Abstract
Cospeciation occurs when interacting groups, such as hosts and parasites, speciate in tandem, generating congruent phylogenies. Cospeciation can be a neutral process in which parasites speciate merely because they are isolated on diverging host islands. Adaptive evolution may also play a role, but this has seldom been tested. We explored the adaptive basis of cospeciation by using a model system consisting of feather lice (Columbicola) and their pigeon and dove hosts (Columbiformes). We reconstructed phylogenies for both groups by using nuclear and mitochondrial DNA sequences. Both phylogenies were well resolved and well supported. Comparing these phylogenies revealed significant cospeciation and correlated evolution of host and parasite body size. The match in body size suggested that adaptive constraints limit the range of hosts lice can use. We tested this hypothesis by transferring lice among hosts of different sizes to simulate host switches. The results of these experiments showed that lice cannot establish viable populations on novel hosts that differ in size from the native host. To determine why size matters, we measured three components of louse fitness: attachment, feeding, and escape from host defense (preening). Lice could remain attached to, and feed on, hosts varying in size by an order of magnitude. However, they could not escape from preening on novel hosts that differed in size from the native host. Overall, our results suggest that host defense reinforces cospeciation in birds and feather lice by preventing lice from switching between hosts of different sizes.
Cospeciation can yield congruent phylogenies in the absence of selection when organisms with limited powers of dispersal, such as endosymbiotic bacteria, repeatedly diverge in parallel with host populations (1). Congruence is more difficult to explain in the case of organisms with greater mobility, such as herbivorous insects (2) or vertebrate endoparasites and ectoparasites (3–7). Host-imposed selection may reinforce cospeciation in these groups by reducing the probability of host switching. Under this scenario of adaptive cospeciation, parasites dispersing to novel hosts suffer reduced fitness, compared to those remaining on the native host. Host chemical defense and the impact of host species on the risk of parasitoid attack are both thought to influence host use in herbivorous insects (8, 9). However, the adaptive significance of these and other possible determinants of host use have seldom been demonstrated in rigorous detail.
Another factor that may influence host use is a match between host and parasite body size. Cross species correlations of host and parasite body size have been documented for a wide variety of taxa, including parasitic worms, crustaceans, fleas, flies, lice, and ticks, as well as herbivorous aphids, thrips, beetles, flies, moths, and flower mites (3, 5–7, 10–12). The adaptive basis of these correlations has not been tested. Parasites adapted to exploit a particular host size may suffer reduced survival and reproductive success on hosts of the “wrong” size. We reconstructed phylogenies for feather lice (Phthiraptera: Ischnocera) and their hosts and used these phylogenies to document significant cospeciation and matching host–parasite body sizes. We then carried out a series of experiments to test the adaptive basis of the body size match and how this match may reinforce past cospeciation by preventing parasites from switching successfully between hosts of different sizes.
Feather lice are host-specific, permanent ectoparasites of birds that complete their entire life cycle on the body of the host, where they feed largely on abdominal contour feathers (13). Species in the genus Columbicola, which are parasites of pigeons and doves (Columbiformes), are so specialized for life on feathers that they do not venture onto the host's skin (14, 15). Transmission between conspecific hosts occurs mainly during periods of direct contact, like that between parents and their offspring in the nest (16). Columbicola lice can also leave the host by attaching to more mobile parasites, such as hippoboscid flies (15, 17, 18). Because the flies are less specific than the lice (10, 19), this dispersal route may explain records of host-specific Columbicola on the wrong host (15), as well as the presence of identical mtDNA haplotypes of some Columbicola on different host species (e.g., Columbicola adamsi in Fig. 1).
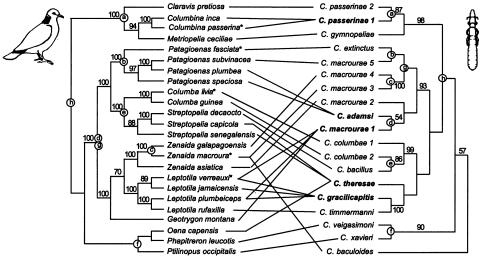
Fig. 1.
Phylogenies of pigeons/doves and their feather lice in the genus Columbicola. Numbers after the names Columbicola passerinae, macrourae, and columbae indicate cryptic species of lice (17). Numbers beside branches are percent of 100-ML bootstrap replicates containing the node; values below 50% are not shown. Thin lines show host–parasite associations (17, 43). Fourteen of the 19 Columbicola species (74%) are host-specific. The remaining five species (bold) occur on two or more species of hosts. Circles with letters show cospeciation events inferred between the host and parasite trees by using reconciliation analysis (44). The eight cospeciation events are more than expected by chance (P = 0.029). Reconciliation analysis of trees derived from parsimony analyses of DNA sequences for doves and lice also inferred eight cospeciation events (P = 0.035). Asterisks show the five host species used in the experimental work.
