Abstract
Murine models of autoimmunity allow the study of the earliest events in disease pathogenesis. Our laboratory has developed a xenobiotic induced model of primary biliary cirrhosis (PBC) following immunization of mice with 2-octynoic acid coupled to bovine serum albumin (2-OA-BSA), an antigen selected following quantitative structure activity relationship analysis of the E2 subunit of the pyruvate dehydrogenase complex (PDC-E2), the immunodominant autoantigen of PBC. Recent data in humans with PBC has suggested that a major component of liver pathology is due to activation of innate immunity. We took advantage of our 2-OA-BSA model and immunized mice with and without the addition of α-galactosylceramide (α-GalCer), an invariant natural T cell activator. Importantly, we report herein that 2-OA-BSA immunized mice exposed to α-GalCer develop a profound exacerbation of their autoimmune cholangitis, including significant increases in CD8+ T cell infiltrates, portal inflammation, granuloma formation and bile duct damage. Furthermore, such mice produce increased levels of anti-mitochondrial antibodies and have evidence of fibrosis, a feature not previously reported in the murine models of PBC. In conclusion, our data suggests a primary role of innate immunity in the exacerbation of autoimmune cholangitis and also become a logical explanation for the recurrence of PBC following liver transplantation in the absence of MHC compatibility. We submit that PBC begins with loss of tolerance to PDC-E2 and a multi-lineage anti-mitochondrial response in which autoreactive CD8+ T cells are critical. However, the perpetuation of disease and its exacerbation will also be modulated by innate immune mechanisms.
Keywords: primary biliary cirrhosis, xenobiotics, iNKT cell, α-GalCer, CD8+ T cell
There have been significant advances in defining the cellular and molecular events that modulate the multi-lineage anti-mitochondrial responses found in primary biliary cirrhosis (PBC)(1-3). However, an understanding of the earliest events that lead to PBC, and those that exacerbate disease severity, have been difficult to understand because of the long latency period of disease onset, the variation of disease severity between patients and, until recently, the absence of appropriate animal models. Several important murine models are now described, including the dnTGF-βRII, NOD-congenic, and IL-2Rα deleted mice (3-6). However, in addition to these models, dependent on the genetic background, we have also reported the induction of a PBC-like disease, including the production of anti-mitochondrial antibodies (AMAs), in mice immunized with a molecular mimic of the inner lipoyl domain of E2 subunits of the pyruvate dehydrogenase complex (PDC-E2) (7-9). This molecular mimic, 2-octynoic acid (2-OA), was based upon a careful structural dissection of the immunodominant autoantigen of PBC by quantitative structure-activity relationship analysis (9-11). Importantly, the autoimmune cholangitis induced by chemical xenobiotic immunization not only recapitulates many of the features of human disease, but, more importantly, affords us the opportunity to study early events.
We have taken advantage of our experience in these murine models and have begun to focus attention on the role of innate immunity and, in particular, the role of natural killer T (NKT) cells on modulating disease activity. Invariant NKT (iNKT) cells, expressing semi-invariant Vα14-Jα28 chain preferentially paired with Vβ2, Vβ7, or Vβ8.2 chain, hypersecrete Th1 and Th2 cytokines and chemokines upon stimulation with an appropriate ligand, such as α-galactosylceramide (α-GalCer). In doing so, these iNKT cells exert considerable and promiscuous immune function, including altering immune regulation by activating dendritic cells (DCs), macrophages, NK cells, T cells, B cells and driving the development of adaptive immunity (12-13). iNKT cells appear to play a critical role in the regulation of several other autoimmune diseases, including type 1 diabetes, multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematosus (13-17).
Previously, our lab has proposed that activation of iNKT cells is a critical factor in accelerating disease (18-20). To explore this issue in depth, we immunized C57BL/6 mice with 2-OA-BSA and activated their iNKT cells with α-GalCer. We report herein that iNKT cell activation by α-GalCer leads to a profound exacerbation of portal disease in 2-OA-BSA immunized mice, including increased AMA production, increased CD8+ T cell biliary infiltration, portal inflammation, granuloma formation, bile duct damage and fibrosis. These results are critical and emphasize the role of innate immunity in the natural history of PBC and they further suggest mechanisms by which biliary disease becomes perpetuated in humans as well as explaining the recurrence of PBC following liver transplantation in the absence of MHC compatibility. These data also emphasize the appearance of fibrosis, a feature thus far lacking in the other murine models of autoimmune cholangitis.
Materials and Methods
Experimental protocol
The protocol for induction of autoimmune cholangitis is similar to our previous reports (7). Briefly, female C57BL/6 mice aged 8-10 weeks were obtained from the National Laboratory Animal Center and maintained in the Animal Center of the College of Medicine, National Taiwan University. Mice were intraperitoneally immunized with 2-OA-BSA (100μg) in the presence of complete Freund’s adjuvant (CFA, Sigma-Aldrich, St. Louis, MO, USA) and subsequently boosted at weeks 2, 4, 6, 8 and 10 with 2-OA-BSA and incomplete Freund’s adjuvant (IFA, Sigma-Aldrich). Two μg of α-GalCer (Alexis, San Diego, CA, USA) was diluted in PBS and injected intravenously one day before 1st, 2nd, and 3rd 2-OA-BSA immunizations (group name: α-GC/CFA/2-OA). A control group injected with PBS instead of α-GalCer was used throughout as a control (group name: PBS/CFA/2-OA). To test the direct effects of α-GalCer on liver damage, mice were injected with α-GalCer only (group name: α-GC) or α-GalCer and then CFA/IFA without 2-OA-BSA (group name: α-GC/CFA) throughout the protocol. Sera were obtained on all mice at 4 and 12 weeks post-immunization and titers of IgM and IgG anti-PDC-E2 autoantibodies were measured by ELISA. All mice were sacrificed at either 4 or 12 weeks post immunization and thence examined for liver histopathology, including mononuclear cell phenotypes. Furthermore, to confirm the biologic effects of α-GalCer administration, a nested group of mice were assayed 24 hours after the α-GalCer injection for cytokine production and liver DC phenotypes. Sera on the same mice were also collected at times 0, 2, 6, 10, 24, and 48 hours following the α-GalCer injection and analyzed for sera levels of IFN-γ and IL-4 by ELISA. All experiments were performed following approval of the Animal Welfare Committees of National Taiwan University and the University of California at Davis. The methodology for all of the surrogate readouts are described below.
