Abstract
The social motivation hypothesis of autism posits that infants with autism do not experience social stimuli as rewarding, thereby leading to a cascade of potentially negative consequences for later development. While possible downstream effects of this hypothesis such as altered face and voice processing have been examined, there has not been a direct investigation of social reward processing in autism. Here we use functional magnetic resonance imaging to examine social and monetary rewarded implicit learning in children with and without autism spectrum disorders (ASD). Sixteen males with ASD and sixteen age- and IQ-matched typically developing (TD) males were scanned while performing two versions of a rewarded implicit learning task. In addition to examining responses to reward, we investigated the neural circuitry supporting rewarded learning and the relationship between these factors and social development. We found diminished neural responses to both social and monetary rewards in ASD, with a pronounced reduction in response to social rewards (SR). Children with ASD also demonstrated a further deficit in frontostriatal response during social, but not monetary, rewarded learning. Moreover, we show a relationship between ventral striatum activity and social reciprocity in TD children. Together, these data support the hypothesis that children with ASD have diminished neural responses to SR, and that this deficit relates to social learning impairments.
Keywords: functional MRI (fMRI), social cognition, reward, learning
Introduction
Autism is a pervasive neurodevelopmental disorder with hallmark deficits in social communication and reciprocity. Whereas typically developing (TD) infants show a preference for social over non-social stimuli [Legerstee, Anderson, & Schaffer, 1998], retrospective studies of videotaped birthday parties indicate that children who develop autism show decreased motivation to attend to social stimuli, as evidenced by reduced attention to faces of others, decreased pointing and showing, and failing to orient to their name, as early as the child’s first birthday [Dawson, Meltzoff, Osterling, Rinaldi, & Brown, 1998b; Osterling & Dawson, 1994]. These observations led to the development of the social motivation hypothesis of autism [Dawson et al., 1998b; Dawson, Webb, & McPartland, 2005; Schultz, 2005], which posits that reduced time spent attending to faces, speech, and other social stimuli leads to a cascade of negative consequences for the development of social cognition and language such as decreased expertise in human face [Grelotti, Gauthier, & Schultz, 2002; Pelphrey, Adolphs, & Morris, 2004; Schultz et al., 2000] and speech [Klin, 1991; Kuhl, Coffey-Corina, Padden, & Dawson, 2005; Pelphrey et al., 2004] processing. This lack of social motivation has been attributed to a decreased reward value for social stimuli. Although it has been established that infants with autism spend less time orienting to social events, it is difficult to directly test whether this is due to a primary dysfunction of the reward system per se using behavioral measures alone. Functional magnetic resonance imaging (fMRI) allows a more direct investigation into the neural correlates of reward processing in humans, including response to social rewards (SR). A number of fMRI studies have highlighted a reward network—comprised primarily of anterior cingulate (ACC), orbitofrontal cortex (OFC), and ventral striatum (VS)—which responds to primary rewards such as food [O’Doherty, Deichmann, Critchley, & Dolan, 2002], as well as to secondary rewards such as money [O’Doherty, Kringelbach, Rolls, Hornak, & Andrews, 2001; Thut et al., 1997; see also Knutson & Cooper, 2005 for a review]. Studies of SR in humans support involvement of these same reward networks while viewing pictures of faces [Bartels & Zeki, 2004; Phillips et al., 1998; Spreckelmeyer et al., 2009].
Surprisingly, only one study thus far has examined the neural correlates of reward processing in adults with autism, using fMRI to examine responses to monetary rewards [MR; Schmitz et al., 2008]. Here, we used both MR and SR to investigate reward processing in children with autism spectrum disorder (ASD). Importantly, we examined the social motivation hypothesis by investigating reward processing in the context of socially rewarded learning, that is, learning reinforced by positive social stimuli such as a smiling face. The social rules of engagement are rarely taught explicitly and are likely acquired through observation, imitation, and implicit learning of stimulus–outcome associations [see Frith, 2008 for review]. For example, individuals can acquire fear of a novel object simply by watching another being conditioned to fear that object (i.e., paring the object with a painful shock), even when the object is masked and the observer is unable to report when the stimulus occurs [Olsson & Phelps, 2004]. It is likely that this learning occurs via mirroring of the fearful expression in the observer, thereby creating a subliminal, implicitly acquired, stimulus–outcome association between the novel object and fear. Impairment in the ability to implicitly acquire stimulus–outcome associations could have clear repercussions for social learning. In fact, computational modeling work suggests the development of certain social behaviors such as gaze following and joint attention may rely on probabilistic reward-related learning [Triesch, Teuscher, Deak, & Carlson, 2006].
In this study, we examined the behavioral and neural correlates of rewarded implicit learning in children with ASD, given conventional rewards (i.e., monetary gain) and SR (i.e., smiling faces). Specifically, we focused on three hypotheses regarding reward-related brain activity in children with and without ASD. First, we examined neural responsiveness of brain areas known to be involved in reward processing (e.g., VS) to both social and non-social rewards to examine whether ASD children would demonstrate an overall reduced neural response to rewards, or a more specific deficit for SR. second, we examined the neural correlates of reward-related learning to test the hypothesis that children with ASD would show decreased activity in brain regions known to support implicit learning and reward processing (i.e., frontostriatal networks). Finally, we investigated whether activity in the reward system is related to the level of social functioning by correlating neural reward response to behavioral measures of social responsiveness.
Methods
Participants
Participants were recruited through referrals from the UCLA Autism Evaluation Clinic and through flyers posted around the UCLA campus and the greater Los Angeles area. Sixteen high-functioning boys with autism spectrum disorder with normal IQ and 16 age- and IQ-matched TD boys (see Table I) underwent fMRI scans. The groups did not significantly differ in age or FSIQ as assessed by the Wechsler Abbreviated Scales of Intelligence—Revised [Wechsler, 1999] or Wechsler Intelligence Scale for Children—Third Edition [Wechsler, 1991]. Receptive language skills were assessed with the Peabody Picture Vocabulary Test—Third Edition to verify the ability to understand the verbal instructions. Additionally, the ASD children had a VIQ well within the normal range (108.8±14.9), further indicating a sufficient level of verbal comprehension. For the ASD group, prior clinical autism diagnosis was confirmed by the Autism Diagnostic Observation Scale—General [ADOS-G; Lord et al., 2000] and Autism Diagnostic Interview—Revised [ADI-R; Lord, Rutter, & Le Couteur, 1994]. All children met criteria for autism as defined by the ADI-R (cutoff=22; mean=44.4, range=28–64). Six participants met criteria for Autism Spectrum (cutoff=7; mean=9.2; range=7–11) and ten met autism criteria (cutoff=10, mean=13.3, range= 10–19) as defined by the ADOS-G. Seven participants were not currently taking any medications. Out of the remaining children, two were taking psychostimulants only, two were taking atypical antipsychotics only, three were taking both antipsychotic and psychostimulant medication, one was taking a selective serotonin reuptake inhibitor, one was taking an atypical antidepressant, and medication status was unknown for one child. We would expect the effects due to medication to reduce the between-group differences in the blood oxygenation level dependent (BOLD) response. By report, none of the participants had any known loss of consciousness longer than 5 min, or any neurological (e.g. epilepsy), genetic (e.g., Fragile X) or major psychiatric (e.g., schizophrenia) disorder other than autism. Written informed consent was obtained from participants and their parents according to the specifications of the UCLA Institutional Review Board.
Table I.
Mean and Standard Deviation of Sample Descriptives
| AGE | FSIQ | VIQ* | PIQ | PPVT-III | |
|---|---|---|---|---|---|
| TD | 12.3 (1.76) | 119.0 (8.4) | 119.4 (12.6) | 111.50 (8.1) | 121.6 (14.4) |
| ASD | 12.4 (2.14) | 112.3 (13.6) | 108.8 (14.9) | 114.13 (13.1) | 116.2 (16.0) |
P=0.037.
Experimental Design
Two rapid event-related mixed-trial rewarded learning tasks were designed by adapting the weather prediction task [Knowlton, Squire, & Gluck, 1994] for use in this population. The task requires participants to classify abstract fractal-like images (created using Art Matic Pro, U&I Software LLC) into “Group 1” and “Group 2” pictures by responding with a simple button press (“1” or “2”), with feedback presented after each classification trial (see Fig. 1). Children were asked to press a button every time they saw a picture, and encouraged to win as many rewards as possible. Simple “Group 1” and “Group 2” classifications were used rather than the traditional “Rain” or “Sunshine” to avoid idiosyncratic responses associated with concrete semantic interpretations in children with ASD (e.g., a child might use the presence of blue in the picture to always predict rain or yellow to always predict sunshine). To discourage memorization of the pairs, children were also told that the computer occasionally “messes up” and sometimes a correct answer would result in the wrong feedback, and vice versa.