Dispersal opportunities notwithstanding, most species of Columbicola are restricted to a single species of host (Fig. 1), suggesting that specificity has an adaptive component. We tested whether body size might be such a component, using a combination of comparative and experimental approaches. First, we sequenced mitochondrial and nuclear genes for Columbicola and their hosts and used these data to reconstruct phylogenies for the two groups. Next, we compared the topology of the phylogenies, which revealed a significant amount of congruence, owing to a history of host–parasite cospeciation. We used the phylogenies to calculate phylogenetically independent contrasts, which demonstrated a match in host–parasite body sizes owing to correlated evolution of size. This correlated evolution suggested that size is an adaptive component of specificity that may constrain host use.
To test the impact of size on host use, we transferred lice to novel hosts of different sizes and measured their fitness (survival and reproductive success) compared to that of lice remaining on their native host. We also used an experimental approach to test three often mentioned adaptive hypotheses that could explain the correlated evolution of host and parasite body size. The first hypothesis is that size influences the ability of a parasite to remain attached to its host (7, 12, 20). The second hypothesis is that size influences the ability of the parasite to feed (3, 10, 18). The third hypothesis is that size influences the ability of the parasite to escape from host defense, which is mainly host preening behavior in the case of feather lice (3, 14, 18).
Methods
Host and Parasite Phylogenies. Specific host and parasite taxa for phylogenetic work were chosen based on the availability of fresh samples for DNA sequencing. For doves, total genomic DNA was extracted from muscle tissue, and PCR was used to amplify and sequence the nuclear β-fibrinogen intron 7 (FIB7) gene and portions of the mitochondrial cytochrome b (Cyt b) and cytochrome oxidase I (COI) genes, according to the protocols described by Johnson and Clayton (21) and Johnson et al. (22). For lice, we extracted DNA and prepared slide-mounted voucher specimens as described by Johnson et al. (22). We amplified portions of the nuclear elongation factor 1 alpha (EF-1α) and mitochondrial COI and 12S ribosomal RNA genes by using primers and protocols from Johnson et al. (22). Complementary chromatograms were reconciled by using sequencher (Gene Codes, Ann Arbor, MI), and the sequences were deposited in GenBank (accession nos. AF279704–AF279743, AF190409–AF190426, and AF278608–AF278643), as well as in Johnson and Clayton (21).
All phylogenetic analyses were conducted by using paup* (23). Trees were rooted as described by Johnson et al. (22). For phylogenetic analyses, we first compared the signal in different gene regions for each group by using the partition homogeneity test (23–25). This test revealed no significant incongruence among gene regions for each group, so we combined the data for analysis. To reconstruct phylogenetic trees for doves and lice, we used a maximum-likelihood approach. For both doves and lice, we estimated the simplest model that could not be rejected in favor of a more complex model (26) by using modeltest (27). In both cases, a model incorporating six substitution categories, with unequal base frequencies, invariant sites, and rate variation according to a gamma distribution was selected (GTR + I + G). The parameters from this model were used in 10 random addition replicate heuristic searches to search for the most likely tree. Branch lengths from these analyses were used as branch lengths in the independent contrasts analyses. We also performed bootstrap analysis (28) by using 100 replicates to test the sensitivity of the trees to character resampling. The results of the maximum likelihood analyses are reported herein. We also constructed trees by using unweighted parsimony and, although the trees changed slightly, the conclusions of the cophylogenetic analysis and independent contrast analyses did not change.
Host and Parasite Body Size. We used host body mass as a measure of overall body size. Body mass was calculated as the mean of ≥10 individuals per species taken from museum records or from Dunning (29). As a measure of parasite body size, we used metathoracic width because it is not subject to distortion during specimen preparation and it is significantly correlated with overall body length (r = 0.64, P < 0.0001; unpublished data). Metathoracic width was calculated as the mean of several female specimens per species taken from slide-mounted material or the literature (30).
Phylogenetically independent contrasts of louse size and ln host mass were calculated in caic (31) by using the Columbicola tree (Fig. 1) and maximum-likelihood branch lengths. Contrasts were also calculated for just those nodes associated with inferred cospeciation events (Fig. 1, n = 8), providing simultaneous independence of both host and parasite phylogenies (6, 11).
Feather Size. The adaptive hypotheses tested below assume that host body size is correlated with feather size, as feathers are the relevant substrate for all three performance measures (attachment, feeding, and escape). We therefore also examined the relationship of feather size to overall host body size. We estimated feather size by measuring a fifth primary feather from one individual of 18 of 24 species (Fig. 1) for which we had a feather sample. We measured the diameter of five barbs in the center of each feather (12) by using a computerized video imaging system affixed to a Nikon DIC microscope. We tested the repeatability of our feather size estimates for each species by using the method of Lessels and Boag (32). Overall, our feather size estimates were highly repeatable (r = 0.81; P < 0.001, Fisher's combined probability for 18 host species). The mean of the five measurements per species was used as an index of feather size for each species.