Determination of serum anti-PDC-E2 antibodies
Serum titers of IgM and IgG anti-PDC-E2 autoantibodies were measured by ELISA using our well standardized recombinant autoantigens, including the use of positive and negative controls. Briefly, purified recombinant PDC-E2 at 1 μg/ml in carbonate buffer (pH 9.6) was coated onto ELISA plates at 4°C overnight. After blocking with 1% casein (Sigma-Aldrich) for 1 hour, diluted sera were added for 2 hours at room temperature. The ELISA plates were washed with PBS-tween 20 followed by the addition of horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (1:10000, Zymed Laboratories, Carlsbad, CA, USA) and IgM (1:10000, Invitrogen, Camarillo, CA, USA). The plates were incubated for another 1 hour and immunoreactivity was detected by measuring the optical density (O.D.) at 450 nm after exposure for 20 minutes to tetramethylbenzidine (TMB) substrate (R&D systems, Minneapolis, MN, USA).
Mononuclear cell preparation
Livers were perfused with PBS containing 0.2% BSA (PBS/0.2% BSA) (Sigma-Aldrich), passed through a 100μm nylon mesh, and re-suspended in PBS/0.2% BSA. The parenchymal cells were removed as pellets after centrifugation at 100 g for 1 minute and the non-parenchymal cells were washed in PBS/0.2% BSA three times (440 g, 5 minutes) to remove hepatocytes. Mononuclear cells were then isolated using Histopaque-1077 (Sigma-Aldrich). After centrifugation, collected cells were washed with PBS/0.2% BSA and viability of cells was confirmed by trypan blue dye exclusion. Cell numbers were determined by a hemacytometer (Hausser Scientific, Horsham, PA, USA).
Flow cytometry
Cell population and cytokine secretion of iNKT cells were measured by flow cytometry. Before staining cells, with a previously defined optimal dilution of monoclonal antibodies (Abs), the cells were pre-incubated with anti-CD16/32 (clone 93) to block non-specific FcRγ binding. The following Abs were used in this study: anti-I-Ab FITC, anti-H-2Kb FITC, anti-CD11c PE, anti-CD8a PE, anti-CD19 PE, anti-CD1d PE, anti-CD4 PerCP/Cy5.5, anti-CD80 PerCP/Cy5.5, anti-CD11c PerCP/Cy5.5, (Biolegend, San Diego, CA, USA), anti-CD3 FITC, anti-CD86 PE, anti-CD40 PE, anti-NK1.1 APC (eBioscience, San Diego, CA, USA). For intracellular staining, liver mononuclear cells were incubated with brefeldin A (10 μg/ml) (BD Biosciences, San Diego, CA, USA) at 37°C for 1 hour then incubated with anti-CD16/32 Abs, followed by staining with PerCP/Cy5.5-conjugated CD3 and PE-conjugated PBS57 loaded CD1d tetramer (originally produced by the NIH tetramer facility, and supplied through Dr. David Serreze), permeabilized with Cytofix/Cytoperm reagent (BD Biosciences), and stained with Alexa Fluor® 488-conjugated anti-IFN-γ (clone XMG1.2), Alexa Fluor® 488-conjugated anti-IL-4 (clone 11B11), or rat IgG1 isotype control (clone R3-34) (BD Biosciences). Stained cells were assessed on a FACSCalibur (BD Biosciences) using FlowJo softwares (Tree Star, Inc., Ashland, OR, USA).
Histopathology
Portions of the liver were excised and immediately fixed with 10% buffered formalin solution for 2 days at room temperature. Paraffin-embedded tissue sections were then cut into 4-μm slices for routine hematoxylin and eosin (H-E), silver, and Azan staining. Scoring of liver inflammation was performed on coded H-E-, silver- or Azan- stained sections of liver using a set of indices by a “blinded” pathologist (K.T.); these indices quantitated the degree of portal inflammation, parenchymal inflammation, bile duct damage, granulomas, and fibrosis. Each section was scored as either 0 = no significant change, 1 = minimal, 2 = mild, 3 = moderate, and 4 = severe pathology. Details of this scoring system have been described elsewhere (21). Finally, to detect the presence of α-smooth muscle actin (α-SMA)-positive cells, an immunochemical analysis was performed with a well characterized mAb for α-SMA (22).
Statistical analysis
Results are expressed as the mean ±standard error of the mean (SEM). All graphing and statistical analyses were performed using the Prism graphing program (GraphPad Software, San Diego, CA, USA). p-values were calculated using a two-tailed unpaired Mann-Whitney test except Table 1. The frequency of liver damage in Table 1 was evaluated using Fisher’s exact test. Significance levels were set at a p-value of 0.05.
Table 1.
Histopathology of Immunized Mice
| Group | Liver inflammation |
Portal inflammation |
Bile duct damage |
Granulomas | Proliferative bile ductules |
Liver fibrosis | |
|---|---|---|---|---|---|---|---|
| 4 weeks | PBS/CFA/2-OA | 4/5 (80%) |
4/5 (80%) |
1/5 (20%) |
0/5 (0%) |
0/5 (0%) |
0/5 (0%) |
|
| |||||||
| α-GC/CFA/2-OA | 5/5 (100%) |
5/5 (100%) |
4/5 (80%) |
0/5 (0%) |
4/5 (80%) * |
3/5 (60%) |
|
|
| |||||||
| 12 weeks | α-GC | 0/5 (0%) |
0/5 (0%) |
0/5 (0%) |
2/5 (40%) |
0/5 (0%) |
0/5 (0%) |
|
| |||||||
| α-GC/CFA | 3/5 (60%) |
2/5 (40%) |
1/5 (20%) |
2/5 (40%) |
1/5 (20%) |
1/5 (20%) |
|
|
| |||||||
| PBS/CFA/2-OA | 9/9 (100%) |
9/9 (100%) |
7/9 (78%) |
3/9 (33%) |
7/9 (78%) |
1/9 (11%) |
|
|
| |||||||
| α-GC/CFA/2-OA | 13/13 (100%) |
13/13 (100%) # |
13/13 (100%) # |
12/13 (92%) *,# |
13/13 (100%) # |
10/13 (77%) *,# |
|
Data are expressed as positive mice out of total mice examined and the frequency of each parameter.
p<0.05 compared to PBS/CFA/2-OA.
p<0.05 compared to α-GC/CFA.
