CpG oligodeoxynucleotides (CpG) are potent normal B-cell immune modulating agents and have been used therapeutically as adjuvants in immunotherapy and vaccines (Klinman, 2004). CpG are also used in clinical laboratories as a mitogenic tool to reveal chromosomal abnormalities in malignant B-cells from chronic lymphocytic leukaemia (CLL) patients (Dicker et al., 2006; Haferlach et al., 2007; Mayr et al., 2006). The latter utility has greatly advanced the cytogenetic analysis of CLL cells beyond what can be achieved by interphase fluorescence in situ hybridization (FISH) analysis. However, it remains unclear whether the stimulatory effects of CpG could also induce de novo genomic abnormalities in CLL B-cells in addition to revealing pre-existing genetic lesions. The answer to this question would also have fundamental B lineage tumourigenesis implications that are relevant to the therapeutic use of CpG in the clinic.
To validate the role of CpG in revealing but not inducing cytogenetic abnormalities in CLL B-cells, we stimulated peripheral blood B-cells from 24 healthy adults with CpG and assessed the incidence of chromosomal abnormalities. These cells are considered to have no or very few pre-existing abnormalities. All 24 B-cell samples lacked CLL FISH panel-specific pre-existing abnormalities (Fink et al., 2005). Following CpG stimulation under the conditions identical to those used by the clinical cytogenetic laboratory, we performed karyotype analysis (Table 1). Of note, none of the 24 samples showed evidence of clonal abnormalities as defined in clinical laboratories as having ≥2 cells with the same cytogenetic abnormality(s). Thus, our data confirm that CpG remains a powerful tool for revealing pre-existing CLL B-cell clonal chromosomal abnormalities.
Table 1.
Effect of CpG stimulation on the cytogenetics of normal B-cells
| ID | CpG | CD40L |
|---|---|---|
| 1 | 46,XY,del(15)(q22)[1]; 46,XY[29] | 47,XY,+5[1]; 46,XY[29] |
| 2 | 46,XX[20] | 46,XX[20] |
| 3 | 46,XX[20] | 46,XX,add(7)(q22), add(18)(p11.2)[1]; 46,XX[29] |
| 4 | 46,XX[20] | 46,XX,t(7;17)(q11.2;q25)[1]; 46,XX[29] |
| 5 | 46,XX[20] | 46,XX[20] |
| 6 | 46,XX[20] | 46,XY,t(12;15)(p13;q11.2)[1]; 46,XY[29] |
| 7 | 46,XX[20] | 45,XY,del(1)(q21), dic(10;16)(q24;q24)[1]; 46,XY[29] |
| 8 | 46,XX[20] | 46,XX[20] |
| 9 | 47,XY,+12,inv(12)(q15q24.1)[1]; 47,XY,+12,inv(12)(q13q22)[1]; 47,XY,+3[1]; 46,XY,t(14;20)(q32;q11.2)[1]; 46,XY[26] | Not determined (nd) |
| 10 | 46,XX[20] | nd |
| 11 | 46,XX,add(1)(p13)[1]; 46,XX,add(4)(q12)[1]; 46,XX[18] | nd |
| 12 | 46,XX[20] | nd |
| 13 | 46,XY,-5,+mar[1]; 46,XY[19] | nd |
| 14 | 46,XX[20] | nd |
| 15 | 46,XX[20] | nd |
| 16 | 49,XY,+3,+18,+18[1]; 46,XY[29] | nd |
| 17 | 48,XX,+X,+X[1]; 46,XY[29] | nd |
| 18 | 46,XY,add(1)(q21)[1]; 47,XY,+3[1]; 46,XY,t(3;10)(q12;p15)[1]; 46,XY,add(8)(p11.2)[1]; 46,XY,add(11)(q13)[1]; 46,XY,t(14;19)(q11.2;p13.3)[1]; 46,XY[44] | nd |
| 19 | 46,XX[20] | nd |
| 20 | 46,XX[20] | nd |
| 21 | 46,XY,t(4;9)(q31.1;p22)[1]; 46,XY[19] | nd |
| 22 | 46,XX,t(1;2)(p13;q21)[1]; 46,XX[19] | nd |
| 23 | 46,XX[20] | nd |
| 24 | 46,XX[20] | nd |
However, 9 of 24 samples (37.5%) stimulated with CpG did show ≥1 cell(s) with nonclonal (single appearance / 20 metaphase cells) karyotypic abnormalities. The type and location of chromosomal abnormalities appeared to be random. Among them, 3 of 24 samples (12.5%) had greater than 10% of the cells (equivalent to 2 abnormal cells/20 cells) displaying nonclonal chromosomal abnormalities. In a smaller subset of normal controls (n=8), B-cells were also stimulated with CD40 ligand (CD40L), a known B-cell activator, and 4 out 8 samples tested (50%) showed nonclonal chromosomal abnormalities. These data suggest that both CpG and CD40L may induce chromosomal abnormalities when B-cells are stimulated in vitro. Of interest, it has also been shown that some CpG-activated CLL cells also exhibit nonclonal cytogenetic abnormalities, which are normally not scored clinically (Put et al., 2009).
