (EIIMtl) component (MtlA), a mannitol-1-phosphate dehydrogenase (MtlD) and a mannitol operon repressor (MtlR). The transport of [3H]mannitol by the cloned mannitol operon in E. coli was 13.8±1.4 nmol/min/mg protein. The insertional inactivation of EIIMtl abolished mannitol and sorbitol transport in V. cholerae O395. Comparison of the mannitol utilization apparatus of V. cholerae with those of Gram-negative and Gram positive bacteria suggests highly conserved nature of the system. MtlA and MtlD exhibit 75% similarity with corresponding sequences of E. coli mannitol operon genes, while MtlR has 63% similarity with MtlR of E. coli. The cloning of V. cholerae mannitol utilization system in an E. coli background will help in elucidating the functional properties of this operon.
Keywords: Mannitol, Vibrio cholerae O395, PTS, IIMtl, MtlD
Introduction
The phosphoenolpyruvate-dependent carbohydrate:phosphotransferase system (PTS) catalyzes concomitant transport and phosphorylation of sugars by a process called group translocation (Hengstenberg et al. 1993; Postma et al. 1993; Lengeler et al. 1994; Barabote and Saier 2005). PTS is a multicomponent system consisting of cytoplasmic and membrane-bound proteins. Two cytoplasmic soluble proteins, HPr (heat-stable protein) and enzyme I, are involved in general phosphorylation of carbohydrates before they are taken up by the membrane bound enzyme II made of A, B and C components, coded contiguously in the genome or assembled functionally after being transcribed from genes located far apart. The general process of carbohydrate transport by the PTS is well known. Enzyme I is phosphorylated first with the utilization of a phosphoenolpyruvate (PEP) resulting in phospho-enzyme I and pyruvate. Phospho-enzyme I in turn phosphorylates an intermediate phosphocarrier protein HPr to form phospho-HPr. The phosphoryl group is finally transferred to the carbohydrate via PTS enzyme II (EII) complex (Postma and Lengeler 1985). The sugar specificity of PTS is attributed to the transmembrane permease component EII.
The 6-carbon sugar alcohol, D-mannitol is abundant in nature, such as in plants, algae, yeasts, fungi and bacteria. Mannitol is a major storage polysaccharide in fungi that also plays important roles in osmoregulation and stress tolerance (Jennings 1984; Stoop and Mooibroek 1998). Many microorganisms have mannitol utilization systems, mainly of PEP-dependent phosphotransferase system (PTS) types. Studies on the mannitol utilization systems from various Gram-negative and -positive bacteria such as Escherichia coli (Lee and Saier 1983), Staphylococcus carnosus (Fischer and Hengstenberg 1992), Bacillus subtilis (Akagawa et al. 1996), B. stearothermophilus (Henstra et al. 1996) and Clostridium acetobutylicum (Behrens et al. 2001) have suggested functional similarities, albeit organizational differences exist in the structural genes of the mannitol operons in Gram-positive and –negative bacteria.
The importance of PTS-mediated carbohydrate utilization in marine carbon cycling has been emphasized long ago and shown experimentally in vibrios (Meadow et al. 1987). The availability of whole genome sequences of many bacteria has facilitated the cloning and characterization of these, generally multicomponent, PT systems. The whole genome sequence of V. cholerae O395 has a putative mannitol operon and we tested the hypothesis that this genetic element plays a role in the utilization of mannitol. This paper describes the cloning of the mannitol operon from V. choelrae O395, its functional characterization and comparative analysis of the Vibrio mannitol utilization apparatus with other bacterial mannitol utilization systems.
Materials and methods
Cloning of mannitol operon, enzyme I and HPr encoding genes
The bacterial strains and plasmids used in this study are shown in Table 1. Genomic DNA was extracted from V. cholerae O395, V. cholerae PS-15 and Escherichia coli DH5α using the CTAB (cetyl trimethyl ammonium bromide) method (Ausubel et al. 1995). The mannitol operon containing a mannitol-specific enzyme IICBA (mtlA), mannitol-1-phosphate dehydrogenase (mtlD) and an operon repressor (mtlR) genes was amplified using primers ManXh and ManEco containing XhoI and EcoRI restriction sites (Table 2). The resultant PCR product was double digested with XhoI and EcoRI restriction enzymes, and ligated to a similarly digested pHSG396 plasmid (TaKaRa Bio Inc., Japan) to obtain pHSG396/Mtl, and transformed into E. coli HB101. The ptsI gene encoding enzyme I and the ptsH gene encoding HPr were amplified separately using primers PTS1 and PTS2 containing BamHI and XhoI restriction sites, and the resultant PCR product was restriction digested and ligated into the plasmid vector pSP72 (Promega, Madison, WI) to obtain pSP72/PTS. The plasmid construct pSP72/PTS was electro transformed into E. coli HB101 harboring pHSG396/Mtl, and the resultant transformant E. coli HB/Mtl-PTS was selected on Luria Bertani (LB) agar containing 100 μg/ml ampicillin and 25 μg/ml chloramphenicol.