Fisher’s exact test.
Results
α-GalCer induced IFN-γ and IL-4 production of iNKT cells and maturation of dendritic cells
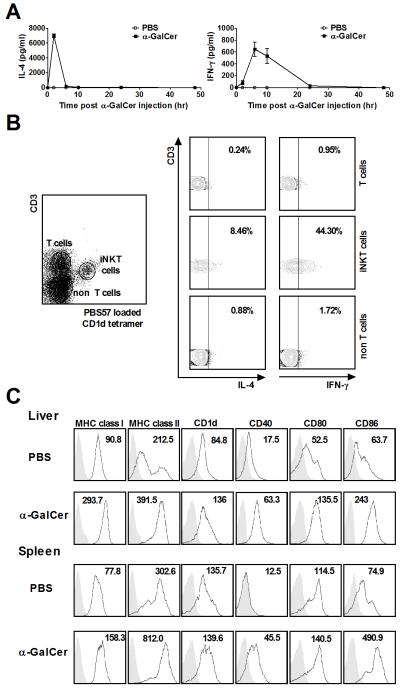
Firstly, to confirm the activity of α-GalCer, a nested substudy was performed in which we intravenously injected α-GalCer to naive mice and analyzed IFN-γ and IL-4 production in serum and in iNKT cells of mice. As shown in Figure 1A, both IFN-γ and IL-4 were increased in mice injected with α-GalCer. Serum IFN-γ was detectable at 2 hours, peaked at approximately 6 hours and was maintained until 24 hours after α-GalCer injection, while IL-4 peaked at 2 hours and became undetectable after 6 hours (Figure 1A). We then used intracellular staining to confirm the cell source of cytokine production at 24 hours after α-GalCer injection. As shown in Figure 1B, IFN-γ and IL-4 were produced dominantly by hepatic iNKT (CD3+CD1d tetramer +) cells. Similarly, to analyze the antigen presenting capacity of DCs, we evaluated surface marker expression 24 hours after α-GalCer injection compared to controls. Significantly increased expression levels of MHC class I (H-2Kb), MHC class II (I-Ab), CD1d, CD80, CD86, and CD40 on DCs (CD11c+NK1.1− cells) in liver and spleen of mice administered with α-GalCer was noted compared to that of mice administered with PBS (Figure 1C), suggesting that α-GalCer intravenously stimulated the full maturation of DCs in liver and spleen.
Figure 1.
α-GalCer induced IFN-γ and IL-4 production of iNKT cells and maturation of dendritic cells. C57BL/6 mice were intravenously injected with α-GalCer or PBS. (A) Serum samples were collected at 0, 2, 6, 10, 24, and 48 hours after α-GalCer or PBS injection. IFN-γ and IL-4 were measured by ELISA. n=5 mice per group. (B) IFN-γ and IL-4 secretion from liver mononuclear cells 24 hours after α-GalCer injection was assayed by staining with surface markers and intracellular anti-mouse IFN-γ and IL-4 Abs. (C) Surface molecule expression on DCs was determined by flow cytometry. Data shown are representative flow cytometric profiles of MHC class I (H-2Kb), MHC class II (I-Ab), CD1d, CD40, CD80, and CD86 expression on the gated DCs (CD11c+ NK1.1− cells) in liver and spleen. The shadow area is an isotype control; the non-shadow area reflects surface molecule expression. The quantity of surface molecules per cell is indicated by the mean fluorescence intensity (MFI). Results are representative of three independent experiments.
Autoimmune cholangitis 4 weeks postimmunization
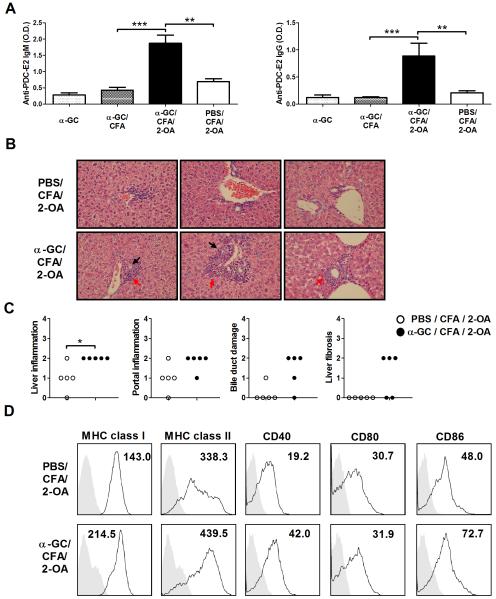
Four weeks postimmunization, sera autoantibodies IgM and IgG to PDC-E2 were significantly increased in α-GC/CFA/2-OA mice as compared to that of PBS/CFA/2-OA, α-GC, and α-GC/CFA control mice (Figure 2A). Importantly, there was a significant increase in liver inflammation, portal inflammation, and bile duct damage in the α-GC/CFA/2-OA group compared to PBS/CFA/2-OA mice (Figure 2B, C and Table 1). Further, ductular proliferation was observed in 4 out of 5 α-GC/CFA/2-OA mice but not in any (0/5) of the PBS/CFA/2-OA mice (Table 1). Furthermore, mild fibrous septa extension (score=2) was observed in 3 out of 5 α-GC/CFA/2-OA mice (Figure 2C and Table 1). In addition, there was significantly increased MHC class I, II and costimulatory molecules CD86 and CD40 expression on the DCs of α-GC/CFA/2-OA mice compared to PBS/CFA/2-OA mice (Figure 2D).