Figure 1.
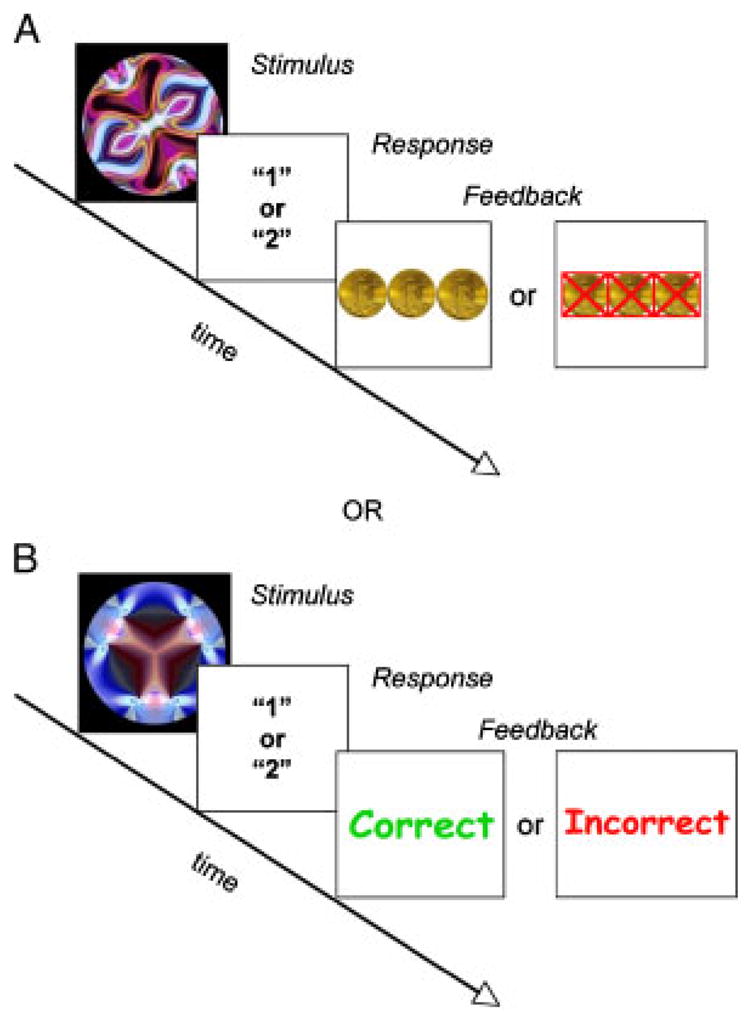
Paradigm design. 4/6 trials were associated with “1” or “2” 100%, 2/6 were random (50%). Panel A depicts an example of a rewarded trial. Panel B is an example of a neutral trial. During the Social version, the coins were replaced with a picture of a woman either smiling, for positive feedback, or frowning, for negative feedback. Neutral trials were replaced with a picture of the same woman with a neutral expression. Trials lasted an average of 5 sec, inter-trial intervals were randomly jittered between 1,250 and 2,500 msec, feedback display was jittered between 1,000 and 1,250 msec after stimulus presentation.
The task was designed to be easy enough to allow improvement in accuracy within one 6 min run, but challenging enough to maintain a reliance on implicit learning of the associations; this was done by limiting the probabilities to either 0, 50 or 100% predictive of one of two outcomes. The presence of randomly (50%) associated pairs in 1/3 of the trials further discouraged a memorization strategy. Each stimulus was associated with either reward or neutral feedback. Therefore, six different abstract stimuli were used for each run, one for each probability and feedback condition (rewarded or neutral). A total of 12 abstract images were used, 6 for the monetary feedback version and 6 for the social feedback version. To prevent item effects, stimuli were counterbalanced across children such that each was equally associated with “1” or “2” as well as with reward or neutral feedback. Each run consisted of 72 interspersed trials optimized for detection of trial-related reward activity [Wager & Nichols, 2003]. Each trial lasted an average of 5 sec, and inter-stimulus intervals were randomly jittered between 1,250 and 2,500 msec. Each stimulus was displayed approximately 2,000 msec, and feedback was displayed for 1,250 msec after each stimulus presentation.
The monetary task provided either MR or monetary neutral (MN) feedback to guide learning of stimulus–response associations and the social version used either SR or social neutral (SN) feedback. Correct MR feedback consisted of a picture of three gold coins, and incorrect MR feedback was presented as the same image with three red X’s through the coins. MN feedback was presented as the words “Correct!” in green or “Incorrect” in red to provide feedback. The SR feedback consisted of a picture of a smiling woman with the words “That’s Right!” in green text for correct trials and a picture of the same woman with a sad face along with the words “That’s Wrong” in red text for incorrect trials. SN feedback consisted of the same woman with a neutral expression and the same text in black. The chosen reward stimuli, faces and coins, are consistent with those used in previous studies of reward processing [e.g., Bray & O’doherty, 2007; Galvan et al., 2006; Izuma, Saito, & Sadato, 2008].
Before the scans, all participants were told that they were going to play two games; during one of them they could earn an extra $5, and for the other they would receive a different kind of “reward” but it was not specifically stated what it would be. To verify that the participants had an equal and realistic idea of the value of money, prior to the scan the experimenter coached the participants to think about what they would buy if they earned the full amount, compared to what they could buy if they did not earn the extra money. All children seemed to fully grasp the concept of potentially gaining more money. Participants received the full $5 bonus regardless of performance at the end of the scanning session. Participants completed both versions of the task, and the presentation order of the two runs was counterbalanced across children.
Data Acquisition
Scans were acquired in a single session on a Siemens Allegra 3 Tesla head-only MRI scanner at the Ahmanson-Lovelace Brain Mapping Center at the University of California, Los Angeles. A pre-scan session in a mock scanner was available to participants to familiarize them with the scanning procedures and sounds. For each participant, a high-resolution structural T2-weighted echo-planar imaging volume (spin-echo, TR=5,000 msec, TE=33 msec, matrix size=128 by 128, FOV=20 cm, 36 slices, 1.56 mm in-plane resolution, 3 mm thick) was acquired coplanar with the functional scans to allow for spatial registration of each participant’s data into a standard coordinate system. For each run 180 functional images were acquired using an echo-planar (EPI) gradient-echo acquisition lasting 6 min and covering the whole cerebral volume (TR=2,000 msec, TE=30 msec, flip angle=90, matrix size 64×64, FOV=20 cm, 33 slices, 3.125 mm in-plane resolution, 4 mm thick). Two volumes at the beginning of each functional run were used to allow equilibration to steady state and were subsequently excluded from the analysis.
Visual stimuli were presented to the subject using 512×512 resolution magnet-compatible 3-D goggles and headphones under computer control (Resonance Technologies, Inc., Northridge, CA). The stimuli were presented using Matlab 7.0.4 psychological experimentation software (The MathWorks Inc., Natick, MA), ran on a Macintosh G4 Powerbook computer. Key press and RTs were recorded for behavioral analysis.
Statistical Analysis
RTs and stimulus classification accuracy for the deterministic trials were collapsed into eight successive trial bins and resulted in three measures each for each trial type (rewarded or neutral) across the duration of the task. Repeated measures analysis of variance analyses were conducted to investigate learning. Two-tailed t-tests were used for group comparisons on demographic variables including age and IQ.
fMRI Data Analysis
fMRI analysis was carried out using FEAT (FMRI Expert Analysis Tool) Version 5.63, part of FSL version 3.3 (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). Functional volumes were motion corrected to the median volume with MCFLIRT [Jenkinson, Bannister, Brady, & Smith, 2002], using a normalized correlation ratio cost function and linear interpolation. Brains were skull stripped using BET (brain extraction tool) [Smith, 2002]. Images were spatially smoothed using a Gaussian kernel of FWHM 5 mm, intensity normalized, and temporally high-pass filtered. Time-series statistical analysis was carried out using FILM with local autocorrelation correction [Woolrich, Ripley, Brady, & Smith, 2001]. Regressors of interest were created by convolving a delta function representing trial onset times with a canonical (double-gamma) hemodynamic response function, along with their temporal derivative. Motion parameters for each subject were entered as covariates of no interest. Groups were equivalent in the amount of head motion during the runs (monetary: TD=0.31 mm (0.27); ASD= 0.33 mm (0.22); t(30)=0.163, P=0.87; social: TD= 0.28 mm (0.11); ASD=0.39 mm (0.25); t(30)=1.68, P=0.103). Functional images were aligned using FMRIB’s Linear Image Registration Tool to high-resolution coplanar images via an affine transformation with six degrees of freedom. The high-resolution coplanar images were then aligned to the standard Montreal Neurological Institute (MNI) average of 152 brains using an affine transformation with 12 degrees of freedom.