Table 1.
Plasmids and bacterial strains used in this study.
| Plasmid construct/Strain | Description/genotype | Source/Reference |
|---|---|---|
| pSP72/PTS | ptsH and ptsI; AmpR | This study |
| pHSG396/Mtl | mtlA, mtlD, mtlR; CmR | This study |
| pSP72/mtlAEC | DH5α mtlA; AmpR | This study |
| pSP72/mtlAO395 | V. cholerae O395 mtlA; AmpR | This study |
| pSP72/mtlAPS15 | V. cholerae PS15 mtlA; AmpR | This study |
| pACD4K/mtlA | Mutagenic plasmid; CmR | This study |
| pAR1219 | Helper plasmid; AmpR | Davanloo et al. (1984) |
| E. coli HB101 | F−, lacY1, galK2, (Strr), xyl-5, mtl-1. | Hanahan (1985) |
| E. coli DH5α | F− endA1 recA1 galE15 galK16 nupG rpsL ΔlacX74 Φ80lacZΔM15 araD139 Δ(ara,leu)7697 mcrA Δ(mrr−hsdRMS- mcrBC) λ− | Invitrogen, Carlsbad, CA. |
| E. coli HB/Mtl | mannitol operon, ptsI, ptsH; AmpR, CmR | This study |
| Vibrio cholerae O395 | Wild-type V. cholerae | ATCC 39541/Ogawa 395 |
| Vibrio cholerae PS15 | Wild-type V. cholerae non-O1 | This study |
| V. cholerae O395 mtlA:: Intron | mtlA mutant, KanR | This study |
Table 2.
Oligonucleotide primers used in this study
| Primer | Nucleotide sequences (5′-3′) | Target gene | Product size (bp) |
|---|---|---|---|
| PTS1 | gcgggatccactattttcatcgcctatctcct | ptsI and ptsH | 2330 |
| PTS2 | gcgctcgaggacggtttacgctcaattgctca | ||
| ManXh | gcgctcgagcggtgtatttttagttgacccgt | Mannitol operon | 7256 |
| ManEco | gcggaattcatcatggcgatggatataaccgg | ||
| Mtl-ECF | gcgggatccatgtcatccgatattaagatcaa | E. coli mtlA | 1914 |
| Mtl-ECR | gcgctcgagttacttacgacctgccagcagttc | ||
| Mtl-VCF | gcgggatccatgatatcatcagacgccaaggt | V. cholerae mtlA | 1950 |
| Mtl-VCR | gcgctcgagttatgccgcttggctggtggcca | ||
| IBS | aaaaaagcttataattatccttaagagacgaaagcgtgcgcccagatagggtg | Mutagenic primers | - |
| EBS1d | cagattgtacaaatgtggtgataacagataagtcgaaagcggtaacttacctttctttgt | ||
| EBS2 | tgaacgcaagtttctaatttcgatttctcttcgatagaggaaagtgtct |
The mtlA of V. cholerae O395, V. cholerae PS15 and E. coli DH5α were amplified using primers listed in Table 2, restriction digested with BamHI and XhoI and ligated into pSP72 to obtain pSP72/mtlAVC, pSP72/mtlAPS15 and pSP72/mtlAEC, respectively. The plasmid constructs were electroporated into E. coli HB101.
Mannitol fermentation phenotypes on indicator media
The mannitol fermentation phenotype of E. coli HB/Mtl-PTS containing the complete mannitol operon, ptsI and ptsH genes (Table 1) was determined by streaking on MacConkey agar containing 1% mannitol, 100 μg/ml ampicillin and 25 μg/ml chloramphenicol. The plates were incubated at 37°C for 48 h and the colonies were observed for pink color due to production of acid from mannitol. E. coli HB101/pHSG396/pSP72 containing the cloning vectors alone was used as the control strain in all the experiments. The minimum mannitol concentration required to exhibit fermentation phenotype was determined by using MacConkey agar plates containing 0.1, 0.20, 0.3, 0.4, 0.5 or 1% mannitol as the sole source of fermentable carbohydrate. The plates were observed for colonies exhibiting red phenotype after 48 h of incubation at 37°C. Similarly, the mannitol fermentation phenotypes of E. coli HB/pSP72/mtlAVC, E. coli HB/mtlAPS15 and E. coli HB/mtlAEC were determined on MacConkey- mannitol-ampicillin agar. E. coli HB101/pSP72 was used as the control.
