Abstract
Background
LX-1031 is an oral, small-molecule tryptophan 5-hydroxylase (TPH) inhibitor that reduces serotonin (5-HT) synthesis peripherally. It has potential for illnesses characterized by excess 5-HT, such as diarrhea-predominant irritable bowel syndrome (IBS-D) and carcinoid diarrhea. In vitro, inhibition of TPH1 occurred in 10−8 – 10−7 M range. In vivo in rodents, LX-1031 has no effect on brain 5-HT while dose-dependently reducing 5-HT, particularly in the small bowel.
Pharmacokinetics
After oral LX1031 in humans, systemic exposure is very low, plasma concentrations are linear in dose range 250 mg QD to 750 mg QID; the median T1/2 for elimination is ~20 hrs, and repeat administration for 14 days doubles Cmax.
Pharmacodynamics
In ascending-single-dose and multiple dose (14 day) trials in healthy volunteers, LX-1031 2g-4g/day significantly reduced urinary 5-hydroxyindoleacetic acid (5-HIAA) starting by day 5, and persisting over the 14 day exposure.
Clinical safety and efficacy
There are no dose limiting toxicities in healthy subjects or remarkable adverse effects in clinical trials to date. Over a 28-day treatment period, LX-1031 was associated with improved weekly global scores (2/4 weeks) and improved stool consistency with lower urinary 5-HIAA excretion.
Conclusion
LX-1031 appears promising for chronic diarrhea associated with increased 5-HT expression including IBS-D. Optimal doses, efficacy and safety in IBS clinical trials need to be fully elucidated; low systemic exposure, selectivity for TPH1 over TPH2, and lack of effect on brain 5-HT in several species suggest that LX-1031 is unlikely to cause affective disorders.
Keywords: irritable bowel, carcinoid
LX-1031 is a heterocyclic substituted phenylalanine analog, an oral small-molecule (molecular weight 538) tryptophan hydroxylase (TPH) inhibitor that reduces synthesis of serotonin (5-HT) peripherally and is being developed for conditions characterized by excess 5-HT expression such as diarrhea-predominant irritable bowel syndrome (IBS-D) and, possibly, carcinoid diarrhea. The goal of blocking the effects of excessive 5-HT is certainly not new (1). However, prior approaches aimed at the inhibition of the synthesis of 5-HT, as with para-chlorophenylalanine, have been impeded by the central adverse effects of inhibition of brain 5-HT synthesis with consequent affective disorders (2,3).
Carcinoid diarrhea is the principal condition resulting from the excessive production of 5-HT usually by metastatic tumor in the liver; it responds to inhibition of 5-HT’s effects as with octreotide treatment (4,5), or to blocking the effects of 5-HT on the motor (6) and secretory (7,8) mechanisms that result in diarrhea as with alosetron (9). Tryptophan hydroxylase is expressed in carcinoid tumors (10) and may potentially be a target for pharmacological inhibition of 5-HT synthesis in this class by an agent that has sufficient systemic exposure; this might constitute an effective treatment for carcinoid diarrhea. However, carcinoid diarrhea is a relatively rare disorder compared to the chronic diarrhea associated with IBS.
The objective of this review is to appraise the pharmacology of LX-1031 and to assess its potential relative to medications in development for the treatment of IBS. IBS is considered to be a disease of the brain-gut axis. It may involve a broad range of physiological and psychological alterations affecting brain-gut regulation, gut function, visceral perception, and mucosal integrity and function (11). Published evidence supports a role of psychosocial (e.g. life event stress) and physical (e.g., enteric infections) stressors as central and peripheral triggers, respectively (12), and a putative role of low-grade chronic inflammation in the pathogenesis of IBS (12).
Current Treatment of IBS
Meta-analyses suggest (13) that several treatments such as peppermint oil, antidepressants, and probiotics based on Bifidobacteria are efficacious in treatment of IBS. The pipeline of medical treatments in IBS is replete with inconsistent or unclear results and failed drug development programs (14). Some targets for therapy are new such as the chloride channels; others are new approaches with promise to safely restore normal bowel function by stimulating motility and secretion. On the other hand, there are still no definite targets or proven therapies for visceral pain.
The predominant mechanism targeted for IBS-D is the 5-HT3 receptor with antagonists like ramosetron. This class of drugs is associated with ischemic colitis and complications of constipation, and, over a 6.5 year period, only ~28,000 patients received the approved drug, alosetron, in the United States after the implementation of a risk management plan (15). A recently proposed use of the agent, colesevelam, is based on sequestration of bile acids and appears mostly applicable in patients with high rates of bile acid synthesis and presumably bile acid malabsorption (16). Rifaximin, a non-absorbable antibiotic, appears efficacious in non-constipated IBS. Current status of the efficacy and safety of new 5-HT4 agonists, 5-HT3 antagonists, intestinal secretagogues (chloride channel activators, guanylate cyclase C agonists), bile acid modulation, anti-inflammatory approaches, κ-opioid agonist, pregabalin and gabapentin is reviewed elsewhere in detail (17), and a summary is provided in Table I. Thus, there is a continued unmet need for new, safe, effective treatment of diarrhea and global symptoms in IBS-D.
Table 1.
