Abstract
In vitro differentiation of embryonic stem cells is tightly regulated by the same key signaling pathways that control pattern formation during embryogenesis. Small molecules that selectively target these developmental pathways, including Wnt, and BMP signaling, may be valuable for directing differentiation of pluripotent stem cells toward many desired tissue types, but to date only few such compounds have been shown to promote cardiac differentiation. Here, we show that XAV939, a recently discovered small molecule inhibitor of Wnt/β-catenin signaling, can robustly induce cardiomyogenesis in mouse ES cells. Our results suggest that a timely administration of XAV939 immediately following the formation of mesoderm progenitor cells promotes cardiomyogenic development at the expense of other mesoderm derived lineages, including the endothelial, smooth muscle and hematopoietic lineages. Given the critical role that Wnt/β-catenin signaling plays in many aspects of embryogenesis and tissue regeneration, XAV939 is a valuable chemical probe to dissect in vitro differentiation of stem cells and to explore their regenerative potential in variety of contexts.
INTRODUCTION
Pluripotent stem cells, capable of self-renewal and differentiation into multiple tissue types, are a promising source of cells for repairing damaged adult tissues, including the heart (1, 2). The prospect for regenerative therapies using stem cells has been greatly advanced by recent breakthroughs in induced pluripotent stem (iPS) cells, derived from adult somatic tissues (3–8). However, numerous formidable challenges remain, before the regenerative potential of stem cells can be harnessed. Foremost of these is dearth of robust methods and inexpensive small molecule reagents to generate sufficient quantities of desired cell types from pluripotent stem cells. A better understanding and small molecule tools to direct differentiation of stem cells will provide the framework for future efforts to harness their regenerative potential.
Since differentiation of pluripotent stem cells in vitro utilizes the same key developmental programs that guide differentiation during embryogenesis; the ability to precisely control these programs would be a powerful approach to achieve directed differentiation in vitro. For instance, the Wnt/β-catenin and BMP (bone morphogenetic protein) pathways play central roles in both embryonic patterning and in guiding differentiation of pluripotent stem cells (9–12). We recently demonstrated that dorsomorphin, a small molecule BMP inhibitor, could be used for inducing of cardiomyocytes in embryonic stem (ES) cells (13). In the context of ES cell differentiation via embryoid body (EB) formation, small molecules offer two major advantages over protein-based cell signaling modulators, such as Noggin and DKK1, or neutralizing antibodies. First, small molecules can readily penetrate multiple cell layers to modulate signaling through out the EB, yielding more consistent results, while much larger proteins may not be able to access the protein core. Another significant advantage of small molecules is that they are less expensive than recombinant proteins, affording greater flexibility in testing of different direct differentiation protocols and scale-up production of desired cell types.
Versatility of a small molecule is particularly important for functional dissection of developmental pathways, such as the Wnt/β-catenin, which plays critical yet complex roles in regulating a number of diverse developmental events in embryogenesis and ES cell differentiation (11, 14, 15). Since inhibition of Wnt/β-catenin signaling appears to be crucial in cardiomyocyte formation across many models including Xenopus and chick embryos, zebrafish and ES cells (9–11, 15); we examined whether XAV939, a recently discovered small molecule inhibitor of Wnt/β-catenin signaling (Figure 1B) (16), could enhance cardiomyocyte induction in ES cells. Here we show that timely administration of XAV939 robustly promoted cardiomyogenesis in mouse ES cells at the expense of other mesoderm derived lineages, including endothelial, smooth muscle and hematopoietic lineages. Small molecule modulators of developmental signaling such as XAV939 and dorsomorphin show promise as valuable chemical reagents to regulate differentiation of pluripotent stem cells and to probe developmental programs involved in differentiation.
Figure 1. Inhibition of Wnt/β-catenin signaling with the small molecule XAV939 promotes formation of spontaneously beating embryoid bodies.
