Abstract
One of the limiting factors in stroke therapeutic development is the use of animal models that do not well represent the underlying medical conditions of patients. In humans, diabetes increases the risk of stroke incidence as well as post-stroke mortality. To understand the mechanisms that render diabetics to increased brain damage, we evaluated the effect of transient middle cerebral artery occlusion (MCAO) in adult db/db mice. The db/db mouse is a model of type-2 diabetes with 4 times higher blood sugar than its normoglycemic genetic control (db/+ mouse). Following transient MCAO, the db/db mice showed significantly higher mortality, bigger infarcts, increased cerebral edema, worsened neurological status compared to db/+ mice. The db/db mice also showed significantly higher post-ischemic inflammatory markers (ICAM1+ capillaries, extravasated macrophages/neutrophils and exacerbated proinflammatory gene expression) compared to db/+ mice. In addition, the post-ischemic neuroprotective heat-shock chaperone gene expression was curtailed in the db/db compared to db/+ mice.
Keywords: Hyperglycemia, Diabetes, Inflammation, Infarction, Stroke, Edema
Type-2 diabetes predisposes humans to stroke, and stroke-induced brain damage is known to be exacerbated with poor functional recovery in these patients (Ergul et al 2009). Although >30% of stroke sufferers are known to be diabetic, the mechanisms that are responsible for the increased post-ischemic brain damage in this population are understudied. Diabetes occurs in 2 major forms; while type-1 diabetes (early onset) is characterized by insulinopenia caused by the destruction of the insulin producing beta islet cells of the pancreas, type-2 diabetes (adult onset in most cases) results from peripheral insulin resistance. A commonality between the 2 conditions is the hyperglycemia which is detrimental to cognition and other brain functions. Of the ~200 million diabetics worldwide, >90% are type-2 diabetics. The genetically altered db/db mouse (Hummel et al 1966) manifests adult onset type-2 diabetes and hence might be a useful animal model to study mechanisms and to test novel therapeutic compounds to prevent ischemic brain damage in diabetic cohort. In the db/db mouse, the diabetic gene (db) which encodes for a G-to-T point mutation of theleptin receptor, leading to abnormal splicing and defective signaling of the adipocyte-derived hormone leptin is transmitted as an autosomal recessive trait. In these mice absence of hypothalamic leptin signaling leads to hyperphagia and obesity culminating in high leptin and insulin levels (Chen et al 1996; Lee et al 1996). The db/db mice start to develop diabetic symptoms around 6 weeks and will become frank diabetics by 12 weeks of age (Sharma et al 2010).
Using adult db/db mice and their normoglycemic genetic control (db/+ mice), we presently evaluated the effect of transient focal ischemia on the mortality rate, infarct volume, neurological dysfunction, cerebral edema, inflammatory indices (ICAM1 expression, neutrophil infiltration, microaglia/macrophage activation and proinflammatory gene expression) and heat-shock protein (HSP) gene expression.
MATERIALS AND METHODS
Focal Ischemia
All the surgical procedures were approved by the Research Animal Resources and Care Committee of the University of Wisconsin-Madison and the animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animals, U.S. Department of Health and Human Services Publication Number. 86–23 (revised). The db/db (C57BLKS/J-m+/+Leprdb/db; type-2 diabetic) and db/+ (normoglycemic genetic controls of db/db) mice (n = 35/genotype) were obtained from the Jackson Laboratories (Bar Harbor, ME). Focal ischemia was induced by transient middle cerebral artery occlusion (MCAO) by an intraluminal suture method as described earlier (Kapadia et al 2006); (Tureyen et al 2007; Tureyen et al 2008). In brief, a mouse was anesthetized with isoflurane (induction: 2%; maintenance: 1.2% in an oxygen and nitrous oxide 50:50 mixture), and the left femoral artery was cannulated for continuous monitoring of arterial blood pressure and to obtain the measurements of pH, Pao2, Paco2, hemoglobin and blood glucose concentration (i-STAT; Sensor Devices, Waukesha, WI). The rectal temperature was maintained at 37.0 ± 0.5°C during surgery with a feedback-regulated heating pad. After a midline skin incision, the left external carotid artery was exposed, a surgical 6–0 monofilament nylon suture blunted at the end was introduced into its lumen and gently advanced to the internal carotid artery until the regional cerebral blood flow (rCBF) was reduced to ~15% of the baseline (recorded by laser Doppler flowmeter; Vasamedics, LLC, St Paul, MN) as described earlier (Vemuganti et al 2004). Following 45 min or 2h of occlusion, the suture was withdrawn (reperfusion was confirmed by laser Doppler), the wound was sutured, mice were allowed to recover from anesthesia and returned to the cage with ad libitum access to food and water. Cohorts of mice were sacrificed at 12h (gene expression studies), 1 day (brain water estimation) or 3 days (all other studies) of reperfusion.
Infarct volume estimation and immunohistochemistry
Infarct volume was measured as described earlier (Kapadia et al 2006; Tureyen et al 2007). In brief, db/db and db/+ mice (n =14/group) subjected to 45 min transient MCAO were perfused transcardially with buffered paraformaldehyde at 3 days of reperfusion. Each brain was postfixed, cryoprotected and sectioned (coronal; 40 μm thick at an interval of 320 μm). The serial sections were stained with Cresyl violet and scanned using the NIH Image program. The volume of the ischemic lesion was computed by the numeric integration of data from 4 serial sections in respect to the sectional interval. To account for edema and differential shrinkage resulting from tissue processing, the injury volumes were corrected by using the Swanson formula: corrected injury volume = contralateral hemisphere volume − (ipsilateral hemisphere volume-measured injury volume) (Swanson et al 1990). Parallel sets of sections from each mouse were immunostained with antibodies against ICAM1 (1:1,000; BD Bioscience Pharmingen, San Jose, CA) and OX42 (CD11b; 1:1,000; BD Pharmingen, San Jose, CA) as described earlier (Kapadia et al., 2006). The OX42+ cells were counted in 3 to 4 X300 fields in the ipsilateral cortex of each mouse. To ensure that the homologous areas of injury was sampled between animals, sections between the coordinates +1 to +1.5 from Bregma in all animals as described earlier Kapadia.