Fixed-effect models were run separately for each subject for each run. The fixed-effect models were taken into a higher-level mixed effects model to investigate within- and between-groups effects. An index of learning (AccSlope) was calculated as the difference between the percent correct during the last eight stimulus presentations and the first eight presentations; as there was a significant difference in accuracy between the TD and ASD groups, this index was used as a covariate in all between-group analyses. First, within-group mixed effects models were run for each condition to identify main effects. Next, two separate two-sample t-tests were run to investigate between-group differences for the Monetary and Social reward runs. All contrasts were cluster corrected at Z>1.96 and P<0.05. Our a priori region of interest, the VS, was corrected for multiple comparisons with an α of 0.005 and a cluster threshold of at least 20 voxels, corresponding to a false-positive probability of less than 0.00001 [Forman et al., 1995]. Correlation analyses with the Social Responsiveness Scale [SRS; Constantino et al., 2003] were masked by a combined mask of areas of activation for TD and ASD groups for correct SR>incorrect SR and confirmed with a Kendall’s rank correlation on extracted parameter estimates from significant clusters in a priori regions of interest (i.e., VS).
Results
Behavioral Results
Overall accuracy, as measured by the percent correct in eight-trial bins for neutral and rewarded trials, increased over time in the TD children for both themonetary (Fig. 2A) and social (Fig. 2B) runs, whereas the ASD children’s performance remained near chance. This suggests a deficit in implicit learning in ASD, regardless of feedback type (i.e., monetary or social feedback). Additionally, within-group analyses showed there were no differences in accuracy between rewarded and neutral trials for either the TD or ASD children, nor were there significant differences in accuracy between the social and monetary runs in either group. These findings indicate that children with ASD demonstrate a general deficit in implicit learning, regardless of reward or feedback type. Overall, RTs were not significantly different between groups (F(1,30) =3.834, P=0.60), indicating that all children attended and responded to the task. RTs for both groups of children significantly decreased over the course of both monetary and social tasks (F(2,29)=17.68, P<0.001). After scanning, subjects were explicitly asked to identify the stimulus–outcome relationships. Analysis of post-test data revealed that both groups performed at chance when asked to identify the outcome (“1” or “2”) for each stimulus (see Table II), indicating that subjects had not gained explicit knowledge about the stimulus–outcome associations. These data suggest that both groups of children were equally engaged during the task, despite the poor learning in the ASD group.
Figure 2.
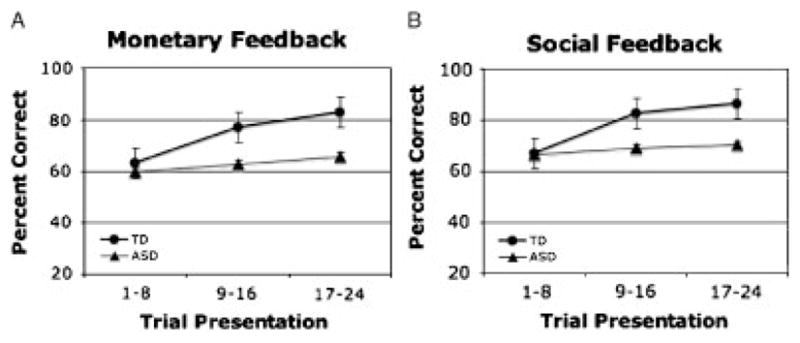
Behavioral performance. Accuracy during Monetary (A) and Social (B) tasks. Error bars represent standard error of the mean (SEM).
Table II.
Behavioral Results: Task Performance for TD and ASD Groups
| TD M (SD) | ASD M (SD) | |
|---|---|---|
| Total percent correct | ||
| Monetary | 74.39 (20.2) | 62.90 (16.0) |
| Social | 79.03 (12.2) | 68.8 (15.5) |
| Post-test number correct (out of 4) | ||
| Monetary | 2.25 (1.2) | 1.63 (1.2) |
| Social | 2.13 (1.5) | 1.88 (1.4) |
| No. of positive rewarded events | ||
| Monetary | 22.43 (5.9) | 19.07 (4.9) |
| Social | 23.0 (4.1) | 22.2 (4.9) |
| No. of negative rewarded events | ||
| Monetary | 15.33 (4.8) | 12.38 (5.1) |
| Social | 11.25 (3.8) | 12.73 (4.7) |
Task accuracy is calculated as the total percent correct over the run for deterministic trials, post-test accuracy is described as the correct number of associations identified out of four possible. The mean number of positive and negative events (i.e., deterministic and random combined) within groups is also reported.
fMRI Results
Stimulus and feedback responses were modeled separately for each trial type (i.e., random/deterministic, neutral/rewarded) for all analyses. The inclusion of random trials in the experimental design curtailed potential confounds by providing sufficient numbers of positive and negative feedback events for all participants although accuracy differed (see Table II). We ran an overall analysis of all events (i.e., positive and negative feedback for rewarded and neutral trials) compared to inter-trial rest (implicit baseline) to verify that both groups attended to and processed sensory aspects of the test similarly (see Table III). To further confirm that both TD and ASD children processed the facial features during the social feedback events, a region of interest analysis was conducted in the fusiform gyrus (FG). Using a face localizer contrast (all facial feedback>all stimulus events) to functionally define the FG ROI in TD children (216 voxels at 42, −48, −18), the percent signal change during social feedback was extracted for each child and compared across groups. Percent signal change was computed from average parameter estimates using the height of an isolated event as the scaling factor, and was relative to the voxel mean. There were no significant differences in the amount of FG activity between groups (TD=3.08 (2.27); ASD=2.29 (2.09); t(30)=1.03, P=0.310). While FG activation in response to facial stimuli is a reliable indicator of attention to the face, we cannot conclude however that face processing in the children with ASD is unimpaired.
Table III.
