Abstract
Voltage-gated calcium channels (CaVs) govern muscle contraction, hormone and neurotransmitter release, neuronal migration, activation of calcium-dependent signalling cascades, and synaptic input integration1. An essential CaV intracellular protein, the β-subunit (CaVβ)1,2, binds a conserved domain (the α-interaction domain, AID) between transmembrane domains I and II of the pore-forming α1 subunit3 and profoundly affects multiple channel properties such as voltage-dependent activation2, inactivation rates2, G-protein modulation4, drug sensitivity5 and cell surface expression6,7. Here, we report the high-resolution crystal structures of the CaVβ2a conserved core, alone and in complex with the AID. Previous work suggested that a conserved region, the β-interaction domain (BID), formed the AID-binding site3,8; however, this region is largely buried in the CaVβ core and is unavailable for protein–protein interactions. The structure of the AID–CaVβ2a complex shows instead that CaVβ2a engages the AID through an extensive, conserved hydrophobic cleft (named the α-binding pocket, ABP). The ABP–AID interaction positions one end of the CaVβ near the intracellular end of a pore-lining segment, called IS6, that has a critical role in CaV inactivation9,10. Together, these data suggest that CaVβs influence CaV gating by direct modulation of IS6 movement within the channel pore.
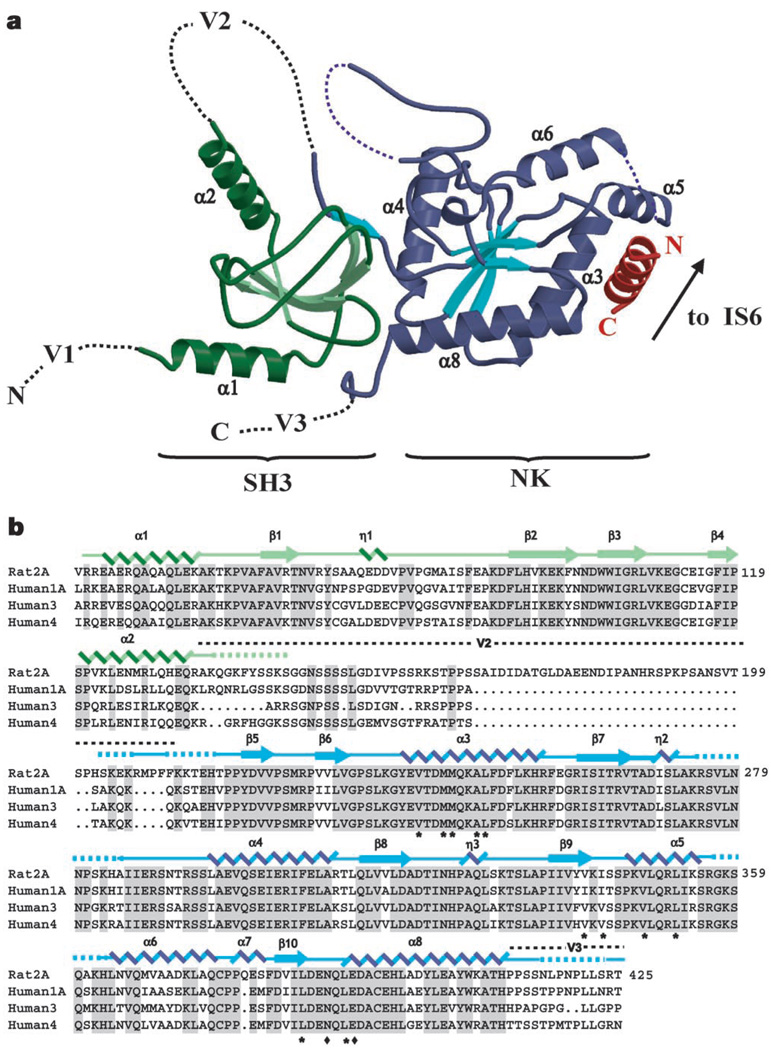
The 1.97 Å resolution structure of the CaVβ2a core shows that CaVβs comprise two well-conserved domains (Fig. 1a). The first, an SH3 fold, contains five antiparallel β-strands (β1–β5), a 310 helix (η1), and two α-helices (α1 and α2) that lie amino-terminal to β1 and carboxy-terminal to β4, respectively. The strand that completes the SH3 fold, β5 (residues 217–224), is separated in the primary structure from the core of the SH3 domain by approximately 70 residues (variable domain 2, V2, a site of splice variation and amino acid insertions and deletions2) that are absent from the structure (Fig. 1b). The second conserved domain consists of a five-stranded parallel β-sheet (β6–β10), surrounded by six α-helices (α3–α8) and two 310 helices (η2 and η3), and is related to the core of nucleotide kinase enzymes.
Figure 1.
