Abstract
Summary
The French Hemovigilance Network has been established in 1994 and records all adverse events associated with the transfusion of a labile blood products (LBP) regardless of their severity. From 1994 to 2006 35,423,172 LBP were issued, 85,812 adverse transfusion reactions notified, and 139 cases of transfusion related acute lung injury (TRALI) observed. The LBP most at risk is fresh frozen plasma (FFP), followed by platelets concentrates (PC) and packed red cells (PRC). However, because the use of FFP is not frequent in France, it only accounts for about 10% of TRALI, whereas PRC and PC are involved in the remaining cases. In no case, pooled FFP treated with solvent-detergent were involved. Patients’ profiles are peculiar with a high disease burden. Therefore, targeting a prevention policy only on FFP would result in a marginal reduction of TRALI in France.
Key Words: TRALI, Hemovigilance, Blood transfusion, Sickle cell disease, Public health
Abstract
Zusammenfassung
Das seit 1994 bestehende French Hemovigilance Network erfasst alle mit der Transfusion von labilen Blutprodukten (LBP) in Verbindung stehenden Nebenwirkungen unabhängig von ihrer Schwere. Von 1994 bis 2006 wurden 35 423 172 LBP ausgegeben, 85 812 negative Transfusionsreaktionen angezeigt und 139 Fälle von transfusionsassoziierter akuter Lungeninsuffizienz (TRALI) beobachtet. Das LBP mit dem höchsten Risiko ist frischgefrorenes Plasma (FFP), gefolgt von Thrombozytenkonzentraten (PC) und Erythrozytenkonzenrtaten (PRC). Da aber FFP in Frankreich nur selten verwendet werden, sind nur 10% der Fälle mit TRALI mit ihnen in Verbindung zu bringen, während PRC- und PC-Transfusionen bei den restliche Fällen eine Rolle spielen. Gepooltes FFP, das mit Solvent/Detergent behandelt wurde, war in keinen der Fälle involviert. Die Patientenprofile waren durch ihre hohe Krankheitslast auffällig. In Frankreich würde daher eine Präventionsstrategie, die lediglich auf die Reduzierung des Einsatzes von FFP abzielt, nur zu einer geringen Reduzierung der TRALI-Inzidenz führen.
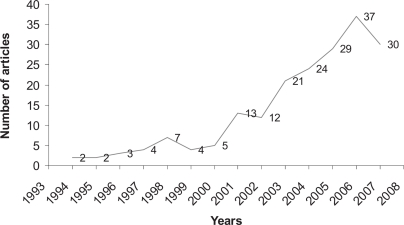
Transfusion related acute lung injury (TRALI) is reported with increasing incidence worldwide. It is interesting to consider the main historical steps: first report in 1951 [1], first pathology data in 1966 [2], first etiologic hypothesis (i.e. leukoagglutinins to HLA and non-HLA antigens) in 1970 [3], first recognition as a distinct clinical entity in 1985 [4], first alternative to immune etiology in 1997 [5], first consensus conference on TRALI in 2004 [6, 7]. To date, there is a clearly growing scientific interest in TRALI (fig 1).
Fig. 1.
Time trends of publications indexed by PubMed. The research was performed on October 16, 2007 with the key word ‘TRALI’.
The French historical milestones are more recent: first French case described in 1979 in the dissertation of Mercuroni [8], individualization as a distinct transfusion reaction in the French Transfusion Incident Report (TIR) form in 2001, first report of the French Hemovigilance Network in 2004 highlighting a peculiar patient profile [9, 10], guidelines for diagnostic and investigation in 2006 [11], most recent report in 2007 [12].
Herein, we present how hemovigilance – the epidemiologic surveillance system of the transfusion chain – can be used to provide information on TRALI. We will focus on three problems: the estimation of incidence rates, the description of the labile blood products (LBP) involved, and the description of patients.
The French Hemovigilance Network
The French Hemovigilance Network has been established in 1994. Briefly, it consists of local, regional and national network levels, where all transfusion reactions are considered regardless of their severity. The Hemovigilance Office is the cornerstone of the network and takes charge of investigations and of the electronic notification via a website called e-FIT (FIT stands for Fiche d'Incident Transfusionnel or TIR).
Data Collection
All adverse transfusion reactions that occurred between January 7, 1994 and December 31, 2006 and possibly, probably or definitely associated with transfusion are considered. The item TRALI is explicitly included since September 1, 2001 and is used for the case selection in e-FIT. For previous incidents, we screened free comments on report forms that mentioned pulmonary edema and fluid overload to be excluded, or comments including TRALI or anti-granulocyte antibodies or non-cardiogenic pulmonary edema or white lung syndrome.
