Abstract
As any new parent knows, having a baby provides opportunities for enrichment, learning and stress –experiences known to change the adult brain. Yet surprisingly little is known about the effects of maternal experience, and even less about the effects of paternal experience, on neural circuitry not directly involved in parenting. Here we discuss how caregiving and the accompanying experiential and hormonal changes influence the hippocampus and prefrontal cortex, brain regions involved in cognition and mood regulation. A better understanding of how parenting impacts the brain is likely to help in devising strategies for treating parental depression, a condition that can have serious cognitive and mental health consequences for children.
Introduction
For all mammalian species, becoming a mother involves remarkable behavioral change driven by a combination of neuroendocrine and experiential factors. Considerable research has been devoted to understanding the neural mechanisms of maternal care in rodents and primates. For a small minority of mammalian species (~6%), including humans, fathers play a significant role in rearing young [1,2]. Less is known about the neural and hormonal mechanisms of paternal care but the limited available evidence suggests that mothers and fathers might recruit similar neural circuitry, hormones and neuromodulators in the service of parenting behavior (Box 1).
Box 1. Mothers and fathers – are they really so different?
Although significantly less is known about the neural mechanisms of parenting behavior in fathers than in mothers, evidence suggests striking similarities between the sexes. Such similarities are particularly surprising because maternal behavior has been linked, in part, to hormonal changes that occur with pregnancy, parturition and lactation, experiences that are not available to fathers. From studies of biparental rodents and primate species that engage in cooperative breeding, we know that caregiving behavior in fathers is similar to that in mothers, suggesting that the same neural pathways might be involved.
Lesion studies, as well as immediate early gene and neuropeptide distribution studies, in rodents have identified similar brain regions involved in maternal and paternal behavior, including the olfactory bulb, medial preoptic area, lateral septum, bed nucleus of the stria terminalis, amygdala and PFC [84–88]. Although less is known about the neural circuitry of parenting in humans, exposure to crying infants activates the amygdala and PFC in fathers and mothers, but not in non-parents [62,63]. Given the hedonic aspects of parenting, it is not surprising that maternal and paternal behavior in rodents involves reward circuitry, namely dopaminergic afferents [89], suggesting neurochemical and neuroanatomical overlap between the sexes. Similar involvement of reward-related brain regions has been observed using neuroimaging in human mothers exposed to photographs of their own happy infants [90], although no study has yet investigated this issue in fathers.
What role do hormones, with their obvious differences between mothers and fathers, play? Here the answer is complex. Mothers exhibit drastic alterations in hormones including decreased estrogen and increased oxytocin and prolactin [11]. These changes are driven by pregnancy, parturition and lactation, as well as by infant contact, and are important for maternal behavior. Glucocorticoid levels also increase and although important for lactation, are not essential for other aspects of maternal behavior [25,91]. Similar but not identical alterations in these hormones are detectable in fathers; these include increased estrogen, oxytocin, prolactin and glucocorticoids [11,33]. Hormonal changes in fathers are induced by contact with the mother and the offspring. For instance, oxytocin levels in human fathers are positively related to the amount of affection the father displays toward his infant [92]. Variations in testosterone levels have also been associated with paternal care, although studies suggest species differences. In primate species, including humans, paternal behavior is related to reduced levels of testosterone [33,93]. The opposite is the case in some rodents for which paternal care is associated with elevated levels of testosterone [94–96]. Reduced testosterone, along with elevated estrogen and oxytocin levels, might facilitate affiliative behavior in primate fathers, whereas increased testosterone levels in rodents seem to support parental aggression toward nest intruders. It remains unclear whether differential hormone responses cause, or are caused by, variation in paternal care, because a definitive link between specific hormones and paternal behavior remains uncertain [97]. Nonetheless, infant contact itself seems to modulate endocrine systems and activate neural circuitry in fathers in a manner that is strikingly similar to that in mothers.
There is an increasing body of literature showing that structural, electrophysiological and molecular changes occur in the maternal and paternal brain. These include modifications not only in brain areas known to be involved in the control of parental behavior, but also in regions not traditionally associated with parenting, but that are instead more widely known to be involved in cognition and mood regulation. Here we highlight maternal and paternal influences on neuroplasticity in two such brain regions, the hippocampus and prefrontal cortex (PFC). We also consider the complex role that hormones and environment play in mediating the impact of parenting on these brain regions and discuss the potential functional consequences of these changes.
Identifying how the brain changes in response to parenthood could be important to better understand mood disturbances, including depression, which are known to be prevalent in new mothers and, as suggested by recent studies, in fathers as well (Box 2). Postpartum mental illness is often associated with inadequate child care, which has been linked to impaired cognitive and emotional function in offspring [3–5], including increased likelihood of developing anxiety and depression later in life [6]. Elucidating the influence of parenting on the brain could also be critical for ensuring the long-term health of offspring.
Box 2. Depression in new parents.
In the US, an estimated 10–15% of new mothers experience postpartum depression [98]; a slightly lower percentage of men (6–12%) also experience depression shortly after the birth of a child [99,100]. Among men whose partners have postpartum depression, the rate is even higher, suggesting a link between maternal and paternal depression [101].
Early parental depression is a significant problem, not just for the afflicted, but also for those in their care. Depression in mothers has been related to persistent disturbances in cognitive and emotional function in offspring [3–5], including increased likelihood of developing anxiety and depression later in life [6]. Depression in fathers can also negatively affect infant development; it has been associated with greater risk of later emotional, behavioral and cognitive difficulties [100,102,103].
Depressed parents seem to provide inadequate care to their offspring, which in turn impairs emotional and cognitive development [104]. Parents with depression are less emotionally attached to their infants, display less affection and interact less overall [3,105], qualities that infants seem to be able to detect. For example, babies become noticeably distressed when an adult (mother, father or unrelated caregiver) maintains a non-interactive still face [106,107]. Lack of social interaction during early life probably contributes heavily to emotional problems that emerge later in the children of depressed parents. Moreover, depressed parents read less often to their children than non-depressed parents, a difference that leads to smaller vocabularies in youngsters [100,103].