Neurological evaluation
Post-ischemic neurological deficits were evaluated on a 5-point scale before transient MCAO and at 3 days of reperfusion by an investigator blinded to the study groups as described earlier (Longa et al 1989); (Kapadia et al 2006; Tureyen et al 2007; Tureyen et al 2008). The number of mice used for the neuroscoring included those used for infarct measurement and MPO activity (n = 14 for the db/db and 17 for the db/+ groups). A score of 0 suggests no neurological deficit (normal), 1 suggests mild neurological deficit (failure to extend right forepaw fully), 2 suggests moderate neurological deficit (circling to the right), 3 suggests severe neurological deficit (falling to the right), and 4 suggests very severe neurological deficit (the mouse could not walk spontaneously; depressed level of consciousness).
Myeloperoxidase (MPO) Assay
MPO activity in brain tissue reflects the neutrophil extravasation. MPO activity was estimated as described previously (Weston et al 2007) in a cohort of db/db and db/+ mice (n = 5/genotype) subjected to 45 min transient MCAO and 3 days of reperfusion. In brief, a mouse was transcardially perfused with isotonic saline, the brain was sliced in matrix to generate 1-mm sections. A section from the coordinates between +1 mm to −1 mm was quickly stained with TTC to confirm infarction. From the adjacent sections the ipsilateral and the contralateral cortex (~50 mg each) were dissected. The wet weight was noted and the tissue was homogenized in ice-cold 5 mM phosphate buffer (pH 6), centrifuged at 30,000g for 30 min at 4°C. The pellet washed briefly with buffer and suspended in 50 mM phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide (Sigma, St Louis, MO). The suspension was subjected to 3 freeze–thaw cycles with sonication (10 sec) between cycles followed by incubation (at 4°C for 20 min) and centrifugation (at 12,500g for 15 min at 4°C). A 50 μl of the supernatant was mixed with 1.45 ml of 50 mM phosphate buffer (pH 6.0) containing o-dianisidine dihydrochloride (0.167 mg/ml; Sigma, St Louis, MO) and hydrogen peroxide (0.0005%). The change in absorbance at 460 nm was recorded for 3 min at 15 sec intervals. MPO activity was calculated using human MPO (Dako, Carpentaria, CA) as a standard. One unit of MPO activity was defined as the amount that degrades 1 μmol of peroxide/min at 25°C.
Brain water content
The brain water content was measured as previously described (Titova et al 2008). Briefly, the ipsilateral and the contralateral cortex were dissected from a cohort of db/db and db/+ mice (n = 5/genotype) killed at 1 day of reperfusion following 45 min MCAO. Tissue samples were weighed to the nearest mg to obtain the wet weight. The tissue was dried at 100°C for 1 day and weighed again to determine the dry weight. Brain water content (%) was calculated as [(wet weight-dry weight)/wet weight] × 100.
Real-time PCR
Gene expression analysis was conducted using quantitative real-time PCR as described earlier (Kapadia et al 2006; Tureyen et al 2007). A cohort of db/db and db/+ mice subjected to 45 min transient MCAO were killed at 12h of reperfusion (n = 4/group). From each mouse, total RNA was extracted from the ipsilateral and the contralateral cortex using the Trizol reagent (Invitrogen, Carlsbad, CA). 1 μg of RNA from each sample was reverse transcribed with oligo(dT)15 and random hexamer primers using M-MuLV reverse transcriptase (Life Technologies, Rockville, MD). 10 ng of cDNA and gene-specific primers were added to SYBR Green PCR Master Mix (SYBR Green I Dye, AmpliTaq DNA polymerase, dNTPs with dUTP and optimal buffer components; Applied Biosystems) and subjected to PCR amplification in a Perkin-Elmer TaqMan 5700 Sequence Detection System (1 cycle at 50°C for 2 min, 1 cycle at 95 °C for 10 min, and 40 cycles at 95°C for 15 sec and 60°C for 1 min). PCR reactions were conducted in duplicate. The amplified transcripts were quantified with the comparative CT method using 18S rRNA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the internal controls as described earlier (Kapadia et al 2006; Tureyen et al 2007). The following transcripts known to be upregulated after focal ischemia were estimated: interleukin (IL)-1β, IL-6, macrophage inflammatory protein-1α (MIP-1α), monocyte chemoattractant protein-1 (MCP-1), P-selectin, E-selectin, HSP27, HSP70 and HSP32 (heme oxygenase-1; HO1). The real-time PCR primers were designed using the Primer Express software (Applied Biosystems) based on the GenBank accession numbers and are same as in our previous papers (Kapadia et al 2006; Tureyen et al 2007).
Statistical analysis
The data are expressed as mean ± SD. Comparisons among groups were performed by one-way ANOVA with Tukey-Kramer multiple comparisons post-test.
RESULTS
Body weight and blood glucose levels
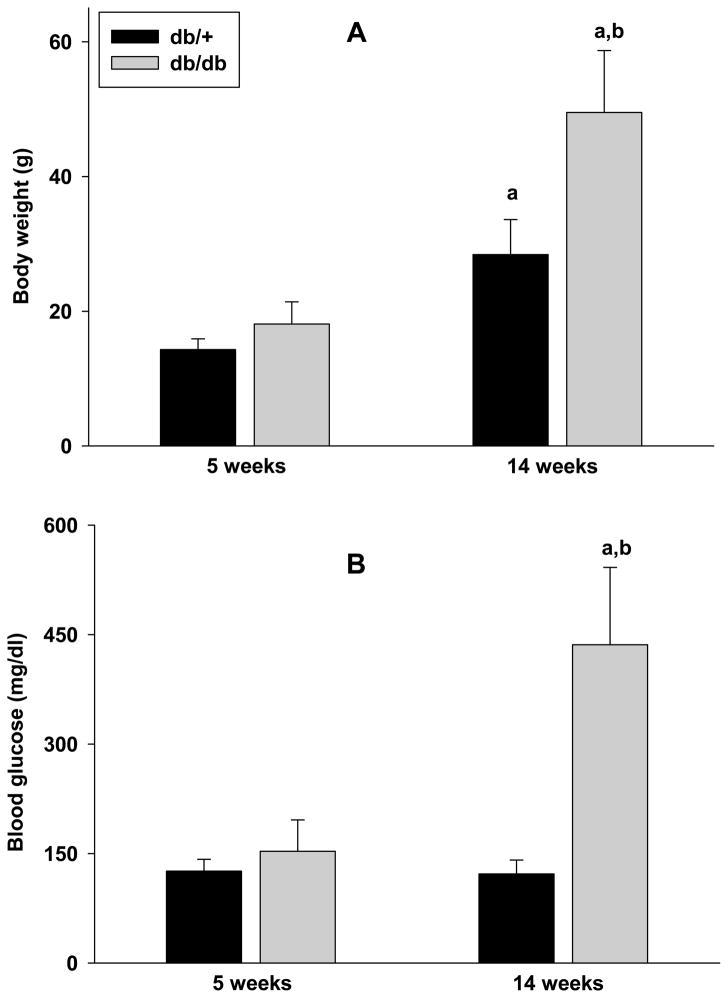
At 5 weeks of age, both db/db and db/+ cohorts showed similar body weight (Fig. 1A; n = 35/group). By 14 weeks of age, the body weight increased by 173% in the db/db mice and by 99% in the db/+ mice (Fig. 1A). Thus, at 14 weeks of age the body weight of db/db mice was 74% higher than db/+ mice (n = 35/group; p<0.05) (Fig. 1A). The blood glucose level was 21% higher in the db/db mice over the db/+ mice at 5 weeks of age (n = 35/group) (Fig. 1B). At 14 weeks of age, the db/db mice showed a 267% higher blood glucose levels than the db/+ mice (n = 35/group; p<0.05) (Fig. 1B).