Structure of the CaVβ2a–CaV1.2 AID complex. a, Ribbon diagram of the complex. Dashed lines indicate regions absent from the structures. SH3 and nucleotide kinase (NK) domains are shown in green and blue, respectively. The AID is shown in red. CaVβ2a α-helices are labelled. Variable regions V1, V2 and V3 are indicated. The CaVβ2a unbound structure is similar to that shown here for the complex. The arrow indicates where the AID connects to transmembrane segment IS6. b, Sequence alignment of representatives of each CaVβ isoform. The top sequence shows residues 40–425 of rat Cavβ2a. Numbers on the right denote each line’s terminal residue. Shading denotes residues identical among isoforms. The two Cavβ2a domains used for crystallization are indicated in green and blue, respectively. Secondary structure elements are indicated: α, α-helix; η, 310 helix; β, β-strand. Dashed lines indicate residues present in the crystallized constructs but absent in the electron density. Location of the V2 and part of the V3 regions are shown. Asterisks identify residues that contribute side-chain contacts to the AID-binding pocket; diamonds mark side chains with direct hydrogen bonds to the AID.
CaVβs share structural features with membrane-associated guanylate kinases (MAGUKs), a protein scaffold family that organizes signalling components near membranes11. MAGUKs contain one or more PDZ domains N-terminal to an SH3 domain, a bridging region known as a HOOK domain and a nucleotide kinase domain11,12. PDZ domains are approximately 100 residues long. The CaVβ2a structure indicates that CaVβs lack N-terminal PDZ domains. There are too few residues N-terminal to the SH3 domain (even in CaVβ1b, the CaVβ with the longest (55 amino acids) N-terminal variable region 1, V1) to fold as a PDZ domain. The absence of a PDZ domain distinguishes CaVβs from canonical MAGUK proteins.
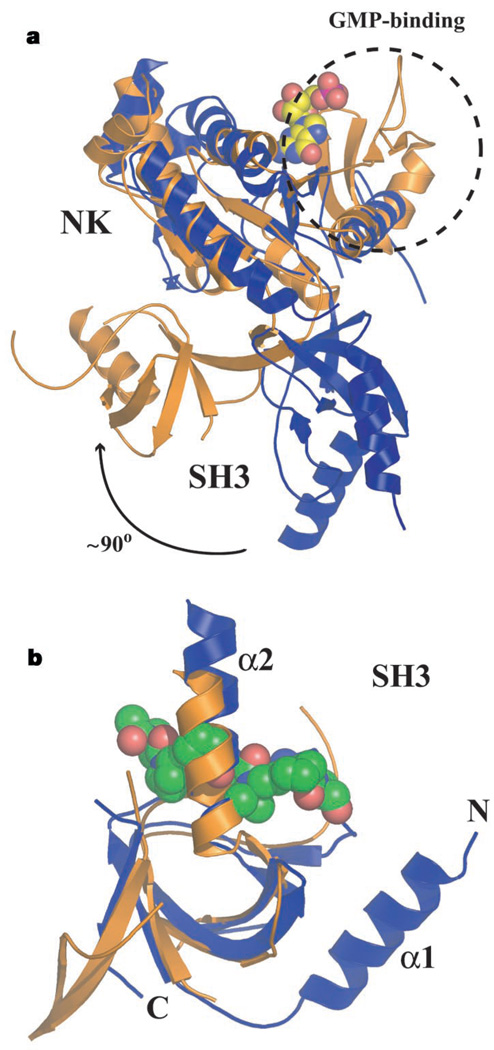
Comparison of CaVβ2a with a representative MAGUK, PSD-95 (refs 12, 13), reveals other differences. Superposition of the nucleotide kinase domains shows that the relative orientations of the SH3 and nucleotide kinase domains differ by approximately 90°, an arrangement that makes CaVβ2a a more elongated structure (Fig. 2a). The nucleotide kinase domain of MAGUKs is homologous to guanylate kinases and retains guanosine monophosphate (GMP) binding, but key residues for enzymatic function are missing12. The four-stranded β-sheet nucleotide kinase subdomain that binds GMP in MAGUKs is absent in Cavβ2a (Fig. 2a). Furthermore, two CaVβ2a loops (between β7–η2 and β8–β9) occlude part of the binding site for the GMP guanosine ring. Thus, the CaVβ nucleotide kinase domain seems to have lost the ability to bind nucleotides.
Figure 2.
Structural comparisons between PSD-95 (gold) and CaVβ2a (blue). a, Superposition of CaVβ2a and PSD-95 nucleotide kinase domains (RMSDCα = 3.9 Å). The dashed circle indicates the guanosine-monophosphate (GMP)-binding domain present in PSD-95 but absent in CaVβ2a. The guanosine monophosphate molecule bound to PSD-95 is displayed in space-filling representation. Nucleotide kinase (NK) and SH3 domains are indicated. The relative change in SH3 domain orientation is indicated. b, Superposition of PSD-95 and CaVβ2a SH3 domains (RMSDCα = 1.6 Å). Position of the polyproline ligand from a superposition with the Sem5 SH3 domain (Protein Data Bank code 2SEM) (RMSDCα = 1.8 Å) is shown in space-filling representation. The Sem5 SH3 is not shown. The DALI server generated the superpositions (http://www.ebi.ac.uk/dali/).