The neural mechanisms by which inadequate parental care contributes to negative emotional and cognitive outcomes in offspring are incompletely understood, but animal studies have provided some suggestions. Early postnatal maternal separation is a widely used paradigm to study maternal influence over offspring development in rats. In adulthood, rat pups subjected to maternal separation exhibit increased anxiety-like behavior, impaired cognitive capabilities and dysregulation of the hypothalamus–pituitary–adrenal axis [108]. These effects stem not from maternal separation itself, but indirectly from abnormal parenting behavior exhibited by mothers on return to their pups [108]. Similar outcomes have been reported in the offspring of mother rats that naturally display low levels of maternal care [109].
Maternal separation is associated with diminished structural plasticity in brain regions linked to cognition and mood regulation, including the hippocampus and PFC. In the hippocampus, maternal separation and low levels of maternal care lead to suppressed postnatal neurogenesis [110,111], reduced dendritic spine density [109,112], and reduced hippocampal BDNF expression and cholinergic innervation [109]. In the PFC, maternal separation, as well as paternal separation (in biparental species), is associated with changes in dendritic spines and synapses [113–115]. If structural changes contribute to behavioral problems associated with inadequate parenting, then the potential to repair these abnormalities by modulating ongoing plastic processes, including adult neurogenesis and dendritic remodeling, might exist. Indeed, studies have shown that environmental enrichment, an experience that enhances structural plasticity in adulthood [28,29,47], restores many of the behavioral abnormalities arising from maternal separation and low maternal care during early life [116,117]. Future studies will be necessary, however, to forge the translational link between animal models of early inadequate parenting and depressed human parents.
Behavioral changes that define parenting
The postpartum period is a time of dramatic behavioral changes for all mammalian species. In rodents, females that were previously unresponsive or aggressive toward pups engage in an elaborate repertoire of caregiving activities at birth and postpartum that includes cleaning the pups, eating the debris of birth, nursing, nest building, licking and grooming, pup retrieval, assuming a nursing (i.e. arched back) posture over the pups and increased aggression toward intruders. In nonhuman primates, maternal behavior includes nursing, infant carrying, grooming and defending the infant from danger. The emergence of maternal behaviors is stimulated by contact with offspring and a complex array of endocrine changes (Box 1). Maternal experience and the accompanying hormonal alterations affect various measures of neural plasticity in numerous brain regions such as the hypothalamus [7,8], amygdala [9] and olfactory bulb [10], areas that are necessary for the full expression of maternal behaviors [11].
For most mammalian species, raising the young is accomplished exclusively by the mother, but for a small minority fathers play a role. Among species in which paternal care occurs (Box 3), there are two general social strategies – a biparental strategy in which the mother–father dyad care for the young and a cooperative breeding strategy in which caregiving is shared by the mother, father, older siblings and sometimes unrelated adults. Regardless of whether the species engages in biparental or cooperative breeding, paternal care typically involves the same behaviors as maternal care, with the exception of nursing. Evidence to date suggests that the neural circuitry underlying paternal behavior is similar to that for maternal behavior (Box 1) in that the same brain regions seem to be activated when fathers and mothers have contact with infants. Moreover, many of the same hormonal changes that accompany the postpartum period also occur in fathers that display parenting behavior (Box 1).
Box 3. Some mammalian species that engage in paternal care.

Maternal and paternal experiences also influence brain regions that are known to be either unnecessary for the behaviors directly associated with parenting or only involved in a modulatory way. Among these are effects on brain regions associated with cognition and mood, including the hippocampus and PFC (Figure 1).
Figure 1.
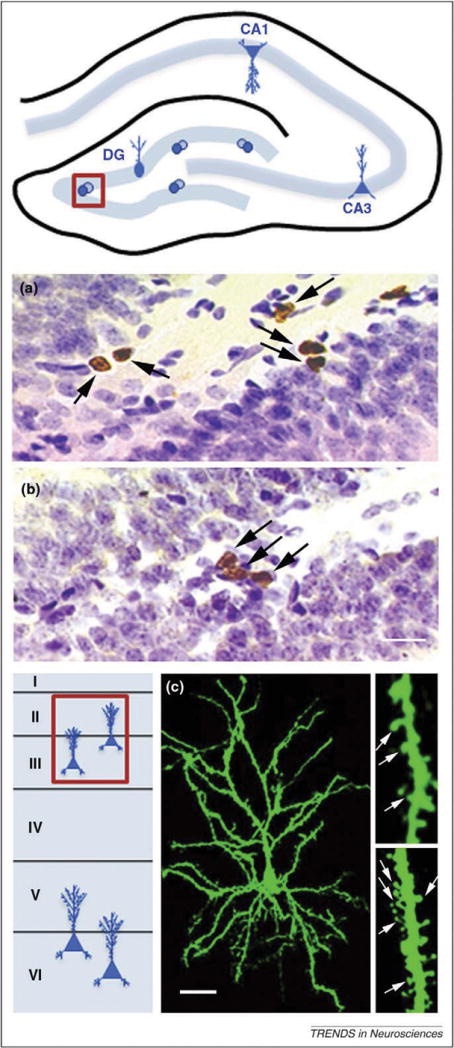
Parental experience produces changes in structural plasticity in the hippocampus and PFC. Top: Schematic diagram of the hippocampus showing the dentate gyrus, the location of adult neurogenesis (boxed area indicates region from which photomicrographs were obtained). Mother rats exhibit suppressed adult neurogenesis in the dentate gyrus prior to weaning of their offspring. (a) Virgin female rats have more proliferating cells, labeled here with the thymidine analog bromodeoxyuridine (BrdU) (arrows), compared with postpartum rats (b). BrdU-labeled cells are stained brown and cells labeled for Nissl are stained purple. Scale bar, 20 μm. Bottom: Schematic diagram of cortical layers (I–VI) in the PFC showing the neuron type affected by parenting (boxed area indicates layers II/III, where changes were detected in pyramidal neurons). Father marmosets exhibit enhanced dendritic spine density on pyramidal neurons of layer II/III PFC compared to non-fathers. (c) Layer II/III PFC pyramidal cell of a marmoset father labeled with the lipophilic tracer DiI (fluorescent green). Magnified views of DiI labeled dendritic segments showing dendritic spines (arrows) in a control (right upper) and a father (right lower). Scale bar, 30 μm for cell, 5 μm for dendritic segments. Adapted, with permission, from Ref. [25,73].