Sugar transport assays
Mannitol transport assay was performed using E. coli HB/Mtl-PTS. HB101/pHSG396/pSP72 (harboring plasmid vectors only) served as the control strain to determine the background radioactive sugar uptake. Cells were grown in 10 ml Luria Bertani (LB) broth containing 1 mM mannitol as an inducer of mannitol metabolizing enzymes, 100 μg/ml ampicillin and 25 μg/ml chloramphenicol to an O.D600 of 1.0, centrifuged and washed twice with an equal volume of Tris chloride buffer (50 mM, pH 8.0) containing 10 mM MgCl2 and resuspended in the assay buffer consisting of 50 mM Tris chloride (pH 8.0), 10 mM MgCl2, 10 mM phosphoenolpyruvate (PEP), 10 mM potassium fluoride and 0.5 mM dithiothreitol. The cells were equilibrated to room temperature for 10 min and radioactive sugar [3H]mannitol (American Radiolabeled Chemicals Inc., MO) was added to a final concentration of 1.0 mM, and incubated for 10 min at 37°C. Following incubation, the mixture was rapidly vacuum filtered through 0.45 μm cellulose nitrate filters (Sartorius, Hayward, CA), washed immediately with 3 ml of Tris-chloride buffer containing 0.5 mM HgCl2 to quench the sugar uptake reaction. The filters were placed in 4 ml of Sigma-Fluor (Sigma, St. Louis, MO) liquid scintillation fluid. The amount of radioactivity was determined using a LS-6500 liquid scintillation counter (Beckman Coulter, Fullerton, CA).
The kinetics of mannitol transport were studied at various concentrations (0.25, 0.5 and 1 mM) of radiolabeled [3H]mannitol. The cells were prepared as described above for transport studies and incubated with 3[H]-mannitol at room temperature. Aliquots of 200 μl were drawn at intervals of 30 sec and 60 sec and rapidly filtered. The values were plotted on Lineweaver-Burk double reciprocal plot and the apparent Vmax and apparent Km values were calculated (Segel 1976).
Inactivation of V. cholerae O395 mtlA
The mtlA gene of V. cholerae O395 was inactivated by intron-mediated insertion using the TargeTron method (Sigma-Aldrich St. Louis, MO). Briefly, primers IBS, EBS1d and EBS2 were designed using TargeTron algorithm (http://www.sigma-genosys.com/targetron/), and used to mutagenize a group II intron by PCR, followed by restriction digestion and ligation into plasmid pACD4K to obtain pACD4K/mtlA. The ligation mixture was transformed into TOP 10 E. coli (Invitrogen, USA). Separately, V. cholerae O395 was electroporated with the helper plasmid pAR1219 (Davanloo et al. 1984). Electrocompetent V. cholerae O395 harboring pAR1219 was transformed with pACD4K/mtlA, and grown in LB containing ampicillin, chloramphenicol and 1% glucose overnight. Following this, 40 μl of the overnight culture was used to inoculate 2 ml of fresh LB broth containing ampicillin, chloramphenicol and 1% glucose to an OD600 of 0.2, followed by the addition of IPTG (isopropyl-β-D-thiogalactopyranoside) to a final concentration of 1 mM. The cells were grown for 30 min at 30°C. IPTG induction results in the intron splicing and integration into target DNA, thus inactivating the target gene. The intron splicing simultaneously activates kanamycin resistance and hence, the mutant colonies were selected on LB plates containing 30 μg/ml kanamycin. The insertion of 2 kb plasmid into mtlA was confirmed by PCR using primers flanking mtlA.
Computer analysis
The deduced amino acid sequences of mannitol PTS proteins of V. cholerae O395 were compared to all other known proteins in the NCBI database by BLASTP analysis (Altschul et al. 1997). The two-dimensional structure of EIIMtl was determined by using the TMHMM sever (Transmembrane helix prediction based on hidden Markov models), the results of which were analyzed using the TMRpres2d (Transmembrane Re-presentation in 2-dimensions). Multiple sequence alignments were derived using the CLUSTALW program (Higgins et al. 1994).
Results
Identification and cloning of mannitol operon
The mannitol operon identified in the whole genome sequence of V. cholerae O395 is arranged as 3 complete open reading frames (ORFs) over a stretch of 3945 bp corresponding to coordinates 1033774-1037718 of the chromosome II (GenBank accession CP001236). The ORFs are organized as mtlA encoding a mannitol enzyme IICBA component (EIIMtl), mtlD encoding a mannitol-1-phosphate dehydrogenase and mtlR, encoding a putative operon repressor (MtlR) (Fig. 1). The 1950 bp mtlA encodes a fused IICB and IIA domains with 649 amino acids. The entire mannitol operon, together with the gene encoding enzyme I and the HPr proteins were separately cloned to obtain pHSG396/Mtl and pSP72/PTS constructs. The two plasmid systems were mobilized into E. coli HB101 to obtain E. coli HB/Mtl-PTS. We used E. coli HB101 for mobilizing our mannitol gene constructs, since this strain lacks EIIMtl.