There were 35,423,172 labile blood products (LBP) issued. In the same time, 85,812 adverse transfusion reactions (ATR) were notified. The ATR rate is therefore 4.4/1,000. 139 ATR fulfil the above mentioned criteria, including 115 cases with ‘TRALI’ as the final diagnosis and 24 cases which were observed prior to the introduction of TRALI as a specific item in the TIR form.
Incidence Rate
From 1994 to 2005, there is a dramatic increase of TRALI notifications, especially since 2002. This is likely due to the extensive information of clinicians by the French Hemovigilance Network. Then the number of TRALI notified reaches a plateau. Since 2005, about 35 TRALI cases are notified each year. For the 2005–2006 period, 64 cases of TRALI occurred and 5,215,892 LBP were issued, resulting in a TRALI incidence rate of 1/81,000. Thus, France can be considered to belong to the low-incidence rate areas for TRALI.
Blood Products Involved
In the 1994–2004 period, fresh frozen plasma (FFP), the classical main LBP at risk, only accounts for about 10% of TRALI cases. About 47% of TRALI cases were related to packed red cells (PRC), 40% to apheresis platelets concentrates (APC) and 1% to pooled platelets concentrates (PPC). In no case, pooled FFP (100 donations) treated with solvent-detergent (FFP S/D) were involved.
In the 2005–2006 period where notifications reach a plateau, the distribution of the LBP involved is quite identical. The incidence rate per type of LBP involved is about 1/110,000 for PRC, 1/15,000 for APC, 1/15,000 for PPC and 1/60,000 for FFP.
Patients’ Profiles
Patients’ ages varies from neonates to 90 years, with identical sex distribution.
e-FIT allows to consider severity because both severe and benign adverse transfusion reactions are included, unlike other hemovigilance systems where only deaths or serious events are considered. Three quarters of patient have a life-threatening TRALI and about 10% died. However, about 15% had a mild form of TRALI who did not require a transfer in an intensive care unit.
Half of the patients had a malignant blood disease. 3 patients had a sickle-cell disease – an interesting aspect because sickle-cell disease is rare in France. One third of the TRALI cases occurred in surgery wards.
In order to render more precisely the disease burden, we also examined the concomitant circumstances of the transfusion. More than two thirds of the patients were transfused either in the operating room or in an intensive care unit (including oncology/hematology wards). In either of them continuous surveillance is required, indicating that most patients who developed TRALI were seriously ill.
Discussion
Patients’ demographic characteristics of the French Hemovigilance Network database are nearly the same as in other series [13].
Exact data will be presented in a forthcoming article. Incidence rates are based on TRALI cases notified and on LBP issued. Thus, these rates are probably underrated. However, for a public health purposes the rates give valuable information because i) both the TRALI cases notified and the LBP issued are stable for the 2005–2006 period and ii) a differential notification bias among the LBP responsible is unlikely. Descriptive epidemiologic methods applied to hemovigilance will be discussed in a forthcoming review article on hemo-vigilance in France.
FFP remains the LBP most at risk, followed by PPC. One may consider that the more plasma a LBP contains, the more increased is the risk of TRALI. In no case FFP S/D was responsible for the development of TRALI., To our knowledge there is no reported case of TRALI related to FFP SD. This may be interpreted as the effect of the dilution of the responsible factor regardless of its nature, immune or non-immune. However, we did not observe any incidence differences between APC and PPC, a situation that may be interpreted as follows: i) because TRALI is a rare event, we did not obtain enough TRALI cases to observe a significant difference; ii) the dilution effect is not large enough to prevent TRALI (PPCs result from the pooling of on average 5 buffy coats of whole blood donations). We hypothesize that the use of pooled FFP S/D could be an appropriate way to lower the TRALI cases due to FFP, which is easier to apply on a nationwide basis than the selection of male donors.
PRCs are the LBP less at risk. However, because they are transfused most frequently, they are involved in one third of TRALI cases [14]. These results are in the same range as those recently presented for Canada [13]. We therefore conclude that, at least in France, targeting a prevention policy only on FFP would result in only a marginal reduction of TRALI.
Patients’ profiles are peculiar with a high disease burden. However, we cannot exclude a recruitment bias. A nested case-control study among e-FIT will allow to test this hypothesis. Another puzzling point is the high proportion of patients with malignant blood diseases, in whom polymorphonuclear counts are lowered by chemotherapies or in whom the blood functions are deficient due to myelodysplasia. On the other hand, such patients often have pulmonary infections that might be the first event in a two events model.