Figure 2.
Increased liver disease, serum AMAs, and maturation of dendritic cells at 4 weeks in α-GalCer injected 2-OA-BSA immunized mice. Mice were immunized with 2-OA-BSA at weeks 0 and 2 and sacrificed at week 4. (A) Serum levels of autoantibodies to PDC-E2 protein were measured by ELISA (1:400 sera dilutions). O.D., optical density. Results are expressed as mean ±SEM. n=10 mice per group. **, p<0.01; ***, p<0.005. (B) Representative results of H-E examination of liver sections (upper panel, PBS/CFA/2-OA mice; lower panel, α-GC/CFA/2-OA mice; x400 magnification). Portal inflammation is shown by red arrows. Bile duct damage is reflected by black arrows. (C) Histopathological scores of individual livers. 0 = no significant change, 1 = minimal, 2 = mild, 3 = moderate, and 4 = severe pathology. *, p<0.05. (D) Surface molecule expression on DCs was determined by flow cytometry. Data shown are representative flow cytometric profiles of MHC class I (H-2Kb), MHC class II (I-Ab), CD40, CD80, and CD86 expression on the gated DCs (CD11c+NK1.1− cells) in liver. The shadow area is an isotype control; the non-shadow area reflects surface molecule expression. The quantity of surface molecules per cell is indicated by the mean fluorescence intensity (MFI). Results are representative of three independent experiments.
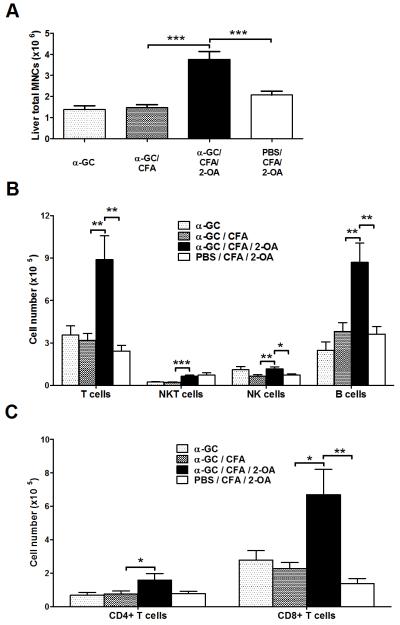
There was a significant increase in liver total mononuclear cells in α-GC/CFA/2-OA mice compared to that of PBS/CFA/2-OA, α-GC, and α-GC/CFA control mice (Figure 3A). In addition, significantly increased numbers of conventional T (CD3+ NK1.1−) cells and B cells were noted in α-GC/CFA/2-OA mice (Figure 3B). Importantly, significantly increased absolute numbers of CD8+ T cells were noted in α-GC/CFA/2-OA mice compared to that of PBS/CFA/2-OA mice (Figure 3C).
Figure 3.
Increased CD3+CD8+ T and B cells in α-GalCer injected 2-OA-BSA immunized mice at 4 weeks. Mice were immunized with 2-OA-BSA at weeks 0 and 2 and sacrificed at week 4. (A) Liver total mononuclear cells (MNCs) were measured. (B) The absolute numbers of individual cell population were measured. (C) CD4+ and CD8+ T cells were measured in each group of mice. Results are expressed as mean ±SEM. n=10 mice per group. *, p<0.05; **, p<0.01; ***, p<0.005.
Autoimmune cholangitis 12 weeks postimmunization
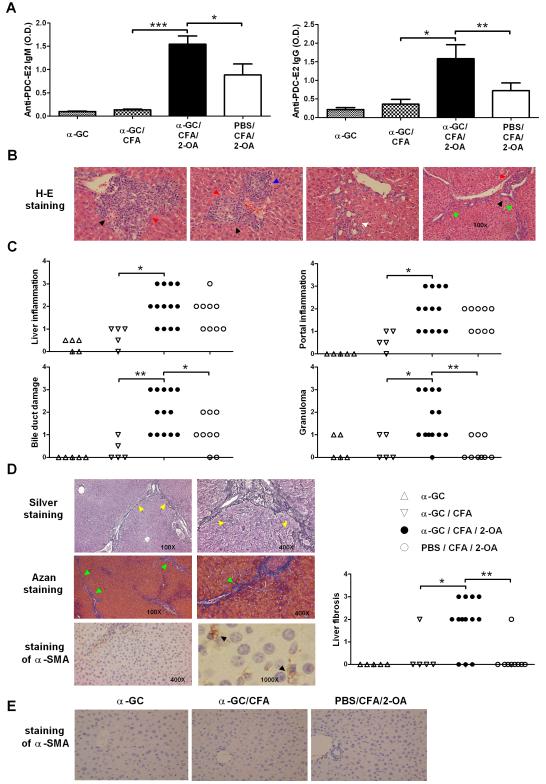
Serum autoantibodies IgM and IgG to PDC-E2 were significantly increased in α-GC/CFA/2-OA mice as compared to PBS/CFA/2-OA, α-GC, and α-GC/CFA control mice (Figure 4A). Examination of H-E-stained liver section revealed portal inflammation, bile duct damage, granulomas, proliferating bile ductules and fibrous septa extension in α-GC/CFA/2-OA group (Figure 4B). In α-GC/CFA/2-OA group, minimal to moderate (score=1-3) liver inflammation, portal inflammation, and bile duct damage were observed (Figure 4C and Table 1). Granulomas were found in 12/13 α-GC/CFA/2-OA mice (Figure 4C and Table 1). In addition, fibrous septa extension was observed in all (13/13) α-GC/CFA/2-OA mice examined (Table 1). It is also important to note, as shown in Figure 4D and Table 1, that 10/13 α-GC/CFA/2-OA mice demonstrated liver fibrosis as highlighted by silver staining and Azan staining. In addition, there were many activated hepatic stellate cells (HSCs) (α-SMA-positive) containing lipid vacuoles in sinusoids and portal area in α-GC/CFA/2-OA mice (Figure 4D). In contrast, there were no detectable α-SMA-positive cells in PBS/CFA/2-OA, α-GC, and α-GC/CFA control mice (Figure 4E). PBS/CFA/2-OA controls had minimal to mild (score=1-2) liver inflammation, portal inflammation and bile duct damage. Three of 9 PBS/CFA/2-OA controls had minimal (score=1) granulomas (Figure 4C and Table 1). Ductular proliferation was also found in 7/9 PBS/CFA/2-OA controls (Table 1). Only 1/9 PBS CFA/2-OA mice had fibrosis (Figure 4D and Table 1). α-GC and α-GC/CFA control mice had none to minimal (score=0-1) liver inflammation, portal inflammation, bile duct damage, or granulomas. Only 1 α-GC/CFA mouse had any evidence of fibrosis, and that was mild.