Fig. 1.
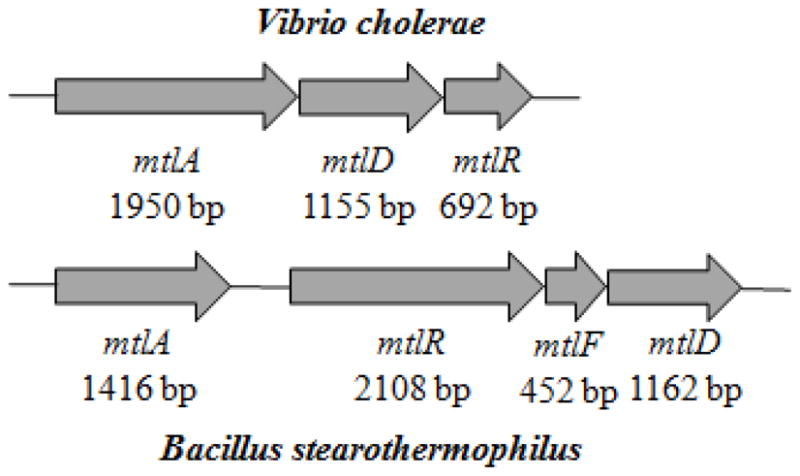
Organization of the mannitol operons of Gram-negative (V. cholerae) and Gram positive bacteria (B. stearothermophilus).
The amino acid sequence of IIMtl from V. cholerae shared high degree of homology with corresponding sequences of several Vibrio species, being 95% identical and 98% similar with putative IIMtl protein of V. mimicus, 83% identical and 90% similar with putative IIMtl protein of V. harveyi, V. alginolyticus, V. orientalis, and V. furnissii. IIMtl sequences with more than 80% identity and 90% similarity with V. cholerae IIMtl were found in several Vibrio spp. including V. metschnikovii, V. coralliilyticus and V. vulnificus. The sequence conservation in EIIMtl across bacterial genera is evidenced by the presence of homologous sequences with high similarity (>75%) in Tolumonas auensis, Providencia rettgeri, Enterobacter sakazakii, Pectobacterium carotovorum, Actinobacillus succinogenes. With the E. coli mannitol specific IICBA component, V. cholerae EIIMtl exhibited 67% identity and 80% similarity.
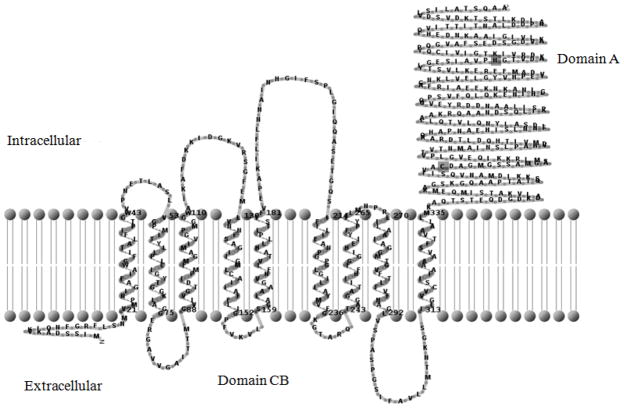
The predicted secondary structure of IIMtl and the putative phosphorylation sites are shown in Fig. 2. The highly conserved nature of the mannitol permease across diverse bacterial groups could be deduced by comparing the amino acid sequence of N-terminal CB domain of IIMtl from V. cholerae with sequences of mannitol permeases from different Gram-positive bacteria (Fig. 3). The C-terminal domain of V. cholerae IIMtl bearing putative phosphorylation sites has high sequence identity with the MtlF domains of Gram-positive bacteria (Fig. 4).
Fig. 2.
Predicted secondary structure of Enzyme IIMtl of V. cholerae O395. The two phosphorylation sites Cys 384 and His 554 in E. coli EIIMtl correspond to Cys-389 and His-564. These two amino acid residues in domain IIA are shown highlighted.
Fig. 3.
Multiple sequence alignment of IICB domain of V. cholerae O395 with the corresponding domains of mannitol permeases from different bacteria. The GenBank accession numbers are shown in parentheses against each bacterial species. E. coli (V01503), V. parahemolyticus (BA000031), Staphylococcus carnosus (YP_002634749), Clostridium acetobutylicum (AAC12848), Bacillus stearothermophilus (P50852).