Attachment Experiment. We tested the ability of Columbicola columbae to remain attached to four species of novel hosts, relative to the native host, by grafting sections of feathers from these hosts onto feathers of the native host, the Rock Pigeon (Columba livia). We used a scalpel to remove a 1-cm2 section of feather vane from the fifth primary feather on each wing of a Rock Pigeon. We then grafted a 1-cm2 section from the same feather of another species to one wing, chosen at random. To the opposite wing we grafted a 1-cm2 section from another (control) Rock Pigeon. Grafts were cemented around the periphery with Scribbles three-dimensional paint, which is harmless to lice when dry (unpublished data). The paint formed a ridge that prevented lice from crawling off the graft onto the adjacent feather vane (see Fig. 3a Inset, feather diagram).
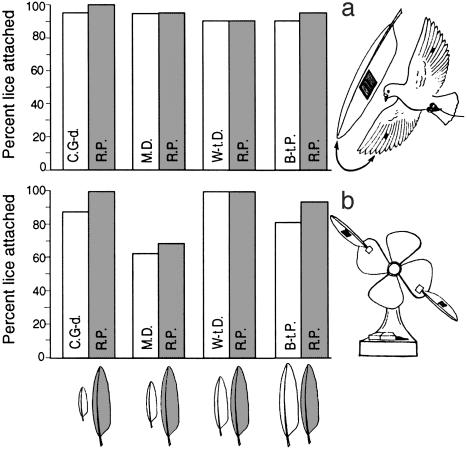
Fig. 3.
Percent of lice remaining attached to grafts from feathers (drawn to scale) of four novel host species, compared to the native host (gray bars and feathers). The host species varied in size by more than an order of magnitude: C.G-d., Common Ground-dove (C. passerina), 30 g; M.D., Mourning Dove (Zenaida macroura), 119 g; W-t.D., White-tipped Dove (Leptotila verreauxi), 153 g; B-t.P., Band-tailed Pigeon (Patagioenas fasciata), 343 g; and the control, R.P., Rock Pigeon (C. livia), 355 g (29). The number of lice remaining attached did not differ significantly on Rock Pigeons allowed to fly a distance of 50–100 m (a) or on feathers taped to a high-speed fan for 20 min (b).
In each trial, two lice were placed on the experimental graft and two lice on the control graft. The Rock Pigeon, attached to a long tether, was released into the air with its wings open and was retrieved before it could close its wings at the end of a 50- to 100-m-long flight. Four Rock Pigeons were used, each with an experimental graft from a different novel host species (10 trials per species × 4 species = 40 trials). The host species varied in size by more than an order of magnitude (Fig. 3 legend).
We also tested the ability of lice to remain attached to novel host feathers by using a high-speed fan. For the fan trials, the experimental and control feathers were removed from the rock pigeons and taped to the blades of the fan (see Fig. 3b Inset, fan diagram). We conducted eight trials per host species, with each trial lasting 20 min on the fan's highest setting (1,260 rpm). Each trial thus simulated a Rock Pigeon flying a distance of 28 km at a velocity of 85 km/h, approximating the velocity of racing pigeons in level flight (80–100 km/h) (33).
Feeding Experiment. We tested the ability of C. columbae to feed on feathers of the four species of novel hosts in vitro. We plucked abdominal contour feathers, the principle diet of this louse (34), from each of the novel host species, as well as from the native host, and put the feathers in 50-ml glass tubes (10 feathers per tube, 18 tubes per host species). The tubes were then placed in a stainless steel lined Percival incubator kept at 33°C and 75% relative humidity on a 12-h light/12-h dark photoperiod (15). After 24 h, the feathers were removed from each tube and weighed to the nearest 0.1 mg three times on an analytical balance, then returned to the tubes. Starting feather mass was taken as the mean value of the three weighings.
Soon after the feathers were weighed, ten haphazardly chosen C. columbae were added to each of 15 of the 18 tubes per host species. The remaining three tubes served as louse-free controls to monitor background changes in feather mass. Lice were obtained from captive “culture” birds by anaesthetizing the lice with CO2 (35). All tubes were returned to the incubator for the duration of the experiment (1 month). At the end of the experiment, the number of live lice in each of the 15 infested tubes was tallied and the lice were removed. The feathers in all 18 tubes were then removed and weighed again three times. Change in feather mass was calculated by comparing the mean mass of feathers at the start of the experiment to mean mass at the end of the experiment.
Escape Experiment. We tested the ability of C. columbae to escape from preening on hosts of different sizes by transferring lice to captive birds with both normal and blocked preening ability. Birds were trapped in the wild and housed individually in 30 × 30 × 56-cm wire mesh cages in our animal facility. They were maintained on a 12-h light/12-h dark photoperiod and provided ad libitum grain (Pigeon Mix, Wheatland Seed, Brigham City, Utah), grit, and water. Natural, background infestations were eliminated by keeping birds at <25% relative humidity for at least 10 weeks after capture (36), which killed all lice and eggs. During the course of the transfer experiments, the relative humidity in the animal rooms was raised to 60–70%.