Figure 4.
Activation of iNKT cells by α-GalCer in 2-OA-BSA immunized mice exacerbates autoimmune cholangitis. Mice were immunized as described and sacrificed at week 12. (A) Serum levels of autoantibodies to PDC-E2 were measured by ELISA (1:400 sera dilutions). O.D., optical density. Results are expressed as mean ±SEM. n=10 mice per group. *, p<0.05; **, p<0.01; ***, p<0.005. (B) Representative results of histopathologic examination of liver sections of α-GC/CFA/2-OA mice (left three panels, x400 magnification; right panel, x100 magnification). Portal inflammation is shown with red arrows. Bile duct damage is shown with black arrows. Granulomas are shown with blue arrows. Proliferating bile ductules are shown with white arrows. Fibrous septa extension is shown with green arrows. (C) Histopathological scores of individual livers. 0 = no significant change, 1 = minimal, 2 = mild, 3 = moderate, and 4 = severe pathology. (D) Silver staining and Azan staining are used to highlight fibrous septa extension (yellow arrows and green arrows, respectively). The brown color in α-SMA staining indicates α-SMA positive cells. Liver fibrosis scores: 0 = no significant change, 1 = minimal, 2 = mild, 3 = moderate, and 4 = severe pathology. (E) α-SMA staining of liver sections in α-GC, α-GC/CFA, and PBS/CFA/2-OA mice.
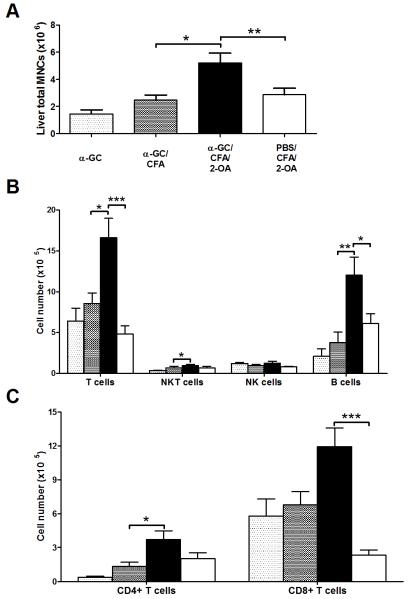
The total liver mononuclear cell infiltrates were higher in α-GC/CFA/2-OA mice as compared to that of PBS/CFA/2-OA, α-GC, and α-GC/CFA control mice (Figure 5A). In addition, significantly increased numbers of conventional T (CD3+ NK1.1−) cells and B cells were noted in α-GC/CFA/2-OA mice (Figure 5B). Importantly, significantly increased frequency and absolute numbers of CD8+ T cells were noted in α-GC/CFA/2-OA mice compared to that of PBS/CFA/2-OA mice (Figure 5C).
Figure 5.
α-GalCer activates iNKT cells which recruit CD3+CD8+ T cells to liver at week 12. Mice were immunized as described and sacrificed at week 12. (A) Liver total mononuclear cells (MNCs) were measured. (B) The absolute numbers of individual cell population were measured. (C) CD4+ and CD8+ T cells were measured in each group of mice. Results are expressed as mean ±SEM. n=10 mice per group. *, p<0.05; **, p<0.01; ***, p<0.005.
Discussion
Our previous work in human PBC and in the dnTGF-βRII mouse model of PBC, has suggested that activation of iNKT cells is a critical factor in accelerating disease (18-20). However, the mechanism is still unknown. In the present study, we investigated the effects of activated iNKT cells stimulated with α-GalCer in the pathogenesis of murine PBC by xenobiotic chemical immunization. α-GalCer injection exacerbated autoimmune cholangitis in 2-OA-BSA immunized mice, including increased AMA production, portal inflammation, and bile duct damage. Our data suggest that iNKT cell activation is a critical factor in modulating the natural history of PBC. We note that human PBC has a long latency time. For example, serum AMAs precede disease by many years (1, 23). The results herein suggest that the evolution from subclinical to clinical disease, i.e. from an adaptive to an overwhelming innate or bystander response, may depend on exposure to a natural ligand that activates NKT cells.
It is important to note that this model has been based on a careful selection of the immunogen, 2-OA. We have previously performed a quantitative structural activity relationship analysis and rigorous epitope analysis of human PBC sera against extensive panels of chemicals that were coupled to the lysine residue (137K) of PDC-E2 (8-10, 24-25). The advantage of this study is the ability to elucidate the early events of autoimmune cholangitis. Our data imply that PBC requires loss of tolerance to PDC-E2 and thus an adaptive multi-lineage anti-mitochondrial response. However, it also includes an overwhelming innate immune response and we submit that the innate immune response, combined with the unique biologic properties of bile duct cells and apotopes, are sufficient to explain the recurrence of PBC following liver transplantation. In other words, in the absence of MHC restriction, disease reoccurrence would depend on a non-adaptive cellular mechanism, i.e. innate immunity. In this respect we note our recent work on apotopes of biliary epithelial cells (26). This latter work, coupled with our data herein, would also explain the success of ursodiol, a drug which appears to have anti-apoptotic properties and also may modulate innate responses. Our data would also explain the relative failure of immunosuppressive drugs to alter PBC, since such agents are ineffective against innate mechanisms.