Fig. 1.
Body weights (A) and blood glucose levels (B) of db/db and db/+ mice at 5 weeks and 14 weeks of age. Values are mean ± SD (n = 35/group). Statistics: ap<0.05 compared with the respective 5 week old group, bp<0.05 compared with the 14 week old db/+ group.
Mortality following transient MCAO
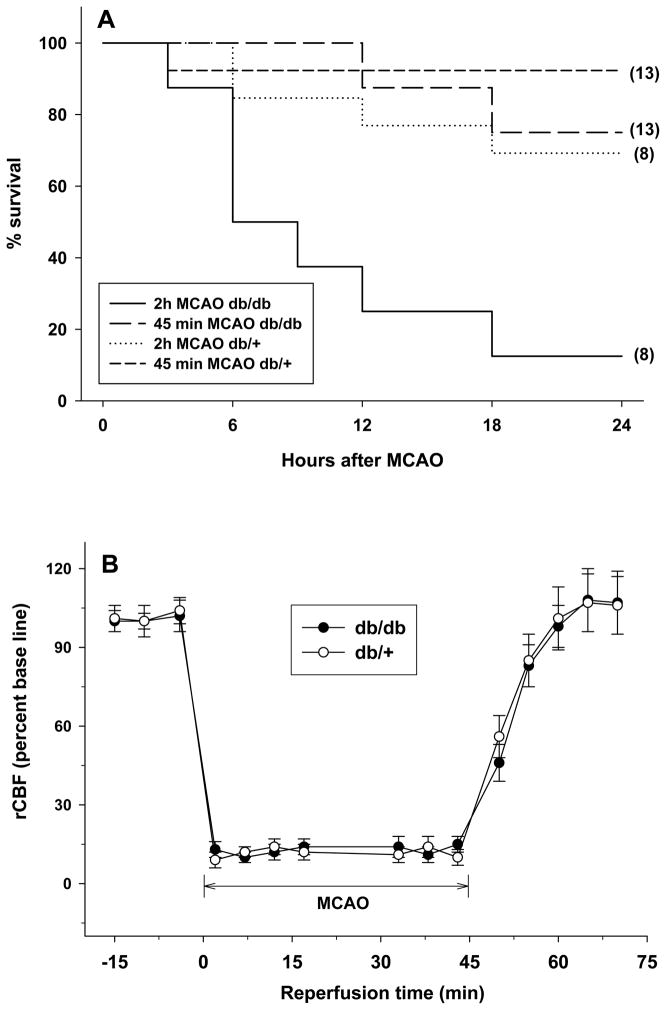
When mice were subjected to a 2h transient MCAO, within 1 day of reperfusion the db/db mice showed a 75% rate of mortality compared to a 13% mortality in the db/+ cohort (n = 8/genotype) (Fig. 2A). When the duration of MCAO was decreased to 45 min, the db/db mice showed a 31% mortality compared to 8% mortality observe in the db/+ cohort (n = 13/genotype) (Fig. 2A). Hence, we used 45 min MCAO duration in the remainder of the experiments. In the 45 min MCAO group, the rCBF (Fig. 2B) and the other physiological parameters (pH, Pao2, Paco2 and hemoglobin; data not shown) were not significantly different between the db/db and db/+ cohorts.
Fig. 2.
Survival rates (A) in 14 week old db/db and db/+ mice within the first 24h of reperfusion following transient MCAO. In panel A, the solid line is 2h MCAO in db/db, dotted line is 2h MCAO in db/+, long dashed line is 45 min MCAO in db/db and short dashed line is 45 min MCAO in db/+ cohorts. The number in the parenthesis after each line is the number of mice used for that group (A). The rCBF was not significantly different between db/db and db/+ mice subjected to 45 min transient MCAO (B). Values are mean ± SD (n = 13/group).
Infarction and neurological dysfunction following focal ischemia
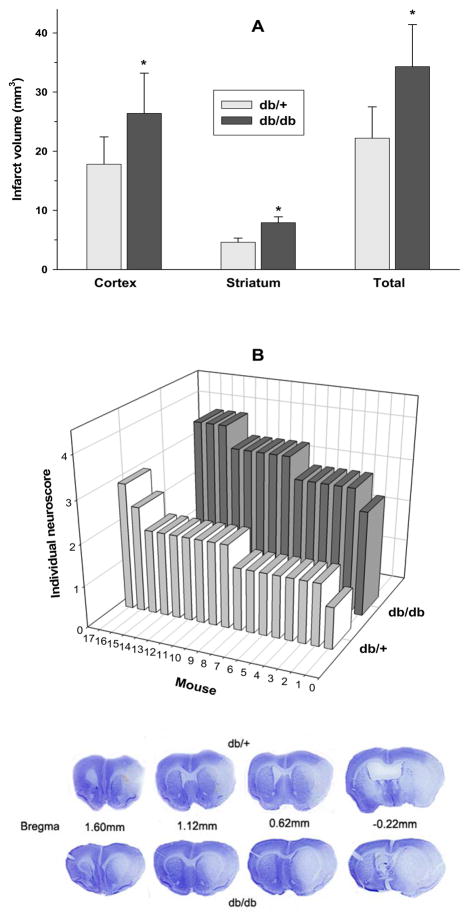
Following 45 min transient MCAO and 3 days of reperfusion, the infarct volume was observed to be 54.5% higher in the db/db compared to the db/+ mice (n = 9 for db/db and 12 for db/+ cohorts; p<0.05) (Fig. 3A). The average post-ischemic neuroscore at 3 days of reperfusion following 45 min transient MCAO was also observed to be 88% higher (p<0.05) in the db/db (3.35 ± 0.45; n =14) over the db/+ (1.76 ± 0.047; n = 17) cohorts (Fig. 3B). This indicates a very severe neurologic deficit in the db/db group compared to the mild neurological deficit in the db/+ group. Cresyl violet stained sections from representative mice from the db/db and db/+ cohorts subjected to 45 min transient MCAO and were shown in Fig. 3.