The structures of Cavβ2a and PSD-95 SH3 domains are similar (Fig. 2b). Neither is compatible with canonical modes of proline-rich ligand binding. Both lack the aromatic residues necessary for ligand engagement13, and the surface that would bind polyproline ligands is blocked by the α2 helix12,13. In PSD-95, residues C-terminal to the nucleotide kinase domain contribute an extra SH3 β-strand that is absent from canonical SH3 domains13 and absent in Cavβ2a. The HOOK domain, present in MAGUKs and CaVβ2a, bridges SH3 β-strands β4 and β5 and comprises α2 and variable domain 2 of CaVβ2a. HOOK domains are important regulatory regions for interactions of MAGUKs with other proteins11,12,14 and may serve a similar function for Cavβ protein–protein interactions.
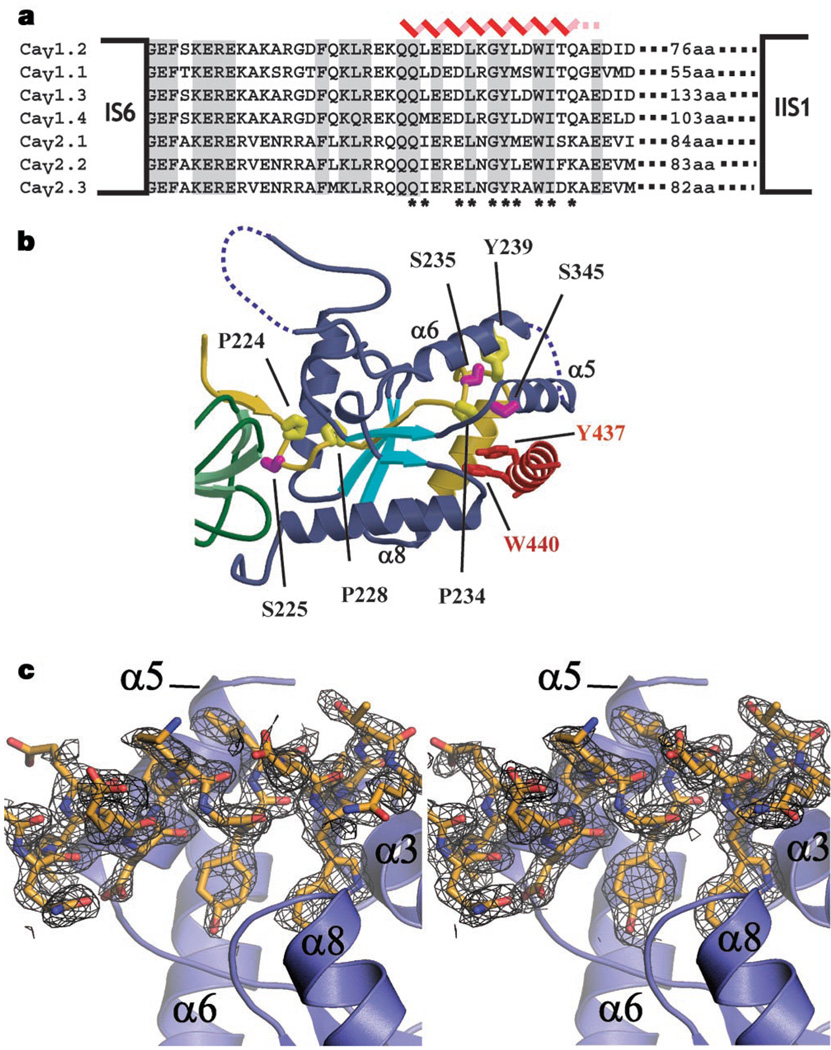
Cavβs exert their effects on CaV function by binding the pore-forming α1 subunit at a conserved, 18-residue sequence located between membrane domains I and II (the AID)3,15 (Fig. 3a). Interpretation of mutagenesis and biochemical studies suggested that Cavβ–AID interactions occur through a 41-residue segment (CaVβ2a residues 212–252) termed the β-interaction domain (BID)8,16. The CaVβ2a structure shows that the central region of the BID, which includes the residues previously thought to be important for BID–AID interactions8, is entirely buried and cannot participate directly in protein–protein interactions (Fig. 3b). Two putative BID phosphorylation sites8,16 are also buried in this region. Given the extent of burial, mutations used to determine the relative importance of residues involved in BID–AID interactions (for example, proline to arginine)8 are likely to have abolished AID binding by disrupting the folded structure of the nucleotide kinase domain rather than by perturbing direct AID contacts. Although it would appear that the CaVβ2a structure conflicts with previous data, most of the supporting evidence for the BID–AID interaction relied on indirect functional experiments and direct BID–AID binding was never demonstrated8,16.