Preening was blocked by using bits, which are C-shaped pieces of plastic inserted between the upper and lower mandibles of the bill. Bits crimp slightly in the nostrils to prevent dislodging, but without damaging the tissue. They create a 1- to 3-mm gap between the mandibles that impairs the forceps-like action of the bill required for efficient preening. However, bits do not interfere with feeding and they have no apparent side effects (14).
Lice were transferred to the same four novel host species used in the previous experiments. Each recipient bird received 25 C. columbae, which is the equilibrium population size on captive Rock Pigeons with normal preening ability (unpublished data). Lice were obtained from captive culture birds by anaesthetizing the lice with CO2 (35). After a period of 2 months (two louse generations), all birds were euthanized and their louse populations were determined by body washing (37). The populations of lice on birds after 2 months included both adult and nymphal lice; thus, population size incorporated both survival and reproductive components of parasite fitness. Population size is relatively easy to measure in feather lice because they complete their entire life cycle on the body of the host. The total number of lice on each bird (y) was determined as y = (1.10x1/2)2, where x was the number of lice recovered by washing. This regression model, modified from the model of Clayton and Drown (37) by forcing it through the origin, provides an extremely accurate measure of C. columbae populations (r2 = 0.99; P < 0.0001).
Results
Host and Parasite Phylogenies. Maximum likelihood analysis of nuclear and mitochondrial DNA sequences for doves (A-C = 1.388, A-G = 7.69, A-T = 0.86, C-G = 0.70, C-T = 11.11, G-T = 1.00, A = 0.29, C = 0.29, G = 0.16, T = 0.27, proportion invariant sites = 0.49, gamma shape parameter = 0.68) produced a single, completely resolved tree (L = 14,509.04, Fig. 1 Left). Likelihood bootstrap analysis recovered 19 of 21 nodes with >50% bootstrap support.
Maximum-likelihood analysis of nuclear and mitochondrial DNA sequences for lice (A-C = 1.21, A-G = 7.39, A-T = 1.29, C-G = 2.94, C-T = 14.43, G-T = 1.00, A = 0.34, C = 0.15, G = 0.20, T = 0.32, proportion invariant sites = 0.34, gamma shape parameter = 0.37) produced a single, completely resolved tree (L = 9285.04, Fig. 1 Right). Likelihood bootstrap analysis recovered 10 of 17 nodes with >50% bootstrap support. This tree, together with that for the hosts, was used to infer the extent of cospeciation and for the independent contrast analyses.
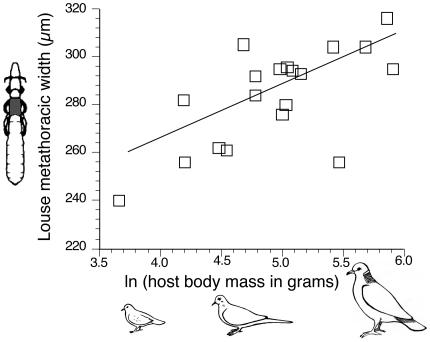
Host and Parasite Body Size. Parasite body size was positively correlated with host body size across species (r = 0.67, n = 19; Fig. 2). Regression through the origin of phylogenetically independent contrasts in louse size against contrasts in host size was highly significant (n = 18, t = 3.97, P = 0.001). When contrasts were restricted to nodes associated with inferred cospeciation events (n = 8), providing reciprocal phylogenetic independence (6), the regression was still significant (t = 2.54, P = 0.039). Regression of contrasts derived from maximum-parsimony trees was also highly significant (t = 4.11, P = 0.0007). Interestingly, the five nonspecific Columbicola (Fig. 1, bold) occurred on hosts that were more similar in size than expected by chance; 8 of 11 host size differences were within the lowest quartile of all possible differences (Wilcoxon signed rank test, P = 0.008).
Fig. 2.
Relationship of parasite body size to host body size across the associations shown in Fig. 1. For the five nonspecific lice (Fig. 1), the mean body mass of their different host species was used. [Reproduced with permission from ref. 45 (Copyright 2003, University of Chicago Press).]
Feather Size. Across host species, feather barb size was correlated with overall body size (r = 0.72, n = 18). This relationship was highly significant when using phylogenetically independent contrasts (t = 3.41, n = 17, P = 0.0035).
Attachment Experiment. There was no significant difference in the number of lice remaining attached to feather grafts of native versus novel species on Rock Pigeons allowed to fly 50–100 m (Fisher's exact test, P = 1.0 for each of four novel species, 10 trials per species). Over 90% of all lice survived the flights, regardless of feather type (Fig. 3a). Attachment was also tested by comparing the survival of lice placed on novel host feathers taped to a high-speed fan for 20 min. Again, the number of lice remaining attached did not differ significantly between novel host feathers and Rock Pigeon controls (P = 0.48 for C.G-d./R.P., 1.0 for M.D./R.P., 1.0 for W-t.D./R.P., and 0.60 for B-t.P./R.P.). Over 60% of all lice survived the 20-min trials, regardless of feather type (Fig. 3b).