To address the remote possibility that CpG-induced cytogenetic abnormalities in healthy donor B-cells reflected pre-existing genetic lesions, we further fractionated B-cells from 6 healthy donors into naïve and memory subsets. Here, we reasoned that memory B-cells were more likely than naive B-cells to harbour certain types of pathological translocations, albeit at very low frequencies (Roulland et al., 2006). However, after CpG stimulation, we observed that both memory and naïve B-cells (3/6 samples in both cases) exhibited nonclonal abnormalities (data not shown) at comparable frequencies. These observations strongly suggest that the nonclonal abnormalities did not pre-exist but instead were induced de novo by CpG stimulation.
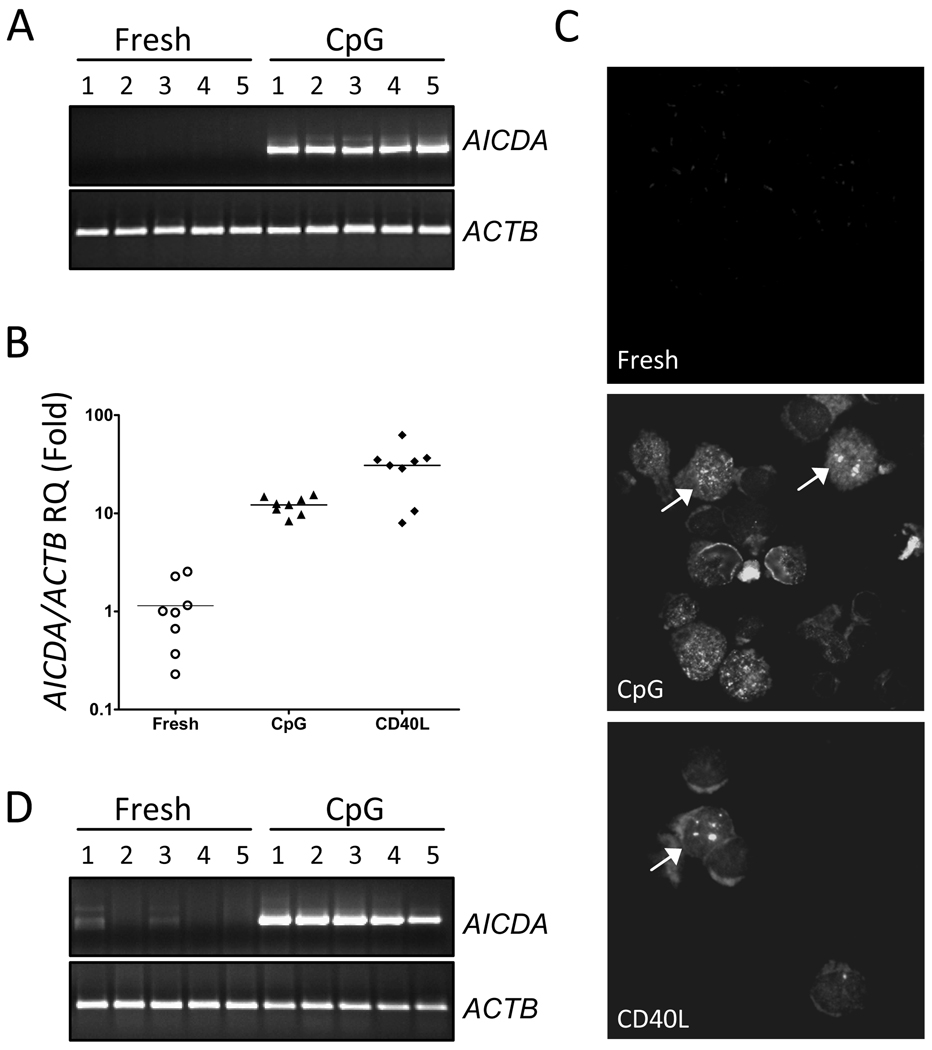
Given that the mutagenic enzyme activation-induced cytidine deaminase (AICDA), which induces mutations in the variable and switch regions of immunoglobulin genes, also induces chromosomal translocations in mouse models (Ramiro et al., 2006; Robbiani et al., 2009), we questioned if CpG stimulation could induce AICDA expression, which in turn may play a role in induction of chromosomal abnormalities. Therefore, we performed polymerase chain reaction (PCR) analysis of AICDA expression on freshly isolated as well as 3-day CpG stimulated B-cells from 5 healthy donors. While freshly isolated normal B-cells expressed little or no detectable AICDA, CpG stimulation resulted in robust induction of AICDA transcripts (Figure 1A). Additional quantitative PCR analyses showed that CpG and CD40L stimulation induced about 15-fold and 25-fold higher levels of AICDA expression, respectively (Figure 1B). These data suggest that CpG activated normal B-cells indeed express significant levels of mutagenic AICDA.
Figure 1. Analysis of CpG activated normal or malignant B-cells.
(A) Conventional PCR analysis of AICDA expression in freshly isolated or 3-day CpG stimulated normal B-cells. (B) Quantitative measurement of AICDA transcripts in 3-day CpG and CD40L stimulated normal B-cells. The final fold changes were calculated after normalization with the ACTB control gene, and unstimulated fresh normal B-cells as control samples. (C) Representative (n=3) immunofluorescence images of γ-H2AX staining for DNA damage foci as marked by yellow arrowheads. (D) Conventional PCR analysis of AICDA expression in freshly isolated or 3-day CpG stimulated CLL B-cells.