Figure 3.
Features of the AID–CaVβ2a interaction and location of the previously described BID. a, Sequence alignment of AID domains (CaV1.2 residues 428–445) and neighbouring residues. The positions of the last transmembrane segment of transmembrane domain I (IS6) and the first transmembrane segment of transmembrane domain II (IIS1) are shown. Secondary structure of the AID from the co-crystal structure is indicated (red). Dashed lines indicate residues absent from the electron density. Asterisks identify side-chain contacts with CaVβ2a closer than 4 Å. b, Position of the previously described BID (residues 212–252; yellow)3,8. Residues previously proposed to mediate AID–BID interactions (P224, P228, P234, Y239) are indicated and have relative accessibilities of 1.4%, 0%, 0% and 32.4%. Putative PKC sites S225, S235 and S345 are also shown (magenta) and have relative accessibilities of 8.8%, 0% and 35.4%, respectively. S345 accessibility reduces to 12% in the complex. Accessibility values are relative to a tripeptide, Gly-X-Gly. c, The left panel shows Fo−Fc electron density, contoured at 2σ, for the AID–CaVβ2a complex before building the AID. The right panel shows final 2Fo−Fc density, contoured at 1σ, for the AID from the refined AID–CaVβ2a structure (right). In both panels the final AID model is shown.
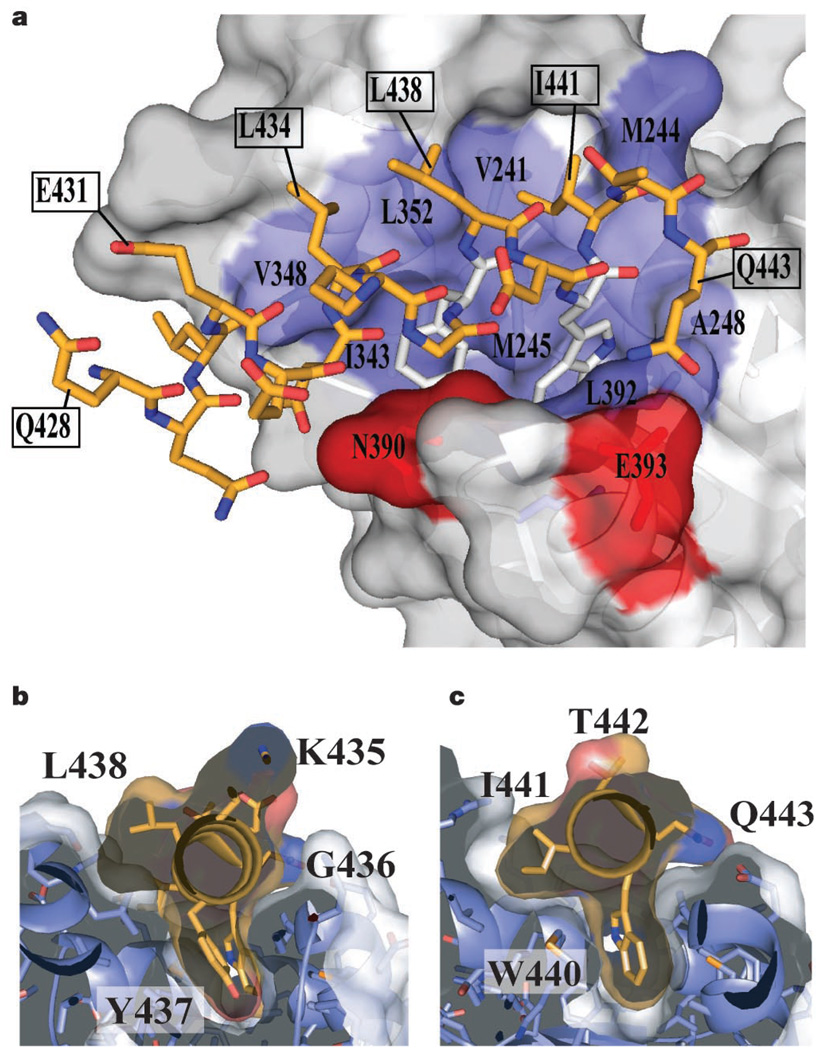
If the BID does not interact directly with the AID, how do Cavβ2a and the AID interact? To answer this, we solved the high-resolution (2.00 Å) structure of a complex between the conserved CaVβ2a core and an 18-residue peptide containing the AID from the L-type channel Cav1.2. The electron density reveals the first 16 residues of the AID and the location of the binding pocket on Cavβ2a (Fig. 3c). Overall, the Cavβ2a structure is very similar to the unbound form (root-mean-square deviation (RMSD)Cα = 0.397 Å) and bears only a few conformational changes in side chains near the AID-binding pocket (Cavβ2a residues M244, N390, E393 and R351). The AID forms an α-helix that is anchored to the binding pocket through a set of conserved residues (AID residues L434, G436, Y437, W440 and I441) that are important for CaVβ binding and for α-subunit modulation by CaVβs8,15,17. These residues bind a deep groove that we call the α-binding pocket (ABP), formed by helices α3, α5 and α8 of the Cavβ2a nucleotide kinase domain at a site distal to the SH3 domain (Figs 1a, 3b and 4a).