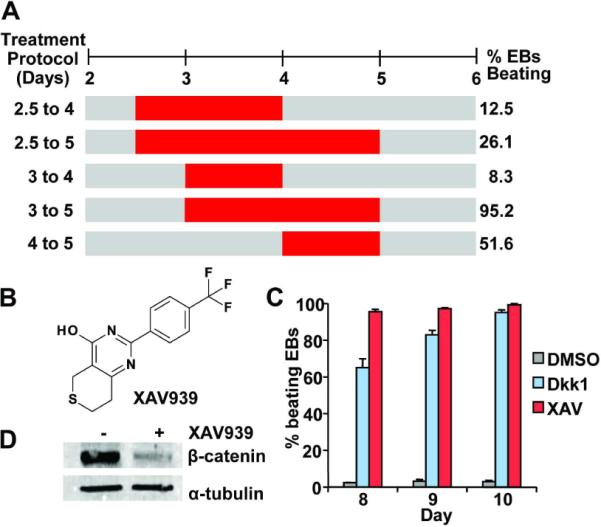
(A) Critical time window for ES cell cardiomyocytes induction with XAV939 (XAV). XAV treatments from Day 2 to 4, Day 2.5 to 5, Day 3 to 4, Day 3 to 5, and Day 4 to 5 were represented by red horizontal bars and the percentages of embryoid bodies (EBs) that beat spontaneously at day 10 of differentiation are shown on the right. Results were obtained from at least 48 EBs for each time point. (B) Chemical structure of XAV939 (3,5,7,8-Tetrahydro-2-[4-(trifluoromethyl)phenyl]-4H-thiopyrano[4,3-d]pyrimidin-4-one). (C) When administered during the day 3 to 5 window, both DKK1 and XAV939 greatly promoted formation of spontaneously beating EBs in comparison to DMSO (P=0.001 for both, P-value was calculated using a two-tailed Student's t test with paired samples throughout this paper, unless otherwise indicated). Results were obtained from at least 48 EBs in 3 independent experiments and standard error was used for graphic plotting throughout this paper, unless otherwise indicated. (D) Western Blot confirmed that β-catenin level was effectively down-regulated in XAV treated EBs (day 5 samples).
RESULTS AND DISCUSSION
Timely chemical inhibition of Wnt/β-catenin signaling induces cardiomyogenesis in mouse ES cells
To assess the impact of XAV939 treatment in a more reproducible and quantitative manner, we utilized the previously reported method of forming EBs in 96-well microtiter plates (13). In brief, aliquots of 500 CGR8 mouse ES cells were distributed in uncoated round bottom microtiter plates in differentiation media, and cells were allowed to aggregate at the bottom of each well by gravity or by brief centrifugation. After 7 to 12 days of differentiation, any EB that contained visible clusters of spontaneously contracting cells was scored as a positive. Using this protocol, we administered XAV939 (1μM) at various time intervals during EB differentiation. Particular attention was paid to the time window from days 2.5 to 5 of differentiation since administration of the protein Wnt antagonist DKK1 starting around day 3 was reported to promote cardiomyogenesis in mouse ES cells (10, 11).
Of the various time intervals examined, we found that XAV939 treatment from day 3 to day 5 of differentiation resulted in over 95% of EBs that beat spontaneously by day 10 (Figure 1A and 1C). Similarly robust cardiomyocyte induction was observed in XAV939-treated R1 mouse ES cell line, indicating that the procardiogenic effects of XAV939 was not cell line-restricted (Supplementary Figure 1). By contrast, only about 3% of DMSO vehicle-treated EBs (Figure 1C) and 5% of EBs treated with RF03922, a structural analog of XAV939 without Wnt inhibitory activity, beat (Supplementary Figure 2A and 2B).
XAV939, a tankyase1/2 inhibitor, stimulates β-catenin degradation by stabilizing Axin, a component of the β-catenin degradation complex (16). As expected for a Wnt inhibitor, treatment with XAV939 (1μM) significantly reduced β-catenin protein level in ES cells, and resulted in 95.8% and 92.8% reduction in expression of the Wnt/β-catenin responsive genes Axin 2 and Cyclin D, in comparison to DMSO treatment (Figure 1D, Supplementary Figure 3). Moreover, consistent with prior reports (10, 11), treatment with DKK1 during the same day 3 to 5 time window also resulted in similarly high percentage of EBs that beat (Figure 1C). Taken together, our results suggest that the XAV939's procardiogenic effects were mediated through Wnt inhibition.