The work herein also demonstrates the presence of not only granulomas but, for the first time and, more importantly, the presence of moderate fibrosis. The induction of fibrosis in this model permits not only dissection of its induction, but also has the potential to be useful in studies of intervention. Liver fibrosis is characterized by an accumulation of extracellular matrix proteins, which are primarily produced by activated HSCs. In the quiescence state, HSCs contain lipid vacuoles with less fibrous features. After activation, HSCs transform to myofibroblastic cells (α-SMA-positive) and migrate to portal area and contribute to fibrosis (27-28). In both human and animal studies, liver inflammation has been suggested as a requisite for the earliest stages of fibrosis (27-28) and clearly several lymphoid subpopulations play a role in regulating this process, including NK cells, DCs, and CD8 T cells (29-31). In this regard, natural activation of iNKT cells by endogenous lipid antigens inhibits fibrosis; while the activation of iNKT cells by α-GalCer promotes the process (32). The results presented herein demonstrate significant presence of fibrosis and increased numbers of activated HSCs in the sinusoid and portal areas of α-GalCer injected 2-OA-BSA immunized mice (α-GC/CFA/2-OA group) compared to PBS injected 2-OA-BSA immunized mice (PBS/CFA/2-OA group). However, only 1 mouse had evidence of fibrosis and none had activated HSCs in α-GC and α-GC/CFA groups of mice. These results suggest that α-GalCer does not induce fibrosis and activation of HSCs but rather promote the process of 2-OA-BSA induced fibrosis. In addition, we have also previously suggested a critical role of CD8+ T cells for the induction of PBC in both humans and mice (33-34). For example, adoptive transfer of CD8+ T cells from dnTGF-βRII PBC mice to Rag−/− mice leads to liver histopathology remarkably similar to human PBC. In contrast, transfer of CD4+ T cells from dnTGF-βRII mice to Rag−/− mice leads to the development of inflammatory bowel disease (34). Hence, the observation herein that α-GalCer increases CD8 hepatic T cells takes on particular significance. In addition, α-GalCer potently enhances antigen presenting ability of DCs in α-GalCer injected 2-OA-BSA immunized mice, which then induce CD4+ and CD8+ T cell immunity (35-38).
NKT cells have been demonstrated to play diverse roles in both the pathogenesis and modulation of type I diabetes, systemic sclerosis, rheumatoid arthritis, multiple sclerosis and autoimmune liver disease (13, 39-41). In PBC, CD1d expression and the frequency of iNKT cells are both increased in the liver of patients (18-19). In our previous work on the dnTGF-βRII mouse model of PBC, we likewise demonstrated the importance of NKT cells for PBC initiation (20). A recent study demonstrates that murine infection with Novosphingobium aromaticivorans, a gram-negative microorganism initiates development of autoimmune cholangitis in both C57BL/6 and a congenic NOD strain (42). This latter observation is particularly noteworthy because Novosphingobium aromaticivorans has four copies of PDC-E2, all of which are remarkable homologues of human PDC-E2 (24, 43). Further, Novosphingobium aromaticivorans also contains abundant levels of glycosphingolipids with an α-linked sugar, similar to α-GalCer (24, 44), which may be a natural ligand of iNKT cells (42). Finally, in the murine model of concanavalin A (Con-A)-induced hepatitis, iNKT cells are required and sufficient for induction of liver injury (45-46).
While there is a multi-orchestrated immune response in patients with PBC, one lesson from the murine models and these data in particular is the profound importance of innate immune responses. We suggest that loss of tolerance to PDC-E2 in humans with PBC is secondary to a genetic predisposition and environmental influences of either xenobiotic chemicals or bacterial mimics. This leads to a multi-lineage anti-mitochondrial response. This multi-lineage loss of tolerance to PDC-E2 would be clinically insignificant were it not for the unique apotopes found on biliary cells. Further, the perpetuation of disease, and perhaps the initiation from the asymptomatic serologically positive patient, may be dependent on activation of NKT cells. The use of α-GalCer demonstrates the ability of this model to develop hepatic fibrosis. Finally, we submit that the use of this model and the other models of murine autoimmune cholangitis are valuable tools to explore new therapeutic options for patients with PBC.
Acknowledgements
We thank Dr. D. Serreze for providing the CD1d-tetramer staining reagent.
This work was supported by grants from the National Science Council, Taiwan (NSC97-2320-B-002-001), Frontier and Innovative Research of National Taiwan University (98HM00285), National Institutes of Health grants DK39588 and DK067003.