Fig. 3.
The db/db mice showed significantly bigger infarcts (A) and worsened neurological function (B) compared to db/+ mice following 45 min transient MCAO (A). Mice were tested at 3 days of reperfusion for both measures. *p<0.05 compared to db/+ group (one-way ANOVA followed by Tukey-Kramer multiple comparisons post-test). Bars in A represents mean ± SD (n = 9 for the db/db and 12 for the db/+ groups). The number of mice used for infarct measurement in the 2 groups was not equal as 3 mice in the db/db and 1 mouse in the db/+ groups died before 3 days of reperfusion. Bars in B represent individual neuroscore of each animal (n = 14 for the db/db and 17 for the db/+ groups). The number of mice for the neuroscoring includes those used for infarct measurement and MPO activity. The mean ± SD neuroscore was 88% higher (p<0.05) in the db/db (3.33±0.46) compared to db/+ (1.76 ±0.47) cohort. The bottom images are Cresyl violet-stained serial section from representative mice from db/db and db/+ cohorts subjected to 45 min transient MCAO and 3 days of reperfusion.
Post-ischemic brain water content
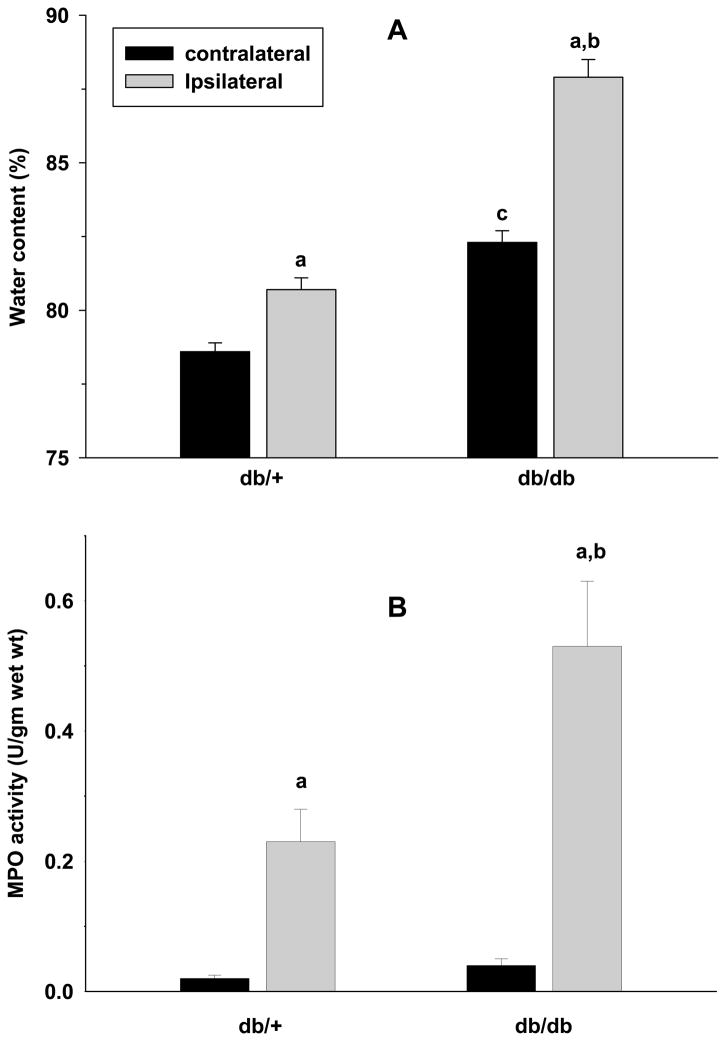
We measured brain water content which reflects edema in db/db and db/+ mice subjected to 45 min MCAO and 1 day of reperfusion (n =5/group). Compared to the respective contralateral cortex, the ipsilateral cortex of both genotypes showed a significant increase in the water content (Fig. 4A). However, the db/+ mice showed a greater percent increase compared to db/+ mice (Fig. 4A). In the db/db mice the contralateral cortex also showed significantly increased water content compared to the contralateral cortex of db/+ mice (Fig. 4A).
Fig. 4.
The cerebral water content was observed to be significantly higher in the ipsilateral cortex in comparison with the respective contralateral cortex in both db/+ and db/db cohorts subjected to 45 min transient MCAO and 1 day of reperfusion (A). The ipsilateral cortex of db/db group also showed significantly increased water content compared to the ipsilateral cortex of the db/+ mice. The MPO activity which is an indicator of the presence neutrophils was observed to be significantly higher in the ipsilateral cortex compared to the respective contralateral cortex of both db/+ and db/db mice subjected to transient MCAO and 3 days of reperfusion (B). In addition, the ipsilateral cortex of db/db showed significantly higher MPO activity than the ipsilateral cortex of the db/+ mice (B). The values are mean ± SD (n = 5/group). Statistics: ap<0.05 compared with the respective contralateral cortex, bp<0.05 compared with the ipsilateral cortex of db/+ and cp<0.05 compared with the contralateral cortex of db/+ (one-way ANOVA followed by Tucky-Kramer multiple comparisons post-test).