Parenting and the hippocampus
Lesions of the hippocampus have minimal effects on maternal behavior [12,13], but this brain region is nonetheless affected by parenting. The hippocampus plays an important role in certain types of learning and memory, anxiety regulation and feedback of the stress response. In adult mammals, the hippocampus also exhibits a capacity for dramatic structural reorganization in the form of adult neurogenesis, dendritic remodeling, and the formation and elimination of dendritic spines and synapses (Figure 1). In virgin animals without reproductive or maternal experience, these forms of structural plasticity are modulated by hormones, including estrogen and glucocorticoids [14–19], levels of which are altered in mothers and fathers (Box 1). Moreover, several experiences that seem intrinsic to parenting, such as stress, learning and environmental enrichment, also influence hippocampal structure in virgins [19–21]. Perhaps it is not surprising that parental experience alters structural plasticity in the hippocampus, although the manner in which this occurs seems to be complex, most probably reflecting the multitude of hormonal and experiential changes associated with parenting.
Adult neurogenesis
The dentate gyrus of the hippocampus continues to produce several thousand new neurons each day in young adulthood [22]. Adult neurogenesis in the hippocampus has been documented in many mammalian species, including humans [23]. The regulation of adult neurogenesis by hormones and experience has been the subject of intensive investigation [19]. Ovarian steroids enhance [15], whereas adrenal steroids suppress [14,18], cell proliferation in the dentate gyrus of virgin rats. Postpartum female rats exhibit decreased estrogen levels [24] and increased glucocorticoid levels [24,25], suggesting that new neuron production might be impaired in mothers. The postpartum period is associated not just with hormonal changes, but also with experiential changes that might impact adult neurogenesis. Stress is known to inhibit, whereas learning and environmental enrichment are known to enhance, adult neurogenesis [19–21]. Despite the fact that some of the stimuli inherent to parenting seem to enhance adult neurogenesis, available evidence suggests that maternal experience is associated with suppression of cell proliferation during the postpartum period [24–26].
In mother rats, decreased cell proliferation is evident as early as 1 day after parturition and occurs in both first-time mothers (primiparous) (Figure 1) and females that have produced more than one litter (multiparous) [24–26]. By the time of weaning and beyond, cell proliferation is restored in postpartum females [25]. Thus, maternal experience has a suppressive, albeit temporary, effect on cell production in the hippocampus that does not seem to be modulated by repeated reproductive experience. The suppressive effect of maternal experience on adult neurogenesis seems to be linked to both hormonal changes and interaction with offspring. Reduced cell proliferation during the postpartum period is dependent on elevated basal glucocorticoid levels associated with lactation; removal of nursing pups reduces corticosterone levels and prevents the decrease in number of proliferating cells [25]. Moreover, prevention of increased basal corticosterone levels in postpartum mothers by means of adrenalectomy and low-dose corticosterone replacement eliminates the reduction in cell proliferation. Thus, it seems that in postpartum females, offspring interactions inhibit hippocampal cell production through changes in adrenal steroids (Figure 2). It should be noted that elevated adrenal steroid levels might not be the sole factor involved and that decreased estradiol levels, which are known to reduce adult neurogenesis, might participate as well [24,27].
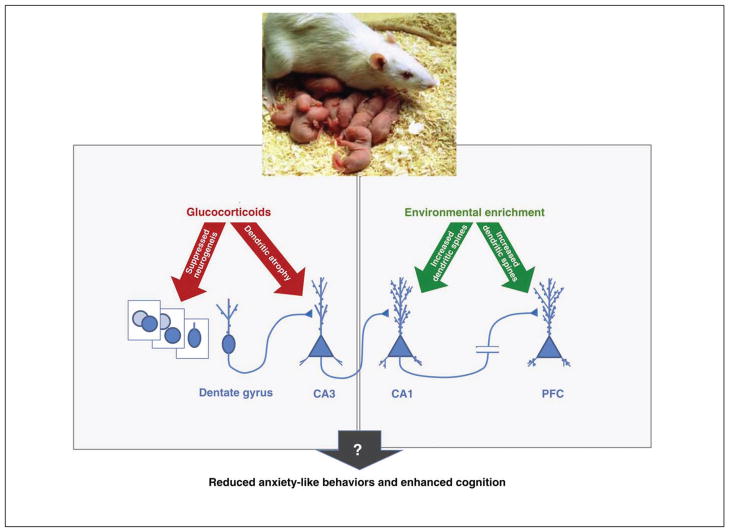
Figure 2.
Parenting can alter anxiety and cognition by inducing structural changes through potentially different mechanisms. This model diagram illustrates some of the structural changes that occur with parenting, including suppressed neurogenesis in the dentate gyrus, reduced dendritic complexity in the CA3 pyramidal cell population of the hippocampus, and enhanced dendritic spine density in pyramidal cells of the CA1 hippocampal region and layer 2–3 of the PFC. Parenting-induced elevated glucocorticoid levels might underlie changes in the dentate gyrus and CA3 regions of the hippocampus, whereas the enriching aspects of infant contact might produce changes in the CA1 region and PFC. Changes in the structure of the hippocampus and PFC might be responsible for parenting-induced alterations in behaviors associated with these brain regions, including reduced anxiety-like behavior and enhanced cognition. Photo credit: J. Alberts (University of Indiana).
Postpartum-induced suppression of adult neurogenesis is linked to glucocorticoid elevations that occur along with lactation, so it seems likely that this effect might be specific to lactating mothers. The glucocorticoid-induced decrease in cell proliferation in lactating females suggests that other aspects of maternal experience, such as enriching effects, which would be expected to enhance adult neurogenesis, are secondary to glucocorticoid changes. Indeed, pup exposure in virgin female rats stimulates adult neurogenesis, an effect that might reflect environmental enrichment in the absence of hormonal changes associated with pregnancy, parturition and lactation [26]. Studies have shown that environmental enrichment stimulates new neuron production in the dentate gyrus of virgin male and female rodents [28,29]. Males do not lactate, so it seems reasonable to predict that fathers would display adult neurogenesis changes that are more akin to enriched environment effects, such as those observed in virgin females. Consistent with this, brief exposure to pups increases cell proliferation in the dentate gyrus of virgin male prairie voles [30]. A similar stimulatory effect of pup exposure on adult neurogenesis has been observed in laboratory mouse fathers, which do not normally engage in parental behavior, but do so after repeated interaction with their offspring [31]. By contrast, in California mice fathers that naturally demonstrate paternal care behaviors, a reduction in new cell production, similar to that detected in mother rats, has been observed [32]. This effect is probably linked to hormonal changes that occur in fathers. For example, fathers exhibit elevated glucocorticoids [33], although this hormonal change is not associated with lactation.