Summary of Current and Most Promising Novel Therapies for IBS
| Drug | Drug Class | Pharmacodynamic | Clinical Efficacy | Adverse effects |
|---|---|---|---|---|
| Antispasmodics, peppermint oil | Antispasmodics, peppermint oil | Muscle relaxant | Episodic pain; older, relatively small trials | - |
| Prucalopride | 5-HT4 receptor agonist | Accelerate colon transit | Extensive clinical trial efficacy for CC, not for IBS | Concerns about 5-HT4 cardiac effects appear unfounded based on dose difference to stimulate IKr |
| Velusetrag | 5-HT4 receptor agonist | Single phase II CC study shows efficacy | ||
| Naronapride | 5-HT4 receptor agonist | Single phase II CC study shows efficacy | ||
| Alosetron | 5-HT3 receptor antagonist | Delay colon transit, reduce visceral sensation | Extensive clinical trial efficacy for IBS-D | Significant constipation, class-related ischemic colitis |
| Ramosetron | 5-HT3 receptor antagonist | 2 trials clinical efficacy for IBS-D | ||
| Dexloxiglumide | CCK1 receptor antagonist | Delay asc. colon transit | Inconsistent efficacy in trials | - |
| Lubiprostone | Cl-channel opener | Accelerate SB and colon transit | Extensive clinical trial efficacy for CC, IBS-C | Nausea in ~25% |
| Linaclotide | Guanylate cyclase C receptor agonist | Accelerate colon transit | Extensive clinical trial efficacy for CC, IBS-C | Diarrhea |
| Colesevelam | Bile acid binder | Slows colon transit based on BA synthesis rate | No clinical trials | - |
| Na cromoglycate | Mast cell stabilizer | Reduced mast cells | Weak clinical trial efficacy | - |
| Ketotifen | Mast cell stabilizer, histamine H1 receptor antagonist | Unclear, ? reduced sensation | Weak clinical trial efficacy | Central effects e.g. fatigue |
| Mesalazine | 5-ASA agent | Reduced inflammation | Weak clinical trial efficacy | - |
| Pregabalin | GABA; α2δ ligand | Reduced pain in animal and human models of IBS | No completed clinical trials | Central effects |
| Tricyclics | Tricyclics | Few small positive trials; Meta-analysis unconvincing | Central effects; constipation; anti-cholinergic effects | |
| SSRI/SNRI | SSRI/SNRI | Central effects; diarrhea | ||
| Dextofisopam | Benzodiazepine | Unclear | Weak clinical trial efficacy | Central effects? |
| Asimadoline | K opioid receptor agonist | Reduced pain in models | Weak clinical trial efficacy | - |
| Probiotics | Unclear | Bifidobacteria, combined probiotics beneficial | - | |
| Rifaximin | Unabsorbed antibiotic | Adequate relief of IBS and bloating | Extensive clinical trial efficacy for IBS-non-C | Efficacy persists for 10 weeks after stopping Rx |
Serotonin Synthesis, Actions in GI Tract and Putative Role in IBS Pathogenesis
It is estimated that 95% of the 5-HT in the human body is located in the GI tract, with 90% being in enterochromaffin (EC) cells in the epithelial layer and 5% in the neural structures intrinsic to the bowel wall. 5-HT is also present in blood platelets. 5-HT is synthesized from tryptophan sequentially by tryptophan hydroxylase (TPH) and aromatic amino acid decarboxylase (AADC). TPH exists as two isoforms: TPH1 and TPH2. These share ~70% identity and they are ~50% identical to phenylalanine and tyrosine hydroxylases. In enterochromaffin (EC) cells of the gut, TPH1 is primarily expressed; in contrast, TPH2 is expressed exclusively in neuronal cells, including the myenteric plexus. 5-HT is involved in motility, sensation and secretion in the gastrointestinal tract (18).
There are seven main classes of 5-HT receptors, with several subclasses that can be differentiated on the basis of their molecular structure, transduction pathways and functions (19). To date, the serotonergic receptors of greatest relevance in the GI tract are the 5-HT3 receptors which are ion channels, and the 5-HT4 receptors which have 7 transmembrane domains. 5-HT reuptake is a mechanism for inactivation of released 5-HT in the digestive tract as well as in the central nervous system (CNS) and platelets. 5-HT reuptake is controlled by a serotonin transporter protein (SERT) which is one of the solute carrier family of proteins (SLC6A4).
The principles linking 5-HT as a critical transmitter involved in the pathophysiology of IBS are summarized here and have been discussed in detail elsewhere (18).
The most consistent findings are the increase in plasma 5-HT in diarrheal diseases such as carcinoid diarrhea and IBS-D, including in children with IBS-D, and reduced levels in IBS-C (20–24). In addition, some reports document alterations in tissue levels of 5-HT and of the reuptake protein SERT in IBS. Thus, for example, post-infectious IBS is associated with increased 5-HT content in rectal biopsies (25,26); on the other hand, tissue expression of SERT in rectal or colonic biopsies is inconsistent in different reports in the literature (27–29). Reduced platelet SERT has been reported with increased plasma 5-HT in IBS-D, and platelet SERT influences the response to the 5-HT3 receptor antagonist, alosetron (30). Several groups investigated whether genetic control of SERT was associated with IBS, in view of the potential role of SERT in determining plasma and tissue 5-HT levels. Meta-analysis shows that 5-HTTLPR genotype (the gene controlling the promoter for SERT protein synthesis) is not significantly associated with IBS in Caucasians or Asians (31). Conversely, Niesler et al. have demonstrated association of a functional variant in the 5-HT3e gene and IBS-D (32).