Conclusion
TRALI determinants appear to be multiple and not fully understood in e-FIT, even though it is clear that the French hemovigilance data fit well with a 2-hit model.
In the field of epidemiology, we can compare TRALI with hypercoagulable states for which the diagram of a balance is often used. Transfusion of a blood product is a risk factor, but the patient has protections against TRALI. There are two ways to tip the scales: a blood product at risk and a patient at risk. The combination of these two factors in different proportions could explain the range of reactions between no TRALI and fatal TRALI. It could also explain why the same donor can be dangerous for some patients [15] but not for others [16].
References
- 1.Barnard RD. Indiscriminate transfusion: a critique of case reports illustrating hypersensitivity reactions. N Y State J Med. 1951;51:2399–2402. [PubMed] [Google Scholar]
- 2.Philipps E, Fleischner FG. Pulmonary oedema in the course of a blood transfusion without overloading the circulation. Dis Chest. 1966;50:619–623. doi: 10.1378/chest.50.6.619. [DOI] [PubMed] [Google Scholar]
- 3.Ward HN. Pulmonary infiltrates associated with leukoagglutinin transfusion reactions. Ann Intern Med. 1970;73:689–694. doi: 10.7326/0003-4819-73-5-689. [DOI] [PubMed] [Google Scholar]
- 4.Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion. 1985;25:573–577. doi: 10.1046/j.1537-2995.1985.25686071434.x. [DOI] [PubMed] [Google Scholar]
- 5.Silliman CC, Paterson AJ, Dickey WO, Stroneck DF, Popovsky MA, Caldwell SA, Ambruso DR. The association of biologically active lipids with the development of transfusion-related acute lung injury: a retrospective study. Transfusion. 1987;37:719–726. doi: 10.1046/j.1537-2995.1997.37797369448.x. [DOI] [PubMed] [Google Scholar]
- 6.Goldman M, Webert K, Arnold D, Freedman J, Hannon J, Blajchman M. Proceedings of a consensus conference towards an understanding of TRALI. Transfus Med Rev. 2005;19:2–31. doi: 10.1016/j.tmrv.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Kleinman S, Caulfield T, Chan P, Davenport R, McFarland J, McPhedran S, Meade M, Morrison D, Pinsent T, Robillard P, Slinger P. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004;44:1774–1789. doi: 10.1111/j.0041-1132.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- 8.Dimercurio JP: Premier cas d'oedème pulmonaire lésionnel au cours d'une plasmaphérèse; contribution à l'amélioration de cette technique. Thèse Med Paris VI Université René Descartes, 1979.
- 9.Bulletin d'Hémovigilance n°8 – Janvier/Février 2004 http://afssaps.sante.fr/pdf/5/hemo8.pdf
- 10.Renaudier P, Vo Mai MP, Azanowsky JM, et al. Epidemiology of transfusion related acute lung injury in Gifit, the French Hemovigilance database: a study of the French Hemovigilance Network. Transfusion. 2004;44:23. [Google Scholar]
- 11.www.afssaps.sante.fr
- 12.Renaudier P, Vo Mai MP, Ounnoughene N, et al. Epidemiology of transfusion related acute lung injury in e-FIT, the French Hemovigilance database: 1994 to 2006. A study of the French Hemovigilance Network. Transfusion. 2007;47(suppl 35):7A. [Google Scholar]
- 13.Robillard P, Hyson C, McCombie N. TRALI, possible TRALI and respiratory complications of transfusions reported to the Canadian Transfusion Transmitted Injuries Surveillance System. Transfusion. 2007;47(suppl 35):5A. [Google Scholar]
- 14.Odent-Malaure H, Quainon F, Ruyer-Dumontier P, Ducroz S, Verdier P, Voitellier E, Raynal MF, Fromont P, Muller JY, Rebibo D, Absi L, Garraud O. Transfusion related acute lung injury (TRALI) caused by red blood cell transfusion involving residual plasma anti-HLA antibodies: a report on two cases and general considerations. Clin Dev Immunol. 2005;4:243–248. doi: 10.1080/17402520500307926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopko PM, Marshall CS, Mackenzie MR, Holland PV, Popovsky MA. Transfusion related acute lung injury: report of a clinical look-back investigation. JAMA. 2002;287:1968–1971. doi: 10.1001/jama.287.15.1968. [DOI] [PubMed] [Google Scholar]
- 16.Toy P, Hollis-Perry K, Jun J, Nakagawa M. Recipients of blood from a donor with multiple HLA antibodies. A look back study of transfusion-related acute lung injury. Transfusion. 2004;44:1683–1688. doi: 10.1111/j.0041-1132.2004.04193.x. [DOI] [PubMed] [Google Scholar]