Abbreviations
- 2-OA
2-octynoic acid
- α-GalCer
α-galactosylceramide
- AMAs
anti-mitochondrial antibodies
- α-SMA
α-smooth muscle actin
- BSA
bovine serum albumin
- CFA
complete Freund’s adjuvant
- DC
dendritic cells
- dnTGF-βRII
TGF-β receptor II dominant-negative
- HSCs
hepatic stellate cells
- iNKT
invariant natural killer T
- PBC
primary biliary cirrhosis
- PDC-E2
E2 subunits of the pyruvate dehydrogenase complex
References
- 1.Gershwin ME, Mackay IR. The causes of primary biliary cirrhosis: Convenient and inconvenient truths. Hepatology. 2008;47:737–745. doi: 10.1002/hep.22042. [DOI] [PubMed] [Google Scholar]
- 2.He XS, Ansari AA, Ridgway WM, Coppel RL, Gershwin ME. New insights to the immunopathology and autoimmune responses in primary biliary cirrhosis. Cell Immunol. 2006;239:1–13. doi: 10.1016/j.cellimm.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Chuang YH, Ridgway WM, Ueno Y, Gershwin ME. Animal models of primary biliary cirrhosis. Clin Liver Dis. 2008;12:333–347. ix. doi: 10.1016/j.cld.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oertelt S, Lian ZX, Cheng CM, Chuang YH, Padgett KA, He XS, Ridgway WM, et al. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J Immunol. 2006;177:1655–1660. doi: 10.4049/jimmunol.177.3.1655. [DOI] [PubMed] [Google Scholar]
- 5.Wakabayashi K, Lian ZX, Moritoki Y, Lan RY, Tsuneyama K, Chuang YH, Yang GX, et al. IL-2 receptor alpha(−/−) mice and the development of primary biliary cirrhosis. Hepatology. 2006;44:1240–1249. doi: 10.1002/hep.21385. [DOI] [PubMed] [Google Scholar]
- 6.Irie J, Wu Y, Wicker LS, Rainbow D, Nalesnik MA, Hirsch R, Peterson LB, et al. NOD.c3c4 congenic mice develop autoimmune biliary disease that serologically and pathogenetically models human primary biliary cirrhosis. J Exp Med. 2006;203:1209–1219. doi: 10.1084/jem.20051911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakabayashi K, Lian ZX, Leung PS, Moritoki Y, Tsuneyama K, Kurth MJ, Lam KS, et al. Loss of tolerance in C57BL/6 mice to the autoantigen E2 subunit of pyruvate dehydrogenase by a xenobiotic with ensuing biliary ductular disease. Hepatology. 2008;48:531–540. doi: 10.1002/hep.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung PS, Quan C, Park O, Van de Water J, Kurth MJ, Nantz MH, Ansari AA, et al. Immunization with a xenobiotic 6-bromohexanoate bovine serum albumin conjugate induces antimitochondrial antibodies. J Immunol. 2003;170:5326–5332. doi: 10.4049/jimmunol.170.10.5326. [DOI] [PubMed] [Google Scholar]
- 9.Amano K, Leung PS, Rieger R, Quan C, Wang X, Marik J, Suen YF, et al. Chemical xenobiotics and mitochondrial autoantigens in primary biliary cirrhosis: identification of antibodies against a common environmental, cosmetic, and food additive, 2-octynoic acid. J Immunol. 2005;174:5874–5883. doi: 10.4049/jimmunol.174.9.5874. [DOI] [PubMed] [Google Scholar]
- 10.Rieger R, Leung PS, Jeddeloh MR, Kurth MJ, Nantz MH, Lam KS, Barsky D, et al. Identification of 2-nonynoic acid, a cosmetic component, as a potential trigger of primary biliary cirrhosis. J Autoimmun. 2006;27:7–16. doi: 10.1016/j.jaut.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Long SA, Quan C, Van de Water J, Nantz MH, Kurth MJ, Barsky D, Colvin ME, et al. Immunoreactivity of organic mimeotopes of the E2 component of pyruvate dehydrogenase: connecting xenobiotics with primary biliary cirrhosis. J Immunol. 2001;167:2956–2963. doi: 10.4049/jimmunol.167.5.2956. [DOI] [PubMed] [Google Scholar]
- 12.Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu L, Van Kaer L. Natural killer T cells and autoimmune disease. Curr Mol Med. 2009;9:4–14. doi: 10.2174/156652409787314534. [DOI] [PubMed] [Google Scholar]
- 14.Sharif S, Arreaza GA, Zucker P, Mi QS, Sondhi J, Naidenko OV, Kronenberg M, et al. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat Med. 2001;7:1057–1062. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- 15.Singh AK, Wilson MT, Hong S, Olivares-Villagomez D, Du C, Stanic AK, Joyce S, et al. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1801–1811. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang B, Geng YB, Wang CR. CD1-restricted NK T cells protect nonobese diabetic mice from developing diabetes. J Exp Med. 2001;194:313–320. doi: 10.1084/jem.194.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng D, Liu Y, Sidobre S, Kronenberg M, Strober S. Activation of natural killer T cells in NZB/W mice induces Th1-type immune responses exacerbating lupus. J Clin Invest. 2003;112:1211–1222. doi: 10.1172/JCI17165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kita H, Naidenko OV, Kronenberg M, Ansari AA, Rogers P, He XS, Koning F, et al. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology. 2002;123:1031–1043. doi: 10.1053/gast.2002.36020. [DOI] [PubMed] [Google Scholar]
- 19.Tsuneyama K, Yasoshima M, Harada K, Hiramatsu K, Gershwin ME, Nakanuma Y. Increased CD1d expression on small bile duct epithelium and epithelioid granuloma in livers in primary biliary cirrhosis. Hepatology. 1998;28:620–623. doi: 10.1002/hep.510280303. [DOI] [PubMed] [Google Scholar]
- 20.Chuang YH, Lian ZX, Yang GX, Shu SA, Moritoki Y, Ridgway WM, Ansari AA, et al. Natural killer T cells exacerbate liver injury in a transforming growth factor beta receptor II dominant-negative mouse model of primary biliary cirrhosis. Hepatology. 2008;47:571–580. doi: 10.1002/hep.22052. [DOI] [PubMed] [Google Scholar]
- 21.Tsuneyama K, Nose M, Nisihara M, Katayanagi K, Harada K, Nakanuma Y. Spontaneous occurrence of chronic non-suppurative destructive cholangitis and antimitochondrial autoantibodies in MRL/lpr mice: possible animal model for primary biliary cirrhosis. Pathol Int. 2001;51:418–424. doi: 10.1046/j.1440-1827.2001.01223.x. [DOI] [PubMed] [Google Scholar]