Serotonergic agents are used in therapy of lower functional gastrointestinal disorders. In IBS-D, alosetron (which blocked the effects at 5-HT3 receptors that are relevant to stimulation of motility and secretion and transmission of pain in the gut) shows considerable efficacy in the relief of urgency, diarrhea and abdominal pain. These agents did not reduce the 5-HT content, production or release from the gut. The early generation 5-HT4 receptor agonists, such as cisapride and tegaserod, reversed slow motility and relieved constipation, but they have been withdrawn because of cardiac or vascular adverse effects. These agents activate receptors on intrinsic cholinergic neurons to stimulate motility without increasing the levels of 5-HT which appears to be deficient in patients with IBS-C or diseases associated with constipation (33). Newer 5-HT3 receptor antagonists (34–36) and 5-HT4 receptor agonists (37–39) are efficacious, appear to be safer than earlier generation agents in these classes, and promise to provide relief for IBS symptoms in patients (40).
A novel class of compounds (of which the prototype is LX-1031) is being developed that directly inhibits 5-HT synthesis in enterochromaffin cells, potentially reversing the underlying pathogenetic factor in conditions like IBS-D. This could be an alternative to the application of 5-HT3 receptor antagonists in IBS-D.
Serotonin Synthesis Inhibition
Direct blockade of 5-HT synthesis can be achieved through inhibition of TPH with para-chlorophenylalanine (pCPA); however, this results in depletion of brain 5-HT, and it is linked to depression and other alterations in CNS-mediated functions such as weight loss ataxia and debilitation (41), thus precluding therapeutic use.
LX-1031 is an oral, small-molecule TPH inhibitor that reduces synthesis of 5-HT peripherally. LX-1031 is one of a series of substituted 3-(4-(1,3,5-triazin-2-yl)-phenyl)-2-aminopropanoic acids that were optimized through extensive structure-activity relationship studies (42,43) to act locally in the intestine with minimal systemic exposure after oral administration. The class of drugs does not penetrate the blood-brain barrier, in part, as a result of its molecular size.
Preclinical Development
Preclinical pharmacology
The series of aminopropanoic acid compounds (that include LP-533401 and its ethyl ester prodrug LP-615819) inhibit TPH1 at low concentrations: the in vitro IC50 value of LP-533401 was 0.7μM against purified human TPH1 in enzyme and cell-based assays, and IC50 value was 0.4 μM in inhibition of 5-HT synthesis in the rat mastocytoma cell line RBL-2H3 (44). This class of compounds, exemplified by effects in vitro in enzyme and cell-based assays (44), inhibits both TPH1 and TPH2. The selectivity for inhibition of TPH1 in the gastrointestinal tract rather than the brain is, therefore, based on distribution of the compound and failure to cross the blood-brain barrier. Thus, Shi et al. reported that the concentration of LP533401 in the brain is approximately 1% of that in the plasma after dosing at 10mg/kg by oral gavage (42, 44).
In mice, administered doses of 0, 30 and 90 mg/kg in vivo LP-533401 had no effect on 5-HT levels in the brain, but it dose-dependently reduced 5-HT levels in the duodenum, jejunum and ileum. At equal doses, LP-615819 was more potent than para-chlorophenylalanine in reducing 5-HT in the jejunum. However, unlike para-chlorophenylalanine, LP-615819 did not reduce brain 5-HT (44).
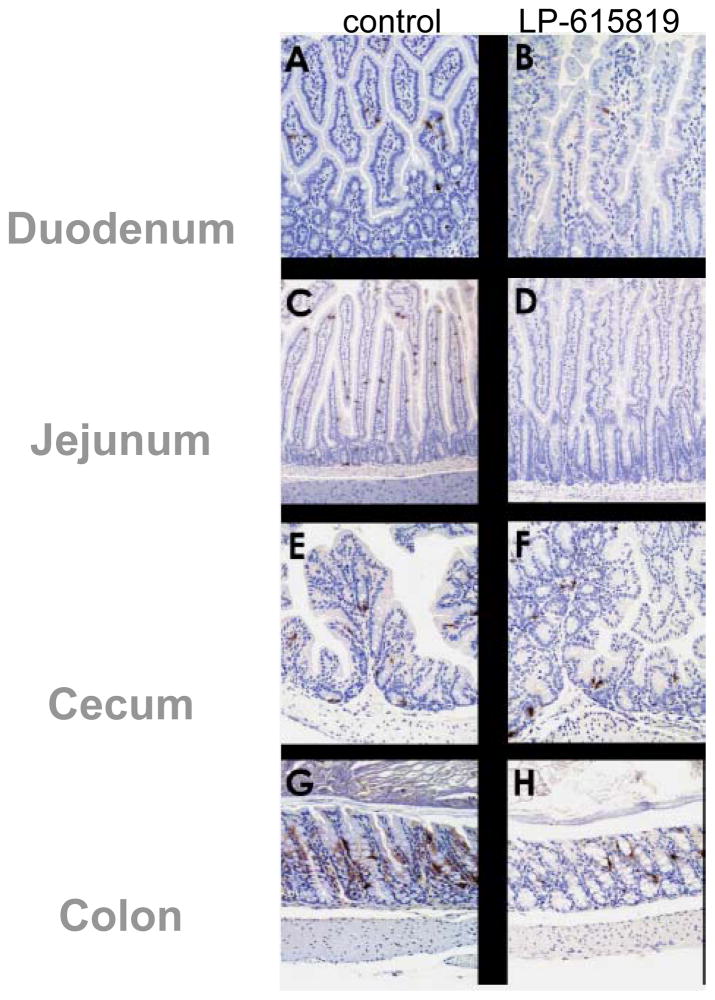
Dose-dependent 5-HT reduction in the gastrointestinal tract by LP-615819 was also demonstrated in the mouse in studies that compared vehicle, 20, 45 and 90 mg/kg. Effects in duodenum, jejunum and ileum were consistent (44); effects in the antrum and colon were less dramatic (about 25 and 50% relative reductions compared to vehicle); in contrast, there were no effects on brain 5-HT expression. The effects on intestinal 5-HT expression were confirmed by immunohistochemistry (Figure 1).