Finally, we investigated whether CpG or CD40L-stimulated B-cells exhibit evidence of double stranded DNA breaks (DSBs), known intermediates of chromosomal abnormalities (Ramiro et al, 2006). To that end, we evaluated CpG and CD40L-stimulated B-cells for phospho-histone 2AX (γH2AX) foci, the hallmark of DSBs. As presented in Figure 1C, although freshly isolated B-cells did not display intranuclear γH2AX foci, CD40L-activated B-cells exhibited a number of large and coarse γH2AX foci and CpG-stimulated B-cells exhibited a number of γH2AX foci, albeit more diffuse in appearance. Therefore, B-cells activated in vitro with CpG as well as CD40L do indeed show evidence of DNA damage, in addition to the induction of the DNA mutating enzyme AICDA.
These observations are consistent with the explanation that CpG activation has the potential to induce genetic instability in B-cells through the mutagenic activity of AICDA. Recently, in a separate study, we found that the activation of B-cells with CpG or CD40L induced a robust induction of AICDA expression, echoing the data presented in this study. More importantly, while AICDA expression is induced, CpG stimulation does not induce expression of various DNA repair activities that we believe are necessary to offset AICDA mutagenic activity (unpublished observations).
Given that CpG stimulation induces robust expression of AICDA and nonclonal cytogenetic abnormalities in vitro, it is reasonable to question what would happen to these B-cells if they were activated in vivo when CpG is used as a therapeutic agent. Similarly, what is the role of natural bacterial CpG in the development and progression of B-cell malignancies? Indeed, a recent report showing that CpG could induce aberrant class switch recombination in mature or immature B-cells is consistent with our findings (Edry et al., 2008). Although most CpG-induced chromosomal abnormalities may be incompatible with cell survival, it is possible that some rarely occurring abnormalities could indeed be pathogenic and play a role in lymphomagenesis. Thus, CpG activation of malignant B-cells could also play a role in clonal evolution and further progression of B-cell malignancies such as CLL. Therefore, our study suggests the need for more in-depth studies to address these important issues.