Many of the behavioral and neuroendocrine adaptations of the postpartum period are also evident during late pregnancy [34]. Thus, it seems plausible that suppression of the production of new neurons would emerge before parturition. Decreased hippocampal volume has been observed in pregnant rats and humans [35,36], suggesting that reduced adult neurogenesis contributes to these volumetric changes. Cell proliferation in the hippocampus does not seem to be altered during pregnancy in rats or mice [10,37–39] and the current evidence on pregnancy-induced alterations in cell survival is mixed, with some work suggesting a possible enhancement [37] and other studies showing a decrease [40] or no effect [39]. It is possible that these inconsistencies are related to the different gestational timepoints examined and could suggest that neurogenesis is altered, but only at specific times during pregnancy.
Despite the unresolved effects of pregnancy, some evidence suggests that maternal experience has a lasting effect on progenitor cells in the dentate gyrus that extends into aging. Whereas middle-aged virgin female rats exhibit no change in cell proliferation with estrogen treatment, middle-aged mothers exhibit an increase in cell proliferation, an effect similar to that observed in young adults [41]. This suggests that maternal experience slows the aging process, at least insofar as estrogen sensitivity of progenitor cells is concerned. The duration of this effect remains unknown. Long-term benefits of being a mother on age-related cognitive decline and other measures of brain aging have also been reported [42].
It is important to note that maternal experience also has effects on neurogenesis in the subventricular zone (SVZ), an area that gives rise to neurons of the olfactory bulb, a brain region important for maternal care [43]. Increased neurogenesis has been reported in mice and rats during pregnancy and the early postpartum period [10,38]. Pup exposure in virgin female rats also stimulates neurogenesis in the SVZ [44]. In contrast to the involvement of glucocorticoids in the postpartum reduction in neurogenesis in the hippocampus, increased neurogenesis in the SVZ seems to be mediated by prolactin [10]. Thus, maternal experience can have opposite effects on the same form of structural plasticity in two different brain regions through distinct mechanisms.
Dendritic and synaptic remodeling
The effects of parenting on hippocampal plasticity are not limited to alterations in cell proliferation and neurogenesis, but also extend to dendritic spines and architecture. Like the effects of maternal experience on adult neurogenesis, changes in these morphological measures are complex (Figure 2).
Increased spine densities on apical dendrites of hippocampal CA1 pyramidal neurons have been reported in late pregnant and postpartum (primiparous) rats [45,118]. The postpartum period is also associated with increases in spine density on dendrites of dentate gyrus granule cells [118]. Hippocampal dendritic spine density is controlled by circulating levels of ovarian hormones [17], so enhanced spine density in late pregnancy might be related to elevated estrogen levels that occur at this time. In support of this, virgin females treated with a pregnancy-like regimen of estradiol and progesterone exhibit similar spine enhancements [45]. Estrogen levels are low after birth, so it is likely that estrogen does not mediate the enhanced spine density postpartum. It is possible that the spines generated during late pregnancy are maintained postpartum or that the latter diminish and new spines are generated [46]. The specific hormonal or environmental stimuli that maintain or induce spine growth postpartum have yet to be determined. One possibility is that postpartum increases in spine density result from the enriching effects of maternal experience (Figure 2). In addition to its positive effect on the production of new neurons, environmental enrichment is known to have a beneficial effect on dendritic spines in the hippocampus [19,47].
To date, no time-course studies have examined the duration of dendritic spine changes throughout the post-partum period. It does not seem that spine density remains elevated in primiparous females at the time of weaning, indicating that enhanced dendritic spine density might be transient and dependent on the presence of offspring [48]. Another possibility is that the persistence of dendritic spines may be modulated by reproductive experience. Consistent with this are data showing that unlike primiparous females, multiparous females at weaning have more hippocampal dendritic spines compared to virgins, although this effect is specific to CA1 basal dendrites [48,49].
Dendritic architecture in the hippocampus is also influenced by maternal experience. Primiparous rats exhibit decreased dendritic length and fewer dendritic branch points on pyramidal neurons in both the CA1 and CA3 regions of the hippocampus relative to virgin and multiparous females [48]. It is important to note that these results were observed in postpartum females after weaning, leaving open the possibility that alterations in dendritic architecture emerge earlier in the postpartum period when pups are present. The factors responsible for dendritic tree shrinkage have yet to be determined. High levels of circulating glucocorticoids have been associated with dendritic atrophy of pyramidal cells in the hippocampus [16,50], but did not correlate with hippocampal dendritic remodeling around the postpartum period [48]. A role for glucocorticoids cannot be ruled out because levels were measured at the time of weaning and not during pregnancy or postpartum, when hormonal changes are likely to be more evident and contribute to dendritic remodeling.
Synaptic plasticity and gene expression
In addition to structural changes, alterations in hippocampal synaptic plasticity also occur in the postpartum period. Compared to virgin females, long-term potentiation (LTP) is enhanced in hippocampal slices from multiparous mice during the early postpartum period of their second litter [51]. Oxytocin might be an important mediator of this effect, because LTP was also enhanced in hippocampal slices of virgins treated with this neuropeptide [51]. Similarly, an enhancement in LTP is evident in primiparous females after weaning [52]. These effects are surprising because other hormonal changes associated with the post-partum period, such as reduced estrogen and increased glucocorticoid levels, have been linked to impaired LTP [53,54]. Taken together, the results suggest that factors in the maternal brain might buffer against some of the negative influences associated with hormonal changes.
Maternal experience is also associated with changes in the expression of genes, such as those encoding GABAA receptor subunits and neuropeptides, in the hippocampus and other brain regions that are likely to influence synaptic plasticity [55–57]. Whether parenting-induced changes have a positive, neutral or negative effect on behavior remains largely undetermined, although a recent study has demonstrated abnormal postpartum behaviors in mice with deficiencies in δ-subunit-containing GABAA receptors [58].
Parenting and the prefrontal cortex
The PFC is activated by parenting behavior in both rodents and humans [59–63]. Lesions of the PFC do not have a profound effect on the initiation of parenting behavior, although some evidence in rodents suggest that pup retrieval can be affected [64]. The PFC has been implicated in many functions that might indirectly influence parenting, including working memory, cognitive flexibility and mood regulation [65,66].