Figure 1.
Effect of LP-651819 on expression of 5-HT in mice, based on immunohistochemistry. Reproduced with permission from ref. 44, Liu et al. JPET 2008;325:47–55.
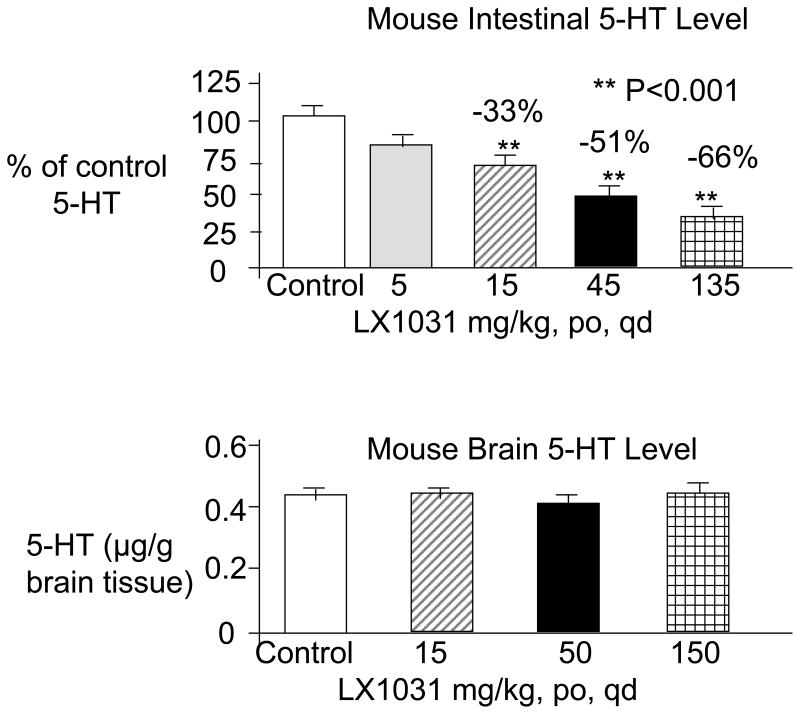
With oral administration of LX-1031 in mice, the average 5-HT reductions in the jejunum relative to control were ~33, 51, and 66% with the 15, 45 and 135 mg/kg/day doses respectively (Figure 2); the effect of 5 mg/kg/day on GI 5-HT content was not significantly different from control. In separate studies, 15, 50 and 150 mg/kg p.o. daily did not alter brain 5-HT content [(45) Figure 2]. In a preliminary report (in abstract form), the effects of LX-1031, 100mg/kg daily, on 5-HT levels in jejunal mucosa were reversible within 2 days of discontinuation in mice (45).
Figure 2.
Administration of LX-1031 reduces 5-HT in the GI tract but not in the brain. Reproduced from ref. 45, Brown PM et al. Am J Gastroenterology 2007;102:S961 (abstract).
Animal pharmacology
In a dose-response study of 10, 30 and 90 mg/kg, LP-615819 reduced by 50% (relative to the active comparator, ondansetron) the cisplatin-induced, centrally-mediated emesis associated with release of gut 5-HT in ferrets. The anti-emetic effects of LP-615819 were associated with the reduced duodenal and jejunal 5-HT content and no effect on brain 5-HT content.
Pharmacokinetics
Pharmacokinetics
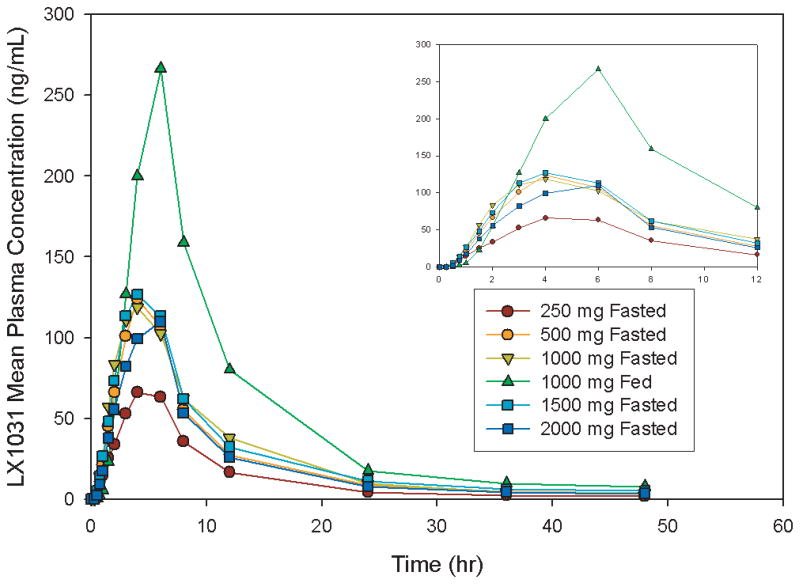
After oral administration of LX-1031, systemic exposure is very low; the mean t1/2 of elimination is ~20 hours across all dose groups tested. Cmax ranged from 84.4 to 384 ng/mL under fed conditions. LX-1031 was detected in plasma ~30 minutes after a single oral dose on Day 1. Plasma concentrations and exposures increased over the range 250 mg q.d. to 750 mg q.i.d., but plasma concentrations were similar between 750 mg q.i.d. and 1000 mg q.i.d. (Figure 3). A preliminary report indicates the mean AUC (0–6h) was approximately 3-fold higher and the Cmax was 2-fold higher on Day 14 than Day 1 for all dose groups, and the elimination pattern of LX-1031 from plasma was multiphasic (45). A full report is awaited to assess the potential for accumulation of medication, as well as the relationship of trough and peak levels to pharmacodynamic effects.