Fig. 4.
Comparison of C-terminal part of EIIMtl from V. cholerae O395, E. coli (GenBank accession V01503) and V. parahemolyticus (GenBank accession BA000031) with MtlF of Gram-positive bacteria Staphylococcus carnosus (GenBank accession YP_002634751), Bacillus stearothermophilus (GenBank accession Q45420) and Clostridium acetobutylicum (GenBank accession AAC12850)
Mannitol transport
E. coli HB/Mtl-PTS exhibited a mannitol fermentation phenotype (red) on MacConkey agar containing mannitol. The minimum mannitol concentration required to show a fermentation phenotype on MacConkey agar was 0.20% (11 mM). The mannitol transport was measured using [3H]mannitol. At 1 mM final concentration of [3H]mannitol in the assay mixture, the uptake was determined to be 13.8±1.4 nmol/min/mg protein. The kinetics of uptake was measured over a concentration range of [3H]mannitol and an average Km value of 2.74±0.2 μM was obtained, while the Vmax value was 16.6±0.21 nmol/min/mg protein. With the wild type O395 strain, the Km and Vmax values were 0.97±0.02 μM and 8.6±0.11 nmol/min/mg protein, while in the case of V. cholerae non-O1 strain PS15, the mannitol transport kinetic studies detected a Km and a Vmax of 1.18±0.13 μM and 48.1±1.8 nmol/min/mg protein, respectively.
The mtlA gene of V. cholerae O395 was successfully disrupted by intron-mediated insertion. The mutant strain V. cholerae O395 mtlA::intron was unable to transport mannitol, as evidenced by the non-fermenting phenotype of the mutant colonies on MacConkey-mannitol agar. In addition, V. cholerae O395 mtlA::intron failed to ferment sorbitol.
Discussion
Microorganisms evolve to utilize diverse substrates to survive under conditions of low nutrient availability. The polyol sugar mannitol is a carbon source widely utilized by many species of bacteria. Apart from being a carbon source, mannitol is known to contribute to osmoregulation and stress tolerance (Efiuvwevwere et al. 1999). The metabolism of mannitol via PTS involves genes organized into an operon in Gram-positive and Gram-negative bacteria (Fischer et al. 1989; Fischer and Hengstenberg 1992; Akagawa et al. 1995; Behrens et al. 2001). The PTS permeases consist of homologous proteins that may have evolved from a common ancestral PTS (Postma and Lengeler 1985). The organization of the mannitol operon in V. cholerae consists of an Enzyme IIMtl (mtlA) -encoding IICBA complex, followed by mtlD and mtlR, similar to that reported from many other Gram-negative bacteria (Lee and Saier 1983). In Gram-positive bacteria such as B. stearothermophilus (Fig. 1), the enzyme IICB and IIA components are encoded by separate genes, mtlA and mtlF, respectively (Fischer et al. 1989).
We demonstrated that the IIMtl encoded by mtlA is the only mannitol-specific uptake system in V. cholerae O395 by insertional inactivation of the gene. The intron insertion into the mtlA gene resulted in V. cholerae with a mannitol non-fermenting phenotype. The intron insertion into mtlA resulted in the disruption of the entire operon as evidenced by the absence of mtlA, mtlD and mtlR RNA transcripts when tested by real-time PCR (data not shown). Further, our results also conclusively prove that a single operon functions to transport and utilize both mannitol and sorbitol in V. cholerae O395, since the inactivation of mtlA abolished the transport of sorbitol. However, cloning of the 7.2 kb mtlADR with promoter sequences did not confer a mannitol fermentation phenotype in E. coli HB101, suggesting that E. coli enzyme I and HPr components poorly interact with V. cholerae EIIMtl. A second plasmid system consisting of V. cholerae ptsH and ptsI gene sequences was designed, and complementation by this second plasmid system was apparent from red colonies of E. coli HB101 on MacConkey-mannitol agar.