Post-ischemic inflammatory markers
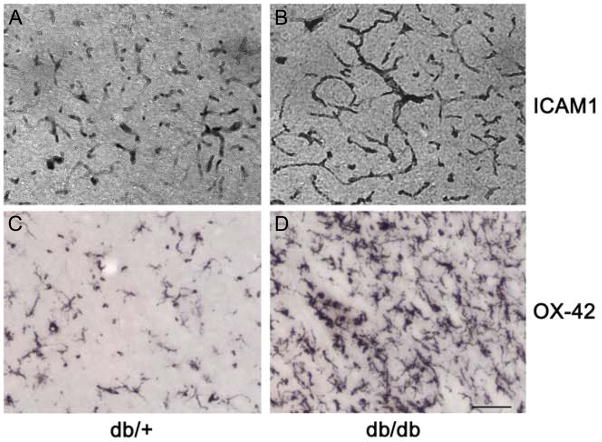
The MPO activity which indicates the presence of neutrophils was negligible in the contralateral cortex of db/db and db/+ mice subjected to transient MCAO and 3 days of reperfusion (n =5/group) (Fig. 4B). The ipsilateral cortex of both genotypes showed significantly elevated MPO activity compared to the respective contralateral cortex but the fold increase was significantly higher in the db/db cohort (db/+ by 10.4 fold and db/db by 18.3 fold; n =5/group) (Fig. 4B). In addition, OX42 immunostaining (a measure of activated microglia/macrophages) and the ICAM1 immunostaining (indicates increased extravasation of neutrophils and macrophages into brain parenchyma) were higher in the ipsilateral cortex of the db/db compared to db/+ mice (n = 5/genotype) (Fig. 5).
Fig. 5.
ICAM-1 immunopositive capillaries (A and B) and the OX-42 immunopositive activated microglia/macrophages (C and D) were higher in the ipsilateral cortex of db/db (B and D) compared to the db/+ (A and C) mice subjected to 45 min transient MCAO and 3 days of reperfusion. Scale bar is 100 μm. The images were taken from the ipsilateral cortex from the coordinates +1.0 from Bregma. The OX42+ cells were observed to be significantly higher (by 67% ± 11%; p<0.05 by Student’s t test) in the ipsilateral cortex of db/db (85 ± 15 cells/high power field) compared to db/+ (44 ± 8 cells/high power field) mice (n = 3/genotype).
At 12h of reperfusion following 45 min transient MCAO, the ipsilateral cortex of both db/+ and db/db mice showed significantly increased expression of several inflammatory genes compared to the respective contralateral cortex (n = 4/genotype; Table 1). However, db/db group showed significantly higher increases over db/+ group in the levels of pro-inflammatory cytokines IL-6 and IL-1β (by 84% and 146%, respectively; p<0.05), chemokines MIP-1α and MCP-1 (by 135% and 65%, respectively; p<0.05), adhesion molecules P-selectin and E-selectin (by 64% and 96%, respectively; p<0.05) (Table 1).
Table 1.
Post-ischemic cortical inflammatory gene expression in the db/db and db/+ mice
| Transcript | GenBank# | Δfold over contralateral cortex | ||
|---|---|---|---|---|
| db/+ | db/db | % difference | ||
| IL-1β | NM_008361 | 3.6±0.4 | 7.4±0.8 | +146* |
| IL-6 | NM_031168 | 5.8±0.8 | 9.8±1.0 | +84* |
| MIP-1α (CCL3) | NM_011337 | 4.1±0.7 | 8.3±1.0 | +135* |
| MCP-1 (CCL2) | AF065933 | 8.1±1.1 | 12.7±1.6 | +65* |
| E-selectin | NM_011345 | 3.5±0.5 | 5.1±0.6 | +96* |
| P-selectin | NM_011347 | 6.6±1.1 | 10.2±1.1 | +64* |
| HSP70 | NM_010478 | 106±12 | 41.2±9.1 | −62* |
| HSP27 | U03561 | 39.1±7.3 | 12.3±2.6 | −71* |
| HO-1 (HSP32) | NM_010442 | 24.2±4.1 | 5.1±1.9 | −78* |
18S rRNA and GAPDH used as house-keeping controls showed no difference in expression between the two genotypes subjected to sham-operation or transient MCAO. IL, interleukin; MIP, macrophage inflammatory protein; MCP, monocyte chemoattractant protein; HSP, heat shock protein; HO, heme oxygenase. The tissue was obtained from the ipsilateral cortex at 12h of reperfusion following a 45 min transient MCAO. The Δfold is in comparison to the respective contralateral cortex. The percent difference = (Δfold over sham in MCAO of db/+ group − 1) −(ΔFold over sham in db/db group − 1)/( fold over sham in MCAO of db/+ group − 1)*100.
p<0.05 (db/+ versus db/db by one-way ANOVA followed by Tucky-Kramer multiple comparisons post-test).
HSP gene expression after focal ischemia
The expression of the neuroprotective HSPs HSP27, HSP70 and HSP32/HO-1 are known to be upregulated after focal ischemia (Dhodda et al 2004). The ipsilateral cortex of both db/db and db/+ mice subjected to 45 min transient MCAO and 12h reperfusion showed significantly increased mRNA levels of HSP70, HSP27 and HSP32/HO-1 compared to the respective contralateral cortex (n =4/group; Table 1). However, the increased gene expression of all the 3 HSPs was significantly less in the db/db compared to db/+ mice by 62 to 78% (Table 1).
DISCUSSION
Results of the present study show that transient focal ischemia induces bigger infarcts, worsened neurological status, increased cerebral water accumulation, higher inflammation and curtailed induction of HSPs in type-2 diabetic db/db mice compared to the normoglycemic db/+ mice. Furthermore, type-2 diabetic mice manifested a significantly higher rate of mortality following focal ischemia.
One of the limiting factors in stroke therapeutic development is the use of healthy rodents to understand the mechanisms of post-ischemic brain damage. As >30% of the stroke sufferers are diabetic with several metabolic changes in the body, it is important to understand the pathophysiologic characteristics and further test the new stroke drugs in diabetic subjects. The present study reiterates this by showing that type-2 diabetic mice respond very differently to focal ischemia with increased edema, inflammation and mortality. Our studies show that the db/db mouse manifests many other characteristics of human diabetics following stroke. The db/db mice is known to develop neuropathy (Calcutt et al 1988), nephropathy (Sharma et al 2003), retinopathy (Clements et al 1998) and impaired wound healing (Kumari et al 2007) similar to human type-2 diabetic patients. Hence, these mice might be well-suited to study the effects of stroke with all the confounding pathological features of diabetes. One of the limitations of the present study is that we haven’t studied the effect of focal ischemia in prediabetic (5 weeks old) db/db mice as those were too small in size to induce MCAO.