- 22.Fujimoto M, Tsuneyama K, Kainuma M, Sekiya N, Goto H, Takano Y, Terasawa K, et al. Evidence-based efficacy of Kampo formulas in a model of non alcoholic fatty liver. Exp Biol Med (Maywood) 2008;233:328–337. doi: 10.3181/0707-RM-207. [DOI] [PubMed] [Google Scholar]
- 23.Oertelt S, Rieger R, Selmi C, Invernizzi P, Ansari AA, Coppel RL, Podda M, et al. A sensitive bead assay for antimitochondrial antibodies: Chipping away at AMA-negative primary biliary cirrhosis. Hepatology. 2007;45:659–665. doi: 10.1002/hep.21583. [DOI] [PubMed] [Google Scholar]
- 24.Selmi C, Balkwill DL, Invernizzi P, Ansari AA, Coppel RL, Podda M, Leung PS, et al. Patients with primary biliary cirrhosis react against a ubiquitous xenobiotic-metabolizing bacterium. Hepatology. 2003;38:1250–1257. doi: 10.1053/jhep.2003.50446. [DOI] [PubMed] [Google Scholar]
- 25.Rieger R, Gershwin ME. The X and why of xenobiotics in primary biliary cirrhosis. J Autoimmun. 2007;28:76–84. doi: 10.1016/j.jaut.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lleo A, Selmi C, Invernizzi P, Podda M, Coppel RL, Mackay IR, Gores GJ, et al. Apotopes and the biliary specificity of primary biliary cirrhosis. Hepatology. 2009;49:871–879. doi: 10.1002/hep.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117:539–548. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Safadi R, Ohta M, Alvarez CE, Fiel MI, Bansal M, Mehal WZ, Friedman SL. Immune stimulation of hepatic fibrogenesis by CD8 cells and attenuation by transgenic interleukin-10 from hepatocytes. Gastroenterology. 2004;127:870–882. doi: 10.1053/j.gastro.2004.04.062. [DOI] [PubMed] [Google Scholar]
- 30.Connolly MK, Bedrosian AS, Mallen-St Clair J, Mitchell AP, Ibrahim J, Stroud A, Pachter HL, et al. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-alpha. J Clin Invest. 2009;119:3213–3225. doi: 10.1172/JCI37581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 32.Park O, Jeong WI, Wang L, Wang H, Lian ZX, Gershwin ME, Gao B. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology. 2009;49:1683–1694. doi: 10.1002/hep.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kita H, Matsumura S, He XS, Ansari AA, Lian ZX, Van De Water J, Coppel RL, et al. Quantitative and functional analysis of PDC-E2-specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis. J Clin Invest. 2002;109:1231–1240. doi: 10.1172/JCI14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang GX, Lian ZX, Chuang YH, Moritoki Y, Lan RY, Wakabayashi K, Ansari AA, et al. Adoptive transfer of CD8(+) T cells from transforming growth factor beta receptor type II (dominant negative form) induces autoimmune cholangitis in mice. Hepatology. 2008;47:1974–1982. doi: 10.1002/hep.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujii S, Shimizu K, Hemmi H, Fukui M, Bonito AJ, Chen G, Franck RW, et al. Glycolipid alpha-C-galactosylceramide is a distinct inducer of dendritic cell function during innate and adaptive immune responses of mice. Proc Natl Acad Sci U S A. 2006;103:11252–11257. doi: 10.1073/pnas.0604812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taraban VY, Martin S, Attfield KE, Glennie MJ, Elliott T, Elewaut D, Van Calenbergh S, et al. Invariant NKT cells promote CD8+ cytotoxic T cell responses by inducing CD70 expression on dendritic cells. J Immunol. 2008;180:4615–4620. doi: 10.4049/jimmunol.180.7.4615. [DOI] [PubMed] [Google Scholar]
- 38.Semmling V, Lukacs-Kornek V, Thaiss CA, Quast T, Hochheiser K, Panzer U, Rossjohn J, et al. Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell-licensed DCs. Nat Immunol. 2010;11:313–320. doi: 10.1038/ni.1848. [DOI] [PubMed] [Google Scholar]
- 39.Grajewski RS, Hansen AM, Agarwal RK, Kronenberg M, Sidobre S, Su SB, Silver PB, et al. Activation of invariant NKT cells ameliorates experimental ocular autoimmunity by a mechanism involving innate IFN-gamma production and dampening of the adaptive Th1 and Th17 responses. J Immunol. 2008;181:4791–4797. doi: 10.4049/jimmunol.181.7.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang JQ, Wen X, Liu H, Folayan G, Dong X, Zhou M, Van Kaer L, et al. Examining the role of CD1d and natural killer T cells in the development of nephritis in a genetically susceptible lupus model. Arthritis Rheum. 2007;56:1219–1233. doi: 10.1002/art.22490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferri S, Longhi MS, De Molo C, Lalanne C, Muratori P, Granito A, Hussain MJ, et al. A multifaceted imbalance of T cells with regulatory function characterizes type 1 autoimmune hepatitis. Hepatology. 2010;52:999–1007. doi: 10.1002/hep.23792. [DOI] [PubMed] [Google Scholar]
- 42.Mattner J, Savage PB, Leung P, Oertelt SS, Wang V, Trivedi O, Scanlon ST, et al. Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe. 2008;3:304–315. doi: 10.1016/j.chom.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padgett KA, Selmi C, Kenny TP, Leung PS, Balkwill DL, Ansari AA, Coppel RL, et al. Phylogenetic and immunological definition of four lipoylated proteins from Novosphingobium aromaticivorans, implications for primary biliary cirrhosis. J Autoimmun. 2005;24:209–219. doi: 10.1016/j.jaut.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 44.Kawahara K, Moll H, Knirel YA, Seydel U, Zahringer U. Structural analysis of two glycosphingolipids from the lipopolysaccharide-lacking bacterium Sphingomonas capsulata. Eur J Biochem. 2000;267:1837–1846. doi: 10.1046/j.1432-1327.2000.01189.x. [DOI] [PubMed] [Google Scholar]
- 45.Kaneko Y, Harada M, Kawano T, Yamashita M, Shibata Y, Gejyo F, Nakayama T, et al. Augmentation of Valpha14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J Exp Med. 2000;191:105–114. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci U S A. 2000;97:5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]