General Task Effects: MNI Anatomical Coordinates for All Events as Compared to Rest for Monetary and Social Runs by Group
| Anatomical region | Monetary: all events—Rest |
Social: all events—Rest |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TD |
ASD |
TD |
ASD |
||||||||||||||||||
| BA | x | y | z | Z | BA | x | y | z | Z | BA | x | y | z | Z | BA | x | y | z | Z | ||
| Caudate | L | ||||||||||||||||||||
| R | 12 | 10 | 6 | 2.94 | 14 | 12 | 6 | 3.19 | 16 | 4 | 14 | 2.72 | |||||||||
| Putamen | L | ||||||||||||||||||||
| R | 18 | 4 | 6 | 2.87 | |||||||||||||||||
| Globus Pallidus | L | ||||||||||||||||||||
| R | 20 | 4 | −2 | 2.38 | |||||||||||||||||
| Orbitofrontal | L | 11 | −40 | 38 | −10 | 3.04 | |||||||||||||||
| R | 11 47 |
24 28 |
42 18 |
−14 −18 |
2.75 3.26 |
||||||||||||||||
| Inferior frontal gyrus | L | 46 | −40 | 36 | 8 | 3.78 | 45 47 |
−46 −26 |
24 14 |
20 −20 |
3.68 2.50 |
||||||||||
| R | 46 | 42 | 38 | 18 | 3.98 | 46 | 48 | 32 | 16 | 4.14 | |||||||||||
| Middle frontal gyrus | L | 8 | −40 | 12 | 40 | 3.53 | 9 | −48 | 24 | 34 | 2.94 | ||||||||||
| R | 6/8 | 50 | 8 | 40 | 4.26 | 9 | 44 | 26 | 32 | 3.33 | 9 | 52 | 26 | 34 | 3.36 | ||||||
| Precentral gyrus | L | 6 | −44 | 4 | 32 | 3.71 | 4 | −44 | 4 | 32 | 3.30 | 6 | −40 | 2 | 34 | 2.87 | |||||
| R | 4 | 48 | 6 | 32 | 4.14 | 6 | 46 | 4 | 34 | 3.12 | 6 | 52 | 0 | 48 | 3.47 | ||||||
| Frontal Pole | L | 10 | −28 | 58 | −6 | 4.02 | 10 | −42 | 52 | −6 | 3.14 | ||||||||||
| R | 10 | 42 | 50 | 4 | 3.60 | 10 | 28 | 54 | −2 | 4.27 | |||||||||||
| Hippocampus/Parahippocampal gyrus | L | 30 | −16 | −36 | −4 | 3.98 | 35 | −18 | −32 | −6 | 3.46 | ||||||||||
| R | 30 | 18 | −32 | −8 | 3.61 | 28 | 22 | −30 | −4 | 3.57 | |||||||||||
| Cingulate | |||||||||||||||||||||
| Anterior | 24 | 8 | 30 | 20 | 3.96 | ||||||||||||||||
| Paracingulate | 6/8 | 4 | 24 | 44 | 3.50 | ||||||||||||||||
| Posterior | |||||||||||||||||||||
| Insula | L | −28 | 18 | −8 | 3.50 | −32 | 16 | 4 | 3.42 | −30 | 16 | −8 | 2.81 | ||||||||
| R | 34 | 20 | 2 | 4.06 | 36 | 16 | −4 | 4.05 | |||||||||||||
| Fusiform gyrus | L | 37 | −32 | −76 | −20 | 5.85 | 37 | −30 | −68 | −16 | 5.61 | 37 | −28 | −70 | −18 | 5.42 | 37 | −40 | −70 | −18 | 5.45 |
| R | 37 | 22 | −72 | −16 | 5.71 | 37 | 40 | −60 | −14 | 5.15 | 37 | 32 | −60 | −20 | 5.01 | 37 | 30 | −80 | −20 | 5.36 | |
| Occipital cortex | L | ||||||||||||||||||||
| R | 18 | 10 | −98 | 16 | 5.70 | 18 | 32 | −90 | −6 | 5.47 | 18 | 12 | −100 | 16 | 5.70 | ||||||
| Superior parietal lobule | L | ||||||||||||||||||||
| R | 7 | 28 | −60 | 52 | 3.96 | 7 | 34 | −60 | 54 | 3.37 | |||||||||||
| Inferior parietal lobule | L | 40 | −38 | −52 | 48 | 3.85 | |||||||||||||||
| R | 40 | 40 | −52 | 42 | 3.37 | ||||||||||||||||
| Supramarginal gyrus | L | 40 | −48 | −52 | 50 | 4.30 | |||||||||||||||
| R | 40 | 48 | −44 | 54 | 3.94 | ||||||||||||||||
BA refers to putative Brodmann’s Area; L and R refer to left and right hemispheres; x, y, and z refer to the left–right, anterior–posterior, and inferior–superior dimensions, respectively; Z refers to the Z-score at those coordinates (local maxima or submaxima). Cluster corrected for multiple comparisons, Z>1.96, P<0.05.
Due to the significant differences in accuracy between groups, the slope of each participant’s learning curve was used as a covariate in the between-group analyses to insure that any observed differences did not merely reflect a difference in the rate of learning. This index of learning (AccSlope) was calculated as the difference in percent accuracy between the first eight and last eight deterministic trials. The AccSlope measure, rather than overall accuracy, was chosen in order to capture the individual differences in the rate of learning.
Children With ASD Show Pronounced Deficits in VS Response to SR
Monetary Reward Response
Within-group analyses
Within-group analyses for the response to positive MR feedback (i.e., coins) as compared to negative MR feedback (i.e., crossed-out coins) revealed a large cluster of activity in regions restricted to the VS (see Fig. 3, Table IV) in TD children only. Neither group demonstrated VS activity in response to correct vs. incorrect neutral feedback, suggesting a specific and robust response to MR in the VS for the TD group only.
Figure 3.
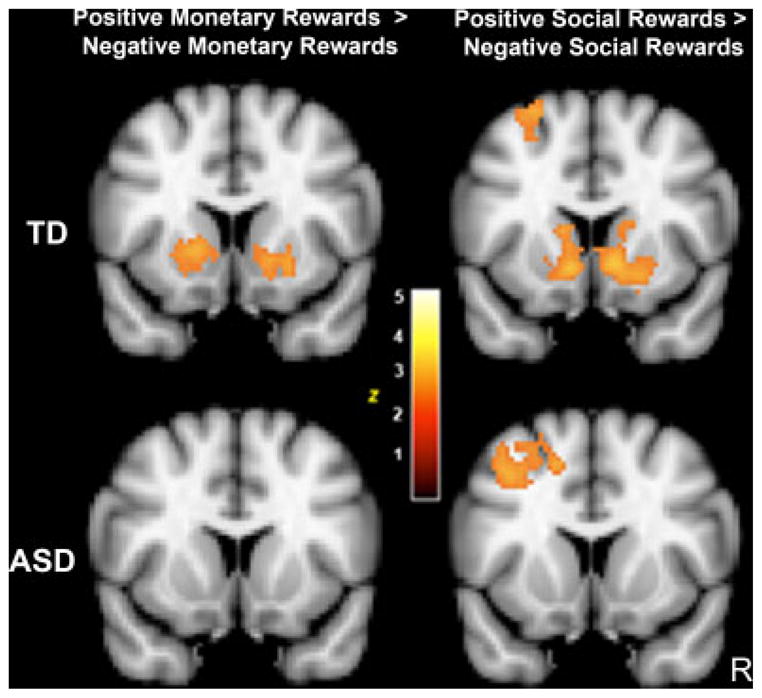
Reward response within groups for positive vs. negative reward feedback. MNI coordinates, y=12, Z>1.96, P<0.05 cluster corrected.
Table IV.
Neural Responses to Rewards: MNI Anatomical Coordinates for the Contrasts Positive Reward–Negative Reward Feedback for Monetary and Social Runs by Group
| Anatomical region | Monetary: positive reward>negative reward feedback |
Social: positive reward>negative reward feedback |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TD |
ASD |
ASD>TD | TD |
ASD |
||||||||||||||||||||||
| BA | x | y | z | Z | BA | x | y | z | Z | BA | x | y | z | Z | BA | x | y | z | Z | BA | x | y | z | Z | ||
| Caudate | L | −12 | 14 | 10 | 3.18 | −10 | 22 | 0 | 2.97 | |||||||||||||||||
| R | 16 | 14 | 14 | 2.46 | ||||||||||||||||||||||
| Putamen | L | −16 | 10 | −2 | 3.32 | −20 | 4 | −2 | 2.55 | |||||||||||||||||
| R | 26 | 6 | 2 | 2.36 | ||||||||||||||||||||||
| Nucleus accumbens | L | −12 | 4 | −10 | 3.22 | −10 | 14 | −4 | 3.44 | |||||||||||||||||
| R | 12 | 6 | −8 | 3.19 | 6 | 14 | −4 | 3.37 | ||||||||||||||||||
| Medial Prefrontal | L | 10 | 0 | 60 | 2 | 3.02 | ||||||||||||||||||||
| R | ||||||||||||||||||||||||||
| Orbitofrontal | L | 11 | −26 | 24 | −18 | 2.99 | ||||||||||||||||||||
| R | 11 | 14 | 52 | −14 | 3.15 | |||||||||||||||||||||
| Inferior frontal gyrus | L | |||||||||||||||||||||||||
| R | 47 | 32 | 32 | −14 | 2.72 | |||||||||||||||||||||
| Middle frontal gyrus | L | 8 | −28 | 26 | 46 | 3.01 | 6/8 | −30 | 8 | 42 | 3.21 | |||||||||||||||
| R | ||||||||||||||||||||||||||
| Precentral gyrus | L | 4 | −40 | −8 | 60 | 3.08 | 6 | −38 | 0 | 30 | 3.47 | |||||||||||||||
| R | ||||||||||||||||||||||||||
| Frontal Pole | L | |||||||||||||||||||||||||
| R | 10/11 | 14 | 50 | −14 | 3.03 | 10 | 30 | 64 | 6 | 2.99 | 10 | 28 | 54 | −12 | 2.79 | |||||||||||
| Cingulate | ||||||||||||||||||||||||||
| Anterior | 32/24 | 2 | 42 | −4 | 3.12 | |||||||||||||||||||||
| Paracingulate | 32 | −6 | 38 | −16 | 2.98 | |||||||||||||||||||||
| Posterior | 31 | 0 | −48 | 46 | 3.26 | 31 | −2 | −34 | 34 | 3.44 | ||||||||||||||||
| Subcallosal | L | 25/11 | −2 | 26 | −18 | 3.10 | 32 | −8 | 36 | −12 | 3.03 | |||||||||||||||
| R | ||||||||||||||||||||||||||
| Occipital cortex | 19 | 30 | −72 | 38 | 2.84 | |||||||||||||||||||||
| Superior parietal lobule | L | 7 | 24 | −56 | 64 | 3.40 | ||||||||||||||||||||
| R | 7 | 36 | −46 | 60 | 3.26 | |||||||||||||||||||||
| Superior temporal gyrus | L | |||||||||||||||||||||||||
| R | 41 | 58 | −32 | 20 | 3.10 | |||||||||||||||||||||
BA refers to putative Brodmann’s Area; L and R refer to left and right hemispheres; x, y, and z refer to the left–right, anterior–posterior, and inferior–superior dimensions, respectively; Z refers to the Z-score at those coordinates (local maxima or submaxima). Cluster corrected for multiple comparisons, Z>1.96, P<0.05.