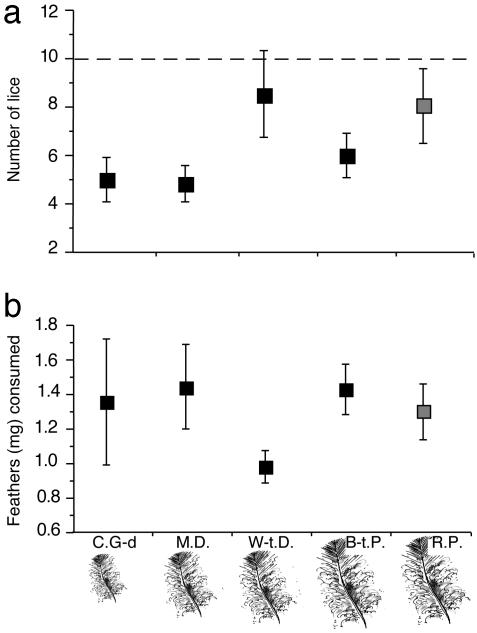
Feeding Experiment. The overall mean (±SE) number of live C. columbae in tubes with feathers was 6.5 (±0.57) after 1 month (Fig. 4a). There was no significant difference in the number of lice among the five host species (ANOVA, F = 1.9, df = 4, P = 0.12; Fig. 4a). Similarly, there was no significant difference in feather mass among host species (F = 0.75, df = 4, P = 0.56; Fig. 4b). All five species showed a substantial decrease in feather mass in tubes containing lice (mean decrease = 6.41 ± 0.26 mg, n = 15 vials per species) compared to control tubes without lice (mean decrease = 0.11 ± 0.03 mg, n = 3 vials per species; F = 41.01, df = 5, P < 0.0001). Decreased feather mass was caused by lice feeding on the feathers. The decreases were accompanied by weekly deposits of frass (louse feces) on the bottoms of all louse-infested tubes. Furthermore, 10 lice placed in each of 15 tubes without any feathers all died within a few days, demonstrating the need for a steady food supply.
Fig. 4.
(a) Number of C. columbae (mean ± SE) after 1 month in tubes containing feathers (drawn to scale) from one of four novel host species (black squares) or the native host (gray squares). Dotted line shows the number of lice placed in each tube at the start of the trial. (b) Mean (±SE) feather mass consumed per louse per tube during the trials. Host abbreviations are as in Fig. 3.
Escape Experiment. Host species and preening treatment each had a significant overall effect on louse population size (Fig. 5, two-way ANOVA, ln transformed data; host species F = 9.3, df = 4, P < 0.0001; preening F = 147.5, df = 1, P < 0.0001). The interaction between host species and preening treatment was also significant (F = 3.2, df = 4, P = 0.022), indicating that the impact of preening on lice depended on the host species to which they were transferred. Comparing lice on each individual (preening) host species further showed that C. columbae populations were lower on small-bodied hosts than on the native host (Fig. 5). In contrast, louse populations on the native host, as well as those on Band-tailed Pigeons, did not change significantly over the course of the experiment (ANOVA, F = 0.095, df = 2, P = 0.91).
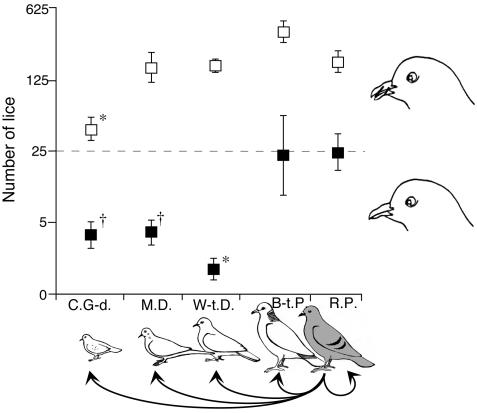
Fig. 5.
Population sizes (mean ± SE) of Rock Pigeon lice (C. columbae) transferred to novel host species, relative to the native host (gray bird). Host abbreviations are as in Fig. 3. Note log scale on y axis. Open squares are bitted birds that could not preen; closed squares are birds with normal preening ability (n = 6 birds per square, except W-t.D., n = 5 per square). Dotted line shows the number of lice transferred to each bird at the start of the experiment, which lasted 2 months. *, P < 0.01; †, P ≤ 0.05; for Dunnett's post hoc comparisons of the number of lice on each novel host species, relative to the number on Rock Pigeons (within preening treatments).
Species of Columbicola escape from preening by hiding in furrows between the adjacent barbs of the large flight feathers on the wings and tail (14). However, lice on novel hosts that differed in size from the native host were unable to hide, making them vulnerable to preening. Significantly fewer lice inserted between feather barbs on the three small hosts, compared to the two large hosts (visual examination method, ref. 37; G test, Gadj = 5.94, df = 1, P = 0.015).
Discussion
The louse parasite and avian host phylogenies we reconstructed were well resolved and well supported. Comparing the phylogenies revealed more congruence than expected by chance, owing to a history of cospeciation (Fig. 1). Phylogenetically independent contrast analyses revealed that louse body size matches host body size, owing to correlated evolution of parasite and host body size over macroevolutionary time (Fig. 2). Similar correlations have been shown for lice on mammals (6, 11).