Interestingly, IWR-1-endo (17), another recently reported small molecule Wnt inhibitor which also promotes β-catenin degradation by stabilizing Axin-scaffolded destruction complex, failed to significantly induce cardiomyogenesis in ES cells (Supplementary Figure 2C and 2D). Reason for this difference is unknown, but since DKK1 could also induce cardiomyogenesis in this model, we do not believe that the difference reflects off-target effects of XAV939. Rather, we speculate that IWR-1-endo, which does not yet have a known cellular target, unveils additional cell transduction events that counteract the procardiogenic effects of the canonical Wnt signaling.
Robust induction of cardiomyogenesis by XAV939
To aid in gauging the extent of cardiomyogenesis, we used CGR8-Dsred cells, a derivative of the CGR8 mouse ES cells which stably express the nuclear red fluorescent protein gene (DsRed-Nuc) under the control of alpha-myosin heavy chain (α-MHC) promoter. ES cells exposed to XAV939 from day 3 to 5 of differentiation formed large, synchronously beating areas containing numerous DsRed+ cells by day 10 of differentiation (Figure 2A), whereas DMSO-treated ES cells produced tiny beating areas containing very few DsRed+ cells (Figure 2B). Overall, an average of 55.6% of cells in XAV939-treated cells were DsRed+, whereas 1.8% of DMSO-treated cells were DsRed+, representing an approximately 30-fold increase in the relative abundance of cardiomyocytes following treatment with XAV939 (Figure 2C). Increased formation of cardiomyocytes was confirmed by immunostaining for the cardiac sarcomere proteins Troponin-T and α-actinin (Figure 2D).
Figure 2. ES cells treated with XAV939 (XAV) from day 3 to 5 formed large areas consisting of spontaneously beating cardiomyocytes.
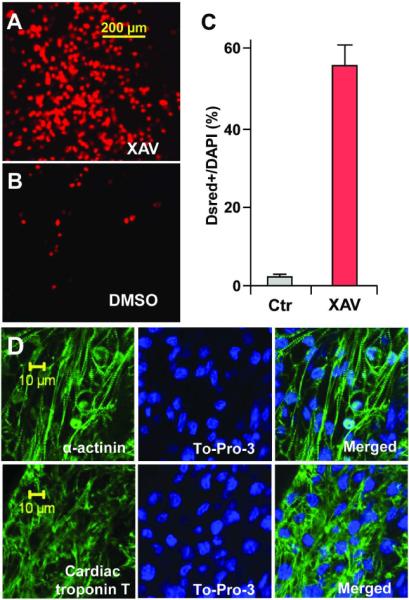
CGR-DsRed ES cells, which express DsRed-Nuc fluorescent protein under the control of cardiac α-MHC promoter, formed numerous red fluorescent nuclei following XAV treatment (A), versus relative few fluorescent nuclei following DMSO treatment (B). (C) Approximately 55.6% of DAPI+ nuclei co-expressed DsRed following XAV treatment, versus 1.8% following DMSO treatment (P=0.009). Results were obtained from 6 fields of XAV-treated EBs (on average, 59.6 DsRed+ cells out of 107.2 DAPI+ cells), and 6 fields of DMSO-treated controls (on average, 1.6 DsRed+ cells out of 92.2 DAPI+). (D) XAV939 treated ES cells formed larger areas of cardiomyocytes that expressed sarcomere proteins α-actinin (above) and cardiac Troponin-T (below). Left panels, immunostaining for sarcomere proteins (green). Middle panels, DAPI stained nuclei (blue). Right panels, merged images. Confocal images were taken using a Zeiss inverted LSM 510 confocal microscope (40×).
Consistent with a robust cardiac induction, the XAV treatment of ES cells from day 3 to 5 of differentiation led to huge increases in the expression of several cardiac genes, as measured by quantitative real-time PCR (Q-PCR). For example, XAV treatment resulted in a 22-fold increase in the cardiac myosin heavy chain gene (Myh6) expression and a 6.1-fold increase in the cardiac marker Nkx2.5 expression at day 10 of differentiation, in comparison to DMSO treatment (Figure 3A and 3B). Moreover, western blotting with cardiac Troponin-T antibody showed a much higher cardiac Troponin-T protein levels in XAV939-treated EBs than in DMSO control-treated EBs (Figure 3C).