Between-Group Analyses
Although there were qualitative differences between the TD and ASD children in the VS response to MR, the between-group comparison (TD>ASD) was not significant (Z=1.84, P=0.66). The converse contrast (ASD>TD) revealed a significant difference in paracingulate cortex and OFC (Table IV). These results suggest increased activity in prefrontal cortex may compensate for a blunted VS response to MR in children with ASD.
Social Reward Response
Within-group analyses
The social feedback run was analyzed in an identical manner to the monetary feedback run. As seen in Figure 3, the contrast between positive SR (i.e., smiling face) and negative SR (i.e., sad face) revealed a significant cluster of activity in VS in the TD group only. In addition to the VS response, there were additional clusters in regions associated with processing socially relevant information including medial prefrontal cortex and superior temporal gyrus. Additional regions are listed in Table IV. Neither group showed significant activation in the contrast of correct vs. incorrect SN feedback. A contrast between positive SR and positive SN expression feedback revealed a significant cluster in pregenual cingulate cortex (Z=3.19, P<0.05 cluster corrected, MNI coordinates: 8, 38, 8) in the TD group only (Fig. 4), consistent with an increased subjective value for social reward feedback in this group.
Figure 4.
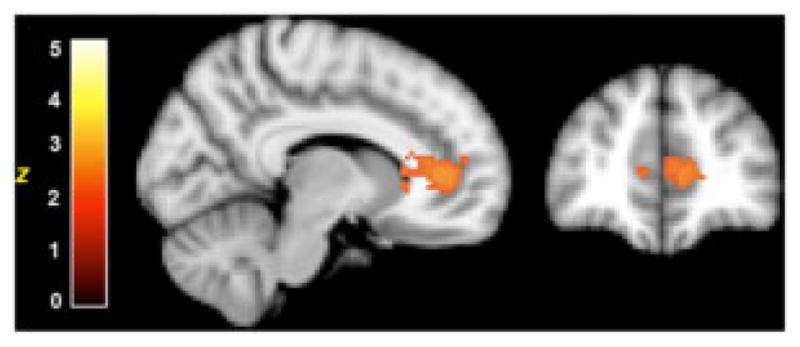
Outcome valuation signal in TD children for social rewards. MNI maximum coordinates 8, 38, 8; Z=3.19, P<0.05 cluster corrected.
Between-group analyses
Between-group comparisons on the positive SR compared to negative SR (i.e., smiling vs. frowning face) revealed significantly greater activity in bilateral VS in TD compared to ASD children (right VS magnitude=P<0.005, 75 voxels; left VS magnitude=P<0.005, 41 voxels). There were no regions of activation for the converse contrast (ASD>TD). This indicates a significantly reduced neural response to SR, such as smiling faces, in the VS in children with ASD.
Children With ASD Show Decreased Frontostriatal Activity During Social Rewarded Learning
We then investigated the neural correlates of reward-guided learning. Feedback for trials that were 100% predictive (deterministic) and trials that predicted an outcome 50% of the time (random) were analyzed separately. This allowed us to investigate the effect of instructive feedback processing (i.e., feedback which can guide learning of stimulus–outcome associations) via correct deterministic trials, as opposed to random positive feedback. See Table V for a list of significant activation clusters.
Table V.
Neural Responses to Reward-Based Learning: MNI Anatomical Coordinates for Instructive Feedback (Deterministic Positive Rewards as Compared to Rest) for Monetary and Social Runs by Group
| Anatomical region | Monetary positive deterministic rewards>rest |
Social positive deterministic rewards>rest |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TD |
ASD |
TD |
ASD |
TD>ASD |
||||||||||||||||||||||
| BA | x | y | z | Z | BA | x | y | z | Z | BA | x | y | z | Z | BA | x | y | z | Z | BA | x | y | z | Z | ||
| Caudate | L | −8 | 16 | 0 | 2.52 | −14 | 18 | 6 | 2.97 | |||||||||||||||||
| R | 8 | 18 | 0 | 2.53 | 16 | 4 | 12 | 2.75 | ||||||||||||||||||
| Putamen | L | −26 | 14 | 0 | 2.60 | |||||||||||||||||||||
| R | 32 | −16 | 2 | 3.67 | 20 | 10 | −2 | 2.29 | ||||||||||||||||||
| Nucleus accumbens | L | −8 | 10 | −8 | 2.23 | |||||||||||||||||||||
| R | ||||||||||||||||||||||||||
| Orbitofrontal | L | 47 | −24 | 10 | −18 | 3.19 | ||||||||||||||||||||
| R | 47 | 38 | 24 | −18 | 3.18 | |||||||||||||||||||||
| Inferior frontal gyrus | L | |||||||||||||||||||||||||
| R | 45 | 52 | 26 | 14 | 2.91 | |||||||||||||||||||||
| Middle frontal gyrus | L | 9 | −42 | 16 | 36 | 3.13 | ||||||||||||||||||||
| R | ||||||||||||||||||||||||||
| Precentral gyrus | L | 4 | −44 | 8 | 34 | 3.03 | ||||||||||||||||||||
| R | 4 | 34 | −24 | 42 | 3.06 | 4 | 48 | 2 | 30 | 3.48 | ||||||||||||||||
| Frontal Pole | L | 10 | −16 | 66 | 20 | 2.29 | ||||||||||||||||||||
| R | 10/11 | 44 | 50 | 2 | 3.33 | 10 | 30 | 50 | −6 | 3.32 | ||||||||||||||||
| Hippocampus/Parahippocampal gyrus | L | |||||||||||||||||||||||||
| R | 35 | 24 | −6 | −26 | 2.35 | 30 | −20 | −14 | 2.44 | |||||||||||||||||
| Cingulate | ||||||||||||||||||||||||||
| Anterior | 24 | −2 | 38 | 6 | 3.39 | 24 | −4 | 36 | 12 | 3.23 | ||||||||||||||||
| Paracingulate | 32 | 6 | 44 | 24 | 3.09 | |||||||||||||||||||||
| Posterior | 31 | 2 | −22 | 46 | 3.08 | |||||||||||||||||||||
| Subcallosal | L | |||||||||||||||||||||||||
| R | 32 | −2 | 24 | −12 | 2.41 | |||||||||||||||||||||
| Insula | L | |||||||||||||||||||||||||
| R | ||||||||||||||||||||||||||
| Fusiform gyrus | L | 37 | −20 | −74 | −10 | 4.03 | 37 | −38 | −68 | −18 | 3.94 | 37 | −42 | −50 | −20 | 3.46 | 37 | −32 | −76 | −12 | 3.99 | −26 | 18 | −10 | 3.45 | |
| R | 37 | 34 | −68 | −14 | 4.16 | 37 | 26 | −70 | −16 | 3.84 | 37 | 42 | −52 | −18 | 3.61 | 37 | 20 | −12 | −12 | 3.74 | ||||||
| Occipital cortex | L | 17 | 2 | −92 | 6 | 4.18 | 18 | −22 | −96 | −8 | 3.59 | |||||||||||||||
| R | 34 | −66 | 46 | 3.68 | 18 | 8 | −94 | 16 | 3.57 | |||||||||||||||||
| Inferior parietal lobule | L | 7 | −46 | −52 | 50 | 3.44 | ||||||||||||||||||||
| R | ||||||||||||||||||||||||||
| Supramarginal gyrus | L | 40 | −44 | −52 | 50 | 3.09 | ||||||||||||||||||||
| R | 40 | 50 | −46 | 50 | 2.91 | |||||||||||||||||||||
BA refers to putative Brodmann’s Area; L and R refer to left and right hemispheres; x, y, and z refer to the left–right, anterior–posterior, and inferior–superior dimensions, respectively; Z refers to the Z-score at those coordinates (local maxima or submaxima). Cluster corrected for multiple comparisons, Z>1.96, P<0.05.