The match in body size between lice and their hosts suggests that lice are limited to a narrow range of host body size. This proved to be the case for Columbicola; the five nonspecific Columbicola in our study (Fig. 1, bold) were present on hosts that are more similar in size than expected by chance, despite ecological opportunities to disperse among hosts of different sizes. Of the five host species used in our transfer experiments (Fig. 1, asterisks), all but one, the Band-tailed Pigeon (Patagioenas fasciata), are sympatric and syntopic (sharing habitat) at our field sites in Texas (17).
We tested the adaptive significance of the match in parasite and host body size by using the well studied species C. columbae, a host-specific parasite of the Rock Pigeon. We measured the fitness (survival and reproductive success) of C. columbae transferred to four novel host species of different sizes, relative to the fitness of lice transferred to new (control) individuals of the native host. The largest of the four novel hosts, the Band-tailed Pigeon, is similar in size to the Rock Pigeon (<5% difference in body mass). It was included as a positive control allowing us to compare the fitness of C. columbae on a novel host that is similar in size to the native host. The transfer experiments showed that lice cannot establish viable populations on novel hosts that differ in size from the native host. To determine why size matters, we tested three often mentioned components of louse fitness: attachment, feeding, and escape from host defense (preening).
The impact of size on attachment was tested by comparing the survival of lice placed on sections of novel host feathers grafted onto feathers of Rock Pigeons trained to fly along a 100-m playing field. Over 90% of all lice survived these flights, regardless of feather type (Fig. 3a). Attachment was also tested by comparing the survival of lice placed on novel host feathers taped to a high-speed fan set to simulate a racing pigeon in flight. Over 60% of all lice survived the 20-min trials, regardless of feather type (Fig. 3b). The ability of lice to remain attached to feathers was independent of host size, contrary to the expectations of previous workers, who argued that large lice would have difficulty clinging to small feathers, particularly in heavy airflow (12, 18).
The impact of size on feeding was tested by comparing the population sizes of C. columbae cultured on feathers from different species in an incubator. At the end of the month-long trial, the number of lice did not differ significantly among the five host species (Fig. 4a), nor did feather mass consumption differ (Fig. 4b). This experiment shows that C. columbae is capable of using feathers from smaller novel host species as food. Other experiments currently in progress (unpublished data) show that lice are also capable of using feathers from larger novel hosts as food. Our results contradict the conventional wisdom that mandible size, which is correlated with overall size (18), limits the range of feather sizes on which lice can feed.
The impact of size on escape from host defense was tested by comparing the relative fitness (survival and reproduction) of lice transferred to novel hosts with and without normal preening ability. We measured the population size of lice on each bird 2 months after the initial transfer of 25 lice per bird; 2 months is approximately two louse generations (15). When preening was prevented, louse populations on all five host species increased, showing that lice not only survived, but also reproduced (Fig. 5, □). The increase was smallest on Common Ground-doves, which had a mean of 41 lice per bird (range = 17–70) by the end of the experiment. Louse populations increased more on the other three novel host species but did not differ significantly from the native host, which had a mean of 182 lice per bird (range = 75–363). The ability of lice to survive and reproduce on the four novel hosts confirms that the feathers of these species provide adequate food and habitat for C. columbae in the absence of host preening.
The results differed dramatically for lice transferred to birds with normal preening (Fig. 5, ▪). C. columbae populations decreased sharply on the three smallest host species, which all had means of ≤4 lice per bird per species (range for three host species = 0–8). Although preening affected lice to varying degrees on all five hosts, its impact was greatest on the three small species. There was also a significant interaction between preening and host species (Fig. 5), confirming that the impact of preening depended on the host species to which lice were transferred. Preening had a much stronger effect on lice transferred to small-bodied species of novel hosts than it did on lice transferred to the Band-tailed Pigeon, a novel host that does not differ in size from the native host, the Rock Pigeon.
The negative effect of preening on lice transferred to smaller novel hosts begs the question whether preening would select against lice transferred to larger hosts. Lice presumably can hide between furrows that are wider than those on the native host. Thus, if preening selects only in favor of small body size, then the correlated evolution of body size we documented between Columbicola and their hosts (Fig. 2) presumably reflects a balance between preening and other opposing forces that select for increased size in Columbicola (14).
Our results suggest that the Band-tailed Pigeon should be a suitable host for C. columbae. Why, then, does C. columbae not occur on this host under natural conditions, given that Band-tailed Pigeons and Rock Pigeons are sympatric? We believe the answer lies partly with the fact that Band-tailed Pigeons live primarily in montane, forested habitat that is typically not occupied by Rock Pigeons. Thus, the absence of C. columbae from Band-tailed Pigeons may reflect a lack of ecological opportunity for lice to disperse between Rock Pigeons and Band-tailed Pigeons, despite sympatry of the two host species. Rock Pigeons are also a European species introduced to North America in the 1600s (33), which is recent from a macroevolutionary perspective.