Structural reorganization has been observed in the PFC of rodents and primates. As in the hippocampus, these changes can be induced by alterations in hormones and experience. Manipulation of estrogen and glucocorticoids affects dendritic architecture and dendritic spine density on pyramidal neurons of the medial PFC [67–70]. Moreover, experiences that seem to be related to parenting, such as stress and enriched environment living, also alter dendritic complexity and spine density in this brain region [47,71,72].
Structural reorganization occurs in the PFC of marmoset fathers. Marmoset fathers engage in extensive caregiving behavior by carrying, protecting and feeding the young (Box 3) [73]. Marmoset fathers, regardless of whether they are first-time or experienced, have enhanced dendritic spine density on pyramidal neurons of the PFC [73]. A possible involvement of vasopressin, a neuropeptide implicated in paternal care [33], was also suggested by the demonstration that parallel increases in the abundance of vasopressin V1a receptors, and the proportion of dendritic spines that were labeled for this receptor, occurred in the PFC of fathers. The abundance of V1a receptors correlated negatively with offspring age, so these effects might not be permanent, but instead might be driven by father–offspring interactions, which lessen as offspring mature. As with some of the effects of maternal experience on hippocampal structure, hormones and offspring probably work together in some way to alter the PFC of fathers. In this regard, it is worth noting that living in an enriched environment, in the absence of caregiving experience, is sufficient to induce similar changes in dendritic spine density of pyramidal neurons in the PFC of marmosets [47]. These findings suggest that the enriching aspect of parenting might be important for driving changes in the brains of marmoset fathers (Figure 2).
Female rats also exhibit increased dendritic spine density on pyramidal neurons of the medial PFC during the postpartum period [118]. In addition, glial changes have been reported in specific PFC regions of postpartum rats [74]. The extent to which these structural changes directly influence cognitive function remains unknown, but recent evidence indicates that mother rats perform better than virgins on attentional set shifting, a cognitive behavioral task that requires the PFC [118].
Functional consequences of parenting-induced neuroplasticity
The fact that maternal and paternal experience can remodel neural systems not directly related to parental care suggests that functions mediated by these brain regions might also be altered. Numerous studies in non-parents have linked adult neurogenesis and dendritic remodeling to hippocampal functions, including learning and memory, anxiety regulation and stress responses [19,21,75,76]. An increasing body of evidence suggests that maternal experience alters behaviors associated with the hippocampus, including enhanced spatial navigation learning and reduced anxiety-like behaviors [24,52,77–82]. The extent to which maternal experience-induced changes in hippocampal structure contribute to changes in hippocampal function, however, remains unexplored. Likewise, the influence of paternal experience on hippocampal function has been almost completely ignored, but given the similarities observed between mothers and fathers, it seems likely that changes in hippocampal function occur. Recent evidence suggests that changes in adult neurogenesis in fathers might be linked to kin recognition, at least in some species [31]. It is also likely that functions mediated by the PFC are altered by parenting, because structural plasticity in this brain region has been linked to alterations in PFC-dependent cognitive behavior [72,118]. Finally, given the connections between the hippocampus and the PFC, as well as the involvement of this pathway in the regulation of anxiety-like behavior [83], it is possible that the combined action of parenting on structural plasticity in both the hippocampus and the PFC could mediate modulation of mood in mothers and fathers.
Conclusion
Although it has long been recognized that parental care can have a profound influence on offspring wellbeing, only recently has there been an appreciation of the impact of offspring on parents. The brains of parents are clearly different from those of non-parents, having been changed by the presence of offspring and corresponding hormonal fluctuations. Available evidence suggests that structural reorganization occurs in the hippocampus and PFC of mothers and fathers, but these studies are incomplete and the link between changes in brain structure and function remains unexplored (Box 4). Future studies focused on identifying brain changes, as well as the related behaviors, that are influenced in both mothers and fathers will fill the gaps in our knowledge of how the brain is influenced by childrearing.
Box 4. Unanswered questions.
What are the hormonal and neural mechanisms that underlie reduced anxiety and improved cognition in parenting?
How do changes in structural plasticity influence brain function in parents?
Are there fundamental differences in the brains of mothers and fathers and, if so, to what extent are these differences driven by experience and hormones?
Do experienced parents undergo additional modifications in brain structure or are parenting-related brain changes specific to first-time parents?
How does the parenting brain cope with high levels of glucocorticoids?
Are parenting changes induced primarily by newborn cues or do older offspring elicit similar changes?
Do changes in brain structure contribute to mood disorders in parents?
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (MH54970 to E.G. and MH084148 to B.L), a NARSAD Young Investigator Award (B.L.) and a Ruth L. Kirschstein postdoctoral NRSA fellowship from the National Institute on Aging (E.R.G.).