Slow fermentation of D-mannitol by toxigenic strains of V. cholerae has been used to distinguish them from non-toxigenic strains that ferment mannitol rapidly (Wang et al. 2007). In our study, we used two V. cholerae strains for comparison of the functioning of mannitol operon. V. cholerae O395 is a toxigenic strain that ferments mannitol very slowly. When streaked on MacConkey-mannitol agar, V. cholerae O395 exhibited a white colony phenotype suggestive of non-sorbitol fermentation. When the white colonies were streaked again, light pink colonies appeared, that turned red upon a third streaking on MacConkey-mannitol agar (data not shown). In contrast, V. cholerae PS15, a non-O1 serotype, exhibited dark red colonies in the first streak plating, suggesting rapid mannitol utilization. We wanted to determine if this difference could be demonstrated in an E. coli background by cloning EIIMtl (mtlA) from V. cholerae O395 and V. cholerae PS15. Interestingly, HB/mtlAO395 and HB/mtlAPS15 were both white colonies on MacConkey-mannitol agar. As a positive control, we cloned mtlA from E. coli DH5α and transformed HB101 (HB/mtlAEC) which exhibited dark red colonies on MacConkey-mannitol agar. Since mtlA was constitutively expressed from a high copy plasmid, the non-functioning of V. cholerae mtlA, from both toxigenic and non-toxigenic strains in E. coli, provides indirect evidence to suggest that the differences in rate of fermentation between toxigenic and non-toxigenic strains are not due to differences in the levels of mtlA expression. A recent study showed that mtlR is not responsible for differences in mannitol fermentation rates between toxigenic and non-toxigenic strains (Wang et al. 2007). These observations together with the results of our study compel us to propose that in V. cholerae, the functioning of the mannitol operon is distinct compared to E. coli, and the differences in the mannitol utilization between toxigenic and non-toxigenic strains could be due the differences in expression of ptsH and ptsI genes.
EIIMtl with 649 amino acid residues has a predicted molecular weight of 68.69 kDa. Analysis of the N-terminal amino acid sequence of IIMtl did not reveal the presence of a signal peptide. Using bioinformatic tools, we derived a secondary structure for EIIMtl, which exhibited a hydrophobic transmembrane N-terminal domain and a hydrophilic cytoplasmic domain, akin to the IIMtl of E. coli (Lengeler et al. 1994) (Fig. 2). The membrane bound N-terminal portion is 334 amino acids long with 9 predicted transmembrane domains. The cytoplasmic domain corresponds to C-terminal portion of the protein from amino acids 335–649. The larger EIIMtl of E. coli has a fused C-terminal domain, which functions independently as EIII (MtlF) in Gram-positive bacteria (Saier et al. 1985). In E. coli IImtl, His-554 and Cys-384 have been shown to be the first and second sites of phosphorylation, respectively (Pas and Robillard 1988; Pas et al. 1988). In V. cholerae EIIMtl, based on the sequence alignment, the potential phosphorylation sites are predicted to be Cys-389 and His-564 (Fig. 3). We also compared the C-terminal 147 amino acids of V. cholerae EIIMtl with corresponding sequences from E. coli and V. parahemolyticus, and with MtlF from Staphylococcus carnosus, Clostridicum acetobutylicum and Bacillus stearothermophilus (Fig. 4). The first phosphorylation site within a highly conserved PHGT peptide was identified in sequences compared here, except in the MtlF of C. acetobutylicum in which tyrosine is replaced by a methionine. However, phosphorylation at these sites in the mannitol permease of V. cholerae remains to be experimentally demonstrated. The MtlF domains of S. carnosus, C. acetobutylicum and B. stearothermophilus were 35–44% similar to the corresponding sequence in the C-terminal domain of V. cholerae IIMtl.
MtlA simultaneously takes part in phosphorylation and transport of mannitol as mannitol 1-phosphate, that is subsequently oxidized to fructose 6-phosphate by the cytoplasmic enzyme mannitol-1-phosphate dehydrogenase (Wolff and Kaplan 1956; Saier 1977; Lee et al. 1981). Since we cloned the entire mannitol operon containing mtlD encoding mannitol phosphate dehydrogenase, the transport of mannitol was essentially “downhill”. The mannitol transport kinetics were determined in E. coli HB101 using [3H]mannitol. In the presence of PEP, [3H]mannitol was transported at a rate of 13.8±1.4 nmol/min/mg protein. The apparent Km value of 2.74±0.2 μM suggests that the mannitol PTS of V. cholerae has a very high affinity for its substrate. Since, E. coli HB101 has a native MtlD, this will not be a definitive determination of mannitol utilization. Hence, we compared the mannitol transport kinetics of wild type V. cholerae O395 and V. cholerae PS15, the latter being a non-O1 strain. The Km of mannitol transport was not significantly different between these two strains, being 0.97±0.02 μM and 1.18±0.13 μM, respectively for V. cholerae O395 and PS15 strains, suggesting similar affinities for the substrate. However, the Vmax value of V. cholerae PS15 was 48.1±1.8 nmol/min/mg, much higher than the Vmax of 16.6±0.21 nmol/min/mg protein obtained with V. cholerae O395.
