Abstract
Summary
This article describes the TRALI reduction policies which were introduced by the National Blood Service in late 2003. Consideration was given to the reasons for their introduction and how the changes were implemented. The observed effects which followed the introduction of these policies were examined by analysis of reports to the Serious Hazards of Transfusion (SHOT) Scheme.
Key Words: Transfusion-related acute lung injury, Transfusion, Leucocyte antibodies, HLA antibodies, TRALI
Abstract
Zusammenfassung
Der vorliegende Artikel beschreibt die Verfahrensweisen zur Reduktion von TRALI, die Ende des Jahres 2004 durch den National Blood Service eingeführt wurden. Die Gründe für deren Umsetzung und in welcher Form diese Veränderungen durchgeführt wurden, werden dargestellt. Die beobachteten Effekte im Anschluss an die Einführung dieser Verfahrensweisen wurden anhand einer Analyse der Berichte an das Serious-Hazards-of-Transfusion(SHOT)-Programm untersucht.
Serious Hazards of Transfusion
This UK Haemovigilance Scheme was established in 1996 and has included TRALI as an adverse event category since reporting began. The case definition of TRALI has been acute dyspnoea with hypoxia and bilateral pulmonary infiltrates occurring during or in the 24 h after transfusion with no other apparent cause. This definition remained unchanged until 2006 when the timing was changed from ‘during or in the 24 h after transfusion’ to ‘during or in 6 h after transfusion’. All reported cases which met the Serious Hazards of Transfusion (SHOT) TRALI definition are included in annual reports. Additionally the likelihood of TRALI in each case has been graded according to clinical factors and the serological results of investigations for leucocyte antibodies.
The National Blood Service TRALI Risk Reduction Project
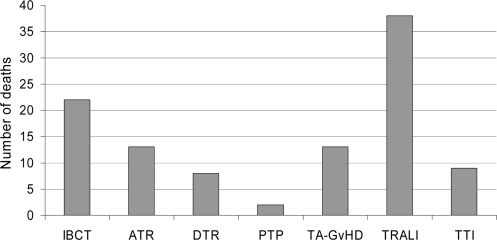
This initiative was stimulated by National Blood Service (NBS) and user concerns based on increasing numbers of reports of TRALI to SHOT. An NBS TRALI risk reduction project group was set up in March 2003 to assess whether the risk of this very serious adverse event could be reduced. At that time TRALI was the leading cause of death at least possibly attributable to transfusion. More recent cumulative data have confirmed this. Between 1996 and 2005 inclusive, a total of 3,239 reports were made covering all categories of adverse events; during this time 30 million components were issued in the UK. TRALI reports represented only a relatively small proportion of the total (5.7%), with the largest proportion of reports (71.5%) relating to incorrect blood component transfused (IBCT). When we look at deaths, at least possibly due to transfusion, in this period of time we observe that, although TRALI reports represented a small proportion of the total, it was associated with the highest number of deaths (38). The second leading cause of death was IBCT with 22 deaths in this category (fig 1).
Fig. 1.
Deaths reported to SHOT as at least possibly due to transfusion 1996–2005 analysed according to category of adverse event.
IBCT = Incorrect blood component transfused; ATR = acute transfusion reaction; DTR = delayed transfusion reaction; PTP = post transfusion purpura, TA GvHD = transfusion associated graft versus host disease; TTI = transfusion transmitted infection.
The project group also considered data provided from SHOT by Dr Lorna Williamson on the risk of TRALI according to implicated component and looked at the characteristics of the implicated donors associated with these cases.
Risk
The risk of specific components being implicated in reported TRALI cases had been analysed per total number of each component issued in the UK during the period 1996–2002 (table 1). It was observed that the risk of a component being implicated in a TRALI case reported to SHOT was 5.7 times higher for components containing relatively high quantities of plasma (250–300 ml) compared with those containing lower plasma volumes (<30 ml). The analysed components with higher plasma content comprised fresh frozen plasma (FFP) and platelets; those with lower plasma content included cryoprecipitate and red cell concentrates. It was concluded that TRALI risk was higher following transfusion of FFP or platelets than of red cell concentrates or cryoprecipitate.
Table 1.