References
- 1.Lonstein JS, De Vries GJ. Sex differences in the parental behavior of rodents. Neurosci Biobehav Rev. 2000;24:669–686. doi: 10.1016/s0149-7634(00)00036-1. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez-Duque E, et al. The biology of paternal care in human and non-human primates. Annu Rev Anthropol. 2009;38:115–130. [Google Scholar]
- 3.Nadel J, et al. Two-month-old infants of depressed mothers show mild, delayed and persistent change in emotional state after non-contingent interaction. Infant Behav Dev. 2005;28:418–425. [Google Scholar]
- 4.Hay DF, et al. Antepartum and postpartum exposure to maternal depression: different effects on different adolescent outcomes. J Child Psychol Psychiatry. 2008;49:1079–1088. doi: 10.1111/j.1469-7610.2008.01959.x. [DOI] [PubMed] [Google Scholar]
- 5.Fihrer I, et al. The impact of postnatal and concurrent maternal depression on child behaviour during the early school years. J Affect Disord. 2009;119:116–123. doi: 10.1016/j.jad.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Halligan SL, et al. Maternal depression and psychiatric outcomes in adolescent offspring: a 13-year longitudinal study. J Affect Disord. 2007;97:145–154. doi: 10.1016/j.jad.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Featherstone RE, et al. Plasticity in the maternal circuit: effects of experience and partum condition on brain astrocyte number in female rats. Behav Neurosci. 2000;114:158–172. doi: 10.1037//0735-7044.114.1.158. [DOI] [PubMed] [Google Scholar]
- 8.Keyser-Marcus L, et al. Alterations of medial preoptic area neurons following pregnancy and pregnancy-like steroidal treatment in the rat. Brain Res Bull. 2001;55:737–745. doi: 10.1016/s0361-9230(01)00554-8. [DOI] [PubMed] [Google Scholar]
- 9.Rasia-Filho AA, et al. Influence of sex, estrous cycle and motherhood on dendritic spine density in the rat medial amygdala revealed by the Golgi method. Neuroscience. 2004;126:839–847. doi: 10.1016/j.neuroscience.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Shingo T, et al. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- 11.Numan M, Insel T. The Neurobiology of Parental Behavior. Springer-Verlag; 2003. [Google Scholar]
- 12.Kimble DP, et al. Hippocampal lesions disrupt maternal, not sexual, behavior in the albino rat. J Comp Physiol Psychol. 1967;63:401–407. doi: 10.1037/h0024605. [DOI] [PubMed] [Google Scholar]
- 13.Terlecki LJ, Sainsbury RS. Effects of fimbria lesions on maternal behavior in the rat. Physiol Behav. 1978;21:89–97. doi: 10.1016/0031-9384(78)90281-0. [DOI] [PubMed] [Google Scholar]
- 14.Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 15.Tanapat P, et al. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol. 2005;481:252–265. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- 16.Woolley CS, et al. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- 17.Gould E, et al. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gould E, et al. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leuner B, Gould E. Structural plasticity and hippocampal function. Annu Rev Psychol. 2010;61:111–140. doi: 10.1146/annurev.psych.093008.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- 21.Leuner B, et al. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- 22.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 23.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 24.Darnaudery M, et al. Early motherhood in rats is associated with a modification of hippocampal function. Psychoneuroendocrinology. 2007;32:803–812. doi: 10.1016/j.psyneuen.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Leuner B, et al. Maternal experience inhibits the production of immature neurons in the hippocampus during the postpartum period through elevations in adrenal steroids. Hippocampus. 2007;17:434–442. doi: 10.1002/hipo.20278. [DOI] [PubMed] [Google Scholar]
- 26.Pawluski JL, Galea LA. Reproductive experience alters hippocampal neurogenesis during the postpartum period in the dam. Neuroscience. 2007;149:53–67. doi: 10.1016/j.neuroscience.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 27.Green AD, Galea LA. Adult hippocampal cell proliferation is suppressed with estrogen withdrawal after a hormone-simulated pregnancy. Horm Behav. 2008;54:203–211. doi: 10.1016/j.yhbeh.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 28.Kempermann G, et al. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 29.Tashiro A, et al. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruscio MG, et al. Pup exposure elicits hippocampal cell proliferation in the prairie vole. Behav Brain Res. 2008;187:9–16. doi: 10.1016/j.bbr.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mak GK, Weiss S. Paternal recognition of adult offspring mediated by newly generated CNS neurons. Nat Neurosci. 2010;13:753–758. doi: 10.1038/nn.2550. [DOI] [PubMed] [Google Scholar]
- 32.Kozorovitskiy Y, et al. Fatherhood influences neurogenesis in the hippocampus of California mice. Proceedings of the 2007 Neuroscience Meeting, Abstract 626.21, Society for Neuroscience.2007. [Google Scholar]
- 33.Wynne-Edwards KE. Hormonal changes in mammalian fathers. Horm Behav. 2001;40:139–145. doi: 10.1006/hbeh.2001.1699. [DOI] [PubMed] [Google Scholar]
- 34.Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nat Rev Neurosci. 2008;9:11–25. doi: 10.1038/nrn2280. [DOI] [PubMed] [Google Scholar]
- 35.Galea LA, et al. Spatial working memory and hippocampal size across pregnancy in rats. Horm Behav. 2000;37:86–95. doi: 10.1006/hbeh.1999.1560. [DOI] [PubMed] [Google Scholar]
- 36.Oatridge A, et al. Change in brain size during and after pregnancy: study in healthy women and women with preeclampsia. Am J Neuroradiol. 2002;23:19–26. [PMC free article] [PubMed] [Google Scholar]
- 37.Banasr M, et al. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. Eur J Neurosci. 2001;14:1417–1424. doi: 10.1046/j.0953-816x.2001.01763.x. [DOI] [PubMed] [Google Scholar]
- 38.Furuta M, Bridges RS. Gestation-induced cell proliferation in the rat brain. Brain Res Dev Brain Res. 2005;156:61–66. doi: 10.1016/j.devbrainres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Pawluski JL, et al. Pregnancy decreases ERα-expression and pyknosis, but not cell proliferation or survival, in the hippocampus. J Neuroendocrinol. 2010;22:248–257. doi: 10.1111/j.1365-2826.2010.01960.x. [DOI] [PubMed] [Google Scholar]
- 40.Rolls A, et al. Decrease in hippocampal neurogenesis during pregnancy: a link to immunity. Mol Psychiatry. 2008;13:468–469. doi: 10.1038/sj.mp.4002126. [DOI] [PubMed] [Google Scholar]