Figure 3. XAV939 treatment from day 3 to 5 of ES cell differentiation strongly induces cardiomyogenesis.
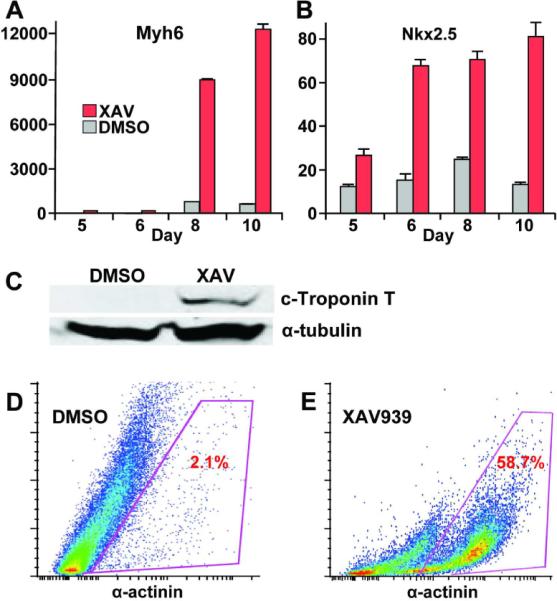
(A, B) XAV treatment led to huge increase in expression of cardiac markers Myh6 and Nkx2.5. Q-PCR results represent relative expression normalized to that of DMSO-treated cells at Day 0. Measurements were obtained from at least three independent experiments for each time-point. Red bars, XAV939-treated. Gray bars, DMSO-vehicle treated. P-values for Myh6 expression at day 8 and 10 following XAV treatment were both <0.0001, in comparison to DMSO-vehicle treatment as negative controls. P-values for Nkx2.5 expression at day 8 and 10 following XAV treatment were 0.0004 and 0006, respectively, in comparison to DMSO treatment. (C) Western blot showing much higher levels of the cardiac Troponin T protein in XAV-treated ES cells on day 10 in comparison to DMSO-treated controls. Antibody against α-tubulin was used as loading control. (E) Representative FACS analysis showing an approximately 28-fold increase in the fraction of α-actinin+ cells following XAV939 treatment vs. DMSO controls.
Cardiomyocyte induction by XAV939 treatment was further quantified by FACS analysis. At day 10 of differentiation, EBs treated with XAV939 or DMSO were dissociated, and stained with anti-α-actinin and secondary AlexaFluor-488 antibodies. FACS analyses showed that approximately 58% of XAV939 -treated ES cells were positive for sarcomere protein α-actinin, whereas only about 2.1% of DMSO-treated cells were positive for α-actinin, representing an approximately 28-fold increase in the abundance of α-actinin+ cells (Figure 3D and 3E). Thus, by a number of measures, cardiac induction by a timely treatment of ES cells with XAV939 is very robust (Figures 2C, 3D and 3E).