One of the hallmarks of diabetes is recurrent hyperglycemia which might be a contributor of the presently observed exacerbated post-ischemic brain damage in diabetic mice. However, a previous study from our laboratory observed no neuroprotection when focal ischemia was induced in db/db mice in which the blood glucose levels were controlled with a 3 week metformin treatment (Tureyen et al 2007). Surprisingly, treating db/db mice with transcription factor PPARγ agonist rosiglitazone for 3 weeks (which also controlled the blood glucose levels) prior to the induction of focal ischemia led to a moderate decrease in the infarct volume (Tureyen et al 2007). Thus, normalizing blood glucose level might not be protective and rosiglitazone-induced neuroprotection can be attributed to its anti-inflammatory effects. Recent studies also showed that treatment of obese, diabetic ob/ob mice with PPARγ agonist darglitazone for 7 days before the induction of hypoxic-ischemia reduced the infarct size and exacerbated the inflammatory response at 8h and 24h after ischemia onset (Kumari et al 2010). It is well known that post-treatment with PPARγ agonists rosiglitazone and pioglitazone significantly decreased CNS damage following focal ischemia, traumatic brain injury and spinal cord injury in normoglycemic rodents (Okada et al 2002; Park et al 2007; Sundararajan et al 2005; Tureyen et al 2007; Yi et al 2008; Zhao et al 2006). However, disappointingly rosiglitazone post-treatment had no protective effect in db/db mice subjected to transient MCAO (Tureyen et al 2007). This lack of efficacy with the acute post-treament might be due to low levels of PPARγ in diabetics and as rosiglitazone treatment induces PPARγ expression, the long-term pretreatment might have allowed the drug action. Darglitazone is a much more robust PPARγ agonist with 7 times more efficacy than rosiglitazone and hence might be a better choice as a therapeutic, but it is not known if this drug can protect the diabetic brain if given after stroke treatment.
The majority of the experimental studies to date that evaluated the effect of diabetes in stroke outcome used type-1 diabetes models. The post-ischemic brain damage was also observed to be exacerbated in type-1 diabetic rodents following global or focal ischemia (Elewa et al 2009; Ergul et al 2009; Kamada et al 2007; Kusaka et al 2004; Shen et al 2010). Studies that used type-2 diabetic animals to understand the mechanisms of stroke-induced brain damage were much fewer. Vannucci et al., (2001) showed that female db/db mice are more resistant to hypoxic ischemia than male db/db mice. A recent study showed that exacerbated post-ischemic pathological symptoms observed in db/db mice can be alleviated by knocking-out the enzyme aldose reductase which is the first enzyme in the polyol pathway that converts excess glucose to sorbitol and further metabolizes to fructose (Yeung et al 2010). In addition to db/db mice, the effect of focal ischemia was studied in few other rodent models of type-2 diabetes. Mayanagi et al. (2008) showed that treating 8 week old obese diabetic ob/ob mice with rosuvastatin induces neuroprotection after focal ischemia. The ob/ob and db/db mouse models are different in the leptin status, the former lacks leptin while the latter lacks leptin receptor (Halle and Persson, 2002). While db/db and ob/ob mice are obese, type-2 diabetes models, the Goto-Kakizaki (GK) rat is a lean type-2 diabetes model (Goto et al 1976). The GK rat is an important resource for experimental studies as many type-2 diabetics in the world are not obese. Furthermore, in diabetic population in addition to infarction, edema and hemorrhagic transformation (HT) are also major problems following stroke (Ergul et al 2009). In the present study, we observed exacerbated edema and infarction, but not HT in db/db mice subjected to 45 min transient MCAO. We also failed to observe any HT in the db/db mice that died within the first 12h after 2h MCAO. On the other hand GK rats show increased cerebrovascular matrix metalloprotease activity and tortuosity, and hence focal ischemia in these animals induces HT (Elgebaly et al 2007; Ergul et al 2007; Ergul et al 2009; Li et al 2010). However, due to high collateral circulation the GK rats develop smaller infarcts (Ergul et al 2007). Thus, db/db mice and GK rats together offer a unique resource to study post-stroke brain damage and therapies that need to be tested to minimize infarction and HT in diabetic patients. A recent study showed that in high fat diet combined with streptozotocin rat model of type-2 diabetes, exacerbated post-ischemic brain damage and deteriorated cognitive impairment are associated with the β-secretase (BACE1) activation and increased Aβ generation (Zhang et al 2009).
Uncontrolled inflammation during the acute period after stroke is thought to be a major mediator of cerebrovascular failure and brain damage (del Zoppo 2009). Increased expression of cell adhesion molecules enabling the extravasation of WBCs and further induction of pro-inflammatory transcription factors and other inflammatory genes are thought to be the major mediators of post-ischemic inflammation (Yi et al 2007). We currently observed that the gene expression of pro-inflammatory cytokines, chemokines and adhesion molecules was much higher in the db/db mouse brain compared to normoglycemic db/+ control at 12h of reperfusion following transient MCAO. Thus, the exacerbated inflammation might be a contributing factor to the increased post-stroke brain damage observed in the diabetic brain. Furthermore, the macrophages and neutrophils will release the oxygen and nitrogen free radicals which are extremely toxic to neurons. Interestingly, db/db mice are known to manifest systemic inflammation as well as an impaired ability to curtail inflammation (Dula et al 2010; Li et al 2009; Lu et al 2004; Meng et al 2010).
Following transient focal ischemia, HSPs are known to be induced in brain as an endogenous neuroprotective response. HSP70 induced in neurons acts as a molecular chaperone that mediates the ischemic tolerance, prevents protein aggregation/denaturation and attenuates the excitotoxic neurotransmitter release after ischemia (Dhodda et al 2004; Takagi et al 1994). HSP27 induced in astrocytes is also thought to be neuroprotective by mediates protein chaperoning in addition to increasing intracellular glutathione levels, reducing the reactive oxygen species and preventing the apoptosis (Currie et al 2000; Garrido et al 1999; Mehlen et al 1996). HSP32/HO-1 is a stress-inducible astroglial form of HO which is known to prevent the cell death by regulating the cellular iron levels and by controlling the inflammation (Baranano and Snyder 2001; Ferris et al 1999; Lee and Chau 2002). We presently observed that the post-ischemic induction of HSP70, HSP27 and HSP32/HO1 was significantly curtailed in the brains of db/db mice indicating a disability to prevent cell death and failure to induce plasticity in the diabetic brain. The presently observed inability to induce neuroprotective HSPs following focal ischemia in type-2 diabetic mice was not reported earlier and this might be an important feature that might be responsible for the increased ischemic brain damage in these animals.