The match of Columbicola body size to host body size is selectively favored for escape from host preening defense. Clayton et al. (14) showed that, within species, preening selects against C. columbae that are too large to fit between feather barbs on the native host. Over macroevolutionary time, the selective effect of preening leads to a match between parasite size and host size across species, providing a bridge between microevolutionary process and macroevolutionary pattern. The selective effect of preening has additional macroevolutionary consequences by selecting against parasites switching between hosts of different sizes. Preening thus reinforces phylogenetic congruence, providing a further link between microevolutionary process and broad scale microevolutionary pattern.
Accompanying the selective effect of host preening on C. columbae is a reciprocal selective effect of C. columbae on host bill morphology, which is a critical component of preening efficiency (14). This reciprocal selective effect suggests that lice influence the adaptive radiation of avian bill morphology. Recent comparative studies show that bill shape is correlated with louse load among species (38) and with louse load among populations within species (39). A specific feature of bill morphology, the maxillary overhang, has also been shown to be a specific adaptation for controlling lice and other ectoparasites (ref. 38 and unpublished data). The results of these studies suggest that louse body size and host bill morphology may coevolve in a fashion typical of host–parasite arms races (10, 40).
Body size has a profound effect on the form and function of organisms and is one of the most direct links between macroevolution and microevolution (41, 42). Macroevolutionary trends in body size can be a challenge to interpret because it is difficult to reconstruct the history of selection acting on size. The long-term association implied by congruent parasite–host phylogenies makes it possible to reconstruct the selective context in which the parasite evolved. Our comparative results confirm a strong macroevolutionary pattern of correlated body size evolution between Columbicola and their hosts, suggesting that size plays an important role in cospeciation between birds and feather lice. Our experimental results support this hypothesis by indicating that host defense constrains host switching by exerting strong selection against lice transferred to novel hosts differing in size from the native host. Overall, our results suggest that host defense reinforces cospeciation in birds and feather lice by preventing lice from switching between hosts of different sizes.
Acknowledgments
We thank P. Coley, F. Goller, R. Jarvis, R. Minckley, B. Moyer, W. Potts, A. Read, D. Reed, E. Sohn, and two anonymous reviewers for comments on the manuscript and other assistance. For permission to trap birds, we thank the U.S. Fish and Wildlife Service, Arizona Game and Fish, Oregon Fish and Wildlife, Texas Parks and Wildlife, and the Utah Division of Wildlife Resources. For parasite and host tissues or feather samples, we are grateful to S. Barton, R. Palma, J. Weckstein, R. Moyle, B. Marks, C. Witt, R. Faucett, S. de Kort, The Field Museum, Kansas Museum of Natural History, Louisiana State University Museum of Natural Science, and Tracy Aviary. This work was supported by National Science Foundation Awards DEB-9703003 and DEB-0107947 (to D.H.C.) and National Science Foundation Award DEB-0118794 (to D.H.C. and K.P.J.).
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AF190409–AF190426, AF278608–AF278643, and AF279704–AF279743).
References
- 1.Moran, N. A. & Baumann, P. (1994) Trends Ecol. Evol. 9, 15–20. [DOI] [PubMed] [Google Scholar]
- 2.Becerra, J. X. (1997) Science 276, 253–256. [DOI] [PubMed] [Google Scholar]
- 3.Kirk, W. D. J. (1991) Ecol. Entomol. 16, 351–359. [Google Scholar]
- 4.Timms, R. & Read, A. F. (1999) Trends Ecol. Evol. 14, 333–334. [DOI] [PubMed] [Google Scholar]
- 5.Poulin, R. (1998) Evolutionary Ecology of Parasites (Chapman & Hall, London).
- 6.Harvey, P. H. & Keymer, A. E. (1991) Philos. Trans. R. Soc. London B 332, 31–39. [Google Scholar]
- 7.Sasal, P., Trouvé, S., Müller-Graf, C. & Morand, S. (1999) J. Anim. Ecol. 68, 437–444. [Google Scholar]
- 8.Musser, R. O., Hum-Musser, S. M., Eichenseer, H., Peiffer, M., Ervine, G., Murphy, J. B. & Felton, G. W. (2002) Nature 416, 599–600. [DOI] [PubMed] [Google Scholar]
- 9.Lill, J. T., Marquis, R. J. & Ricklefs, R. E. (2002) Nature 417, 170–173. [DOI] [PubMed] [Google Scholar]
- 10.Thompson, J. N. (1994) The Coevolutionary Process (Univ. Chicago Press, Chicago).
- 11.Morand, S., Hafner, M. S., Page, R. D. M. & Reed, D. L. (2000) Biol. J. Linn. Soc. 70, 239–249. [Google Scholar]
- 12.Tompkins, D. M. & Clayton, D. H. (1999) J. Anim. Ecol. 68, 489–500. [Google Scholar]
- 13.Price, R. D., Hellenthal, R. A., Palma, R. L., Johnson, K. P. & Clayton, D. H. (2003) The Chewing Lice: World Checklist and Biological Overview, Illinois Natural History Survey Special Publication no. 24.