Inhibition ofWnt/β-catenin signaling promotes cardiomyogenesis at the expense of other mesoderm-derived cell lineages
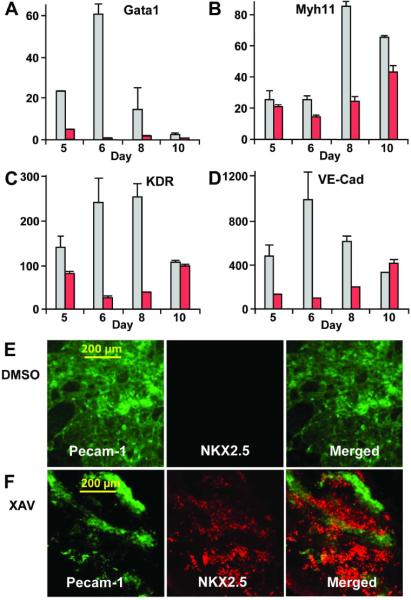
Since day 3 of mouse ES cell differentiation corresponds to the period immediately after the initial appearance of BryT+ mesoderm cells (13), we reasoned that XAV939 treatment from day 3 to 5 was inducing cardiomyogenesis by perturbing the developmental repertoire of mesoderm progenitor cells. To gain further insights into how XAV939 treatment promotes cardiomyogenesis, we examined the impact of XAV939 treatment on the RNA expression levels of the mesoderm-derived hematopoietic, endothelial and smooth muscle cell-specific markers. In contrast to the cardiomyocyte markers, we found that XAV939 treatment significantly decreased the expression of the hematopoietic progenitor marker Gata1 (Figure 4A) and the smooth muscle-specific myosin heavy chain gene Myh11 (Figure 4B). Furthermore, XAV939 treatment resulted in a drastic reduction in the expression of the vascular marker KDR (Flk-1/Vegfr2) and vascular endothelial-cadherin (VE-Cad) expression at days 6 and 8 suggesting that XAV939 treatment decreases endothelial cell differentiation (Figure 4C, Figure 4D).
Figure 4. XAV939 treatment promotes cardiomyogenesis at the expense of other mesoderm lineages.
XAV939 treatment decreased the expressions of mesoderm-derived cell lineage markers, including the hematopoietic marker Gata1 (A), the smooth muscle-specific myosin heavy chain gene Myh11 (B), and the endothelial markers Flk-1 and vascular endothelium-cadherin (VE-cad) (C, D). XAV treatment led to huge increase in expression of cardiac markers Myh6 and Nkx2.5. Q-PCR results represent relative expression normalized to that of DMSO-treated cells at Day 0. Measurements were obtained from at least three independent experiments for each time-point. Red bars, XAV939-treated. Gray bars, DMSO-vehicle treated. (E) DMSO-treated ES cells rarely formed cells that express the cardiac Nkx2.5 protein (red immunostaining, right panel), but rather formed large patches of cells that express the endothelial Pecam-1 protein (green immunostaining, middle panel). (F) By contrast, XAV939-treated ES cells formed large areas of Nkx2.5 expressing cardiomyocytes (left panel), and considerably smaller areas of Pecam-1 expressing endothelial cells (middle panel). (E, F) Right panels, merged images.
Next, we examined whether the substantial cardiomyocyte induction by XAC939 was associated with a similarly impressive induction of the ectoderm or the endoderm. Because the temporal window for efficient cardiomyocyte induction by XAV939 (day 3 – 5; Figure 1A) falls beyond the timeframe for expression of the earliest endoderm and ectoderm markers (18, 19), we examined the impact of XAV939 on expression of Foxa1 (endoderm) and Nestin (ectoderm), which are present from early embryonic germ layers to later derivatives. Our Q-PCR analyses indicate that the day 3 – 5 treatment with XAV939 had only a modest effect (less than 1.5 fold difference) on either the Nestin or the Foxa2 expression (Supplementary Figure 4). Thus, while these results do not rule out an indirect role played by ectodermal or endodermal derivatives, the XAV939-induced cardiomyocyte formation does not appear to involve stoichiometric increases in either lineages.
On immunohistochemistry, DMSO-treated EBs formed large contiguous patches of cells expressing the endothelial marker Pecam-1 with very few cells expressing the cardiac marker Nkx2.5 (Figure 4E). By contrast, XAV-treated EBs formed large patches of Nkx2.5+ cells juxtaposed to considerably smaller patches of Pecam-1+ cells (Figure 4F). Taken together, our results suggest that inhibition of Wnt/β-catenin signaling soon after the initial formation to early mesoderm cells at day 3 of differentiation leads to the huge induction of cardiomyocyte formation at the expense of cells of other mesoderm lineages, such as endothelial cells.
A possible explanation for the relative enrichment of cardiomyocytes and reduction of other mesoderm-derived cell lineages is that the XAV939 treatment led to preferential apoptosis of non-cardiac cell types. However, we did not detect significant apoptosis in differentiating ES cells that were treated with either DMSO control or XAV939 (Supplementary Figure 5).