Figure 3.
LX-1031 mean plasma concentration over time.
Inset: The plasma concentrations are plotted over the first 12 hours after oral administration. Reproduced from ref. 45, Brown PM et al. Am J Gastroenterology 2007;102:S961 (abstract).
In view of the low systemic exposure of LX-1031, treatment of carcinoid diarrhea may require development of an analog [LX-1032 (46)] with greater systemic exposure to target synthesis of 5-HT in metastatic carcinoid tumor cells. Review of LX-1032 is outside the scope of the current review of LX-1031.
Pharmacodynamics in healthy volunteers
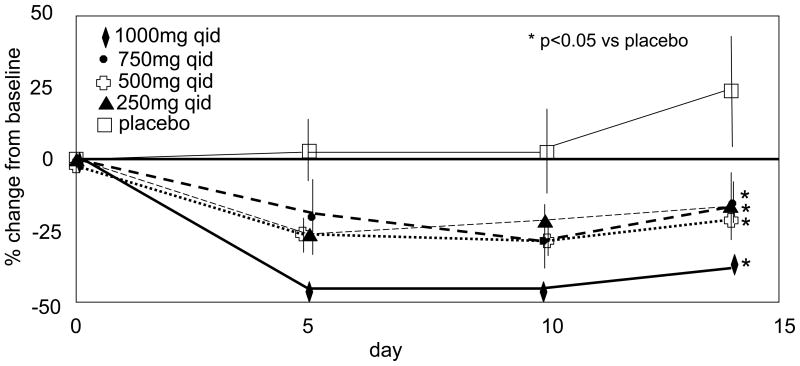
In double-blind, serial, multiple-ascending dose tolerance studies (doses of LX-1031 ranging from 250 mg q.d. to 1000 mg q.i.d.), mean 5HIAA reductions of 33% were noted (47). In phase I multiple-dose studies, 54 healthy volunteers received the drug for up to 14 days while the diet was strictly controlled to avoid 5-HT-rich foods. Urine 24-hour collections were obtained at baseline and on days 5, 10 and 14. Significant reductions in urinary 5-HIAA, observed by day 5, persisted over the duration of exposure [(47) Figure 4].
Figure 4.
Inhibition of 5-HT synthesis shown by reduced urinary 5-HIAA. Reproduced from reference 47, Brown P et al. Gastroenterology 2009;136(Suppl 1):A237 (abstract).
Safety
In studies conducted in healthy normal participants receiving 250 mg up to 2000 mg, single daily doses in 39 volunteers (30 male, 9 female), and multiple doses in 72 volunteers (54 males and 18 females) over 2 weeks were well tolerated with no dose limiting toxicities reported. LX-1031 was well tolerated at doses up to 4g/day over 14 days (47).
Clinical Development: Phase II Study
LX-1031 was tested in a multicenter, double-blind, placebo-controlled, randomized clinical trial (NCT00813098) of 250 or 1000 mg q.i.d. vs. placebo for 28 days in 155 patients with non-constipated IBS (48). The results appear in an abstract (48): Urine and blood samples were collected pre-dose, on Day 28, and 2 weeks after last dose in a subset of patients (n=80) to evaluate blood 5-HT and urinary 5-HIAA. A dose-dependent reduction in 24-hour urinary 5-HIAA was observed (49), indicating pharmacologic inhibition of peripheral 5-HT production. A reduction in whole-blood 5-HT was also observed. These reductions in 5-HIAA correlated with improvements in global assessment of adequate relief and stool consistency in patients with non-constipating IBS. The study endpoint was global assessment of relief of pain/discomfort weekly, and responders were defined based on weekly global relief in 2/4 weeks: 61% of 1000 mg q.i.d. LX-1031-treated and 45% of placebo-treated patients achieved this primary study endpoint. It is unclear how the drug performed using the more stringent definition of weekly responders based on 75% of weeks of the trial. LX-1031, 1000 mg dose, improved stool consistency. This effect occurred from week 1 and there was significant correlation with urine 5-HIAA reduction (49).
The preliminary report provided no data on the effects of the 250 mg q.i.d. dose in the phase II trial.
No Phase III trials have been reported to date.
Adverse Effects
Data available in an abstract (47) suggest that, in two small clinical trials (LX.1031.102, n=30 and LX.1031.103, n=24), LX-1031 was well tolerated, with no imbalance in adverse effects other than incidental injury (4/30 vs. 0/18 for placebo), skin or subcutaneous disorders (4/30 vs. 0/18 for placebo) and vascular disorders (2/30 vs. 0/18 for placebo) in one of the two clinical trials. In the other clinical trial with 24 participants, none of these adverse effects were recorded.