Monetary Reward-Related Learning
Within-group analyses
Within-group analysis of positive MR feedback for deterministic trials compared to inter-trial rest revealed activity in regions involved in both reward processing, such as VS and OFC, as well as regions involved in learning and memory, including right hippocampus and putamen in TD children. For the same contrast, the ASD group also showed significant clusters in the right hippocampus, frontal regions including IFG and MFG, and the inferior parietal lobule. Despite the apparent differences in activity across groups as revealed by the within-group comparisons, direct statistical contrasts between the two groups indicated no significant between-group differences in the neural response to positive monetary rewarded feedback for deterministic trials.
Social Reward-Related Learning
Within-group analyses
Analysis of positive SR feedback for deterministic trials within the TD group revealed involvement of bilateral anterior caudate head, rostral ACC and FG, supporting recruitment of implicit learning and reward-processing networks. Conversely, for the same contrast the ASD group demonstrated only FG and lateral occipital cortex activity, suggesting only basic processing of the stimulus features for this type of feedback. These results may point to a reliance on different processing strategies between TD and ASD children, such that TD children show activity in canonical implicit learning and reward regions such as VS, dorsal striatum, and PFC, whereas ASD children primarily utilize visual processing regions without showing activity in reward-related regions such as the VS.
Between-group analyses
A between-group comparison demonstrated significantly greater activity in ACC, ventral PFC and striatum for TD as compared to ASD children. There were no significant regions of activation for the converse contrast (ASD>TD). The between-group comparisons are consistent with the hypothesis that the TD, but not ASD, children engage frontostriatal networks during socially rewarded learning (see Fig. 5), without evidence of compensatory activity in the ASD children outside of canonical rewarded-learning networks.
Figure 5.
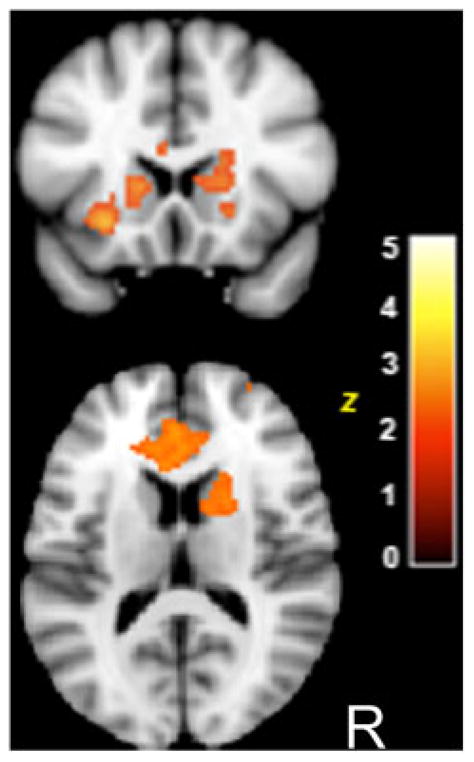
Between-group differences in response to socially rewarded learning trials. TD>ASD deterministic social rewards vs. rest. MNI coordinates, z=14. Z>1.96, P<0.05 cluster corrected.
VS Response to SR Relates to the Level of Social Functioning
To test the hypothesis that response to positive social feedback relates to the development of social reciprocity, we examined the relationship between neural responses in the VS to different reward types and level of social functioning as measured by the SRS [Constantino et al., 2003]. We hypothesized that poorer social reciprocity would predict lower VS response to SR. The SRS is a continuous measure of social behaviors for use in typical and atypical populations. Poorer social functioning is reflected in a higher score. In our sample, the mean TD score was 12.4±8.7 and the mean ASD score was 103±26.4. A regression masked by the combined TD and ASD mean activation to positive SR>negative SR was conducted within the TD and ASD groups on the response to positive deterministic rewards as compared to rest for each run. A significant negative correlation between the SRS score and the response to positive deterministic SR was seen only in the TD group in dorsal striatum (Z=3.14; MNI coordinates=14, 8, 4), VS (Z=2.69; MNI coordinates=14, 22, −4), precuneus (Z=2.99; MNI coordinates=4, −56, 14), and the right temporal–parietal junction (Z=2.65; MNI coordinates=50, −30, 28). No significant correlations were observed for positive deterministic MR for TD children, nor for ASD children for either feedback condition, suggesting a specific relationship between VS responsiveness to SR and levels of social reciprocity in TD children (see Fig. 6). Though the absence of a relationship between SRS and VS response to SR in children with ASD could also be due to the lack of significant activation in the VS in these subjects, the Bartlett test of homogeneity of variances between the TD and ASD children on the amount of VS activity was non-significant (P=0.257).
Figure 6.
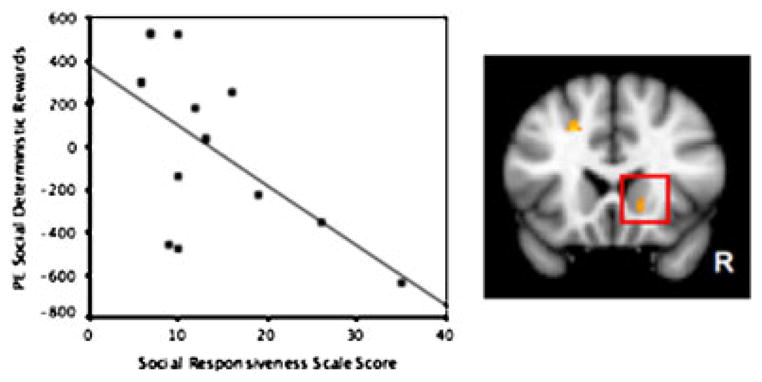
Ventral striatum response to positive social feedback relates to a measure of social responsiveness in TD children. Negative correlation between SRS and parameter estimates (PE) in ventral striatum adjacent to nucleus accumbens for deterministic social rewards in TD children. Z = 2.68, P<0.005; Kendall’s rank correlation PEsocial, τ = −0.53, P = 0.007; PEmoney, τ = −0.31, P = 0.11.
Discussion
In this study, we found that children with ASD show both a behavioral impairment in implicit learning, as well as a reduced neural response to SR and socially rewarded learning in canonical reward-processing brain regions. First we examined whether TD and ASD children were able to implicitly learn the stimulus–outcome associations. TD children demonstrated significant learning over the course of the paradigm for both neutral and rewarded trials within both the social and monetary tasks. However, classification accuracy within the ASD group remained near chance for rewarded and neutral trials for the duration of both social and monetary tasks. These findings indicate impaired implicit learning in ASD, independent from reward processing. Next, we examined the neural response to rewards, independent of learning, by examining responses to both random and deterministic rewards. Although significant VS activity for both monetary and SR was observed in TD, but not ASD, children, significant between-group differences were found only for SR, indicating reduced neural responses to SR in children with ASD. We then investigated whether there were differences in the networks associated with rewarded learning between ASD and TD children. Again, we found that children with ASD showed significantly reduced activation of frontostriatal networks relative to TD children during socially rewarded learning. Finally, we examined the degree to which the neural response to reward was related to measures of social functioning and found a positive correlation in the VS in TD children only, such that better social functioning was related to greater activity in the VS in response to positive social feedback.
Our goal was to test the social motivation hypothesis by examining rewarded learning within monetary and social contexts in children with ASD. Using traditional behavioral measures, such as looking preferences, the reward response to social stimuli in autism is difficult to assess. With fMRI we were able to investigate both the behavioral and neural correlates of rewarded learning in autism. Our findings are consistent with the prediction that children with ASD do not find social stimuli rewarding, as evidenced by reduced neural responses to SR in regions associated with reward processing. When we examined the general neural response to monetary and social reward events, we discovered that only TD children showed VS activity for both reward types, whereas ASD children did not demonstrate a significant response to either monetary or SR. However, significant between-group differences were shown only for SR, suggesting that children with ASD may be specifically impaired on processing SR. These findings are consistent with the behavioral evidence that children with autism do not find social stimuli rewarding. Furthermore, comparisons between positive reward feedback and positive neutral feedback indicate a strong valuation signal in pregenual cingulate cortex in TD children specifically in response to SR. This suggests that during typical development, positive social feedback may be particularly salient and have a high intrinsic reward value [de Araujo, Kringelbach, Rolls, & McGlone, 2003; Hare, O’Doherty, Camerer, Schultz, & Rangel, 2008; Plassmann, O’Doherty, Shiv, & Rangel, 2008]. This signal was not seen for either reward type in ASD children, nor for MR in TD children, thus further supporting the hypothesis of abnormal social reward processing in children with ASD.