Components implicated in TRALI cases reported to SHOT. Risk of each component calculated according to number of relevant components issued in the UK 1996–2002 (n = 103)
| TRALI cases reported to SHOT | Incidence | |
|---|---|---|
| High plasma (250–300 ml) | ||
| FFP/CSP | 31/2.3 million | 1:74,000 |
| Platelets | 17/1.5 million | 1:88,000 |
| Low plasma (<30 ml) | ||
| Cryoppt | 1/0.5 million | 1:500,000 |
| Red cells | 28/15.6 million | 1:557,000 |
CSP = Cryosupernatant plasma, Cryoppt = cryoprecipitate.
Which Donors Were Implicated?
All donors found to have leucocyte antibodies recognising cognate recipient antigens in SHOT TRALI cases have been female. More recent analysis of SHOT reports between 2000 and 2005 found such concordant donor leucocyte antibodies in 59 of 89 (66%) complete donor investigations. The HLA antibody specificities which were most frequently identified in donors in cases with concordant antibody were HLA-DR4,-DR52 and-A2. Concordant granulocyte antibodies were less frequently identified in TRALI cases, and HNA-1a was the most common specificity identified.
Assumptions for TRALI Risk Reduction
The assumptions which were made by the project group based on donor and component risk were as follows:
-
–
Donor HLA and HNA antibodies are important causes of TRALI.
-
–
Highest risk components are FFP and platelets.
-
–
Highest risk donors are previously pregnant women.
-
–
Other possible causes of TRALI were not addressed by the project.
Approaches to Risk Reduction
Based on these assumptions, different approaches to risk reduction were considered by the group. Exclusion of female donors with a history of pregnancy from production of higher risk components was discussed. This would have entailed asking additional questions of donors and changes to the computer system. It was decided that an additional question for female donors relating to pregnancies during the health checking process at sessions was undesirable because additional questions had already been added for other reasons, and feedback from donor focus groups had informed the NBS that many donors considered the health check was too long already. The group concluded that a more practical and acceptable alternative approach would be to use male plasma as far as possible for FFP production and for the plasma contribution to buffy coat derived platelet pools.
Feasibility of Preferential Use of Male Plasma
This new policy was first introduced in July 2003 at a single pilot site in Newcastle Blood Centre. It was found feasible to exclude female plasma from over 95% of FFP and subsequently a similar percentage of platelet pools. This policy was then extended to the whole of the NBS in October 2003.
An additional donor exclusion was added for other reasons in April 2004 as a result of vCJD in the UK and the risk of transfusion transmission of this disease. This excluded any donor who had been transfused since 1980. This will have reduced further the number of donors who have been potentially immunised against white cells.
Implementation
The practicalities of this risk reduction measure were simple. At blood donor sessions all donations from males were marked with an ‘M’ and all female donations were marked ‘F’ by hand on the top of the blood pack label. When these donations were received at the Blood Centre for processing, the donations marked ‘M’ were used preferentially for the production of FFP or for providing the plasma contribution to buffy coat derived platelet pools. Plasma from the donations marked ‘F’ was discarded; UK plasma is not fractionated because of vCJD risk.
Previously issued female FPP was not recalled from hospital stocks and at that time had a 1-year expiry. This means that in 2004 the proportion of female FFP would have been higher than in subsequent years. Recent NBS data showed that in the first financial quarter of 2007 over 90% of FFP and plasma contributions to platelet pools were derived from male donations. It has not been possible for the NBS to achieve 100% male FFP or plasma for platelet pools because of current UK requirements which make it necessary to manufacture FFP and buffy coats on the same day that the blood is collected. A change to an overnight hold would allow the NBS to achieve 100% FFP and plasma for platelet pooling.
Impact of Deferring Donors Transfused since 1980
This donor exclusion criterion has caused the loss of 82,911 (5.5%) of NBS donors to date. As in other parts of the world, the NBS has seen a gradual but steady decline in donor numbers over the last 5 years to which this new exclusion has clearly contributed.
What Were the Effects?