In the phase II clinical trial (48), LX-1031 was well tolerated at both dose levels (250 and 1000 mg q.i.d.) over the 28-day treatment period with no evidence of dose-limiting toxicity.
Summary and a Look to the Future
LX-1031 represents a novel class of drugs that has the potential to reverse one of the mechanisms that mediates symptoms of IBS, including diarrhea and, potentially, also pain. Efficacy studies have demonstrated that an increased proportion of LX-1031-treated patients with IBS experienced global relief and improved bowel function (e.g. stool consistency) compared with patients who received placebo. The multiple studies in animal species suggest the inhibition is selective to the periphery and the central effects attributed to the drug, para-chlorophenylalanine, are avoided.
The proof of concept studies to data have focused on the use of a biochemical marker, that is, urinary 5-HIAA excretion, which reflects metabolism of 5-HT. Dose selection to date appears based on the urinary 5-HIAA excretion and plasma 5-HT. There are no reports of dose-response studies using pharmacodynamic measurements that more closely reflect the manifestations of IBS, such as colonic transit or sensation. The drug development program has fairly extensively tested in humans the 250 mg and 1000 mg q.i.d. doses. However, in the phase IIB clinical trial, the preliminary report did not provide data on efficacy of the lower dose. Hence, the lowest and highest effective doses are as yet unclear. Further studies are needed using the endpoints proposed for regulatory approval (50); in particular, the potential benefit of TPH1 inhibition for the pain component of IBS requires further study. Whereas, the 5-HT3 receptor antagonist, alosetron, significantly impacted the pain of IBS by targeting visceral afferents that have 5-HT3 receptors (51) or central mechanisms in IBS patients (52), it is uncertain whether global inhibition of 5-HT synthesis by LX-1031 will have direct effects on pain pathways.
While we await the results of phase III clinical trials, the potential adverse effects and safety from inhibition of peripheral 5-HT synthesis in large numbers of patients are similarly important. Will TPH inhibitors induce severe constipation? Will the imbalance of 5-HT alter platelet function or function of vascular smooth muscle? Will this class of drugs induce ischemic colitis, which remains a disincentive to prescription of 5-HT3 receptor antagonists in IBS patients? On the other hand, there appears to be a potential advantage for women with IBS-D receiving TPH1 inhibitors. Thus, this class of compounds appears to have an effect on osteoporosis, based on the observation that LP533401, another small molecule inhibitor of TPH1, administered orally daily for up to six weeks acts prophylactically or therapeutically, in a dose-dependent manner, to treat osteoporosis in ovariectomized rodents by increase in bone formation (53).
In conclusion, small molecule TPH1 inhibitors, of which the prototype is LX-1031, constitute an intriguing class of novel compounds which has the potential to improve bowel dysfunction in conditions associated with increased production of 5-HT. This class of compounds has potential in IBS-D and carcinoid diarrhea. Optimal dose, efficacy and safety require further elucidation.
Acknowledgments
Dr. Camilleri’s work in IBS is supported by NIH grants DK79866 and DK86182.
Footnotes
Author’s Contributions: Dr. Camilleri conceived, researched, and wrote this paper.
Disclosures: Dr. Camilleri has no disclosures relevant to this subject.
References
- 1.Editorial: Serotonin now: clinical implications of inhibiting its synthesis with para-chlorophenylalanine. Ann Intern Med. 1970;73:607–30. doi: 10.7326/0003-4819-73-4-607. [DOI] [PubMed] [Google Scholar]
- 2.Shopsin B, Friedman E, Gershon S. Parachlorophenylalanine reversal of tranylcypromine effects in depressed patients. Arch Gen Psychiatry. 1976;33:811–9. doi: 10.1001/archpsyc.1976.01770070041003. [DOI] [PubMed] [Google Scholar]
- 3.Ruhé HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12:331–59. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 4.Kvols LK, Moertel CG, O’Connell MJ, Schutt AJ, Rubin J, Hahn RG. Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue. N Engl J Med. 1986;315:663–6. doi: 10.1056/NEJM198609113151102. [DOI] [PubMed] [Google Scholar]
- 5.Saslow SB, O’Brien MD, Camilleri M, et al. Octreotide inhibition of flushing and colonic motor dysfunction in carcinoid syndrome. Am J Gastroenterol. 1997;92:2250–6. [PubMed] [Google Scholar]
- 6.von der Ohe MR, Camilleri M, Kvols LK, Thomforde GM. Motor dysfunction of the small bowel and colon in patients with the carcinoid syndrome and diarrhea. N Engl J Med. 1993;329:1073–8. doi: 10.1056/NEJM199310073291503. [DOI] [PubMed] [Google Scholar]
- 7.Donowitz M, Charney AN, Heffernan JM. Effect of serotonin treatment on intestinal transport in the rabbit. Am J Physiol. 1977;232:E85–94. doi: 10.1152/ajpendo.1977.232.1.E85. [DOI] [PubMed] [Google Scholar]
- 8.Kisloff B, Moore EW. Effect of serotonin on water and electrolyte transport in the in vivo rabbit small intestine. Gastroenterology. 1976;71:1033–8. [PubMed] [Google Scholar]