As there is a demonstrated relationship between learning and reward, we also examined the neural correlates of instructive feedback processing (i.e., feedback which can guide learning of stimulus–outcome associations) via correct deterministic reward trials (i.e. trials in which the stimulus–outcome association are constant). At the behavioral level, TD children were able to learn the stimulus–outcome associations for both reward types, whereas the children with ASD were unable to learn the associations during the task and overall accuracy remained near chance. Post-test data confirmed that neither group explicitly memorized the stimulus–outcome associations. These behavioral results indicate impaired implicit learning in children with ASD, though this is largely an unexplored area in the field. To control for this difference in learning between the ASD and TD children, an estimate of each child’s learning rate was included as a covariate in all between-group analyses. At the neural level, TD children demonstrated activity in networks involved in reward processing and implicit learning, including dorsal and VS, frontal cortices, and hippocampus for both monetary and social tasks. Children with ASD demonstrated activity in these regions during the monetary-rewarded learning task but not during the social condition. Between-group comparisons revealed greater frontostriatal activity for TD than ASD children for social reward learning trials. Together, the between-group differences in basic reward processing and rewarded learning specifically within the social context supports impaired socially rewarded learning in children with ASD. Thus, this finding provides empirical support for the social motivation hypothesis of autism [Dawson, Meltzoff, Osterling, Rinaldi, & Brown, 1998a; Dawson et al., 2004, 2005; Schultz, 2005] which posits that decreased reward value of social stimuli in children with ASD can negatively impact the development of social behaviors.
Finally, we investigated whether activity in the reward system, particularly the response to SR, related to the children’s level of social functioning. We found a relationship between a previously validated measure of reciprocal social behaviors [SRS; Constantino et al., 2003] and the amount of VS activity for SR that guide learning in TD children only. This correlation was not seen in children with ASD, supporting the hypothesis that appropriate response to SR, especially those that inform learning, are related to the development of social skills in children. The lack of a correlation in the ASD group may also reflect decreased variance in the percent signal change due to the overall small amount of activity observed in the VS. Notably, however, our findings are not likely due to a failure on the part of the children with ASD to attend to the task, as there was activation of similar neural networks for all events (as compared to rest) across groups, as well as comparable RT data. Furthermore, equivalent amounts of activity in fusiform cortices during trials involving the presentation of facial expressions suggest that differences between groups during processing of social feedback are not likely due to a failure of ASD children to attend to or process the faces.
One previous fMRI study examined reward responsiveness in adults with ASD using MR and did not find between-group differences in reward-related areas such as VS or OFC [Schmitz et al., 2008]. By also examining responses to SR, we were able to reveal significant reduction in reward circuitry activity during processing of SR in children with ASD. Our findings are also consistent with previous structural [Haznedar et al., 2006; Hollander et al., 2005; Kates et al., 1998; Langen, Durston, Staal, Palmen, & van Engeland, 2007; Sears et al., 1999] and functional [Haznedar et al., 2006; Takarae, Minshew, Luna, & Sweeney, 2007] MRI studies demonstrating abnormalities in the striatum in individuals with autism. However, it is unclear to what extent these functional and structural differences are primary or secondary to social abnormalities. While the current study is limited in the ability to draw conclusions about causality, the evidence supporting early differences in social motivation and response to rewarding social stimuli in children with autism are suggestive of abnormal function and structure early in life contributing to the development of abnormal social behaviors. This hypothesis will need to be addressed in younger cohorts in future studies. An additional limitation of our study is the potential confound due to the medication status of nine of our participants with ASD. However, when we examined activity in our primary region of interest, the VS, we did not find evidence for an association between medication status and activity in this region. In fact, unmedicated children demonstrated some of the lowest VS responses. The children in this study were primarily taking psychostimulants, which have demonstrated no effects on the hemodynamic response [Rao et al., 2000] and antipsychotics, which have been shown to normalize BOLD responses [Lencz et al., 2000; Schlosser et al., 2003; Snitz et al., 2005]. Thus, the use of medication in several ASD participants may have actually decreased group differences between ASD and TD children. However, both stimulants and antipsychotics act on the dopamine system, albeit in opposite directions, which is one of the neurotransmitters involved in reward signaling. As such, the presence of these medications may alter the neural response to reward. Future studies on reward processing controlling for the effects of medication should be pursued.
By placing rewards within an implicit learning paradigm, we were able to examine the relationship between reward processing and implicit learning, functions which have been shown to rely on neighboring frontostriatal networks [Shohamy, Myers, Kalanithi, & Gluck, 2008]. As suggested by computational models, impaired implicit learning may have repercussions for the development of social behaviors such as joint attention [Triesch et al., 2006]. A previous investigation by Mostofsky et al. [2000] found impaired procedural learning—a type of implicit learning—in individuals with ASD which the authors interpreted as reflective of cerebellar dysfunction. Our behavioral findings show an implicit learning deficit in children with ASD, providing additional evidence of impaired implicit learning in this population. However, future studies should be pursued to better characterize the nature of this impairment. Behaviors that arise from rewarded learning trials are often conceptualized in terms of classical conditioning [Pavlov, 1927] and modeled as a function of a prediction error, that is, the difference between expected rewards and actual reward receipt. For instance, the Rescorla–Wagner learning model [Rescorla & Wagner, 1972] presumes that prediction error estimates will converge towards 0 irrespective of accuracy in a deterministic context, effectively assuming that subject accuracy is not dependent on the prediction error. Currently, there are no data that investigate the applicability of this model for learning impaired subjects, and for this reason we did not employ that prediction error model. Future studies should further examine implicit learning and prediction error in individuals with ASD.
Our investigation into the neural correlates of rewarded learning in ASD is a direct test of the social motivation hypothesis of autism. In at least some animals, it appears that social stimuli serve as important primary rewards that influence behaviors important for survival. For example, the same neural networks involved in other forms of reward processing (e.g., food, drugs, etc.), underlie social processes such as pair-bonding [Young, Murphy Young, & Hammock, 2005; Young & Wang, 2004] and mother–offspring bonding [Levy, Kendrick, Goode, Guevara-Guzman, & Keverne, 1995] in small rodents [Febo, Numan, & Ferris, 2005] and voles [Young et al., 2005]. Impaired reward processing and learning may be the underlying factor for the abnormal development of some social behaviors in children with ASD, and targeting brain regions involved in social rewarded learning for possible therapeutic intervention in this population may prove to be a valuable early treatment approach. For example, oxytocin, a neurohypophyseal hormone linked to pro-social behaviors, has a high density of receptors within the nucleus accumbens. Administration of this neurohormone to individuals with ASD has been shown to decrease repetitive behaviors [Hollander et al., 2003] and increase affective speech comprehension [Hollander et al., 2007]. Furthermore, oxytocin administration has been shown to modulate BOLD activity in regions associated with social cognition and reward in human [Kirsch et al., 2005] and rodent [Febo et al., 2005]. Conversely, our results may reflect differences in the structural integrity of a distributed reward processing and learning network, in which case future studies should examine the developmental trajectory of these structures and their connectivity. Our data would suggest that increasing reward responsiveness in ASD, perhaps through pharmacotherapy, might augment social learning. Future studies may investigate the degree to which manipulating VS activity affects social responsiveness, and ultimately autistic symptomatology, in children with autism spectrum disorders.
Acknowledgments
This work was in part supported by grants from the NICHD (PO1 HD035470 and NIH/NICHO 1P50 HD055784; 1R01 HD065280-01; UL1 RR025774), the National Alliance for Autism Research, Autism Speaks, Whitehall Foundation, as well as by the Training Program in Neurobehavioral Genetics (T32 MH073526), and a NRSA predoctoral fellowship (F31 MH079645) and Dickinson Fellowship to Ashley Scott-Van Zeeland. The authors wish to thank J. Cohen for her technical assistance. For generous support the authors also wish to thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family and Northstar Fund. The project described was in part also supported by grants (RR12169, RR13642 and RR00865) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCR or NIH.
Grant sponsors: National Alliance for Autism Research; Autism Speaks; Whitehall Foundation; Brain Mapping Medical Research Organization; Brain Mapping Support Foundation; Pierson-Lovelace Foundation; Ahmanson Foundation; Northern Piedmont Community Foundation; Tamkin Foundation; Jennifer Jones-Simon Foundation; Capital Group Companies Charitable Foundation; Robson Family and Northstar Fund; NICHD; Grant number: POI HD035470; Grant sponsor: The National Center for Research Resources; Grant numbers: RR12169; RR13642; RR00865; Grant sponsor: Training Program in Neurobehavioral Genetics; Grant number: T32 MH073526; Grant sponsor: NRSA; Grant number: F31 MH079645; Grant sponsor: NIH/NICHD; Grant numbers: 1P50 HD055784; 1R01 HD065280-01; Grant sponsors: Dickinson Fellowship; NIH; Grant number: UL1 RR025774.