Figure 4.
AID–ABP interactions. a, Surface representation of the CaVβ2a ABP, bound to the AID. The AID (gold) is shown in stick representation. Y437 and W440 are white. CaVβ2a residues that contribute hydrophobic (blue) and hydrogen bond (red) side-chain contacts to the AID are labelled. Select residues of the AID are labelled to orient the reader. b, c, Slices through the AID–ABP interaction at AID positions Y437 and W440 (gold). Labels indicate the AID residues.
The complex buries approximately 730 Å2 of ABP surface, of which about 350 Å2 are hydrophobic. AID side chains D433, W440 and Q443 make direct hydrogen bonds to the ABP (Supplementary Fig. 1). The depth and extent of burial of the aromatic AID positions Y437 and W440 is particularly striking (Fig. 4b, c). The AID Y437 hydroxyl group is central to a buried hydrogen bond network comprising three water molecules, AID residue D433 and five CaVβ2a residues (Supplementary Fig. 1). Mutation of this tyrosine to phenylalanine greatly diminishes AID–CaVβ binding15. The extensive AID–ABP interactions are consistent with biochemical experiments demonstrating strong AID–CaVβ interaction (dissociation constant ~6–20 nM)18. The CaVβ side chains that contact the AID are highly conserved among CaVβ isoforms (see Figs 1b, 3a and 4a; see also Supplementary Fig. 1). Thus, both binding partners engage each other through conserved residues to create the AID–ABP interaction.
Interactions between the CaVα1 and CaVβ subunits markedly influence the cell surface expression of functional channels6,7. Control of CaV trafficking by regulating CaVα1–CaVβ interactions is emerging as an important means of modulating cellular excitability7. CaV channel subtypes are major clinical targets for drugs that treat cardiovascular disease, migraine and pain19. Development of compounds that could interfere with the AID–ABP binding interactions might provide new ways to modulate CaV function in pathological states.
The CaVβ–AID structure provides a starting point for understanding how CaVβ modulates numerous channel properties. G-protein βγ subunits (Gβγ) inhibit CaV function1,4; however, the sites of Gβγ–CaV interactions and precise inhibitory mechanisms remain highly controversial4. Biochemical experiments show that Gβγ binds the CaV2 AID and that mutations at AID positions Q1, Q2, R5, L7, G9 and Y10 (corresponding to Cav1.2 AID residues 428, 429, 432, 434, 436 and 437) abolish Gβγ–AID binding20. In contrast, other studies suggest that the in vitro Gβγ–AID interaction is functionally irrelevant and that the relevant Gβγ-binding determinants lie elsewhere in the channel cytoplasmic domains21,22. The structure of the CaVβ2a–AID complex shows that three of the putative Gβγ–AID interacting positions (L7, G9 and Y10), which are invariant in CaV1 and CaV2 channels (Fig. 3a), are deeply buried by interactions with CaVβ (Fig. 4a, b; see also Supplementary Fig. 2 and Table 2). The extent of burial of these residues, which are critical for maintaining CaVβ–AID association3,15, suggests that Gβγ and CaVβ cannot bind to the AID simultaneously. Taken together with the observation that the Gβγ–AID affinity is at least 10–20-fold weaker than the CaVβ–AID20 affinity, the structure also indicates that it is unlikely that Gβγ could effectively compete with CaVβ for AID binding without drastic structural rearrangement of the CaVβ–AID complex. Thus, our data lend support to the view that the major Gβγ interaction sites lie in other CaV cytoplasmic domains21–23.