- 9.Saslow SB, Scolapio JS, Camilleri M, et al. Medium-term effects of a new 5HT3 antagonist, alosetron, in patients with carcinoid diarrhoea. Gut. 1998;42:628–34. doi: 10.1136/gut.42.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert JA, Bates LA, Ames MM. Elevated aromatic-L-amino acid decarboxylase in human carcinoid tumors. Biochem Pharmacol. 1995;50:845–50. doi: 10.1016/0006-2952(95)02006-x. [DOI] [PubMed] [Google Scholar]
- 11.Camilleri M. Editorial: Mechanisms in IBS: something old, something new, something borrowed…. Neurogastroenterol Motil. 2005;17:311–6. doi: 10.1111/j.1365-2982.2004.00632.x. [DOI] [PubMed] [Google Scholar]
- 12.Spiller RC. Infection, immune function, and functional gut disorders. Clin Gastroenterol Hepatol. 2004;2:445–55. doi: 10.1016/s1542-3565(04)00159-4. [DOI] [PubMed] [Google Scholar]
- 13.Brandt LJ, Chey WD, Foxx-Orenstein AE, et al. An evidence-based systematic review on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;10:S1–35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 14.Camilleri M, Chang L. Challenges to the therapeutic pipeline for irritable bowel syndrome: end points and regulatory hurdles. Gastroenterology. 2008;135:1877–91. doi: 10.1053/j.gastro.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang L, Tong K, Ameen V. Ischemic colitis and complications of constipation associated with the use of alosetron under a risk management plan: clinical characteristics, outcomes, and incidences. Am J Gastroenterol. 2010;105:866–75. doi: 10.1038/ajg.2010.25. [DOI] [PubMed] [Google Scholar]
- 16.Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol. 2010;8:159–65. doi: 10.1016/j.cgh.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camilleri M. Review Article: New receptor targets for medical therapy in irritable bowel syndrome. Aliment Pharmacol Ther. 2010;31:35–46. doi: 10.1111/j.1365-2036.2009.04153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camilleri M. Serotonin in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obesity. 2009;16:53–9. doi: 10.1097/med.0b013e32831e9c8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim D-Y, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol. 2000;95:2698–709. doi: 10.1111/j.1572-0241.2000.03177.x. [DOI] [PubMed] [Google Scholar]
- 20.Dunlop SP, Coleman NS, Blackshaw PE, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–57. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 21.Houghton LA, Atkinson W, Whitaker RP, Whorwell PJ, Rimmer MJ. Increased platelet depleted plasma 5-hydroxytryptamine concentration following meal ingestion in symptomatic female subjects with diarrhoea predominant irritable bowel syndrome. Gut. 2003;52:663–70. doi: 10.1136/gut.52.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuo XL, Li YQ, Yang XZ, et al. Plasma and gastric mucosal 5-hydroxytryptamine concentrations following cold water intake in patients with diarrhea-predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2007;22:2330–7. doi: 10.1111/j.1440-1746.2006.04772.x. [DOI] [PubMed] [Google Scholar]
- 23.Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006;130:34–43. doi: 10.1053/j.gastro.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 24.Faure C, Patey N, Gauthier C, Brooks EM, Mawe GM. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology. 2010;139:249–58. doi: 10.1053/j.gastro.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–11. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miwa J, Echizen H, Matsueda K, Umeda N. Patients with constipation-predominant irritable bowel syndrome (ibs) may have elevated serotonin concentrations in colonic mucosa as compared with diarrhea-predominant patients and subjects with normal bowel habits. Digestion. 2001;63:188–94. doi: 10.1159/000051888. [DOI] [PubMed] [Google Scholar]
- 27.Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin re-uptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–64. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Kerckhoffs AP, Ter Linde JJ, Akkermans LM, Samsom M. Trypsinogen IV, serotonin transporter transcript levels and serotonin content are increased in small intestine of irritable bowel syndrome patients. Neurogastroenterol Motil. 2008;20:900–7. doi: 10.1111/j.1365-2982.2008.01100.x. [DOI] [PubMed] [Google Scholar]
- 29.Camilleri M, Andrews CN, Bharucha AE, et al. Alterations in expression of p11 and SERT in mucosal biopsy specimens of patients with irritable bowel syndrome. Gastroenterology. 2007;132:17–25. doi: 10.1053/j.gastro.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellini M, Rappelli L, Blandizzi C, et al. Platelet serotonin transporter in patients with diarrhea-predominant irritable bowel syndrome both before and after treatment with alosetron. Am J Gastroenterol. 2003;98:2705–11. doi: 10.1111/j.1572-0241.2003.08669.x. [DOI] [PubMed] [Google Scholar]
- 31.Van Kerkhoven LA, Laheij RJ, Jansen JB. Meta-analysis: a functional polymorphism in the gene encoding for activity of the serotonin transporter protein is not associated with the irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:979–86. doi: 10.1111/j.1365-2036.2007.03453.x. [DOI] [PubMed] [Google Scholar]