SUMMARY
Pluripotent stem cells, especially patient-derived iPS cells, show great promise as source of cells for regenerative therapy as well as a platform for drug discovery and patient-specific diagnostics. However, given the inherent complexity of stem cell biology, it is imperative to gain a better understanding of the mechanism of stem cell differentiation and to develop robust and inexpensive methods to direct differentiation of stem cells toward desired tissue types. In principle, small molecules that selectively modulate key nodal points in embryonic development, such as the BMP and Wnt signaling pathways, are valuable tools for dissecting signaling pathways involved in lineage commitment of pluripotent stem cells; and as pharmaceutical agents to direct stem cell differentiation toward desired cell types (20, 21).
Here, we report an efficient method to robustly induce cardiomyocytes in mouse ES cells using XAV939, a recently described small molecule inhibitor of the Wnt/β-catenin signaling. Thus, XAV939 joins a handful of small molecules that can robustly promote cardiomyogenesis in stem cells. Using this small molecule to precisely control the timing of Wnt/β-catenin signaling during ES cell differentiation, we showed that Wnt inhibition limited to a narrow temporal window of day 3 and 5 differentiation was sufficient for huge induction in cardiomyocyte formation over DMSO controls. Interestingly, this time window corresponds to the initiation of mesoderm formation and specification into various mesoderm lineages. Consistent with earlier reports, we find that inhibiting Wnt signaling during this stage promotes the formation of cardiomyocyte at the expense of other mesoderm-derived cells, such as endothelial, hematopoietic and smooth muscle cells. Indeed, our results suggest that, in the in vitro model of mouse ES cell differentiation, the predominant differentiation program for newly formed mesoderm cells appear to be vascular endothelial cells. Blocking Wnt signaling during the critical period appears to shift developmental program toward cardiomyogenesis.
Since developmental pathways such as Wnt/β-catenin and BMP signaling function at multiple nodes in embryogenesis to specify formation of many different tissue types in the body, small molecules that selectively modulate them will be useful for directed differentiation of variety of cell types from pluripotent stem cells. For example, dorsomorphin, a small molecule BMP signaling inhibitor was used to induce differentiation of ES cells into cardiomyocytes as well as neurons (13, 22). Since Wnt/β-catenin signaling is critical for the formation of multiple tissues in the embryo, XAV939 could also be valuable for directing in vitro differentiation of stem cells toward many additional cell types. Finally, should XAV939 or its future structural analogs be proven to have favorable in vivo bioavailability, they could serve as important pharmacologic tools to explore the regenerative potential of stem cells in live animals.
MATERIALS AND METHODS
Cell culture and Chemicals
Murine ES cell lines, CGR8, CGR8-Dsred and R1, were grown in feeder-free conditions as monolayers. CGR8 cells were maintained in GMEM (Sigma-Aldrich) supplemented with 10% FBS (Gibco), 2 mM L-glutamine, (Cellgro), 0.05 mM 2-mercaptoethanol (Sigma-Aldrich), and 200 U/ml murine LIF (Chemicon International). R1 cells were maintained in High Glucose DMEM (Gibco) supplemented with 15% FBS, 2 mM L-glutamine, 1× nonessential amino acids, 100 U/ml penicillin-100 μg/ml streptomycin (Cellgro), 0.05 mM 2-mercaptoethanol, 1 mM sodium pyruvate (Sigma-Aldrich), and 200 U /ml murine LIF. Both cell lines were cultured on 0.2% gelatin-coated dishes. Every 24 hours, cells were washed in 1× PBS and culture media was replaced. Cells were passaged when confluence reached 50–60% to preserve the undifferentiated phenotype. XAV939 and RF03922 were purchased from Maybridge (Cornwall, England). IWR-1-endo was purchased from Caymam Chemical (Ann Arbor, MI).