Mannitol fermentation has been used as an important biochemical assay in the identification of of vibrios (West and Colwell 1984). Vibrios, which live in nutrient-limiting oligotrophic waters may vastly benefit physiologically by having an efficient uptake system for mannitol either by biofilm form of association with mannitol-producing algae or the utilization of mannitol released into seawater from decaying seaweeds. The mannitol fermentation phenotype has been correlated with virulence in V. vulnificus (Drake et al. 2010). However, the exact mechanism by which mannitol utilization contributes to virulence is not elucidated at the molecular level. While being involved in phosphorylation and uptake of specific sugars, PTS is known to be involved in global regulation of other carbohydrate utilization genes and the Kreb’s cycle (Postma and Lengeler 1985). To achieve mannitol transport, all V. cholerae PTS components were required in E. coli. Transformation of E. coli HB101 with mtlA (IIMtl) alone did not confer a positive mannitol fermentation phenotype. When ptsH and ptsI genes were mobilized in a separate plasmid into E. coli HB101 with EIIMtlA, the host cells utilized mannitol. A similar cloning strategy was previously reported for obtaining a functional PTS system of Staphylococcus carnosus (Fischer and Hengstenberg 1992).
This study will be the first step towards understanding the structure-function relationship of the mannitol transporter in V. cholerae and its physiological significance in vibrios. We are currently attempting to express and purify EIIMtl and to determine if i) Cys-389 and His-564 are active phosphorylation sites by site directed mutagenesis ii) differences in levels of ptsH and ptsI gene expression are responsible for differences in mannitol transport rates and utilization between the toxigenic and non-toxigenic strains of V. cholerae.
Acknowledgments
This work was made possible in part by National Institutes of Health grants R15 GM070562 and P20 RR016480, the latter of which is from the NM-INBRE program of the National Center for Research Resources, a contribution from Calton Research Associates in honor of George and Clytie Calton, and an Internal Research Grant from ENMU. We thank Dr. Chythanya Rajanna, Emerging Pathogens Institute, University of Florida for Vibrio cholerae O395 and Dr. Judith A. Johnson, Professor of Pathology and Director of Core Laboratories, Emerging Pathogens Institute, University of Florida for V. cholerae PS15.
References
- Akagawa E, Kurita K, Sugawara T, Nakamura K, Kasahara Y, Ogasawara N, Yamane K. Determination of a 17,484 bp nucleotide sequence around the 39 degrees region of the Bacillus subtilis chromosome and similarity analysis of the products of putative ORFs. Microbiology. 1995;141:3241–3245. doi: 10.1099/13500872-141-12-3241. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingsten RE, Moore DD, Seidman JG, Smith JA, Struhl K. Short Protocols in Molecular Biology. 3. John Wiley and Sons; New York: 1995. [Google Scholar]
- Barabote RD, Saier MH., Jr Comparative genomic analyses of the bacterial phosphotransferase system. Microbiol Mol Biol Rev. 2005;69:608–634. doi: 10.1128/MMBR.69.4.608-634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens S, Mitchell W, Bahl H. Molecular analysis of the mannitol operon of Clostridium acetobutylicum encoding a phosphotransferase system and a putative PTS-modulated regulator. Microbiology. 2001;147:75–86. doi: 10.1099/00221287-147-1-75. [DOI] [PubMed] [Google Scholar]
- Davanloo P, Rosenberg AH, Dunn JJ, Studier FW. Cloning and expression of the gene for bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1984;81:2035–2039. doi: 10.1073/pnas.81.7.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake SL, Whitney B, Levine JF, Depaola A, Jaykus LA. Correlation of mannitol fermentation with virulence-associated genotypic characteristics in Vibrio vulnificus isolates from oysters and water samples in the Gulf of Mexico. Foodborne Pathog Dis. 2010;7:97–101. doi: 10.1089/fpd.2009.0362. [DOI] [PubMed] [Google Scholar]
- Efiuvwevwere BJO, Gorris LGM, Smid EJ, Kets EPW. Mannitol-enhanced survival of Lactococcus lactis subjected to drying. Appl Microbiol Biotechnol. 1999;51:100–104. [Google Scholar]
- Fischer R, Hengstenberg W. Mannitol-specific enzyme II of the phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus carnosus. Sequence and expression in Escherichia coli and structural comparison with the enzyme IImannitol of Escherichia coli. Eur J Biochem. 1992;204:963–969. doi: 10.1111/j.1432-1033.1992.tb16717.x. [DOI] [PubMed] [Google Scholar]