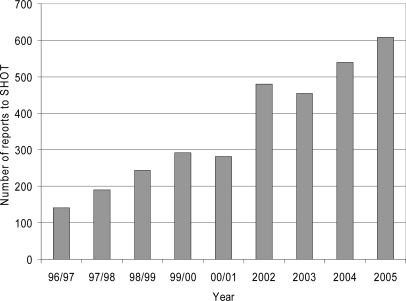
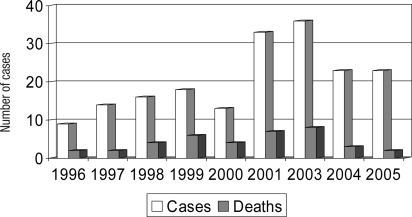
SHOT data were analysed to assess whether there have been changes in reports of TRALI since the introduction of the policy of preferential use of male plasma (table 2). The annual number of TRALI cases reported to SHOT decreased from 36 in 2003 to 23 in 2004 and 23 in 2005. In the same period cases which were assessed as highly likely or probably to be TRALI reduced from 22 in 2003 to 6 in 2005. This decrease in TRALI reports occurred at a time when the total number of reports of all adverse events analysed by SHOT was steadily increasing each year from 457 in 2003 to 609 in 2005 (fig 2). This general increase in reporting to SHOT followed increasing awareness of SHOT through annual reports and an emphasis being placed on haemovigilance in UK hospitals by the Department of Health.
Table 2.
Change in TRALI profile since 2003
| TRALI | Reporting year |
||
|---|---|---|---|
| 2003 | 2004 | 2005 | |
| Number of cases analysed | 36 | 23 | 23 |
| Highly likely | 20 | 10 | 3 |
| Probable | 2 | 3 | 3 |
| Possible | 6 | 4 | 3 |
| Unlikely | 8 | 6 | 14 |
Fig. 2.
Annual numbers of reports to SHOT, including all categories of adverse events 1996-2005.
The number of reported deaths at least possibly due to TRALI decreased from 8 in 2003 to 3 in 2004 and 2 in 2005 (fig 3).
Fig. 3.
Annual numbers of TRALI cases and deaths at least possibly due to TRALI 1996–2005 (n = 185).
Implicated Components in Donors
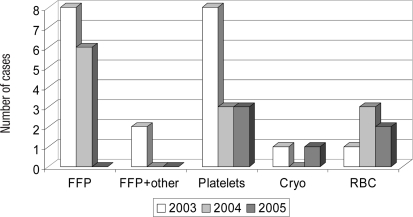
TRALI cases proven to involve a donor with leucocyte antibodies with specificity for patient antigen have been analysed according to the implicated transfused component in 2003, 2004 and 2005. Ten such cases relating to FFP transfusion were seen in 2003, whereas none was seen in 2005. Platelet related cases showed a decrease from 8 to 3. Cases related to red cells have, in contrast, shown only little change (fig 4).
Fig. 4.
TRALI cases with a donor with a leucocyte antibody, positive by cross-match or specific for cognate patient antigen, analysed by implicated component.
Overall Findings
Following the introduction of the TRALI risk reduction policies in 2003, SHOT data to the end of 2005 had shown:
-
–
fewer total reports of suspected TRALI,
-
–
fewer deaths at least possibly due to TRALI,
-
–
a disappearance of TRALI cases due to FFP with a donor with concordant antibody,
-
–
a reduction in TRALI cases due to platelets with a donor with concordant antibody.
Preliminary 2006 TRALI SHOT data have also been consistent with these observations.
Further Risk Reduction?
Additional measures to reduce risk further have also been considered. Attention has been given to platelets obtained by apheresis. These are currently obtained from both male and female donors but there has been a policy of preferential male recruitment since 2006, and currently approximately 75% of donations are obtained from male donors. A feasibility project is planned for screening apheresis donors for HLA and possibly HNA antibodies. The NBS is also considering whether it would be possible to allow overnight holds for plasma to allow more flexibility in use of male donors for frozen plasma and plasma for buffy coat derived platelet pools. Another approach which is in development is replacement of platelet plasma by platelet additive solution.
In conclusion, the NBS TRALI Risk Reduction Project has been associated with an observed reduction in TRALI cases and deaths reported to SHOT. Further work is in progress to minimise the occurrence of this serious adverse event.
Acknowledgements
Hospital staff for reporting cases to SHOT; members of the SHOT team and in particular Dorothy Stainsby, Lorna Williamson, Hilary Jones, Hannah Cohen; colleagues involved in individual case assessments: Edwin Massey, Nay Winn, Sheila MacLennan, Monty Mythen, Cliff Morgan and Neil Soni and the NBS Risk Reduction Project Group members: Michelle Ashford, Gordon Nicholson, Lindsey Lewis and Neil Beckman.