ACKNOWLEDGEMENTS
This work was supported by grant from the National Institutes of Health (R01CA136591 and P01CA062242 awarded to DFJ; and R01CA95241 awarded to NEK).
Footnotes
AUTHORSHIP CONTRIBUTIONS
XW, DFJ, NEK, GSN, SAS and DLVD designed the research; XW, MAP, SAS, DKW and BKA performed the experiments and analysed the data; and XW and DFJ wrote the paper. All authors reviewed and approved the manuscript.
CONFLICTS OF INTEREST DISCLOSURE
The authors declare no conflicts of interest in connection to this work.
REFERENCES
- Dicker F, Schnittger S, Haferlach T, Kern W, Schoch C. Immunostimulatory oligonucleotide-induced metaphase cytogenetics detect chromosomal aberrations in 80% of CLL patients: A study of 132 CLL cases with correlation to FISH, IgVH status, and CD38 expression. Blood. 2006;108:3152–3160. doi: 10.1182/blood-2006-02-005322. [DOI] [PubMed] [Google Scholar]
- Edry E, Azulay-Debby H, Melamed D. TOLL-like receptor ligands stimulate aberrant class switch recombination in early B cell precursors. International Immunology. 2008;20:1575–1585. doi: 10.1093/intimm/dxn117. [DOI] [PubMed] [Google Scholar]
- Fink SR, Paternoster SF, Smoley SA, Flynn HC, Geyer SM, Shanafelt TD, Lee YK, Jelinek DF, Kay NE, Dewald GW. Fluorescent-labeled DNA probes applied to novel biological aspects of B-cell chronic lymphocytic leukemia. Leukemia Research. 2005;29:253–262. doi: 10.1016/j.leukres.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Haferlach C, Dicker F, Schnittger S, Kern W, Haferlach T. Comprehensive genetic characterization of CLL: a study on 506 cases analysed with chromosome banding analysis, interphase FISH, IgV(H) status and immunophenotyping. Leukemia. 2007;21:2442–2451. doi: 10.1038/sj.leu.2404935. [DOI] [PubMed] [Google Scholar]
- Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004;4:249–258. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- Mayr C, Speicher MR, Kofler DM, Buhmann R, Strehl J, Busch R, Hallek M, Wendtner CM. Chromosomal translocations are associated with poor prognosis in chronic lymphocytic leukemia. Blood. 2006;107:742–751. doi: 10.1182/blood-2005-05-2093. [DOI] [PubMed] [Google Scholar]
- Put N, Konings P, Rack K, Jamar M, Van Roy N, Libouton JM, Vannuffel P, Sartenaer D, Ameye G, Speleman F, Herens C, Poirel HA, Moreau Y, Hagemeijer A, Vandenberghe P, Michaux L. Improved detection of chromosomal abnormalities in chronic lymphocytic leukemia by conventional cytogenetics using CpG oligonucleotide and interleukin-2 stimulation: A Belgian multicentric study. Genes, Chromosomes & Cancer. 2009;48:843–853. doi: 10.1002/gcc.20691. [DOI] [PubMed] [Google Scholar]
- Ramiro AR, Jankovic M, Callen E, Difilippantonio S, Chen HT, McBride KM, Eisenreich TR, Chen J, Dickins RA, Lowe SW, Nussenzweig A, Nussenzweig MC. Role of genomic instability and p53 in AID-induced c-myc-Igh translocations. Nature. 2006;440:105–109. doi: 10.1038/nature04495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbiani DF, Bunting S, Feldhahn N, Bothmer A, Camps J, Deroubaix S, McBride KM, Klein IA, Stone G, Eisenreich TR, Ried T, Nussenzweig A, Nussenzweig MC. AID produces DNA double-strand breaks in non-Ig genes and mature B cell lymphomas with reciprocal chromosome translocations. Mol Cell. 2009;36:631–641. doi: 10.1016/j.molcel.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulland S, Navarro JM, Grenot P, Milili M, Agopian J, Montpellier B, Gauduchon P, Lebailly P, Schiff C, Nadel B. Follicular lymphoma-like B cells in healthy individuals: a novel intermediate step in early lymphomagenesis. The Journal of Experimental Medicine. 2006;203:2425–2431. doi: 10.1084/jem.20061292. [DOI] [PMC free article] [PubMed] [Google Scholar]