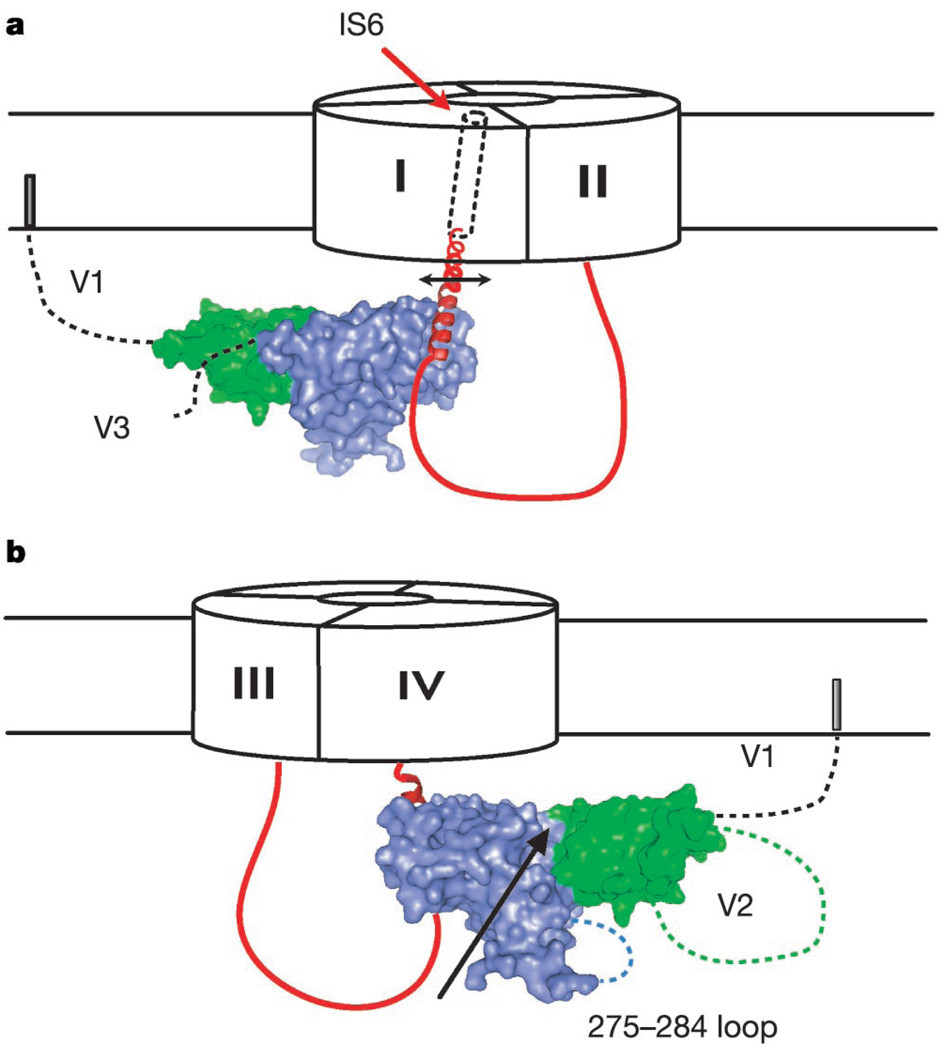
How might CaVβs affect channel gating? Although the detailed mechanisms of CaV inactivation processes remain unknown, functional experiments show that the IS6 pore-lining segment has a key role10. Motions of pore-lining transmembrane segments are a common theme emerging in ion channel gating24,25. Twenty-two residues separate the AID helix N terminus from the cytoplasmic end of IS6. We do not know the structure, but sequence evaluation suggests that these residues have a high helix propensity and could readily form a continuous helix between the AID helix and the presumed transmembrane helix of IS6 (Fig. 5a). AID residue 5 (E432, here) slows inactivation when negatively charged (as in CaV1 channels) and speeds inactivation when positively charged (as in CaV2 channels)26,27. This residue is exposed on the surface of the AID helix (Fig. 4a) where it would be available to interact with other parts of the channel and could influence the rates of movement of the CaVβ–AID complex and therefore IS6. The profound inactivation rate slowing caused by CaVβ2a requires anchoring of the N terminus to the membrane by palmitoylation28. Orientation of the AID helix towards IS6 places the N-terminal membrane anchor on the periphery of the α1 subunit and suggests that CaVβ2a slows channel inactivation by restricting the movement of the IS6 transmembrane domain. CaVβs have a deep groove between the SH3 and nucleotide kinase domains that is on the same face as the V2 domain. These features may be used to engage other cytoplasmic parts of the channel29,30 and allow CaVβ to couple motions in other channel domains directly to IS6 through AID attachment.
Figure 5.
Cartoon of proposed model for how CaVβ affects CaVα1 gating. a, Orientation of CaVβ2a with respect to the I–II loop (red), pore-forming subunit, and connection to IS6. In CaVβ2a, variable region 1 (V1) is tethered to the membrane. The I–II loop between the AID N terminus and IS6 is depicted as a helix. CaVβ2a SH3 and nucleotide kinase domains are coloured green and blue, respectively. The arrow indicates that CaVβ couples to IS6 movements (rotations, translations or both). b, View from the opposite side of a. The groove between SH3 and nucleotide kinase domains (demarcated by the arrow) and two flexible Cavβ2a regions, the 275–284 loop and variable region 2 (V2), are on the same CaVβ face, opposite the ABP. These regions may interact with other pore-forming subunit cytoplasmic domains.