- 14.Clayton, D. H., Lee, P. L. M., Tompkins, D. M. & Brodie, E. D., III (1999) Am. Nat. 154, 261–270. [DOI] [PubMed] [Google Scholar]
- 15.Martin, M. (1934) Can. Entomol. 66, 6–16. [Google Scholar]
- 16.Clayton, D. H. & Tompkins, D. M. (1994) Proc. R. Soc. London Ser. B 256, 211–217. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, K. P., Williams, B. L., Drown, D. M., Adams, R. J. & Clayton, D. H. (2002) Mol. Ecol. 11, 25–38. [DOI] [PubMed] [Google Scholar]
- 18.Clay, T. (1949) Evolution (Lawrence, Kans.) 3, 279–299. [Google Scholar]
- 19.Price, P. W. (1980) Evolutionary Biology of Parasites (Princeton Univ. Press, Princeton).
- 20.Kennedy, C. E. J. (1986) Ecol. Entomol. 11, 291–300. [Google Scholar]
- 21.Johnson, K. P. & Clayton, D. H. (2000) Mol. Phylogenet. Evol. 14, 141–151. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, K. P., Adams, R. J., Page, R. D. M. & Clayton, D. H. (2003) Syst. Biol. 52, 37–47. [DOI] [PubMed] [Google Scholar]
- 23.Swofford, D. L. (2001) paup*: Phylogenetic Analysis Using Parsimony (*and Other Methods) (Sinauer, Sunderland, MA), Version 4.0 beta.
- 24.Farris, J. S., Kallersjo, M., Kluge, A. G. & Bult, C. (1994) Cladistics 10, 315–320. [DOI] [PubMed] [Google Scholar]
- 25.Farris, J. S., Kallersjo, A. G., Kluge, A. G. & Bult, C. (1995) Syst. Biol. 44, 570–572. [Google Scholar]
- 26.Huelsenbeck, J. P. & Crandall, K. A. (1997) Annu. Rev. Ecol. Syst. 28, 437–466. [Google Scholar]
- 27.Posada, D. & Crandall, K. A. (1998) Bioinformatics 14, 817–818. [DOI] [PubMed] [Google Scholar]
- 28.Felsenstein, J. (1985) Evolution (Lawrence, Kans.) 39, 783–791. [DOI] [PubMed] [Google Scholar]
- 29.Dunning, J. B. (1993) CRC Handbook of Avian Body Masses (CRC, Boca Raton, FL).
- 30.Tendeiro, J. (1965) Mem. Junta Invest. Ultramar 32, 1–460. [Google Scholar]
- 31.Purvis, A. & Rambaut, A. (1994) Comparative Analysis by Independent Contrasts (CAIC) (Oxford Univ. Press, Oxford), 2nd Ed. [DOI] [PubMed]
- 32.Lessells, C. M. & Boag, P. T. (1987) Auk 104, 116–121. [Google Scholar]
- 33.Johnston, R. F. & Janiga, M. (1995) Feral Pigeons (Oxford Univ. Press, New York).
- 34.Nelson, B. C. & Murray, M. D. (1971) Intl. J. Parasitol. 1, 21–29. [DOI] [PubMed] [Google Scholar]
- 35.Moyer, B. R., Gardiner, D. W. & Clayton, D. H. (2002) Oecologia 131, 203–210. [DOI] [PubMed] [Google Scholar]
- 36.Moyer, B. R., Drown, D. M. & Clayton, D. H. (2002) Oikos 97, 223–228. [Google Scholar]
- 37.Clayton, D. H. & Drown, D. M. (2001) J. Parasitol. 87, 1291–1300. [DOI] [PubMed] [Google Scholar]
- 38.Clayton, D. H. & Walther, B. A. (2001) Oikos 94, 455–467. [Google Scholar]
- 39.Moyer, B. R., Peterson, A. T. & Clayton, D. H. (2002) Condor 104, 675–678. [Google Scholar]
- 40.Geffeney, S., Brodie, E. D., Jr., Ruben, P. C. & Brodie, E. D., III (2002) Science 297, 1336–1339. [DOI] [PubMed] [Google Scholar]
- 41.Jablonski, D. (1996) in Evolutionary Biology, eds. Jablonski, D., Erwin, D. H. & Lipps, J. H. (Univ. Chicago Press, Chicago).
- 42.Brown, J. H. & West, G. B. (2000) Scaling in Biology (Oxford Univ. Press, Oxford).
- 43.Clayton, D. H. & Price, R. D. (1999) Ann. Entomol. Soc. Am. 92, 675–685. [Google Scholar]
- 44.Page, R. D. M. (1990) Syst. Zool. 39, 205–226. [Google Scholar]
- 45.Clayton, D. H., Al-Tamimi, S. & Johnson, K. (2003) in Tangled Treees: Phylogeny, Cospeciation, and Coevolution, ed. Page, R. D. M. (Univ. of Chicago Press, Chicago).