ES cell Differentiation
ES cells were trypsinized and embryoid bodies (EBs) were generated by the three-dimensional hanging drop method (Day 0). Briefly, EBs were grown in hanging drops for two days (Day 0 to Day 2), each of which initially consisted of 500 cells in 20 μL of EB differentiation media. The EB differentiation media was composed of IMDM (Gibco) supplemented with 20% FBS, 1.6 mM L-glutamine, 1× nonessential amino acids, 0.08 mM 2-mercaptoethanol, and either 2 μM dorsomorphin or DMSO. For R1 cells, differentiation media additionally contained 1 mM sodium pyruvate. At day 2 of differentiation (Day 2), The EBs were washed down and transferred to uncoated Petri dishes and suspended in differentiation media. EBs were treated with XAV939 or DMSO from day 3 to day5, and On Day 4, the EBs were moved to gelatin-coated 6-well plates, allowed to attach and incubated in differentiation media until Day 14. Throughout this time, the media was replaced every 48–72 hours. Each day, differentiating cell clusters were microscopically examined for the presence of contracting cardiomyocytes and, in the case of CGR8-DsRed cells, red fluorescence.
In the second culture technique, aliquots of 500 ES cells were distributed in uncoated 96-well round bottom plates with 100 μL differentiation media. XAV939- or DMSO was added in the medium from day 3 and ashed out on day 5. EBs were microscopically examined for contracting cardiomyocytes on days 8 through 12. Any well containing spontaneously beating cells was recorded as 1 positive result.
Dsred/DAPI cell counting
DsRed CGR8 ES cells treated with dorsomorphin or DMSO were trypsinized on day 12 and resuspended in EB medium. DAPI was added into the cells at final concentration of 5μM and the cells are ready to count after 10 minutes' staining. 2μl cell solution was loaded on coverslips and Dsred+ and DAPI+ were counted under fluorescence microscopy. Each area was counted three times to get average numbers.
FACS analysis
EBs were dissociated into single cell suspensions after trypsinization. Following a wash with 10%FBS/DMEM, cells were permeabilized with 0.05% saponin/PBS buffer for 20 minutes on ice. Cells were then stained with an α-Actinin antibody (Sigma; 1:100 dilution in 10%FBS/DMEM) for 1 hour. Following washes with 10%FBS/DMEM, cells were incubated with an anti-mouse secondary antibody conjugated to AlexaFluor-488 (1:400 dilution in 10%FBS/DMEM) for 30 minutes in dark. After additional washes in 10%FBS/DMEM, cells were resuspended in 300 μL 10%FBS/DMEM and analyzed on the 5-laser BD LSRII FACS instrument.
Immunostaining and confocal microscopy
EBs treated with dorsomorphin or DMSO (day 0~1) were plated at day 4 on glass coverslip culture chambers coated with 1% gelatin. At day 10, EBs were fixed in 5% formaldehyde at room temperature for 30 minutes, and then permeabilized with 0.2% Triton X-100 in PBS. After blocking with 1 mg/ml BSA in PBS, cells were incubated with mouse monoclonal anti-α-Actinin (Sigma), or mouse cardiac Troponin T (Santa Cruz) antibodies at concentrations recommended by the manufacturers. After overnight incubation, cells were washed several times with PBS and then incubated with the AlexaFluor-488-conjugated rabbit anti-mouse IgG (Molecular Probes) and 5 μM 4'-6-Diamidino-2-phenylindole (DAPI). Immunostaining images were obtained using both a Leica inverted microscope (10×) and a Zeiss inverted LSM 510 confocal microscope (40×).
Quantitative real-time PCR
Cells were harvested on days 0, 2, 3, 4, 6, 8, 10, 12 of EB differentiation and stored at −80°C in cell lysis buffer RLT (Qiagen). Three independent samples were collected for each time point studied. Total RNA was extracted using the RNeasy Mini Kit according to the manufacturer's instructions and treated with RNase-free DNase I (Qiagen). First-strand cDNA was synthesized with the SuperScript III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen). Using cDNA as template, TaqMan real-time PCR assays was performed in triplicates on the ABI Prism 7900 HT sequence detection system (Applied Biosystems) according to the manufacturer's instructions. Data were normalized to GAPDH, and levels of gene expression were normalized to that of Day 0 DMSO-treated cells. The following TaqMan probe and primer sets (Applied Biosystems) were used: nk×2.5 (Mm00657783_m1), myh6 (Mm00440354_m1), Axin2 (Mm00443610_m1), Cyclin D (Mm00432360_m1), Nestin (Mm00450205_m1), Foxa2 (Mm00839704_mH) and GAPDH (Mm99999915_g1).