- Fischer R, Eisermann R, Reiche B, Hengstenberg W. Cloning, sequencing and overexpression of the mannitol-specific enzyme-III-encoding gene of Staphylococcus carnosus. Gene. 1989;82:249–257. doi: 10.1016/0378-1119(89)90050-4. [DOI] [PubMed] [Google Scholar]
- Hanahan D. In: DNA Cloning. Glover D, editor. Vol. 1. IRL Press, Ltd; 1985. [Google Scholar]
- Hengstenberg W, Kohlbrecher D, Witt E, Kruse R, Christiansen I, Peters D, Pogge von Strandmann R, Städtler P, Koch B, Kalbitzer HR. Structure and function of proteins of the phosphotransferase system and of 6-phospho-beta-glycosidases in gram-positive bacteria. FEMS Microbiol Rev. 1993;12:149–163. doi: 10.1111/j.1574-6976.1993.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Henstra SA, Tolner B, ten Hoeve Duurkens RH, Konings WN, Robillard GT. Cloning, expression, and isolation of the mannitol transport protein from the thermophilic bacteriumBacillus stearothermophilus. J Bacteriol. 1996;178:5586–5591. doi: 10.1128/jb.178.19.5586-5591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings DH. Polyol metabolism in fungi. Adv Microb Physiol. 1984;25:149–193. doi: 10.1016/s0065-2911(08)60292-1. [DOI] [PubMed] [Google Scholar]
- Lee CA, Saier MH., Jr Mannitol-specific enzyme II of the bacterial phosphotransferase system. III. The nucleotide sequence of the permease gene. J Biol Chem. 1983;258:10761–10767. [PubMed] [Google Scholar]
- Lee CA, Jacobson GR, Saier MH., Jr Plasmid-directed synthesis of enzymes required for D-mannitol transport and utilization in Escherichia coli. Proc Natl Acad Sci USA. 1981;78:7336–7340. doi: 10.1073/pnas.78.12.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler JW, Jahreis K, Wehmeier UF. Enzymes II of the phosphoenol pyruvate-dependent phosphotransferase systems: their structure and function in carbohydrate transport. Biochim Biophys Acta. 1994;1188:1–28. doi: 10.1016/0005-2728(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Meadow ND, Revuelta R, Chen VN, Colwell RR, Roseman S. Phosphoenolpyruvate:glycose phosphotransferase system in species of Vibrio, a widely distributed marine bacterial genus. J Bacteriol. 1987;169:4893–4900. doi: 10.1128/jb.169.11.4893-4900.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pas HH, Robillard GT. S-phosphocysteine and phosphohistidine are intermediates in the phosphoenolpyruvate-dependent mannitol transport catalyzed by Escherichia coli IIMtl. Biochemistry. 1988;27:5835–5839. doi: 10.1021/bi00416a002. [DOI] [PubMed] [Google Scholar]
- Pas HH, ten Hoeve-Duurkens RH, Robillard GT. Bacterial phosphoenolpyruvate-dependent phosphotransferase system: mannitol-specific EII contains two phosphoryl binding sites per monomer and one high-affinity mannitol binding site per dimer. Biochemistry. 1988;27:5520–5525. doi: 10.1021/bi00415a020. [DOI] [PubMed] [Google Scholar]
- Postma PW, Lengeler JW. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985;49:232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma PW, Lengeler JW, Jacobson GR. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993;57:543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier MH, Jr, Grenier FC, Lee CA, Waygood EB. Evidence for the evolutionary relatedness of the proteins of the bacterial phosphoenolpyruvate:sugar phosphotransferase system. J Cell Biochem. 1985;27:43–56. doi: 10.1002/jcb.240270106. [DOI] [PubMed] [Google Scholar]
- Saier MH., Jr Bacterial phosphoenolpyruvate:sugar phosphotransferase systems: structural, functional, and evolutionary interrelationships. Bacteriol Rev. 1977;41:856–871. doi: 10.1128/br.41.4.856-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segel IH. Biochemical calculations: how to solve mathematical problems in general biochemistry. 2. John Wiley & Sons; New York, NY: 1976. [Google Scholar]
- Stoop JM, Mooibroek H. Cloning and characterization of NADP-mannitol dehydrogenase cDNA from the button mushroom, Agaricus bisporus, and its expression in response to NaCl stress. Appl Environ Microbiol. 1998;64:4689–4696. doi: 10.1128/aem.64.12.4689-4696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Yan MY, Zhao YW, Kan B. Transcriptional repressor gene--mtlR of mannitol PTS operon in Vibrio cholerae. Wei Sheng Wu Xue Bao. 2007;47:522–525. [PubMed] [Google Scholar]
- West PA, Colwell RR. Identification and classification of Vibrionaceae-an overview. In: Colwell RR, editor. Vibrios in the environment. John Wiley & Sons Inc; New York, N.Y: 1984. pp. 285–363. [Google Scholar]
- Wolff JB, Kaplan NO. D-Mannitol-l-Phosphate dehydrogenase from Escherichia coli. J Biol Chem. 1956;218:849–869. [PubMed] [Google Scholar]