Unfortunately, many diabetic patients also show chronic hypertension. Elevated blood pressure is known to be associated with increased HT and edema after stroke and treatment with the anti-hypertensive drug candesartan was shown to curtail ischemic brain damage in type-1 diabetic rodents (Kusaka et al 2004). The db/db mice show mild hypertension, but its contribution to the presently observed exacerbated ischemic brain damage observed in these animals is not known. Hypercholesteremia is another complication associated often with type-2 diabetes and previous studies showed that treatment of GK rats subjected to focal ischemia with atorvastatin reduces HT (Elewa et al 2009). Thus, a combination therapy that can curtail multiple pathophysiological mechanisms including, but not limited to inflammation, hypertension, hyperglycemia, edema and oxidative stress might be a good choice for preventing post-stroke brain damage in diabetics.
Acknowledgments
These studies were partly funded by NIH grant NS049448.
Abbreviations used
- db
diabetic gene
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GK
Goto-Kakizaki
- HO1
heme oxygenase-1
- HSP
heat shock protein
- HT
hemorrhagic trnasformation
- ICAM1
intracellular adhesion molecule
- IL
inerleukin
- MCAO
Middle cerebral artery occlusion
- MCP
monocyte chemoattractant protein
- MIP
macrophage inflammatory protein
- MPO
myeloperoxidase
- ob
obese gene
- PPAR
peroxisome proliferator-activated receptor
- rCBF
regional cerebral blood flow
- TTC
triphenyl tetrazolium choloride
- WBC
white blood cell
References
- Baranano DE, Snyder SH. Neural roles for heme oxygenase: contrasts to nitric oxide synthase. Proc Natl Acad Sci U S A. 2001;98:10996–1002. doi: 10.1073/pnas.191351298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcutt NA, Willars GB, Tomlinson DR. Axonal transport of choline acetyltransferase and 6-phosphofructokinase activities in genetically diabetic mice. Muscle Nerve. 1988;11:1206–10. doi: 10.1002/mus.880111204. [DOI] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–5. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- Clements RS, Jr, Robison WG, Jr, Cohen MP. Anti-glycated albumin therapy ameliorates early retinal microvascular pathology in db/db mice. J Diabetes Complications. 1998;12:28–33. doi: 10.1016/s1056-8727(97)00051-2. [DOI] [PubMed] [Google Scholar]
- Currie RW, Ellison JA, White RF, Feuerstein GZ, Wang X, Barone FC. Benign focal ischemic preconditioning induces neuronal Hsp70 and prolonged astrogliosis with expression of Hsp27. Brain Res. 2000;863:169–81. doi: 10.1016/s0006-8993(00)02133-8. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience. 2009;158:972–82. doi: 10.1016/j.neuroscience.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodda VK, Sailor KA, Bowen KK, Vemuganti R. Putative endogenous mediators of preconditioning-induced ischemic tolerance in rat brain identified by genomic and proteomic analysis. J Neurochem. 2004;89:73–89. doi: 10.1111/j.1471-4159.2004.02316.x. [DOI] [PubMed] [Google Scholar]
- Dula SB, Jecmenica M, Wu R, Jahanshahi P, Verrilli GM, Carter JD, Brayman KL, Nunemaker CS. Evidence that low-grade systemic inflammation can induce islet dysfunction as measured by impaired calcium handling. Cell Calcium. 2010;48:133–42. doi: 10.1016/j.ceca.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elewa HF, Kozak A, El-Remessy AB, Frye RF, Johnson MH, Ergul A, Fagan SC. Early atorvastatin reduces hemorrhage after acute cerebral ischemia in diabetic rats. J Pharmacol Exp Ther. 2009;330:532–40. doi: 10.1124/jpet.108.146951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgebaly MM, Portik-Dobos V, Sachidanandam K, Rychly D, Malcom D, Johnson MH, Ergul A. Differential effects of ET(A) and ET(B) receptor antagonism on oxidative stress in type 2 diabetes. Vascul Pharmacol. 2007;47:125–30. doi: 10.1016/j.vph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Ergul A, Elgebaly MM, Middlemore ML, Li W, Elewa H, Switzer JA, Hall C, Kozak A, Fagan SC. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurol. 2007;7:33. doi: 10.1186/1471-2377-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergul A, Li W, Elgebaly MM, Bruno A, Fagan SC. Hyperglycemia, diabetes and stroke: focus on the cerebrovasculature. Vascul Pharmacol. 2009;51:44–9. doi: 10.1016/j.vph.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Wolosker H, Baranano DE, Dore S, Poss KD, Snyder SH. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol. 1999;1:152–7. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- Garrido C, Bruey JM, Fromentin A, Hammann A, Arrigo AP, Solary E. HSP27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB J. 1999;13:2061–70. doi: 10.1096/fasebj.13.14.2061. [DOI] [PubMed] [Google Scholar]
- Goto Y, Kakizaki M, Masaki N. Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med. 1976;119:85–90. doi: 10.1620/tjem.119.85. [DOI] [PubMed] [Google Scholar]
- Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153:1127–8. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke. 2007;38:1044–9. doi: 10.1161/01.STR.0000258041.75739.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia R, Tureyen K, Bowen KK, Kalluri H, Johnson PF, Vemuganti R. Decreased brain damage and curtailed inflammation in transcription factor CCAAT/enhancer binding protein beta knockout mice following transient focal cerebral ischemia. J Neurochem. 2006;98:1718–31. doi: 10.1111/j.1471-4159.2006.04056.x. [DOI] [PubMed] [Google Scholar]
- Kumari R, Willing LB, Krady JK, Vannucci SJ, Simpson IA. Impaired wound healing after cerebral hypoxia-ischemia in the diabetic mouse. J Cereb Blood Flow Metab. 2007;27:710–8. doi: 10.1038/sj.jcbfm.9600382. [DOI] [PubMed] [Google Scholar]