The structures presented here represent the first high-resolution view of any part of the voltage-gated calcium channel, and provide an important step towards understanding the detailed molecular mechanism models for how CaVs function. This work strongly suggests that CaVβ affects CaV gating properties by directly influencing conformational changes that are likely to occur in the channel pore10.
Methods
The crystal structures of recombinant CaVβ2a and CaVβ2a–AID complexes were solved to resolutions of 1.97 Å and 2.00 Å, respectively. The final R/Rfree values are 18.55%/21.32% for CaVβ2a and 19.97%/24.15% for the CaVβ2a–AID complex. Figures were prepared with PyMOL, MOLSCRIPT and RASTER3D. The experimental details for protein expression, purification, crystallization, structure solution, statistics of data collection, phasing and refinement are available as Supplementary Information.
Supplementary Material
Acknowledgements
We thank J. M. Berger, K. Brejc, D. Fass, D. Julius, E. A. Lumpkin and B. A. Schulman for comments on the manuscript; J. Holton at beamline 8.3.1 at the Advanced Light Source for help with data collection; R. W. Tsien and D. T. Yue for the calcium channel clones; and members of the Minor laboratory for support at all stages of this work. This work was supported by awards to D.L.M. from the McKnight Foundation for Neuroscience, the March of Dimes Basil O’Connor Scholar program, the Alfred P. Sloan Foundation, and the Rita Allen Foundation. D.L.M. is a McKnight Foundation Scholar, an Alfred P. Sloan Research Fellow and a Rita Allen Foundation Scholar.
Footnotes
Supplementary Information accompanies the paper on www.nature.com/nature.
Competing interests statement The authors declare that they have no competing financial interests.
Coordinates and structure factors have been deposited in the Protein Data Bank under accession codes 1T0H and 1T0J.
References
- 1.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 2.Dolphin AC. β-subunits of voltage-gated calcium channels. J. Bioenerg. Biomembr. 2003;35:599–620. doi: 10.1023/b:jobb.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- 3.Pragnell M, et al. Calcium channel β-subunit binds to a conserved motif in the I–II cytoplasmic linker of the α1-subunit. Nature. 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- 4.Dolphin AC. G protein modulation of voltage-gated calcium channels. Pharmacol. Rev. 2003;55:607–627. doi: 10.1124/pr.55.4.3. [DOI] [PubMed] [Google Scholar]
- 5.Hering S. β-Subunits: fine tuning of Ca2+ channel block. Trends Pharmacol. Sci. 2002;23:509–513. doi: 10.1016/s0165-6147(02)02104-1. [DOI] [PubMed] [Google Scholar]
- 6.Bichet D, et al. The I–II loop of the Ca2+ channel α1 subunit contains an endoplasmic reticulum retention signal antagonized by the β subunit. Neuron. 2000;25:177–190. doi: 10.1016/s0896-6273(00)80881-8. [DOI] [PubMed] [Google Scholar]
- 7.Beguin P, et al. Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature. 2001;411:701–706. doi: 10.1038/35079621. [DOI] [PubMed] [Google Scholar]
- 8.De Waard M, Scott VE, Pragnell M, Campbell KP. Identification of critical amino acids involved in α1-β interaction in voltage-dependent Ca2+ channels. FEBS Lett. 1996;380:272–276. doi: 10.1016/0014-5793(96)00007-5. [DOI] [PubMed] [Google Scholar]
- 9.Stotz SC, Hamid J, Spaetgens RL, Jarvis SE, Zamponi GW. Fast inactivation of voltage-dependent calcium channels. A hinged-lid mechanism? J. Biol. Chem. 2000;275:24575–24582. doi: 10.1074/jbc.M000399200. [DOI] [PubMed] [Google Scholar]
- 10.Zhang JF, Ellinor PT, Aldrich RW, Tsien RW. Molecular determinants of voltage-dependent inactivation in calcium channels. Nature. 1994;372:97–100. doi: 10.1038/372097a0. [DOI] [PubMed] [Google Scholar]
- 11.Dimitratos SD, Woods DF, Stathakis DG, Bryant PJ. Signaling pathways are focused at specialized regions of the plasma membrane by scaffolding proteins of the MAGUK family. Bioessays. 1999;21:912–921. doi: 10.1002/(SICI)1521-1878(199911)21:11<912::AID-BIES3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 12.Tavares GA, Panepucci EH, Brunger AT. Structural characterization of the intramolecular interaction between the SH3 and guanylate kinase domains of PSD-95. Mol. Cell. 2001;8:1313–1325. doi: 10.1016/s1097-2765(01)00416-6. [DOI] [PubMed] [Google Scholar]