References
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Bray S, O’doherty J. Neural coding of reward-prediction error signals during classical conditioning with attractive faces. Journal of Neurophysiology. 2007;97:3036–3045. doi: 10.1152/jn.01211.2006. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of Autism and Developmental Disorders. 2003;33:427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders. 1998a;28:479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of autism and developmental disorders. 1998b;28:479. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, et al. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Developmental Psychology. 2004;40:271. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Developmental Neuropsychology. 2005;27:403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- de Araujo IET, Kringelbach ML, Rolls ET, McGlone F. Human cortical responses to water in the mouth, and the effects of thirst. Journal of Neurophysiology. 2003;90:1865–1876. doi: 10.1152/jn.00297.2003. [DOI] [PubMed] [Google Scholar]
- Febo M, Numan M, Ferris CF. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. Journal of Neuroscience. 2005;25:11637–11644. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Frith CD. Social cognition. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2008;363:2033–2039. doi: 10.1098/rstb.2008.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grelotti DJ, Gauthier I, Schultz RT. Social interest and the development of cortical face specialization: what autism teaches us about face processing. Developmental Psychobiology. 2002;40:213. doi: 10.1002/dev.10028. [DOI] [PubMed] [Google Scholar]
- Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. Journal of Neuroscience. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Hazlett EA, LiCalzi EM, Cartwright C, Hollander E. Volumetric analysis and three-dimensional glucose metabolic mapping of the striatum and thalamus in patients with autism spectrum disorders. American Journal of Psychiatry. 2006;163:1252–1263. doi: 10.1176/ajp.2006.163.7.1252. [DOI] [PubMed] [Google Scholar]
- Hollander E, Anagnostou E, Chaplin W, Esposito K, Haznedar MM, et al. Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biological Psychiatry. 2005;58:226. doi: 10.1016/j.biopsych.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, et al. Oxytocin increases retention of social cognition in autism. Biological Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, et al. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger’s disorders. Neuropsychopharmacology. 2003;28:193–198. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kates WR, Mostofsky SH, Zimmerman AW, Mazzocco MMM, Landa R, et al. Neuroanatomical and neurocognitive differences in a pair of monozygous twins discordant for strictly defined autism. Annals of Neurology. 1998;43:782–791. doi: 10.1002/ana.410430613. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. Journal of Neuroscience. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A. Young autistic children’s listening preferences in regard to speech: a possible characterization of the symptom of social withdrawal. Journal of Autism and Developmental Disorders. 1991;21:29–42. doi: 10.1007/BF02206995. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR, Gluck MA. Probabilistic classification learning in amnesia. Learning & Memory. 1994;1:106–120. [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Current Opininon in Neurology. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Coffey-Corina S, Padden D, Dawson G. Links between social and linguistic processing of speech in preschool children with autism: behavioral and electrophysiological measures. Developmental Science. 2005;8:F1–F12. doi: 10.1111/j.1467-7687.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- Langen M, Durston S, Staal WG, Palmen SJMC, van Engeland H. Caudate nucleus is enlarged in high-functioning medication-naive subjects with autism. Biological Psychiatry. 2007;62:262. doi: 10.1016/j.biopsych.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Legerstee M, Anderson D, Schaffer A. Five- and eight-month-old infants recognize their faces and voices as familiar and social stimuli. Child Development. 1998;69:37–50. [PubMed] [Google Scholar]
- Lencz T, Bilder RM, Ashtari M, Szeszko PR, Gunduz H, et al. Atypical antipsychotic effects on fMRI in drug-naive schizophrenia. Biological Psychiatry. 2000;47:S99–S99. [Google Scholar]
- Levy F, Kendrick KM, Goode JA, Guevara-Guzman R, Keverne EB. Oxytocin and vasopressin release in the olfactory bulb of parturient ewes: changes with maternal experience and effects on acetylcholine, gamma-aminobutyric acid, glutamate and noradrenaline release. Brain Research. 1995;669:197–206. doi: 10.1016/0006-8993(94)01236-b. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Goldberg MC, Landa RJ, Denckla MB. Evidence for a deficit in procedural learning in children and adolescents with autism: implications for cerebellar contribution. Journal of the International Neuropsychological Society. 2000;6:752–759. doi: 10.1017/s1355617700677020. [DOI] [PubMed] [Google Scholar]
- Olsson A, Phelps EA. Learned fear of “unseen” faces after pavlovian, observational, and instructed fear. Psychological Science. 2004;15:822–828. doi: 10.1111/j.0956-7976.2004.00762.x. [DOI] [PubMed] [Google Scholar]
- Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. Journal of Autism and Developmental Disorders. 1994;24:247–257. doi: 10.1007/BF02172225. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. London: Oxford University Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey K, Adolphs R, Morris JP. Neuroanatomical substrates of social cognition dysfunction in autism. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10:259–271. doi: 10.1002/mrdd.20040. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Bullmore ET, Howard R, Woodruff PW, Wright IC, et al. Investigation of facial recognition memory and happy and sad facial expression perception: an fMRI study. Psychiatry Research. 1998;83:127–138. doi: 10.1016/s0925-4927(98)00036-5. [DOI] [PubMed] [Google Scholar]
- Plassmann H, O’Doherty J, Shiv B, Rangel A. Marketing actions can modulate neural representations of experienced pleasantness. Proceedings of the National Academy of Sciences. 2008;105:1050–1054. doi: 10.1073/pnas.0706929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Salmeron BJ, Durgerian S, Janowiak JA, Fischer M, et al. Effects of methylphenidate on functional MRI blood-oxygen-level-dependent contrast. American Journal of Psychiatry. 2000;157:1697–1699. doi: 10.1176/appi.ajp.157.10.1697. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. New York: Appleton Century Crofts; 1972. [Google Scholar]
- Schlosser R, Gesierich T, Kaufmann B, Vucurevic G, Hunsche S, et al. Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. Neuroimage. 2003;19:751–763. doi: 10.1016/s1053-8119(03)00106-x. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Rubia K, van Amelsvoort T, Daly E, Smith A, Murphy DG. Neural correlates of reward in autism. British Journal of Psychiatry. 2008;192:19–24. doi: 10.1192/bjp.bp.107.036921. [DOI] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience. 2005;23:125. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Archives of General Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Sears LL, Vest C, Mohamed S, Bailey J, Ranson BJ, Piven J. An MRI study of the basal ganglia in autism. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1999;23:613. doi: 10.1016/s0278-5846(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Kalanithi J, Gluck MA. Basal ganglia and dopamine contributions to probabilistic category learning. Neuroscience and Biobehavioral Reviews. 2008;32:219–236. doi: 10.1016/j.neubiorev.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, MacDonald A, III, Cohen JD, Cho RY, Becker T, Carter CS. Lateral and medial hypofrontality in first-episode schizophrenia: functional activity in a medication-naive state and effects of short-term atypical antipsychotic treatment. American Journal of Psychiatry. 2005;162:2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, Rademacher L, Irmak A, et al. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience. 2009 doi: 10.1093/scan/nsn051. %R 10.1093/scan/nsn051: nsn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takarae Y, Minshew NJ, Luna B, Sweeney JA. Atypical involvement of frontostriatal systems during sensorimotor control in autism. Psychiatry Research: Neuroimaging. 2007;156:117. doi: 10.1016/j.pscychresns.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Schultz W, Roelcke U, Nienhusmeier M, Missimer J, et al. Activation of the human brain by monetary reward. Neuroreport. 1997;8:1225–1228. doi: 10.1097/00001756-199703240-00033. [DOI] [PubMed] [Google Scholar]
- Triesch J, Teuscher C, Deak GO, Carlson E. Gaze following: why (not) learn it? Developmental Science. 2006;9:125–147. doi: 10.1111/j.1467-7687.2006.00470.x. [DOI] [PubMed] [Google Scholar]
- Wager TD, Nichols TE. Optimization of experimental design in fMRI: a general framework using a genetic algorithm. Neuroimage. 2003;18:293–309. doi: 10.1016/s1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children. 3. San Antonio, TX: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nature Neuroscience. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Young LJ, Murphy Young AZ, Hammock EA. Anatomy and neurochemistry of the pair bond. The Journal of Comparitive Neurology. 2005;493:51–57. doi: 10.1002/cne.20771. [DOI] [PubMed] [Google Scholar]