- Kumari R, Willing LB, Patel SD, Krady JK, Zavadoski WJ, Gibbs EM, Vannucci SJ, Simpson IA. The PPAR-gamma agonist, darglitazone, restores acute inflammatory responses to cerebral hypoxia-ischemia in the diabetic ob/ob mouse. J Cereb Blood Flow Metab. 2010;30:352–60. doi: 10.1038/jcbfm.2009.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka I, Kusaka G, Zhou C, Ishikawa M, Nanda A, Granger DN, Zhang JH, Tang J. Role of AT1 receptors and NAD(P)H oxidase in diabetes-aggravated ischemic brain injury. Am J Physiol Heart Circ Physiol. 2004;286:H2442–51. doi: 10.1152/ajpheart.01169.2003. [DOI] [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–5. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–6. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- Li J, Wang JJ, Chen D, Mott R, Yu Q, Ma JX, Zhang SX. Systemic administration of HMG-CoA reductase inhibitor protects the blood-retinal barrier and ameliorates retinal inflammation in type 2 diabetes. Exp Eye Res. 2009;89:71–8. doi: 10.1016/j.exer.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Prakash R, Kelly-Cobbs AI, Ogbi S, Kozak A, El-Remessy AB, Schreihofer DA, Fagan SC, Ergul A. Adaptive cerebral neovascularization in a model of type 2 diabetes: relevance to focal cerebral ischemia. Diabetes. 2010;59:228–35. doi: 10.2337/db09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Lu H, Raptis M, Black E, Stan M, Amar S, Graves DT. Influence of diabetes on the exacerbation of an inflammatory response in cardiovascular tissue. Endocrinology. 2004;145:4934–9. doi: 10.1210/en.2004-0737. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Kretz-Remy C, Preville X, Arrigo AP. Human hsp27, Drosophila hsp27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFalpha-induced cell death. EMBO J. 1996;15:2695–706. [PMC free article] [PubMed] [Google Scholar]
- Meng X, Tancharoen S, Kawahara KI, Nawa Y, Taniguchi S, Hashiguchi T, Maruyama I. 1,5-Anhydroglucitol attenuates cytokine release and protects mice with type 2 diabetes from inflammatory reactions. Int J Immunopathol Pharmacol. 2010;23:105–19. doi: 10.1177/039463201002300110. [DOI] [PubMed] [Google Scholar]
- Okada M, Yan SF, Pinsky DJ. Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) activation suppresses ischemic induction of Egr-1 and its inflammatory gene targets. FASEB J. 2002;16:1861–8. doi: 10.1096/fj.02-0503com. [DOI] [PubMed] [Google Scholar]
- Park SW, Yi JH, Miranpuri G, Satriotomo I, Bowen K, Resnick DK, Vemuganti R. Thiazolidinedione class of peroxisome proliferator-activated receptor gamma agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. J Pharmacol Exp Ther. 2007;320:1002–12. doi: 10.1124/jpet.106.113472. [DOI] [PubMed] [Google Scholar]
- Sharma AN, Elased KM, Garrett TL, Lucot JB. Neurobehavioral deficits in db/db diabetic mice. Physiol Behav. 2010 doi: 10.1016/j.physbeh.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, McCue P, Dunn SR. Diabetic kidney disease in the db/db mouse. Am J Physiol Renal Physiol. 2003;284:F1138–44. doi: 10.1152/ajprenal.00315.2002. [DOI] [PubMed] [Google Scholar]
- Shen B, Vetri F, Mao L, Xu HL, Paisansathan C, Pelligrino DA. Aldose reductase inhibition ameliorates the detrimental effect of estrogen replacement therapy on neuropathology in diabetic rats subjected to transient forebrain ischemia. Brain Res. 2010;1342:118–26. doi: 10.1016/j.brainres.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130:685–96. doi: 10.1016/j.neuroscience.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–3. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Takagi K, Ginsberg MD, Globus MY, Martinez E, Busto R. Effect of hyperthermia on glutamate release in ischemic penumbra after middle cerebral artery occlusion in rats. Am J Physiol. 1994;267:H1770–6. doi: 10.1152/ajpheart.1994.267.5.H1770. [DOI] [PubMed] [Google Scholar]
- Titova E, Ostrowski RP, Kevil CG, Tong W, Rojas H, Sowers LC, Zhang JH, Tang J. Reduced brain injury in CD18-deficient mice after experimental intracerebral hemorrhage. J Neurosci Res. 2008;86:3240–5. doi: 10.1002/jnr.21762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tureyen K, Kapadia R, Bowen KK, Satriotomo I, Liang J, Feinstein DL, Vemuganti R. Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J Neurochem. 2007;101:41–56. doi: 10.1111/j.1471-4159.2006.04376.x. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Brooks N, Bowen K, Svaren J, Vemuganti R. Transcription factor early growth response-1 induction mediates inflammatory gene expression and brain damage following transient focal ischemia. J Neurochem. 2008;105:1313–24. doi: 10.1111/j.1471-4159.2008.05233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuganti R, Dempsey RJ, Bowen KK. Inhibition of intercellular adhesion molecule-1 protein expression by antisense oligonucleotides is neuroprotective after transient middle cerebral artery occlusion in rat. Stroke. 2004;35:179–84. doi: 10.1161/01.STR.0000106479.53235.3E. [DOI] [PubMed] [Google Scholar]
- Weston RM, Jones NM, Jarrott B, Callaway JK. Inflammatory cell infiltration after endothelin-1-induced cerebral ischemia: histochemical and myeloperoxidase correlation with temporal changes in brain injury. J Cereb Blood Flow Metab. 2007;27:100–14. doi: 10.1038/sj.jcbfm.9600324. [DOI] [PubMed] [Google Scholar]
- Yeung CM, Lo AC, Cheung AK, Chung SS, Wong D, Chung SK. More severe type 2 diabetes-associated ischemic stroke injury is alleviated in aldose reductase-deficient mice. J Neurosci Res. 2010;88:2026–34. doi: 10.1002/jnr.22349. [DOI] [PubMed] [Google Scholar]
- Yi JH, Park SW, Kapadia R, Vemuganti R. Role of transcription factors in mediating post-ischemic cerebral inflammation and brain damage. Neurochem Int. 2007;50:1014–27. doi: 10.1016/j.neuint.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi JH, Park SW, Brooks N, Lang BT, Vemuganti R. PPARgamma agonist rosiglitazone is neuroprotective after traumatic brain injury via anti-inflammatory and anti-oxidative mechanisms. Brain Res. 2008;1244:164–72. doi: 10.1016/j.brainres.2008.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Pan BS, Zhao B, Zhang LM, Huang YL, Sun FY. Exacerbation of poststroke dementia by type 2 diabetes is associated with synergistic increases of beta-secretase activation and beta-amyloid generation in rat brains. Neuroscience. 2009;161:1045–56. doi: 10.1016/j.neuroscience.2009.04.032. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Patzer A, Herdegen T, Gohlke P, Culman J. Activation of cerebral peroxisome proliferator-activated receptors gamma promotes neuroprotection by attenuation of neuronal cyclooxygenase-2 overexpression after focal cerebral ischemia in rats. FASEB J. 2006;20:1162–75. doi: 10.1096/fj.05-5007com. [DOI] [PubMed] [Google Scholar]