- 13.McGee AW, et al. Structure of the SH3-guanylate kinase module from PSD-95 suggests a mechanism for regulated assembly of MAGUK scaffolding proteins. Mol. Cell. 2001;8:1291–1301. doi: 10.1016/s1097-2765(01)00411-7. [DOI] [PubMed] [Google Scholar]
- 14.Paarmann I, Spangenberg O, Lavie A, Konrad M. Formation of complexes between Ca2+.calmodulin and the synapse-associated protein SAP97 requires the SH3 domain-guanylate kinase domain-connecting HOOK region. J. Biol. Chem. 2002;277:40832–40838. doi: 10.1074/jbc.M205618200. [DOI] [PubMed] [Google Scholar]
- 15.Witcher DR, De Waard M, Liu H, Pragnell M, Campbell KP. Association of native Ca2+ channel β subunits with the α1 subunit interaction domain. J. Biol. Chem. 1995;270:18088–18093. doi: 10.1074/jbc.270.30.18088. [DOI] [PubMed] [Google Scholar]
- 16.De Waard M, Pragnell M, Campbell KP. Ca2+ channel regulation by a conserved β subunit domain. Neuron. 1994;13:495–503. doi: 10.1016/0896-6273(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 17.Berrou L, Klein H, Bernatchez G, Parent L. A specific tryptophan in the I–II linker is a key determinant of β-subunit binding and modulation in Ca(V)2.3 calcium channels. Biophys. J. 2002;83:1429–1442. doi: 10.1016/S0006-3495(02)73914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opatowsky Y, Chomsky-Hecht O, Kang MG, Campbell KP, Hirsch JA. The voltage-dependent calcium channel β subunit contains two stable interacting domains. J. Biol. Chem. 2003;278:52323–52332. doi: 10.1074/jbc.M303564200. [DOI] [PubMed] [Google Scholar]
- 19.Kochegarov AA. Pharmacological modulators of voltage-gated calcium channels and their therapeutical application. Cell Calcium. 2003;33:145–162. doi: 10.1016/s0143-4160(02)00239-7. [DOI] [PubMed] [Google Scholar]
- 20.DeWaard M, et al. Direct binding of G-protein βγ complex to voltage-dependent calcium channels. Nature. 1997;385:446–450. doi: 10.1038/385446a0. [DOI] [PubMed] [Google Scholar]
- 21.Zhang JF, Ellinor PT, Aldrich RW, Tsien RW. Multiple structural elements in voltage-dependent Ca2+ channels support their inhibition by G proteins. Neuron. 1996;17:991–1003. doi: 10.1016/s0896-6273(00)80229-9. [DOI] [PubMed] [Google Scholar]
- 22.Qin N, Platano D, Olcese R, Stefani E, Birnbaumer L. Direct interaction of Gβγ with a C-terminal Gβγ-binding domain of the Ca2+ channel α1 subunit is responsible for channel inhibition by G protein-coupled receptors. Proc. Natl Acad. Sci. USA. 1997;94:8866–8871. doi: 10.1073/pnas.94.16.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivanina T, Blumenstein Y, Shistik E, Barzilai R, Dascal N. Modulation of L-type Ca2+ channels by Gβγ and calmodulin via interactions with N and C termini of α1C. J. Biol. Chem. 2000;275:39846–39854. doi: 10.1074/jbc.M005881200. [DOI] [PubMed] [Google Scholar]
- 24.Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;424:949–955. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Y, et al. The open pore conformation of potassium channels. Nature. 2002;417:523–526. doi: 10.1038/417523a. [DOI] [PubMed] [Google Scholar]
- 26.Herlitze S, Hockerman GH, Scheuer T, Catterall WA. Molecular determinants of inactivation and G protein modulation in the intracellular loop connecting domains I and II of the calcium channel α1A subunit. Proc. Natl Acad. Sci. USA. 1997;94:1512–1516. doi: 10.1073/pnas.94.4.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berrou L, Bernatchez G, Parent L. Molecular determinants of inactivationwithin the I–II linker of α1E (CaV2.3) calcium channels. Biophys. J. 2001;80:215–228. doi: 10.1016/S0006-3495(01)76008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Restituito S, et al. The β2a subunit is a molecular groom for the Ca2+ channel inactivation gate. J. Neurosci. 2000;20:9046–9052. doi: 10.1523/JNEUROSCI.20-24-09046.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker D, et al. A new β subtype-specific interaction in α1A subunit controls P/Q-type Ca2+ channel activation. J. Biol. Chem. 1999;274:12383–12390. doi: 10.1074/jbc.274.18.12383. [DOI] [PubMed] [Google Scholar]
- 30.Walker D, Bichet D, Campbell KP, De Waard M. A β4 isoform-specific interaction site in the carboxyl-terminal region of the voltage-dependent Ca2+ channel α1A subunit. J. Biol. Chem. 1998;273:2361–2367. doi: 10.1074/jbc.273.4.2361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.