- 41.Barha CK, Galea LA. Motherhood alters the cellular response to estrogens in the hippocampus later in life. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Gatewood JD, et al. Motherhood mitigates aging-related decrements in learning and memory and positively affects brain aging in the rat. Brain Res Bull. 2005;66:91–98. doi: 10.1016/j.brainresbull.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Levy F, Keller M. Olfactory mediation of maternal behavior in selected mammalian species. Behav Brain Res. 2009;200:336–345. doi: 10.1016/j.bbr.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 44.Furuta M, Bridges RS. Effects of maternal behavior induction and pup exposure on neurogenesis in adult, virgin female rats. Brain Res Bull. 2009;80:408–413. doi: 10.1016/j.brainresbull.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinsley CH, et al. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm Behav. 2006;49:131–142. doi: 10.1016/j.yhbeh.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 46.Woodside B. Morphological plasticity in the maternal brain: comment on Kinsley et al. motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm Behav. 2006;49:129–130. doi: 10.1016/j.yhbeh.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Kozorovitskiy Y, et al. Experience induces structural and biochemical changes in the adult primate brain. Proc Natl Acad Sci U S A. 2005;102:17478–17482. doi: 10.1073/pnas.0508817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pawluski JL, Galea LA. Hippocampal morphology is differentially affected by reproductive experience in the mother. J Neurobiol. 2006;66:71–81. doi: 10.1002/neu.20194. [DOI] [PubMed] [Google Scholar]
- 49.Brusco J, et al. Plasma hormonal profiles and dendritic spine density and morphology in the hippocampal CA1 stratum radiatum, evidenced by light microscopy, of virgin and postpartum female rats. Neurosci Lett. 2008;438:346–350. doi: 10.1016/j.neulet.2008.04.063. [DOI] [PubMed] [Google Scholar]
- 50.Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 51.Tomizawa K, et al. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci. 2003;6:384–390. doi: 10.1038/nn1023. [DOI] [PubMed] [Google Scholar]
- 52.Lemaire V, et al. Motherhood-induced memory improvement persists across lifespan in rats but is abolished by a gestational stress. Eur J Neurosci. 2006;23:3368–3374. doi: 10.1111/j.1460-9568.2006.04870.x. [DOI] [PubMed] [Google Scholar]
- 53.Warren SG, et al. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res. 1995;703:26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- 54.Maggio N, Segal M. Striking variations in corticosteroid modulation of long-term potentiation along the septotemporal axis of the hippocampus. J Neurosci. 2007;27:5757–5765. doi: 10.1523/JNEUROSCI.0155-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Byrnes EM, et al. Alterations in GABAA receptor α2 subunit mRNA expression following reproductive experience in rats. Neuroendocrinology. 2007;85:148–156. doi: 10.1159/000102535. [DOI] [PubMed] [Google Scholar]
- 56.Nephew BC, et al. Enhanced maternal aggression and associated changes in neuropeptide gene expression in multiparous rats. Behav Neurosci. 2009;123:949–957. doi: 10.1037/a0016734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanna E, et al. Changes in expression and function of extrasynaptic GABAA receptors in the rat hippocampus during pregnancy and after delivery. J Neurosci. 2009;29:1755–1765. doi: 10.1523/JNEUROSCI.3684-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maguire J, Mody I. GABAAR plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fleming AS, Korsmit M. Plasticity in the maternal circuit: effects of maternal experience on Fos-Lir in hypothalamic, limbic, and cortical structures in the postpartum rat. Behav Neurosci. 1996;110:567–582. doi: 10.1037//0735-7044.110.3.567. [DOI] [PubMed] [Google Scholar]
- 60.Febo M, et al. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. J Neurosci. 2005;25:11637–11644. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hernandez-Gonzalez M, et al. Electrical activity of prefrontal cortex and ventral tegmental area during rat maternal behavior. Behav Processes. 2005;70:132–143. doi: 10.1016/j.beproc.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 62.Seifritz E, et al. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biol Psychiatry. 2003;54:1367–1375. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- 63.Swain JE, et al. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Afonso VM, et al. Medial prefrontal cortex lesions in the female rat affect sexual and maternal behavior and their sequential organization. Behav Neurosci. 2007;121:515–526. doi: 10.1037/0735-7044.121.3.515. [DOI] [PubMed] [Google Scholar]
- 65.Dalley JW, et al. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 66.Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 68.Seib LM, Wellman CL. Daily injections alter spine density in rat medial prefrontal cortex. Neurosci Lett. 2003;337:29–32. doi: 10.1016/s0304-3940(02)01287-9. [DOI] [PubMed] [Google Scholar]
- 69.Hao J, et al. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wallace M, et al. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006;1126:176–182. doi: 10.1016/j.brainres.2006.07.064. [DOI] [PubMed] [Google Scholar]
- 71.Radley JJ, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 72.Liston C, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kozorovitskiy Y, et al. Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nat Neurosci. 2006;9:1094–1095. doi: 10.1038/nn1753. [DOI] [PubMed] [Google Scholar]
- 74.Salmaso N, et al. Steroid hormones and maternal experience interact to induce glial plasticity in the cingulate cortex. Eur J Neurosci. 2009;29:786–794. doi: 10.1111/j.1460-9568.2009.06627.x. [DOI] [PubMed] [Google Scholar]
- 75.Revest JM, et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14:959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- 76.Schloesser RJ, et al. Suppression of adult neurogenesis leads to an increased hypothalamo–pituitary–adrenal axis response. Neuroreport. 2009;20:553–557. doi: 10.1097/WNR.0b013e3283293e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pawluski JL, et al. First reproductive experience persistently affects spatial reference and working memory in the mother and these effects are not due to pregnancy or ‘mothering’ alone. Behav Brain Res. 2006;175:157–165. doi: 10.1016/j.bbr.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 78.Macbeth AH, Luine VN. Changes in anxiety and cognition due to reproductive experience: a review of data from rodent and human mothers. Neurosci Biobehav Rev. 2009;34:452–467. doi: 10.1016/j.neubiorev.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 79.Kinsley CH, et al. Motherhood improves learning and memory. Nature. 1999;402:137–138. doi: 10.1038/45957. [DOI] [PubMed] [Google Scholar]
- 80.Pawluski JL, et al. Reproductive experience differentially affects spatial reference and working memory performance in the mother. Horm Behav. 2006;49:143–149. doi: 10.1016/j.yhbeh.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 81.Macbeth AH, et al. Pregnant rats show enhanced spatial memory, decreased anxiety, and altered levels of monoaminergic neurotransmitters. Brain Res. 2008;1241:136–147. doi: 10.1016/j.brainres.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Macbeth AH, et al. Effects of multiparity on recognition memory, monoaminergic neurotransmitters, and brain-derived neurotrophic factor (BDNF) Horm Behav. 2008;54:7–17. doi: 10.1016/j.yhbeh.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adhikari A, et al. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65:257–269. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kirkpatrick B, et al. Limbic system fos expression associated with paternal behavior. Brain Res. 1994;658:112–118. doi: 10.1016/s0006-8993(09)90016-6. [DOI] [PubMed] [Google Scholar]