- 32.Kapeller J, Houghton LA, Mönnikes H, et al. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet. 2008;17:2967–77. doi: 10.1093/hmg/ddn195. [DOI] [PubMed] [Google Scholar]
- 33.Gershon MC, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414. doi: 10.1053/j.gastro.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Andresen V, Montori VM, Keller J, West C, Layer P, Camilleri M. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2008;6:545–55. doi: 10.1016/j.cgh.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsueda K, Harasawa S, Hongo M, Hiwatashi N, Sasaki D. A phase II trial of the novel serotonin type 3 receptor antagonist ramosetron in Japanese male and female patients with diarrhea-predominant irritable bowel syndrome. Digestion. 2008;77:225–35. doi: 10.1159/000150632. [DOI] [PubMed] [Google Scholar]
- 36.Matsueda K, Harasawa S, Hongo M, Hiwatashi N, Sasaki D. A randomized, double-blind, placebo-controlled clinical trial of the effectiveness of the novel serotonin type 3 receptor antagonist ramosetron in both male and female Japanese patients with diarrhea-predominant irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1202–11. doi: 10.1080/00365520802240255. [DOI] [PubMed] [Google Scholar]
- 37.Camilleri M, Vazquez-Roque MI, Burton D, et al. Pharmacodynamic effects of a novel prokinetic 5-HT receptor agonist, ATI-7505, in humans. Neurogastroenterol Motil. 2007;19:30–8. doi: 10.1111/j.1365-2982.2006.00865.x. [DOI] [PubMed] [Google Scholar]
- 38.Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008;358:2344–54. doi: 10.1056/NEJMoa0800670. [DOI] [PubMed] [Google Scholar]
- 39.Manini ML, Camilleri M, Goldberg M, et al. Effects of velusetrag (TD-5108) on gastrointestinal transit and bowel function in health and pharmacokinetics in health and constipation. Neurogastroenterol Motil. 2010;22:42–9. e7–8. doi: 10.1111/j.1365-2982.2009.01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Maeyer JH, Lefebvre RA, Schuurkes JA. 5-HT4 receptor agonists: similar but not the same. Neurogastroenterol Motil. 2008;20:99–112. doi: 10.1111/j.1365-2982.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- 41.Redmond DE, Jr, Maas JW, Kling A, Graham CW, Dekirmenjian H. Social behavior of monkeys selectively depleted of monoamines. Science. 1971;174:428–31. doi: 10.1126/science.174.4007.428. [DOI] [PubMed] [Google Scholar]
- 42.Shi Z-C, Devasagayaraj A, Gu K, et al. Modulation of peripheral serotonin levels by novel tryptophan hydroxylase inhibitors for the potential treatment of functional gastrointestinal disorders. J Med Chem. 2008;51:3684–7. doi: 10.1021/jm800338j. [DOI] [PubMed] [Google Scholar]
- 43.Jin H, Cianchetta G, Devasagayaraj A, et al. Substituted 3-(4-(1,3,5-triazin-2-yl)-phenyl)-2-aminopropanoic acids as novel tryptophan hydroxylase inhibitors. Bioorg Med Chem Lett. 2009;19:5229–32. doi: 10.1016/j.bmcl.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Liu Q, Yang Q, Sun W, et al. Discovery and characterization of novel tryptophan hydroxylase inhibitors that selectively inhibit 5-HT synthesis in the gastrointestinal tract. J Pharmacol Exp Ther. 2008;325:47–55. doi: 10.1124/jpet.107.132670. [DOI] [PubMed] [Google Scholar]
- 45.Brown PM, Jackson JI, Frazier KS, Turner CA, Shi ZC, Liu Q. From mouse knockout to investigational drug: LX1031, a novel potential treatment for irritable bowel syndrome. Am J Gastroenterol. 2007;102:S961. (abstract) [Google Scholar]
- 46.Pappas SC, Brown P, Turnage A, Frazier K, Yang QM, Shi Z-C, Liu Q. LX1032: a potential new therapy for chronic diarrhea in cardinoid syndrome. Gastroenterology. 2009;136(Suppl 1):A352. (abstract) [Google Scholar]
- 47.Freiman J, Jackson J, Frazier KS, Yang QM, Liu Q, Brown P. LX1031: Inhibition of 5-HT synthesis as a new target in the management of irritable bowel syndrome (IBS) Neurogastroenterol Motil. 2009;21:250. [Google Scholar]
- 48.Brown P, Jackson J, Shi Z-C. LX1031: A new approach for managing irritable bowel syndrome (IBS) Gastroenterology. 2009;136(Suppl 1):A237. (abstract) [Google Scholar]
- 49.Zambrowicz B, Brown P, Jackson J, et al. 5-HT biomarker levels correlate with clinical response in phase 2 trial of LX1031, a novel 5-HT synthesis inhibitor for non-constipating IBS. Gastroenterology. 2009;136(Suppl 1):S168. (abstract) [Google Scholar]
- 50.Trentacosti AM, He R, Burke LB, Griebel D, Kennedy DL. Evolution of clinical trials for irritable bowel syndrome: issues in end points and study design. Am J Gastroenterol. 2010;105:731–5. doi: 10.1038/ajg.2010.12. [DOI] [PubMed] [Google Scholar]
- 51.Hicks GA, Coldwell JR, Schindler M, et al. Excitation of rat colonic afferent fibres by 5-HT(3) receptors. J Physiol. 2002;544:861–9. doi: 10.1113/jphysiol.2002.025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayer EA, Berman S, Derbyshire SW, et al. The effect of the 5-HT3 receptor antagonist, alosetron, on brain responses to visceral stimulation in irritable bowel syndrome patients. Aliment Pharmacol Ther. 2002;16:1357–66. doi: 10.1046/j.1365-2036.2002.01287.x. [DOI] [PubMed] [Google Scholar]
- 53.Yadav VK, Balaji S, Suresh PS, et al. Pharmacological inhibition of gut-derived 5-HT synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med. 2010;16:308–12. doi: 10.1038/nm.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]