Western Blotting
Lysates of EBs on day 10 were separated by SDS/PAGE and transferred onto PVDF membrane. The mouse cardiac Troponin T expression was detected by Odyssey system (Li-Cor bioscience) following incubation with mouse cardiac Troponin T antibody (Santa Cruz, 1:200 dilution) and IRDye 800CW-conjugated goat anti-mouse IgG (Li-Cor Bioscience, 1:5000 dilution). Mouse α-tubulin antibody (Abcam, 1:2000) was used as a loading control. To detect β-catenin, day 5 EB lysates were collected and western blotting was carried out in similar way as the above except anti-rabbit β-catenin (Santa Cruz, 1:200 dilution) was used.
TUNEL assay
Apoptotic cells within EBs were detected at day 6 and 8 of differentiation using the in situ TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labeling) assay, following the manufacturer's instructions (Roche Applied Science). Apoptotic cells were then analyzed under a Leica inverted microscope. For positive control, EBs were treated with DNase I (3000U/ml) for 25 minutes at room temperature to induce DNA strand breaks prior to the labeling procedure.
Supplementary Material
Acknowledgements
We thank C. Williams for comments on the manuscript. This work was supported by Veterans Affairs and NIH grants 5U01HL100398 and 1R01HL104040.
Footnotes
Competing interests: The authors declare that they have no competing financial interests.
Supporting Information Available: This material is available free of charge via the Internet
REFERENCE
- 1.Fukuda K, Yuasa S. Stem cells as a source of regenerative cardiomyocytes. Circulation research. 2006;98:1002–1013. doi: 10.1161/01.RES.0000218272.18669.6e. [DOI] [PubMed] [Google Scholar]
- 2.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nature biotechnology. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 3.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 4.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 8.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 9.Foley AC, Mercola M. Heart induction by Wnt antagonists depends on the homeodomain transcription factor Hex. Genes Dev. 2005;19:387–396. doi: 10.1101/gad.1279405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci U S A. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuasa S, Itabashi Y, Koshimizu U, Tanaka T, Sugimura K, Kinoshita M, Hattori F, Fukami S.-i., Shimazaki T, Okano H, Ogawa S, Fukuda K. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotech. 2005;23:607–611. doi: 10.1038/nbt1093. [DOI] [PubMed] [Google Scholar]
- 13.Hao J, Daleo MA, Murphy CK, Yu PB, Ho JN, Hu J, Peterson RT, Hatzopoulos AK, Hong CC. Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PloS one. 2008;3:e2904. doi: 10.1371/journal.pone.0002904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emre N, Coleman R, Ding S. A chemical approach to stem cell biology. Curr Opin Chem Biol. 2007;11:252–258. doi: 10.1016/j.cbpa.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H, Shultz M, Rau C, Schirle M, Schlegl J, Ghidelli S, Fawell S, Lu C, Curtis D, Kirschner MW, Lengauer C, Finan PM, Tallarico JA, Bouwmeester T, Porter JA, Bauer A, Cong F. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 17.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman JR, Kaestner KH. The Foxa family of transcription factors in development and metabolism. Cell Mol Life Sci. 2006;63:2317–2328. doi: 10.1007/s00018-006-6095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov KV, Tarasova Y, Wersto RP, Boheler KR, Wobus AM. Nestin expression--a property of multi-lineage progenitor cells? Cell Mol Life Sci. 2004;61:2510–2522. doi: 10.1007/s00018-004-4144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y, Do JT, Desponts C, Hahm HS, Scholer HR, Ding S. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell stem cell. 2008;2:525–528. doi: 10.1016/j.stem.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Yao S, Chen S, Clark J, Hao E, Beattie GM, Hayek A, Ding S. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A. 2006;103:6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wada T, Honda M, Minami I, Tooi N, Amagai Y, Nakatsuji N, Aiba K. Highly efficient differentiation and enrichment of spinal motor neurons derived from human and monkey embryonic stem cells. PloS one. 2009;4:e6722. doi: 10.1371/journal.pone.0006722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.