- 85.Parker KJ, et al. Paternal behavior is associated with central neurohormone receptor binding patterns in meadow voles (Microtus pennsylvanicus) Behav Neurosci. 2001;115:1341–1348. doi: 10.1037//0735-7044.115.6.1341. [DOI] [PubMed] [Google Scholar]
- 86.Wang ZX, et al. Hypothalamic vasopressin gene expression increases in both males and females postpartum in a biparental rodent. J Neuroendocrinol. 2000;12:111–120. doi: 10.1046/j.1365-2826.2000.00435.x. [DOI] [PubMed] [Google Scholar]
- 87.de Jong TR, et al. From here to paternity: neural correlates of the onset of paternal behavior in California mice (Peromyscus californicus) Horm Behav. 2009;56:220–231. doi: 10.1016/j.yhbeh.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 88.Lee AW, Brown RE. Comparison of medial preoptic, amygdala, and nucleus accumbens lesions on parental behavior in California mice (Peromyscus californicus) Physiol Behav. 2007;92:617–628. doi: 10.1016/j.physbeh.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 89.Lonstein JS. Effects of dopamine receptor antagonism with haloperidol on nurturing behavior in the biparental prairie vole. Pharmacol Biochem Behav. 2002;74:11–19. doi: 10.1016/s0091-3057(02)00952-8. [DOI] [PubMed] [Google Scholar]
- 90.Strathearn L, et al. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rees SL, et al. The effects of adrenalectomy and corticosterone replacement on induction of maternal behavior in the virgin female rat. Horm Behav. 2006;49:337–345. doi: 10.1016/j.yhbeh.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 92.Feldman R, et al. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent–infant contact. Psychoneuroendocrinology. 2010;35:1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 93.Fleming AS, et al. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Horm Behav. 2002;42:399–413. doi: 10.1006/hbeh.2002.1840. [DOI] [PubMed] [Google Scholar]
- 94.Brown RE, et al. Hormonal responses of male gerbils to stimuli from their mate and pups. Horm Behav. 1995;29:474–491. doi: 10.1006/hbeh.1995.1275. [DOI] [PubMed] [Google Scholar]
- 95.Reburn CJ, Wynne-Edwards KE. Hormonal changes in males of a naturally biparental and a uniparental mammal. Horm Behav. 1999;35:163–176. doi: 10.1006/hbeh.1998.1509. [DOI] [PubMed] [Google Scholar]
- 96.Trainor BC, Marler CA. Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc Biol Sci. 2002;269:823–829. doi: 10.1098/rspb.2001.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wynne-Edwards KE, Timonin ME. Paternal care in rodents: weakening support for hormonal regulation of the transition to behavioral fatherhood in rodent animal models of biparental care. Horm Behav. 2007;52:114–121. doi: 10.1016/j.yhbeh.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 98.O’Hara MW. Postpartum depression: what we know. J Clin Psychol. 2009;65:1258–1269. doi: 10.1002/jclp.20644. [DOI] [PubMed] [Google Scholar]
- 99.Schumacher M, et al. Bringing birth-related paternal depression to the fore. Women Birth. 2008;21:65–70. doi: 10.1016/j.wombi.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 100.Paulson JF, et al. Early parental depression and child language development. J Child Psychol Psychiatry. 2009;50:254–262. doi: 10.1111/j.1469-7610.2008.01973.x. [DOI] [PubMed] [Google Scholar]
- 101.Goodman JH. Paternal postpartum depression, its relationship to maternal postpartum depression, and implications for family health. J Adv Nurs. 2004;45:26–35. doi: 10.1046/j.1365-2648.2003.02857.x. [DOI] [PubMed] [Google Scholar]
- 102.Ramchandani P, et al. Paternal depression in the postnatal period and child development: a prospective population study. Lancet. 2005;365:2201–2205. doi: 10.1016/S0140-6736(05)66778-5. [DOI] [PubMed] [Google Scholar]
- 103.Stein A, et al. The influence of maternal depression, caregiving, and socioeconomic status in the post-natal year on children’s language development. Child Care Health Dev. 2008;34:603–612. doi: 10.1111/j.1365-2214.2008.00837.x. [DOI] [PubMed] [Google Scholar]
- 104.Jung V, et al. Interventions with depressed mothers and their infants: modifying interactive behaviours. J Affect Disord. 2007;98:199–205. doi: 10.1016/j.jad.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 105.Paulson JF, et al. Individual and combined effects of postpartum depression in mothers and fathers on parenting behavior. Pediatrics. 2006;118:659–668. doi: 10.1542/peds.2005-2948. [DOI] [PubMed] [Google Scholar]
- 106.Tronick E, et al. The infant’s response to entrapment between contradictory messages in face-to-face interaction. J Am Acad Child Psychiatry. 1978;17:1–13. doi: 10.1016/s0002-7138(09)62273-1. [DOI] [PubMed] [Google Scholar]
- 107.Adamson LB, Frick JE. The still face: a history of a shared experimental paradigm. Infancy. 2003;4:451–473. [Google Scholar]
- 108.Huot RL, et al. Foster litters prevent hypothalamic–pituitary–adrenal axis sensitization mediated by neonatal maternal separation. Psychoneuroendocrinology. 2004;29:279–289. doi: 10.1016/s0306-4530(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 109.Liu D, et al. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 110.Bredy TW, et al. Maternal care influences neuronal survival in the hippocampus of the rat. Eur J Neurosci. 2003;18:2903–2909. doi: 10.1111/j.1460-9568.2003.02965.x. [DOI] [PubMed] [Google Scholar]
- 111.Mirescu C, et al. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- 112.Gos T, et al. Stress-induced synaptic changes in the rat anterior cingulate cortex are dependent on endocrine developmental time windows. Synapse. 2008;62:229–232. doi: 10.1002/syn.20477. [DOI] [PubMed] [Google Scholar]
- 113.Bredy TW, et al. Effect of resource availability on biparental care, and offspring neural and behavioral development in the California mouse (Peromyscus californicus) Eur J Neurosci. 2007;25:567–575. doi: 10.1111/j.1460-9568.2006.05266.x. [DOI] [PubMed] [Google Scholar]
- 114.Helmeke C, et al. Paternal deprivation during infancy results in dendrite- and time-specific changes of dendritic development and spine formation in the orbitofrontal cortex of the biparental rodent Octodon degus. Neuroscience. 2009;163:790–798. doi: 10.1016/j.neuroscience.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 115.Ovtscharoff W, Jr, et al. Lack of paternal care affects synaptic development in the anterior cingulate cortex. Brain Res. 2006;1116:58–63. doi: 10.1016/j.brainres.2006.07.106. [DOI] [PubMed] [Google Scholar]
- 116.Francis DD, et al. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci. 2002;22:7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bredy TW, et al. Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience. 2003;118:571–576. doi: 10.1016/s0306-4522(02)00918-1. [DOI] [PubMed] [Google Scholar]
- 118.Leuner B, et al. Dendritic growth in medial prefrontal cortex and cognitive flexibility are enhanced during the postpartum period. J Neurosci. doi: 10.1523/JNEUROSCI.3388-10.2010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]